Abstract
Background
The novel coronavirus disease 2019 (COVID-19) continues to wreak havoc worldwide. This study assessed the ability of chest computed tomography (CT) severity score (CSS) to predict intensive care unit (ICU) admission and mortality in patients with COVID-19 pneumonia.
Materials and Methods
A total of 192 consecutive patients with COVID-19 pneumonia aged more than 20 years and typical CT findings and reverse-transcription polymerase chain reaction positive admitted in a tertiary hospital were included. Clinical symptoms at admission and short-term outcome were obtained. A semi-quantitative scoring system was used to evaluate the parenchymal involvement. The association between CSS, disease severity, and outcomes were evaluated. Prediction of CSS was assessed with the area under the receiver-operating characteristic (ROC) curves.
Results
The incidence of admission to ICU was 22.8% in men and 14.1% in women. CSS was related to ICU admission and mortality. Areas under the ROC curves were 0.764 for total CSS. Using a stepwise binary logistic regression model, gender, age, oxygen saturation, and CSS had a significant independent relationship with ICU admission and death. Patients with CSS ≥12.5 had about four-time risk of ICU admission and death (odds ratio 1.66, 95% confidence interval 1.66 – 9.25). The multivariate regression analysis showed the superiority of CSS over other clinical information and co-morbidities.
Conclusion
CSS was a strong predictor of progression to ICU admission and death and there was a substantial role of non-contrast chest CT imaging in the presence of typical features for COVID-19 pneumonia as a reliable predictor of clinical severity and patient’s outcome.
Keywords: Chest CT, COVID-19, Prognosis, SARS-CoV-2, CT severity score
Introduction
The coronavirus disease 2019 (COVID-19) caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was a newly emerging disease with high infectiousness and mortality. It belongs to the same family of viruses as the SARS-CoV and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), which caused serious outbreaks in 2003 and 2012, respectively [1,2]. The disease has originated in December 2019 in Wuhan, the capital of the Hubei province in China [3], and has been spreading at a rapid rate worldwide, causing the World Health Organization (WHO) to declare it a pandemic [4]. Since the number of infected patients continues to increase rapidly, there is a need for better diagnosis and treatment of COVID-19 to overcome this pandemic [5]. The disease is mild and self-limiting in most patients, but in some patients, it is severe and fatal. Deciding which patients are at high risk of severe disease or mortality is an essential part of understanding this disease.
Non-contrast chest computed tomography (CT) scan was found to be a useful diagnostic instrument for screening COVID-19 pneumonia. The most prevalent CT imaging findings consist of ground-glass opacities (GGO) with or without consolidation as the indicator of viral pneumonia, which mainly tends to be bilateral and poses a peripheral location accompanied by lower lobe predominance [6,7]. There is scarce data available on the prognostic application of chest CT scans.
Governments and societies are now fighting with numerous stimulating problems, including people and medical personnel panic, lack of cheap, accessible, and sensitive diagnostic methods, limited hospital capacities and intensive care unit (ICU) beds, and insufficient public preventive tools, which engendered a significant psycho-socioeconomic burden on communities. Therefore, most diagnostic attempts are now focused on predicting the severity and prognosis of the disease as the main issue of challenge among clinicians. Some studies have suggested that old age, the presence of co-morbidities, the need for mechanical ventilation, interleukin-6, higher serum levels of D-dimer, lactate dehydrogenase, procalcitonin, and C-reactive protein, lymphocyte count, and features from CT scoring as predictors of severity and prognosis of COVID-19 [8–10]. Appropriate and clear prognostic imaging and clinical models could help choose patients with poorer outcomes, which need hospital care from those that can be managed as an outpatient. This strategy also helps decrease the admission load of patients in the healthcare centers and the greatest reduction in mortality rate. This study assessed the prognostic value of chest CT severity score (CSS) to predict the severity of symptoms and outcomes among patients with typical CT findings.
