Abstract
Background
Older age and comorbid burden are both associated with adverse outcomes in SARS-CoV-2, but it is not known whether the association between comorbid burden and adverse outcomes differs in older and younger adults.
Objective
To compare the relationship between comorbid burden and adverse outcomes in adults with SARS-CoV-2 of different ages (18–64, 65–79 and ≥ 80 years).
Design, setting, and participants
Observational longitudinal cohort study of 170,528 patients who tested positive for SARS-CoV-2 in the US Department of Veterans Affairs (VA) Health Care System between 2/28/20 and 12/31/2020 who were followed through 01/31/2021.
Measurements
Charlson Comorbidity Index (CCI); Incidence of hospitalization, intensive care unit (ICU) admission, mechanical ventilation, and death within 30 days of a positive SARS-CoV-2 test.
Results
The cumulative 30-day incidence of death was 0.8% in cohort members < 65 years, 7.1% in those aged 65–79 years and 20.6% in those aged ≥80 years. The respective 30-day incidences of hospitalization were 8.2, 21.7 and 29.5%, of ICU admission were 2.7, 8.6, and 11% and of mechanical ventilation were 1, 3.9 and 3.2%. Median CCI (interquartile range) ranged from 0.0 (0.0, 2.0) in the youngest, to 4 (2.0, 7.0) in the oldest age group. The adjusted association of CCI with all outcomes was attenuated at older ages such that the threshold level of CCI above which the risk for each outcome exceeded the reference group (1st quartile) was lower in younger than in older cohort members (p < 0.001 for all age group interactions).
Limitations
The CCI is calculated based on diagnostic codes, which may not provide an accurate assessment of comorbid burden.
Conclusions
Age differences in the distribution and prognostic significance of overall comorbid burden could inform clinical management, vaccination prioritization and population health during the pandemic and argue for more work to understand the role of age and comorbidity in shaping the care of hospitalized patients with SARS-CoV-2.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-021-02340-5.
Introduction
Older age is the strongest risk factor for infection with the severe acute respiratory virus syndrome coronavirus-2 (SARS-CoV-2) and older adults have been especially hard-hit during the pandemic [1, 2]. Older adults with SARS-CoV-2 are at increased risk for hospitalization [3–5], critical illness [4, 6–13], prolonged hospitalization [11, 14] and mortality [8, 9, 14–29] compared with their younger counterparts. Because a disproportionate number of hospitalizations and deaths in patients with coronavirus disease 2019 (COVID-19) occur among older adults [30–32], the burden of SARS-CoV-2 has been greatest in countries [33–36] and communities [37] with older populations, and in healthcare facilities [38–40] and health systems serving older adults [41].
However, the prevalence of most comorbid conditions increases with age, which can make it challenging to disentangle the separate effects of age and comorbid burden on outcomes among patients infected with SARS-CoV-2. Prior studies have reported an association between Charlson comorbidity score and other measures of comorbid burden with adverse outcomes in SARS-CoV-2 after adjustment for age [41–49] and differences across age groups in clinical presentation and outcomes of SARS-CoV-2 [11, 14, 27, 31, 50–53]. There are also reports of age differences in the association of individual comorbid conditions [54–56] with adverse outcomes in infected patients.
While few studies have examined the relationship between age, comorbid burden and adverse outcomes in SARS-CoV-2, available data suggest that the relationship between these factors may be complex. Among the first 11,122 infected patients in Denmark, mortality rates were noted to be extremely low in those < 80 years old without comorbid conditions but uniformly high in those ≥80 years old regardless of the number of comorbid conditions [57]. Because both age and comorbidity have figured prominently in guidelines for clinical care, vaccination and social distancing during the pandemic, a more detailed understanding of the relationship of age and comorbid burden with a range of adverse outcomes among adults infected with SARS-CoV-2 could inform clinical care, current and future vaccine prioritization and prognostication [58, 59] and efforts to estimate disease burden and service needs related to SARS-CoV-2 [33].
The US Department of Veterans Affairs (VA) supports the largest integrated national health care system in the US and provides care for more than six million veterans annually [60]. Because the VA serves both younger and older adults and many veterans have multiple comorbid conditions [61], the system may offer unique insights into the relationship between age, comorbid burden and adverse outcomes in those infected with SARS-CoV-2. We designed a study to evaluate differences across age groups in the association of comorbid burden with a range of adverse outcomes among patients infected with SARS-CoV-2.
Methods
Data source and study population
The VA uses a single comprehensive electronic healthcare information network. Data elements from patients’ electronic medical records at individual VA facilities are stored centrally in the VA’s Corporate Data Warehouse (CDW) which is maintained by the VA Informatics and Computing Infrastructure (VINCI). To support research on the health system impacts of the SARS-CoV-2 pandemic, VA maintains the VA National Surveillance Tool, which includes updated clinical and administrative data extracts for all patients tested for SARS-CoV-2 within the VA, with adjudication of all positive test results [60]. Using this resource, we assembled a cohort of all Veterans who tested positive for SARS-CoV-2 nucleic acid by polymerase chain reaction (PCR) at least once between February 28, 2020 and December 31, 2020 with complete information on test date (n = 170,528). The index date for the present study was defined as the date of each patient’s earliest positive test result unless this occurred within the first 15 days of a hospital admission, in which case we used the date of hospital admission as the index date. Follow-up for all study outcomes was available through January 31, 2021, allowing for analysis of all outcomes occurring within 30 days after the index date for all cohort members. This study was approved by the Institutional Review Board of the Veterans Affairs Puget Sound Healthcare System which granted a waiver of informed consent as the study was deemed minimal risk because it involved secondary analyses of existing data and could not otherwise have been conducted due to the large size of the cohort and inclusion deceased patients. Our research was conducted in accordance with the Declaration of Helsinki.
Exposure
The primary exposure was each patient’s Charlson Comorbidity Index (CCI) score (categorized by approximate quartile of the distribution within our cohort as 0, 1, 2–3 or ≥ 4) based on International Classification of Diseases 10th Revision (ICD-10) codes recorded in VA administrative data on or within 2 years before the index data [62].
Outcomes
The following outcomes were ascertained for the 30-day period following the index data using the VA National Surveillance Tool [60]: 1) hospitalization; 2) ICU admission; 3) mechanical ventilation; and 4) death.
Covariates
All analyses were stratified by patients’ age group on the index date (categorized as 18–64, 65–79 and ≥ 80 years) and adjusted for sex, race (Black, White, Other), Hispanic ethnicity, body mass index body (BMI) (categorized as underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2) overweight (25–29.9 kg/m2), stage 1 obesity (30–34.9 kg/m2) and stages 2 & 3 obesity (≥35 kg/m2)) and U.S. Federal Region. To account for potential age differences in outcomes within each age group, multivariate analyses were also adjusted for age as a continuous variable.
Statistical analysis
We used Pearson’s χ2 test to compare patient characteristics across age groups. We plotted 30-day cumulative rates of mortality, hospitalization, ICU admission, and mechanical ventilation by age using a lowess smoother with a bandwidth of 80%. We used a time-to-event analysis accounting for censoring at the time of death to calculate the crude cumulative 30-day incidence of hospitalization, ICU admission, mechanical ventilation, and death within each age group.
We used Cox proportional hazards models stratified by age group to measure the adjusted association of CCI with hospitalization, ICU admission, mechanical ventilation, and death within the first 30 days after the index date after adjustment for age (as a continuous variable), sex, race, Hispanic ethnicity, BMI, and region of residence. We used a two-sided p-value threshold of < 0.05 to assess statistical significance. We confirmed there were no violations of the proportional hazard assumption via visual inspection of log-log graphs. All analyses were stratified by VA medical center. A likelihood ratio test (or χ2 test of predicted hazard ratios) was used to evaluate for interaction between age group and CCI in adjusted analyses.
We conducted a supplementary analysis in which we used competing risk models to estimate the 30-day cumulative incidence of hospitalization, ICU admission and mechanical ventilation and to measure the adjusted association of CCI with each of these non-death outcomes [63]. We also repeated the primary analyses using approximate quartiles of the Elixhauser Comorbidity Index (ECI).
All analyses were conducted using Stata/MP Version 15 (StataCorp, LLC. College Station, TX).
Results
Baseline characteristics
Among the 170,528 patients with a positive test for SARS-CoV-2 during the ascertainment period, 99,483 were aged 18–64, 55,013 were aged 65–79, and 26,032 were aged ≥80 years (Table 1). From the youngest to the oldest age group, there were decreases in the percentage of women, patients of Black and Other race, patients of Hispanic ethnicity and overweight patients. The median CCI (interquartile range) ranged from 0.0 (0.0, 2.0) in the youngest to 4 (2.0, 7.0) in the oldest age group. More than half (54.6%) of patients in the youngest age group had a CCI of 0 as compared with 8.8% of those in the oldest age group, while more than half (57.5%) of those in the oldest age group had a CCI ≥ 4 as compared with 10.7% of those in the youngest age group.
Table 1.
Baseline characteristics of patients with a positive test for SARS-CoV-2 in the VA healthcare system, by age group
| Characteristic | 18-64 years N=99,483 | 65-79 years N=55,013 | ≥80 years N=26,032 | All patients N=170,528 | P value |
|---|---|---|---|---|---|
| Sex | < 0.001 | ||||
| Women, % | 26.2 | 3.8 | 2.2 | 16.7 | |
| Men, % | 73.8 | 96.2 | 97.8 | 83.3 | |
| Race | < 0.001 | ||||
| White, % | 51.7 | 72.5 | 77.2 | 60.8 | |
| Black, % | 23.4 | 18.3 | 12.8 | 20.8 | |
| Other, % | 3.3 | 2.1 | 1.7 | 2.7 | |
| Missing/Unknown | 21.5 | 7.1 | 8.3 | 15.6 | |
| Ethnicity | < 0.001 | ||||
| Non-Hispanic | 69.4 | 89.6 | 89.7 | 77.8 | |
| Hispanic | 11.9 | 6.3 | 5.3 | 9.5 | |
| Missing/Unknown | 18.7 | 4.2 | 5 | 12.7 | |
| Body mass index (kg/m2) | < 0.001 | ||||
| <18.5 (underweight), % | 0.4 | 1.2 | 2.7 | 0.9 | |
| 18.5-24.9 (normal weight), % | 9.9 | 16 | 33.4 | 14.1 | |
| 25.29.9 (overweight), % | 25.7 | 34.2 | 37.2 | 29.5 | |
| 30-34.9 (Obese I), % | 26.2 | 27.6 | 17.1 | 25.8 | |
| ≥35 (Obese II and III), % | 23.4 | 19.4 | 6.2 | 20.5 | |
| Missing, % | 14.5 | 1.6 | 3.4 | 9.3 | |
| Charlson Comorbidity Index (median (IQR)) | 0.0 (0.0,2.0) | 3.0 (2.0,6.0) | 4.0 (2.0,7.0) | 1.0 (0.0,4.0) | < 0.001 |
| Charlson Comorbidity Index (%) | < 0.001 | ||||
| 0 | 54.6 | 11.2 | 8.8 | 36.3 | |
| 1 | 18.3 | 12 | 9.5 | 15.5 | |
| 2-3 | 16.4 | 27.5 | 24.2 | 20.7 | |
| ≥4 | 10.7 | 49.3 | 57.5 | 27.6 | |
| Median (IQR) | 18-64 years | 65-79 years | ≥80 years | All patients | |
| Hospital LOS | 5 (3-9) | 7 (4-14) | 9 (5-15) | 7 (4-13) | |
| ICU (LOS) | 4 (2-8) | 5 (3-10) | 5 (2-9) | 5 (3-9) | |
Crude 30-day cumulative incidence of hospital admission, ICU admission, mechanical ventilation, and death by age group
The cumulative incidence of death increased exponentially with increasing age while the incidence of hospitalization, ICU admission, and mechanical ventilation plateaued at older ages, with rates of mechanical ventilation declining beyond the age of 80 (Fig. 1). The 30-day incidence of death increased across age groups and quartiles of CCI for all outcomes except for mechanical ventilation (Fig. 2). In all age groups, the incidence of hospitalization (Fig. 2a), ICU admission (Fig. 2b) and mechanical ventilation (Fig. 2c) were extremely high for those with a CCI in the fourth quartile. The incidence of death increased linearly with CCI quartile for those aged < 65 and 65–79 years but was extremely high in all quartiles of CCI for those aged ≥80 years (Fig. 2d). Death rates were higher for those aged ≥80 years than for any other age group regardless of CCI. The 30-day incidence of death for those aged< 65 years with a CCI in the 4th quartile was similar to that for patients aged 65–79 years with a CCI in the first quartile. Median hospital length of stay ranged from 5 (interquartile range (IQR) 3–9) in the youngest age group to 9 (IQR 5–15) in the oldest age group and median ICU length of stay was 4 (IQR 2–8) in those < 65 years, 5(IQR 3–10) for those aged 65–79 and 5 (IQR 2–9) for those aged ≥80 years.