Materials and Methods
1. Patients
From March 21, 2020 to August 14, 2020 a total of 192 consecutive patients (>20 years old) with classic symptoms of COVID-19 pneumonia and positive reverse transcriptase-polymerase chain reaction (RT-PCR) test attending in tertiary referral hospital, which is affiliated to Isfahan University of Medical Sciences, Iran, were included. The inclusion criteria were consisting of all patients with respiratory symptoms favoring COVID-19 diagnosis in similarity with WHO interim guideline, available laboratory data, RT-PCR positive test, and at least one of the following criteria: oxygen (O2) saturation ≤93%, tachypnea (respiratory rate ≥22 breaths/min), tachycardia (pulse rate >100/min), and hypotension (systolic blood pressure ≤100 mmHg) according to the Iranian national COVID-19 protocol [11]. Patients whose initial scans were not highly suggestive of COVID-19 and those assessed in the emergency room but not admitted, or those that died in the emergency room, were excluded. All the included patients had undergone non-contrast chest CT imaging within the first 24 hours of hospitalization and an RT-PCR test was performed for all patients. The average (standard deviation [SD]) number of days between hospitalization and CT scan was 1.13 (1.2) days and the time gap between initial clinical assessment, laboratory test sampling, and scans was about 24 hours for all enrolled patients. Demographic and clinical data on admission were collected from patient’s records.
2. Ethics statement
Ethical approval was obtained from the Isfahan University of Medical Sciences ethical committee, compliant with the Declaration of Helsinki (approval no. IR.MUI.MED.REC.1399.164). This study was based on routine medical procedures, and additional written consent was not essential. The data were processed and analyzed by official medical personnel only and were anonymized and de-identified before analysis.
3. Clinical symptoms
Clinical symptoms were extracted from patients’ records documented at admission time. According to WHO classification, established based on admission time’s clinical data, we classified patients into mild and severe pneumonia groups. Severe pneumonia is defined by the presence of respiratory rate >30 breaths/min, severe respiratory distress, or O2 Saturation ≤93% with fever or suspected respiratory infection. Non-severe pneumonia and independence from supplemental oxygen administration were considered mild cases [12].
4. Chest CT imaging
All chest CT images were non-contrast and performed during inspiratory breath-hold at the supine position using a 16-detector-row CT scanner (Philips Brilliance 16, Philips Medical Systems, Best, the Netherlands) with the following default settings: 120 kVp, 100 – 200 mAs, all the imaging data were reconstructed with a slice thickness of 1.5 mm and interval of 0.75 mm. All chest CT images were assessed independently and blindly by two board-certificated radiologists with more than five years of experience in chest CT interpretation, who were blind to the clinical and laboratory data. However, if there were any cases with inter-observational disagreement, the third radiologist with 15 years of experience made the final decision. The CT findings were classified using the Radiology Society of North America consensus statement on Reporting Chest CT findings Related to COVID-19 [13]. Chest CT images were assessed for the presence of ground-glass opacities (GGOs), consolidation, GGOs with consolidation, centrilobular nodules, septal thickening, perilobular opacities, reticular pattern, architectural distortion, subpleural bands, traction bronchiectasis, bronchial wall thickening, mediastinal-hilar lymphadenopathy, and pleural and pericardial effusion [14].
To evaluate the extent of pulmonary parenchymal involvement, we used a semi-quantitative scoring system previously used by others [15,16] using a system previously described for severity of acute respiratory distress syndrome on thin section lung CT scan [17]. Briefly, each lung was divided into three regions, (1) upper (above the level of the carina), (2) middle (between the carina and inferior pulmonary vein), and (3) lower (below the level of the inferior pulmonary vein). Each region of the lung was evaluated in terms of the percentage of involvement on a scale of 0 - 4: 0, no involvement; 1) less than 25%; 2) 25% to less than 50%; 3) 50% to less than 75%; and 4) 75% or greater involvement. Overall CT scores was the summation of scores from all 6 lung regions. Then, the total CSS calculated to be a number in the range of 0 (representative of no involvement) to 24 (indicative of diffuse interstitial involvement) and assigned for each patient.