Fig. 1.
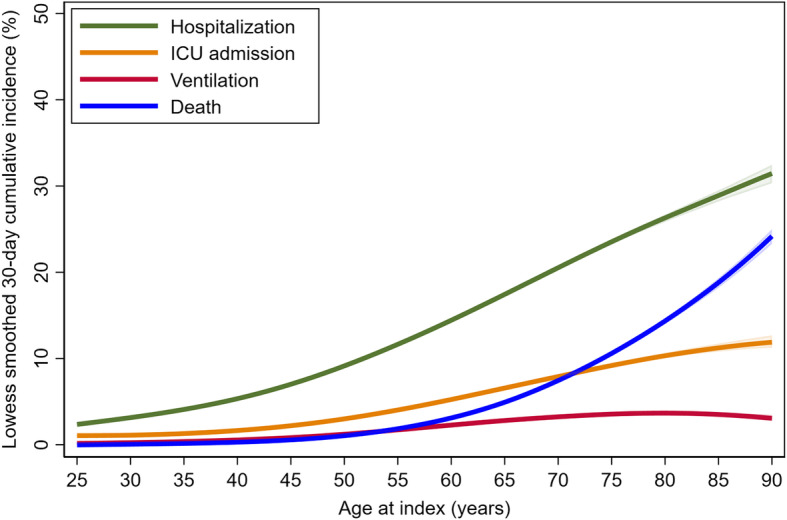
Lowess smoothed 30-day cumulative rates of hospitalization, ICU admission, mechanical ventilation and death by age at baseline
Fig. 2.

30-day cumulative incidence of hospitalization (a), ICU admission (b), mechanical ventilation (c) and death (d) by age group
Adjusted analyses of the association of CCI with hospitalization, ICU admission, mechanical ventilation and death after stratification by age group
The adjusted association of CCI with all outcomes varied systematically across age groups and was attenuated at older ages (p values for all age group interactions < 0.001) (Table 2). Among patients < 65 years, those with a CCI in the 2nd (vs. 1st) quartile (and higher) were at increased risk for hospitalization (adjusted hazard ratio (aHR) 1.43, 95% confidence interval (CI) 1.33, 1.53) and ICU admission (aHR 1.25, 95% CI 1.10, 1.42) and those with a CCI in the 3rd (vs. 1st) quartile (and higher) were at increased risk for mechanical ventilation (aHR 1.41, 95% CI 1.16, 1.70) and death (aHR 1.74, 95% CI 1.38, 2.20). Among those aged 65–79 years, risk of hospitalization was increased for those with a CCI in the 2nd (vs. 1st) quartile of CCI (aHR 1.19, 95% CI 1.07–1.31), risk of ICU admission was increased for those with a CCI in the 3rd (vs. 1st) quartile (aHR 1.33, 95% CI 1.16–1.53), risk of mechanical ventilation was increased for those with a CCI in the 4th (vs.1st) quartile (aHR 1.69, 95% CI 1.43–2.01) and risk of death was increased for those with a CCI in the 2nd (vs. 1st) quartile (aHR 1.34, 95% CI 1.12–1.60). However, among patients ≥80 years, only those with CCI in the 4th (vs.1st) quartile of CCI were at increased risk for hospitalization (aHR 1.55, 95% CI 1.36, 1.78), ICU admission (aHR 1.51, 95% CI 1.21, 1.89) and death (aHR 1.41, 95% CI 1.22, 1.64). Risk for mechanical ventilation did not vary substantially across quartiles of CCI in this oldest age group and was lowest for those with a CCI in the 2nd quartile (aHR 0.55, 95% CI 0.33, 0.93 (Table 2).
Table 2.
Adjusted association of Charlson Comorbidity Index with hospitalization, ICU admission, mechanical ventilation and death, stratified by age group
| 18-64 years N=99,483 | 65-79 years N=55,013 | ≥80 years N=26,032 | All patients N=170,528 | ||
|---|---|---|---|---|---|
| Adjustedab hazard ratio (95% confidence intervals) | Adjustedab hazard ratio (95% confidence intervals) | Adjustedab hazard ratio (95% confidence intervals) | Adjustedab hazard ratio (95% confidence intervals) | P for interaction with age group | |
| Hospitalization | |||||
| Number of hospitalizations | 8,110 | 11,907 | 4,676 | 24,693 | |
| 30-day cumulative incidence of hospitalization (per 100 patients) | 8.2 | 21.7 | 29.5 | 14.5 | < 0.001*** |
| Median length of stay (IQR), days | 4 (2-8) | 6 (3-13) | 8 (4-15) | 6 (3-12) | |
| Charlson Comorbidity Index | < 0.001*** | ||||
| 0 | 1 | 1 | 1 | 1 | |
| 1 | 1.40 (1.31-1.51)*** | 1.19 (1.07-1.31)** | 0.91 (0.77-1.08) | 1.37 (1.29-1.44)*** | |
| 2-3 | 1.86 (1.73-1.99)*** | 1.48 (1.36-1.62)*** | 1.01 (0.88-1.17) | 1.75 (1.66-1.84)*** | |
| ≥4 | 3.35 (3.13-3.58)*** | 2.51 (2.31-2.73)*** | 1.55 (1.36-1.78)*** | 2.91 (2.77-3.05)*** | |
| ICU admission | |||||
| Median Length of stay (IQR), days | 4 (1-7) | 4 (2-10) | 4 (2-8) | 4 (2-9) | |
| Number of ICU admissions | 2,705 | 4,709 | 1,712 | 9,126 | < 0.001*** |
| 30-day cumulative incidence of ICU admission (per 100 patients) | 2.7 | 8.6 | 11 | 5.4 | |
| Charlson Comorbidity Index | < 0.001*** | ||||
| 0 | 1 | 1 | 1 | 1 | |
| 1 | 1.25 (1.10-1.42)*** | 1.11 (0.94-1.30) | 0.92 (0.70-1.23) | 1.25 (1.14-1.37)*** | |
| 2-3 | 1.70 (1.51-1.92)*** | 1.33 (1.16-1.53)*** | 0.91 (0.71-1.16) | 1.60 (1.47-1.74)*** | |
| ≥4 | 3.43 (3.05-3.85)*** | 2.36 (2.08-2.69)*** | 1.51 (1.21-1.89)*** | 2.88 (2.66-3.12)*** | |
| Mechanical ventilation | < 0.001*** | ||||
| Number receiving mechanical ventilation | 1,017 | 2,109 | 490 | 3,616 | |
| 30-day incidence of mechanical ventilation (per 100 patients) | 1 | 3.9 | 3.2 | 2.1 | |
| Charlson Comorbidity Index | < 0.001*** | ||||
| 0 | 1 | 1 | 1 | 1 | |
| 1 | 0.91 (0.73-1.13) | 0.97 (0.78-1.20) | 0.55 (0.33-0.93)* | 1.06 (0.92-1.23) | |
| 2-3 | 1.41 (1.16-1.70)*** | 1.00 (0.83-1.21) | 0.76 (0.51-1.13) | 1.44 (1.27-1.64)*** | |
| ≥4 | 2.72 (2.26-3.26)*** | 1.69 (1.43-2.01)*** | 1.03 (0.71-1.48) | 2.43 (2.16-2.74)*** | |
| Death | < 0.001*** | ||||
| Number of deaths | 770 | 3,897 | 3,255 | 7,922 | |
| 30-day cumulative incidence of death (per 100 patients) | 0.8 | 7.1 | 20.3 | 4.6 | |
| Charlson Comorbidity Index | < 0.001*** | ||||
| 0 | 1 | 1 | 1 | 1 | |
| 1 | 0.98 (0.75-1.29) | 1.34 (1.12-1.60)** | 1.05 (0.88-1.26) | 1.29 (1.15-1.44)*** | |
| 2-3 | 1.74 (1.38-2.20)*** | 1.45(1.25-1.70)*** | 1.08 (0.92-1.27) | 1.53 (1.38-1.69)*** | |
| ≥4 | 3.26 (2.61-4.08)*** | 2.48 (2.14-2.86)*** | 1.41 (1.22-1.64)*** | 2.35 (2.14-2.59)*** | |
a Adjusted for Federal Emergency Management Agency region: 1 (Connecticut, Massachusetts, Maine, New Hampshire, Rhode Island, Vermont), 2 (New Jersey, New York, Puerto Rico), 3 (District of Columbia, Delaware, Maryland, Pennsylvania, Virginia, West Virginia), 4 (Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, Tennessee), 5 (Illinois, Indiana, Michigan, Minnesota, Ohio, Wisconsin), 6 (Arkansas, Louisiana, New Mexico, Oklahoma, Texas), 7 (Iowa, Kansas, Missouri, Nebraska), 8 (Colorado, Montana, North Dakota, South Dakota, Utah, Wyoming), 9 (Arizona, California, Guam, Hawaii, Nevada), 10 (Alaska, Idaho, Oregon, Washington) and all characteristics listed in Table 1 with the exception that age was modeled as a continuous variable.
bStratified by station.
*P<0.05, **P<0.01, ***P<0.001
Results for non-death outcomes were similar when we used a competing risk model (Additional file: Table 1). The results of a sensitivity analysis examining associations of ECI with study outcomes after stratification by age were similar to the primary analysis (Additional file: Table 2).
Discussion
Among VA patients who tested positive for SARS-CoV-2 during the first 10 months of the pandemic, there were marked differences across age groups in the prevalence and prognostic significance of comorbid burden. Associations of comorbid burden with adverse outcomes were generally attenuated at older ages leading to systematic differences across age groups in the threshold level of the CCI (and ECI) associated with an increased risk for adverse outcomes. In cohort members < 65 years and 65–79 years, risk for most outcomes was increased for patients with a CCI in the second or third quartile (CCI of 1 or 2–3, respectively) or higher. On the other hand, among those aged ≥80 years, only a CCI in the 4th quartile (CCI ≥4) was associated with an increased risk for hospitalization, ICU admission and death and risk of mechanical ventilation did not vary greatly with CCI.
The disproportionate number of deaths occurring among older adults is a distinguishing feature of the SARS-CoV-2 pandemic as compared with prior influenza pandemics [32]. The very steep age gradient in risks of death and hospitalization and strong association of comorbid burden with adverse outcomes among younger and middle aged members of our cohort generally supports the US Centers for Disease Control-recommended approach to vaccine prioritization based on age and the presence of comorbid conditions among younger and middle-aged (i.e. < 65 years) adults [64]. However, as we and others have argued elsewhere, there may be opportunities to refine ongoing vaccine prioritization strategies by accounting for the presence of sizeable differences within age groups in risk for adverse outcomes [65–67].
Consistent with the crude mortality rates reported by Reilev et al. for the first 11,122 infected patients in Denmark, risk of death was extremely high for the oldest members of our cohort regardless of CCI [57]. However, there were substantial differences in the relative risk for all adverse outcomes across quartiles of comorbid burden in younger age groups, including those aged 65–79 years. Further, rates of hospitalization, ICU admission and mechanical ventilation for patients with a CCI in the fourth quartile were extremely high regardless of age. Prioritization for vaccination and risk stratification based on comorbid burden may be especially impactful for those aged 65–79 years given that their absolute mortality and hospitalization rates substantially exceed those aged < 65 years. Collectively, these findings suggest there may be opportunities to refine ongoing vaccine allocation strategies to improve risk stratification within this relatively large high-risk group.
While prior studies have described strong associations of CCI and other measures of comorbid burden with adverse outcomes in patients infected with SARS-CoV-2 after adjusting for age [41–48], none to our knowledge have evaluated the adjusted association of comorbid burden with adverse outcomes after stratification by age group. However, our major finding of systematic differences across age groups in the strength and magnitude of the association of comorbid burden with hospitalization, death and ICU admission among patients with SARS-CoV-2 seems consistent with other work describing age differences in the association of individual comorbid conditions [54–56] with adverse outcomes among infected patients. An attenuation of the association of comorbid burden with adverse outcomes in patients with SARS-CoV-2 at older ages also resonates with earlier work demonstrating that in older adults, the presence or absence of particular comorbid conditions and/or abnormalities in specific disease markers often have less prognostic significance than more global measures of functional status and frailty that are not necessarily tied to the presence or severity of specific underlying disease processes [68–70]. Our findings may also suggest the need for alternative approaches to risk stratification within the oldest age group, perhaps based on non-disease-specific measures such as frailty and functional status (71) or different threshold levels of comorbid burden.