5. Short-term outcome
Patients were divided into two groups: the patients with a poor prognosis who received ICU care or died during the hospitalization period and those with a good prognosis who were discharged without ICU care.
6. Discharge criteria
Discharge criteria were defined as the improvement of chest X-ray findings and clinical symptoms, the absence of fever for at least three days’ period, and O2 saturation of more than 93% without mechanical ventilation.
7. Statistical analysis
Continuous and categorical variables are expressed as means with standard error (SE) of the mean or 95% confidence intervals (CIs) and percentages, respectively, unless otherwise specified. Statistical methods used included the Student's t-test, Chi-square test, or Fisher's exact test, one-way analysis of variance followed by the one-way post hoc Tukey test, and stepwise binary logistic regression. Spearman’s rho partial correlation analyses adjusting for age were performed to determine the linear relationship between O2 saturation, respiratory rate, and CSS. Age-standardized means were considered and compared using general linear models. To estimate the predictors of disease short-term outcomes a stepwise binary logistic regression was used with the SPSS for Windows (SPSS Inc., Chicago, IL, USA). Variable age, respiratory rate, and O2 saturation were entered in models as a continuous variable, whereas gender, hypertension, diabetes, cardiovascular, chronic respiratory, and chronic kidney disease were entered in models as categorical. CSS was entered in the model both as categorical and continuous. The ability of CSS to predict short-term outcomes was examined by the receiver- operating characteristic (ROC) curve and their respective areas under the curve, in which sensitivity is plotted as a function of 1-specificity. The area under the curve (AUC) above 0.7 was considered to be useful, while an AUC between 0.8 and 0.9 indicated good diagnostic accuracy.
Results
Out of the 192 patients (114 men and 78 women, mean [SE] age 57.5 [1.11] year), 36 were ICU admitted (18.8%) and unfortunately, 10 (5.2%) died. The incidence of admission to ICU was 22.8% in men and 14.1% in women. Patients with good prognosis had a mean (SE) age of 54.7 (1.28) years, while ICU admitted and deceased patients had mean (SE) age of 68.5 (2.19) and 74.0 (3.44) years, respectively. One patient died before ICU admission. Table 1 shows the differences in the distribution of age, age-adjusted mean CSS, and clinical findings among patients who were discharged without ICU care, and those who received ICU care or died. Those who received ICU care or died during the hospitalization period were older and had higher age-adjusted total CSS, right upper, middle, lower, and left upper and lower lobe CSS, and lower O2 saturation than those who were discharged without ICU care (P <0.001). The mean (SE) CSS was 9.7 (0.45) in the good prognosis group and 14.4 (0.90) in the poor prognosis group. The difference was statistically significant (P <0.001). 88 (56.8%) men were in the good prognosis group and 26 (70.3%) men in the poor prognosis group (P <0.05). The participants of each group were classified into mild and severe pneumonia subgroups by means of admission time symptom severity. As expected, those in the poor prognosis group had more severe pneumonia than participants with a good prognosis (P <0.05). The presence of hypertension as the main co-morbidity affecting severity and prognosis of disease was compared between groups and no significant difference was detected between the good prognosis and poor prognosis groups (P >0.05). Other co-morbidities also compared between the two groups and were not statistically different, except cardiovascular disease, which was higher in the ICU admitted group (P <0.01).