Consistent with earlier studies conducted in a range of different populations infected with SARS-CoV-2 [8, 9, 14–29, 71], the age gradient for crude mortality was extremely steep and mortality rates increased exponentially while slopes for non-death outcomes plateaued at older ages. Age-related increases in rates of ICU admission and mechanical ventilation among members of our cohort were much less steep than those for mortality and hospitalization. Of particular note, crude rates of mechanical ventilation peaked among patients in their late 70’s and declined at older ages and the association of CCI with mechanical ventilation was substantially attenuated among those aged 65–79 years and extinguished in the oldest age group. These findings support the possibility that clinical management of hospitalized patients infected with SARS-CoV-2 might vary depending on the patient’s age and comorbid burden [72]. Collectively, our work argues for studies to understand the impact of age and comorbidity on real-world care practices for patients infected with SARS-CoV-2.
Limitations of our study include first, that our results may not be generalizable to other populations, particularly non-veteran populations and those that include a higher percentage of women. Second, ascertainment of comorbidity was limited to information included in ICD-10 codes which may not fully capture the severity of individual comorbid conditions or comorbid disease burden. Third, we only had information on hospitalizations occurring within the VA or under VA Community Care, thus our results may underestimate rates of non-death outcomes among cohort members. Fourth, due to selective testing for SARS-CoV-2 within our health system, our results may not be generalizable to infected Veterans not tested within the VA and may be subject to bias based on age and comorbidity. Finally, our analyses are adjusted for a limited number of measured patient characteristics and may reflect confounding by unmeasured characteristics.
In conclusion, while prior studies have demonstrated strong associations between older age and comorbid burden with the severe manifestations of COVID-19, none have rigorously evaluated for age differences in the strength and magnitude of the association of comorbid burden with a range of adverse outcomes. Our finding of systematic differences across age groups in the distribution and prognostic significance of comorbid burden among patients infected with SARS-CoV-2 could help to inform clinical care, ongoing vaccine prioritization and prognostication. Age differences in outcomes for hospitalized patients with SARS-CoV-2 also argue for more work to understand the role of age and comorbid burden in shaping clinical care and decision-making.
Supplementary Information
Acknowledgements
We acknowledge the VA Office of Research and Development’s work to develop and support the VA COVID-19 National Surveillance Tool.
Authors’ contributions
All authors approved the final version of the manuscript. AO: Study concept and design (AO, GI, KB), Interpretation of results (AO, KB, GI, JS, KC, ME, JD, VF), analysis of data (KB, PG), drafting of manuscript (AO and KB), Administrative support and approvals (EL), critical revision of manuscript (AO, GI, KB, JS, KC, ME, JD, VF, PG, EL).
Funding
The study was supported by VA CSR&D grant COVID19–8900-11 to GNI.
Availability of data and materials
Our data were obtained under data use agreement and cannot be shared beyond the study team members authorized to access the data. The data sources we used are accessible to other VA researchers for approved projects: https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=3834
Declarations
Ethics approval and consent to participate
The study was approved the Institutional Review Board at the VA Puget Sound Health Care System.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pollan M, Perez-Gomez B, Pastor-Barriuso R, Oteo J, Hernan MA, Perez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell T, Bellin E, Ehrlich AR. Older adults and Covid-19: the Most vulnerable, the hardest hit. Hast Cent Rep. 2020;50(3):61–63. doi: 10.1002/hast.1136. [DOI] [PubMed] [Google Scholar]
- 3.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, Cai Y, Lu Z, Wang J, Wang Y, Liu S, Cheng B, Wang J, Zhang M, Wang L, Niu S, Yao Z, Deng X, Zhou F, Wei W, Li Q, Chen X, Chen W, Yang Q, Wu S, Fan J, Shu B, Hu Z, Wang S, Yang XP, Liu W, Miao X, Wang Z. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W, Liang H, Ou L, Chen B, Chen A, Li C, Li Y, Guan W, Sang L, Lu J, Xu Y, Chen G, Guo H, Guo J, Chen Z, Zhao Y, Li S, Zhang N, Zhong N, He J, for the China Medical Treatment Expert Group for COVID-19 Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, Demertzis Z, Hanna Z, Failla A, Dagher C, Chaudhry Z, Vahia A, Abreu Lanfranco O, Ramesh M, Zervos MJ, Alangaden G, Miller J, Brar I. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3(6):e2012270. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 10.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;17;323(11):1061–9. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- 11.Gold JAW, Wong KK, Szablewski CM, Patel PR, Rossow J, da Silva J, Natarajan P, Morris SB, Fanfair RN, Rogers-Brown J, Bruce BB, Browning SD, Hernandez-Romieu AC, Furukawa NW, Kang M, Evans ME, Oosmanally N, Tobin-D’Angelo M, Drenzek C, Murphy DJ, Hollberg J, Blum JM, Jansen R, Wright DW, Sewell WM, III, Owens JD, Lefkove B, Brown FW, Burton DC, Uyeki TM, Bialek SR, Jackson BR. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 - Georgia, march 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):545–550. doi: 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim L, Garg S, O'Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality among Hospitalized Adults Identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. 2021;72(9):e206–14. 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed]
- 13.Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65(5):533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LRE, Hastie J, Hochman BR, Salazar-Schicchi J, Yip NH, Brodie D, O'Donnell MR. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in new York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasselli G, Zanella A. Critically ill patients with COVID-19 in new York City. Lancet. 2020;395(10239):1740–1741. doi: 10.1016/S0140-6736(20)31190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, march 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu CY, Desai A, de Lima Lopes G, Jr, Grivas P, Painter CA, Peters S, Thompson MA, Bakouny Z, Batist G, Bekaii-Saab T, Bilen MA, Bouganim N, Larroya MB, Castellano D, del Prete SA, Doroshow DB, Egan PC, Elkrief A, Farmakiotis D, Flora D, Galsky MD, Glover MJ, Griffiths EA, Gulati AP, Gupta S, Hafez N, Halfdanarson TR, Hawley JE, Hsu E, Kasi A, Khaki AR, Lemmon CA, Lewis C, Logan B, Masters T, McKay RR, Mesa RA, Morgans AK, Mulcahy MF, Panagiotou OA, Peddi P, Pennell NA, Reynolds K, Rosen LR, Rosovsky R, Salazar M, Schmidt A, Shah SA, Shaya JA, Steinharter J, Stockerl-Goldstein KE, Subbiah S, Vinh DC, Wehbe FH, Weissmann LB, Wu JTY, Wulff-Burchfield E, Xie Z, Yeh A, Yu PP, Zhou AY, Zubiri L, Mishra S, Lyman GH, Rini BI, Warner JL, Abidi M, Acoba JD, Agarwal N, Ahmad S, Ajmera A, Altman J, Angevine AH, Azad N, Bar MH, Bardia A, Barnholtz-Sloan J, Barrow B, Bashir B, Belenkaya R, Berg S, Bernicker EH, Bestvina C, Bishnoi R, Boland G, Bonnen M, Bouchard G, Bowles DW, Busser F, Cabal A, Caimi P, Carducci T, Casulo C, Chen JL, Clement JM, Chism D, Cook E, Curran C, Daher A, Dailey M, Dahiya S, Deeken J, Demetri GD, DiLullo S, Duma N, Elias R, Faller B, Fecher LA, Feldman LE, Friese CR, Fu P, Fu J, Futreal A, Gainor J, Garcia J, Gill DM, Gillaspie EA, Giordano A, Glace (M)G, Grothey A, Gulati S, Gurley M, Halmos B, Herbst R, Hershman D, Hoskins K, Jain RK, Jabbour S, Jha A, Johnson DB, Joshi M, Kelleher K, Kharofa J, Khan H, Knoble J, Koshkin VS, Kulkarni AA, Lammers PE, Leighton JC, Jr, Lewis MA, Li X, Li A, Lo KMS, Loaiza-Bonilla A, LoRusso P, Low CA, Lustberg MB, Mahadevan D, Mansoor AH, Marcum M, Markham MJ, Handy Marshall C, Mashru SH, Matar S, McNair C, McWeeney S, Mehnert JM, Menendez A, Menon H, Messmer M, Monahan R, Mushtaq S, Nagaraj G, Nagle S, Naidoo J, Nakayama JM, Narayan V, Nelson HH, Nemecek ER, Nguyen R, Nuzzo PV, Oberstein PE, Olszewski AJ, Owenby S, Pasquinelli MM, Philip J, Prabhakaran S, Puc M, Ramirez A, Rathmann J, Revankar SG, Rho YS, Rhodes TD, Rice RL, Riely GJ, Riess J, Rink C, Robilotti EV, Rosenstein L, Routy B, Rovito MA, Saif MW, Sanyal A, Schapira L, Schwartz C, Serrano O, Shah M, Shah C, Shaw G, Shergill A, Shouse G, Soares HP, Solorzano CC, Srivastava PK, Stauffer K, Stover DG, Stratton J, Stratton C, Subbiah V, Tamimi R, Tannir NM, Topaloglu U, van Allen E, van Loon S, Vega-Luna K, Venepalli N, Verma AK, Vikas P, Wall S, Weinstein PL, Weiss M, Wise-Draper T, Wood WA, Xu W(V), Yackzan S, Zacks R, Zhang T, Zimmer AJ, West J. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee LYW, Cazier JB, Starkey T, Turnbull CD, Team UKCCMP. Kerr R, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–15. 10.1007/s00125-020-05180-x. Epub 2020 May 29. [DOI] [PMC free article] [PubMed]
- 23.Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health. 2020;8(8):e1018–e1026. doi: 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, and the Northwell COVID-19 Research Consortium. Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, Reiser J, Bansal A, Srivastava A, Zhou Y, Sutherland A, Green A, Shehata AM, Goyal N, Vijayan A, Velez JCQ, Shaefi S, Parikh CR, Arunthamakun J, Athavale AM, Friedman AN, Short SAP, Kibbelaar ZA, Abu Omar S, Admon AJ, Donnelly JP, Gershengorn HB, Hernán MA, Semler MW, Leaf DE, STOP-COVID Investigators. Walther CP, Anumudu SJ, Kopecky KF, Milligan GP, McCullough PA, Nguyen TD, Krajewski ML, Shankar S, Pannu A, Valencia JD, Waikar SS, Hart P, Ajiboye O, Itteera M, Rachoin JS, Schorr CA, Shea L, Edmonston DL, Mosher CL, Karp A, Cohen Z, Allusson V, Bambrick-Santoyo G, Bhatti N, Mehta B, Williams A, Walters P, Go RC, Rose KM, Zhou AM, Kim EC, Lisk R, Coca SG, Altman DR, Saha A, Soh H, Wen HH, Bose S, Leven EA, Wang JG, Mosoyan G, Nadkarni GN, Guirguis J, Kapoor R, Meshberger C, Garibaldi BT, Corona-Villalobos CP, Wen Y, Menez S, Malik RF, Cervantes CE, Gautam SC, Nguyen HB, Ahoubim A, Thomas LF, Sirganagari DR, Guru PK, Bergl PA, Rodriguez J, Shah JA, Gupta MS, Kumar PN, Lazarous DG, Kassaye SG, Johns TS, Mocerino R, Prudhvi K, Zhu D, Levy RV, Azzi Y, Fisher M, Yunes M, Sedaliu K, Golestaneh L, Brogan M, Raichoudhury R, Cho SJ, Plataki M, Alvarez-Mulett SL, Gomez-Escobar LG, Pan D, Lee S, Kirshnan J, Whalen W, Charytan D, Macina A, Ross DW, Leidner AS, Martinez C, Kruser JM, Wunderink RG, Hodakowski AJ, Price-Haywood EG, Matute-Trochez LA, Hasty AE, Mohamed MMB, Avasare RS, Zonies D, Baron RM, Sise ME, Newman ET, Pokharel KK, Sharma S, Singh H, Correa S, Shaukat T, Kamal O, Yang H, Boateng JO, Lee M, Strohbehn IA, Li J, Muhsin SA, Mandel EI, Mueller AL, Cairl NS, Rowan C, Madhai-Lovely F, Peev V, Byun JJ, Vissing A, Kapania EM, Post Z, Patel NP, Hermes JM, Patrawalla A, Finkel DG, Danek BA, Arikapudi S, Paer JM, Puri S, Sunderram J, Scharf MT, Ahmed A, Berim I, Hussain S, Anand S, Levitt JE, Garcia P, Boyle SM, Song R, Zhang J, Sharshir M'A, Rusnak VV, Podoll AS, Chonchol M, Sharma S, Burnham EL, Rashidi A, Hejal R, Judd ET, Latta L, Tolwani A, Albertson TE, Adams JY, Chang SY, Beutler RM, Schulze CE, Macedo E, Rhee H, Liu KD, Jotwani VK, Koyner JL, Shah CV, Jaikaransingh V, Toth-Manikowski SM, Joo MJ, Lash JP, Neyra JA, Chaaban N, Iardino A, Au EH, Sharma JH, Sosa MA, Taldone S, Contreras G, Zerda DDL, Blakely P, Berlin H, Azam TU, Shadid H, Pan M, O'Hayer P, Meloche C, Feroze R, Padalia KJ, Bitar A, Flythe JE, Tugman MJ, Brown BR, Spiardi RC, Miano TA, Roche MS, Vasquez CR, Bansal AD, Ernecoff NC, Kovesdy CP, Molnar MZ, Azhar A, Hedayati SS, Nadamuni MV, Khan SS, Willett DL, Renaghan AD, Bhatraju PK, Malik BA, Joy CM, Li T, Goldberg S, Kao PF, Schumaker GL, Faugno AJ, Hsu CM, Tariq A, Meyer L, Weiner DE, Christov M, Wilson FP, Arora T, Ugwuowo U. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Sanchez FJ, Del Toro E, Cardassay E, Valls Carbo A, Cuesta F, Vigara M, et al. Clinical presentation and outcome across age categories among patients with COVID-19 admitted to a Spanish emergency department. Eur Geriatr Med. 2020;11(5):829–841. doi: 10.1007/s41999-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Incerti D, Rizzo S, Li X, Lindsay L, Yau V, Keebler D, Chia J, Tsai L. Prognostic model to identify and quantify risk factors for mortality among hospitalised patients with COVID-19 in the USA. BMJ Open. 2021;11(4):e047121. doi: 10.1136/bmjopen-2020-047121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Team CC-R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-march 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, Storgaard M, al Khalili S, Simonsen L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, Sanderson C, McKee M, Troeger C, Ong KL, Checchi F, Perel P, Joseph S, Gibbs HP, Banerjee A, Eggo RM, Nightingale ES, O'Reilly K, Jombart T, Edmunds WJ, Rosello A, Sun FY, Atkins KE, Bosse NI, Clifford S, Russell TW, Deol AK, Liu Y, Procter SR, Leclerc QJ, Medley G, Knight G, Munday JD, Kucharski AJ, Pearson CAB, Klepac P, Prem K, Houben RMGJ, Endo A, Flasche S, Davies NG, Diamond C, van Zandvoort K, Funk S, Auzenbergs M, Rees EM, Tully DC, Emery JC, Quilty BJ, Abbott S, Villabona-Arenas CJ, Hué S, Hellewell J, Gimma A, Jarvis CI. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. 10.1001/jama.2020.4683. [DOI] [PubMed]
- 35.Sudharsanan N, Didzun O, Barnighausen T, Geldsetzer P. The contribution of the age distribution of cases to COVID-19 case fatality across countries: a 9-country demographic study. Ann Intern Med. 2020;173(9):714–720. doi: 10.7326/M20-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisman DN, Greer AL, Tuite AR. Age is just a number: a critically important number for COVID-19 case fatality. Ann Intern Med. 2020;173(9):762–763. doi: 10.7326/M20-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millett GA, Jones AT, Benkeser D, Baral S, Mercer L, Beyrer C, Honermann B, Lankiewicz E, Mena L, Crowley JS, Sherwood J, Sullivan PS. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44. doi: 10.1016/j.annepidem.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, Lewis J, Baer A, Kawakami V, Lukoff MD, Ferro J, Brostrom-Smith C, Rea TD, Sayre MR, Riedo FX, Russell D, Hiatt B, Montgomery P, Rao AK, Chow EJ, Tobolowsky F, Hughes MJ, Bardossy AC, Oakley LP, Jacobs JR, Stone ND, Reddy SC, Jernigan JA, Honein MA, Clark TA, Duchin JS, Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team Epidemiology of Covid-19 in a long-term Care Facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham NSN, Junghans C, Downes R, Sendall C, Lai H, McKirdy A, et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020;81(3):411–9. 10.1016/j.jinf.2020.05.073. Epub 2020 June 343. [DOI] [PMC free article] [PubMed]
- 40.Panagiotou OA, Kosar CM, White EM, Bantis LE, Yang X, Santostefano CM, Feifer RA, Blackman C, Rudolph JL, Gravenstein S, Mor V. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439–448. doi: 10.1001/jamainternmed.2020.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ioannou GN, Locke E, Green P, Berry K, O'Hare AM, Shah JA, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christensen DM, Strange JE, Gislason G, Torp-Pedersen C, Gerds T, Fosbol E, et al. Charlson comorbidity index score and risk of severe outcome and death in Danish COVID-19 patients. J Gen Intern Med. 2020;35(9):2801–2803. doi: 10.1007/s11606-020-05991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M, et al. Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension. 2020;76(2):366–72. 10.1161/HYPERTENSIONAHA.120.15324. Epub 2020 Jun 22. PMID: 32564693. [DOI] [PubMed]
- 44.Imam Z, Odish F, Armstrong J, Elassar H, Dokter J, Langnas E, Halalau A. Independent correlates of hospitalization in 2040 patients with COVID-19 at a large hospital system in Michigan, United States. J Gen Intern Med. 2020;35(8):2516–2517. doi: 10.1007/s11606-020-05937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munoz P, Galar A, Catalan P, Valerio M, Aldamiz-Echevarria T, Colliga C, et al. The first 100 cases of COVID-19 in a Hospital in Madrid with a 2-month follow-up. Rev Esp Quimioter. 2020;33(5):369–378. doi: 10.37201/req/072.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou W, Qin X, Hu X, Lu Y, Pan J. Prognosis models for severe and critical COVID-19 based on the Charlson and Elixhauser comorbidity indices. Int J Med Sci. 2020;17(15):2257–2263. doi: 10.7150/ijms.50007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhargava A, Sharma M, Riederer K, Fukushima EA, Szpunar SM, Saravolatz L. Risk factors for in-hospital mortality from COVID-19 infection among black patients - an Urban Center experience. Clin Infect Dis. 2020. 10.1093/cid/ciaa1468. [DOI] [PMC free article] [PubMed]
- 48.Rodilla E, Saura A, Jimenez I, Mendizabal A, Pineda-Cantero A, Lorenzo-Hernandez E, et al. Association of Hypertension with All-Cause Mortality among Hospitalized Patients with COVID-19. J Clin Med. 2020;9(10):3136. 10.3390/jcm9103136. [DOI] [PMC free article] [PubMed]
- 49.Varol Y, Hakoglu B, Kadri Cirak A, Polat G, Komurcuoglu B, Akkol B, Atasoy C, Bayramic E, Balci G, Ataman S, Ermin S, Yalniz E, COVID Study Group The impact of charlson comorbidity index on mortality from SARS-CoV-2 virus infection and a novel COVID-19 mortality index: CoLACD. Int J Clin Pract. 2021;75(4):e13858. doi: 10.1111/ijcp.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercante G, Ferreli F, De Virgilio A, Gaino F, Di Bari M, Colombo G, et al. Prevalence of Taste and Smell Dysfunction in Coronavirus Disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146(8):723–8. 10.1001/jamaoto.2020.1155. PMID: 32556070. [DOI] [PMC free article] [PubMed]