Table 1. Age, Age-adjusted means (SE) and proportions of selected characteristics between 37 patients with COVID-19 pneumonia who admitted to ICU or died and 155 who did not admit to ICU and discharge alive.
| Variables | Age-adjusted Mean (SE) | |||
|---|---|---|---|---|
| Good prognosis | Poor prognosis | Difference (95% CI) | ||
| Age at registration (yr) | 54.8 (1.24) | 68.5 (2.54) | 13.8 (8.21, 19.35)a | |
| Total severity score | 9.7 (0.45) | 14.4 (0.90) | 4.7 (2.70, 6.75)a | |
| Right upper lobe severity score | 1.7 (0.11) | 2.7 (0.23) | 1.0 (0.48, 1.50)a | |
| Right middle lobe severity score | 1.5 (0.14) | 2.0 (0.28) | 0.5 (−0.10, 1.16) | |
| Right lower lobe severity score | 2.5 (0.11) | 3.5 (0.22) | 1.0 (0.52, 1.51)a | |
| Left upper lobe severity score | 1.7 (0.11) | 2.7 (0.22) | 1.0 (0.46, 1.46)a | |
| Left lower lobe severity score | 2.3 (0.11) | 3.5 (0.21) | 1.12 (0.66, 1.61)a | |
| O2 saturation | 90.3 (0.51) | 86.2 (1.08) | −4.1 (−6.50, −1.69)b | |
| Respiratory rate | 21.1 (0.42) | 22.4 (0.90) | 1.3 (−0.72, 3.27) | |
| Age (yr), No. (%) | <65 | 116 (74.8) | 16 (43.2) | −31.6 (−47.4, −14.1)a |
| ≥ 65 | 39 (25.2) | 21 (56.8) | - | |
| Gender, No. (%) | Men | 88 (56.8) | 26 (70.3) | 13.5 (4.2, 28.1)c |
| Women | 67 (43.2) | 11 (29.7) | - | |
| Co-morbidity, No. (%) | Diabetes | 34 (31.9) | 8 (21.6) | −0.3 (−12.8, 16.3) |
| Hypertension | 46 (29.9) | 11 (29.7) | 0.2 (−14.4, 17.4) | |
| Cardiovascular disease | 23 (14.8) | 13 (36.1) | 21.3 (5.5, 37.1) b | |
| Chronic airway disease | 13 (8.4) | 3 (8.3) | −0.1 (−7.9, 13.4) | |
| Cancer | 2 (1.3) | 1 (2.8) | 1.5 (−2.6, 12.6) | |
| Chronic kidney disease | 8 (5.2) | 5 (13.9) | 8.7 (−0.6, 23.0) | |
| Outcomes, No. (%) | ICU admission | 0 (0.0) | 36 (97.3) | 97.3 (85.9, 99.5)a |
| Death | 0 (0.0) | 10 (27.0) | 27.0 (15.2, 43.0)a | |
| Symptom severity, No. (%) | Severe | 94 (60.6) | 29 (78.4) | 17.8 (0.5, 30.6)c |
| Mild | 61 (39.4) | 8 (21.6) | - | |
Age-adjusted means were calculated using general linear models. Patients with a good prognosis were discharged without ICU care and those who received ICU care or died during hospitalization period considered as poor prognosis.
aP <0.001, bP <0.01, cP <0.05,
SE, standard error; COVID-19, coronavirus disease 2019; ICU, intensive care unit; CI, confidence interval.
1. Chest CT findings
Chest CT findings are illustrated in Table 2. GGO and consolidation was the most common finding in both groups and the second most common finding was pure GGO. The GGO rate was higher in the ICU care and consolidation was higher in those with good prognosis. Parenchymal abnormalities were distributed bilaterally in 166 patients (86.4%) (113 patients (72.9%) in the good prognosis group and 36 patients (97.3%) in the ICU care group), whereas unilateral involvement was seen in 43 patients (22.3%) (42 patients (27.1%) in good prognosis group and one patients (2.7%) in ICU care group. The lower zones (50.6%, 86 patients) were the most frequently involved sites in both groups. In the transverse plane, the lung abnormalities mostly showed peripheral (62.0%, 106 patients) involvement in both groups. No significant difference was found in the respiratory rate between the two groups.