- 51.Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmieri L, Vanacore N, Donfrancesco C, Lo Noce C, Canevelli M, Punzo O, Raparelli V, Pezzotti P, Riccardo F, Bella A, Fabiani M, D’Ancona FP, Vaianella L, Tiple D, Colaizzo E, Palmer K, Rezza G, Piccioli A, Brusaferro S, onder G, Italian National Institute of Health COVID-19 Mortality Group. Palmieri L, Andrianou X, Barbariol P, Bella A, Bellino S, Benelli E, Bertinato L, Boros S, Brambilla G, Calcagnini G, Canevelli M, Rita Castrucci M, Censi F, Ciervo A, Colaizzo E, D’Ancona F, del Manso M, Donfrancesco C, Fabiani M, Facchiano F, Filia A, Floridia M, Galati F, Giuliano M, Grisetti T, Kodra Y, Langer M, Lega I, Lo Noce C, Maiozzi P, Malchiodi Albedi F, Manno V, Martini M, Mateo Urdiales A, Mattei E, Meduri C, Meli P, Minelli G, Nebuloni M, Nisticò L, Nonis M, onder G, Palmisano L, Petrosillo N, Pezzotti P, Pricci F, Punzo O, Puro V, Raparelli V, Rezza G, Riccardo F, Cristina Rota M, Salerno P, Serra D, Siddu A, Stefanelli P, de Bella MT, Tiple D, Unim B, Vaianella L, Vanacore N, Vichi M, Rocco Villani E, Zona A, Brusaferro S. Clinical characteristics of hospitalized individuals dying with COVID-19 by age Group in Italy. J Gerontol A Biol Sci Med Sci. 2020;75(9):1796–1800. doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–6. 10.1001/jama.2020.4031. [DOI] [PubMed]
- 54.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17(9):e1003321. doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, Li Z, Shaw SF, Caparosa SL, Nau CL, Saxena T, Rieg GK, Ackerson BK, Sharp AL, Skarbinski J, Naik TK, Murali SB. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson MR, Geleris J, Anderson DR, Zucker J, Nobel YR, Freedberg D, Small-Saunders J, Rajagopalan KN, Greendyk R, Chae SR, Natarajan K, Roh D, Edwin E, Gallagher D, Podolanczuk A, Barr RG, Ferrante AW, Baldwin MR. Body mass index and risk for intubation or death in SARS-CoV-2 infection: a retrospective cohort study. Ann Intern Med. 2020;173(10):782–790. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reilev M, Kristensen KB, Pottegard A, Lund LC, Hallas J, Ernst MT, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49(5):1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farrell TW, Francis L, Brown T, Ferrante LE, Widera E, Rhodes R, Rosen T, Hwang U, Witt LJ, Thothala N, Liu SW, Vitale CA, Braun UK, Stephens C, Saliba D. Rationing limited healthcare resources in the COVID-19 era and beyond: ethical considerations regarding older adults. J Am Geriatr Soc. 2020;68(6):1143–1149. doi: 10.1111/jgs.16539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farrell TW, Ferrante LE, Brown T, Francis L, Widera E, Rhodes R, Rosen T, Hwang U, Witt LJ, Thothala N, Liu SW, Vitale CA, Braun UK, Stephens C, Saliba D. AGS position statement: resource allocation strategies and age-related considerations in the COVID-19 era and beyond. J Am Geriatr Soc. 2020;68(6):1136–1142. doi: 10.1111/jgs.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.2020;Pages. https://www.va.gov/vetdata/docs/Quickfacts/VA_Utilization_Profile_2017.pdf.
- 61.Schuttner L, Reddy A, Rosland AM, Nelson K, Wong ES. Association of the Implementation of the patient-centered medical home with quality of life in patients with multimorbidity. J Gen Intern Med. 2020;35(1):119–125. doi: 10.1007/s11606-019-05429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 63.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 64.CDC;Pages. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19/evidence-table-phase-1b-1c.html.
- 65.Russo AG, Decarli A, Valsecchi MG. Strategy to identify priority groups for COVID-19 vaccination: a population based cohort study. Vaccine. 2021;39(18):2517–2525. doi: 10.1016/j.vaccine.2021.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ioannou GN, Green P, Fan VS, Dominitz JA, O'Hare AM, Backus LI, et al. Development of COVIDVax model to estimate the risk of SARS-CoV-2-related death among 7.6 Million US Veterans for use in vaccination prioritization. JAMA Netw Open. 2021;4(4):e214347. doi: 10.1001/jamanetworkopen.2021.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.King JT, Jr, Yoon JS, Rentsch CT, Tate JP, Park LS, Kidwai-Khan F, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the veterans health administration COVID-19 (VACO) index. PLoS One. 2020;15(11):e0241825. doi: 10.1371/journal.pone.0241825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SJ, Go AS, Lindquist K, Bertenthal D, Covinsky KE. Chronic conditions and mortality among the oldest old. Am J Public Health. 2008;98(7):1209–1214. doi: 10.2105/AJPH.2007.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ben-Ezra M, Shmotkin D. Predictors of mortality in the old-old in Israel: the cross-sectional and longitudinal aging study. J Am Geriatr Soc. 2006;54(6):906–911. doi: 10.1111/j.1532-5415.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 70.Hagg S, Jylhava J, Wang Y, Xu H, Metzner C, Annetorp M, et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21(11):1555–1559. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chatterjee A, Wu G, Primakov S, Oberije C, Woodruff H, Kubben P, Henry R, Aries MJH, Beudel M, Noordzij PG, Dormans T, Gritters van den Oever NC, van den Bergh JP, Wyers CE, Simsek S, Douma R, Reidinga AC, de Kruif MD, Guiot J, Frix AN, Louis R, Moutschen M, Lovinfosse P, Lambin P. Can predicting COVID-19 mortality in a European cohort using only demographic and comorbidity data surpass age-based prediction: an externally validated study. PLoS One. 2021;16(4):e0249920. doi: 10.1371/journal.pone.0249920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nickel CH, Rueegg M, Pargger H, Bingisser R. Age, comorbidity, frailty status: effects on disposition and resource allocation during the COVID-19 pandemic. Swiss Med Wkly. 2020;150:w20269. doi: 10.4414/smw.2020.20269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our data were obtained under data use agreement and cannot be shared beyond the study team members authorized to access the data. The data sources we used are accessible to other VA researchers for approved projects: https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=3834