Table 2. Chest CT findings of 37 patients with COVID-19 who admitted to ICU and 155 who did not admit to ICU.
| Variables | Good prognosis | Poor prognosis | Difference (95% CI) | |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| CT findings | ||||
| GGO | 52 (33.5) | 18 (48.6) | 15.1 (−2.0, 32.1) | |
| GGO and consolidation | 61 (39.4) | 17 (45.9) | 6.5 (−10.3, 23.9) | |
| Consolidation | 17 (11.0) | 0 (0.0) | −11.0 (−16.9, −0.7)a | |
| Bronchial thickening | 10 (6.5) | 3 (8.1) | 1.6 (−5.7, 15.2) | |
| Centrilobular nodules | 7 (4.5) | 1 (2.7) | −1.8 (−6.8, 9.6) | |
| Vascular enlargement | 11 (7.1) | 2 (5.4) | −1.7 (−8.2, 11.0) | |
| Pleural effusion | 10 (6.5) | 2 (5.4) | 1.1 (−7.4, 11.6) | |
| Reticular pattern | 57 (36.8) | 16 (43.2) | 6.4 (−10.1, 23.9) | |
| Total severity score ≥12.5 | 37 (27.0) | 24 (66.7) | 39.7 (23.3, 55.6)b | |
| Total severity score <12.5 | 100 (73.6) | 12 (33.3) | - | |
Patients with a good prognosis were discharged without ICU care and those who received ICU care or died during hospitalization period considered as poor prognosis
aP <0.05, bP <0.001.
CT, computed tomography; COVID-19, coronavirus disease 2019; ICU, intensive care unit; GGO, ground-glass opacities; CI, confidence interval.
2. ROC curve analysis
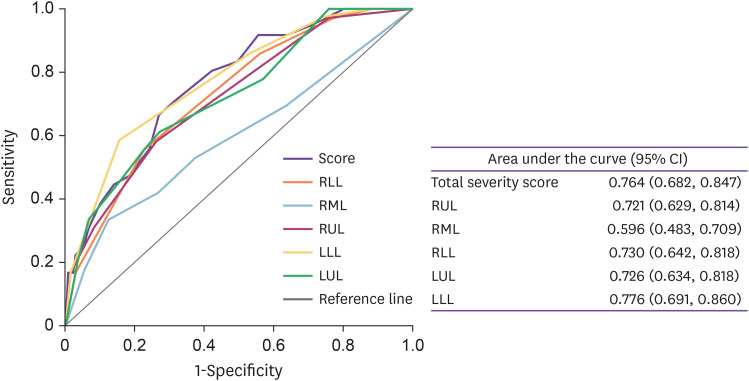
The ROC curves for predicting poor prognosis for total CSS, right upper lobe, right middle lobe, right lower lobe, left upper lobe, and left lower lobe CSS in terms of disease severity are shown in Figure 1. The areas under the ROC curves were 0.764 (95% confidence interval [CI]: 0.682, 0.847) for total CSS, 0.721 (95% CI: 0.629, 0.814) for the right upper lobe, 0.596 (95% CI: 0.483, 0.709) for the right middle lobe, 0.730 (95% CI: 0.642, 0.818) for the right lower lobe, 0.726 (95% CI: 0.634, 0.818) for the left upper lobe, and 0.776 (95% CI: 0.691, 0.860) for the left lower lobe CSS indicating a useful discrimination for patients with higher risk of ICU care and mortality. All CSS subgroups were significant predictors of future risk of ICU admission and mortality (P <0.001). Total CSS had areas slightly but not significantly larger than that of subgroup CSSs. However, in these patients with COVID-19 pneumonia, the subgroup CSS was similar to predict future risk of ICU admission. The optimal total CSS threshold for identifying poor prognosis was 12.5, with 66.7% sensitivity and 73.0% specificity.
Figure 1. Receiver operating characteristic curves for total severity score, the right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), left upper lobe (LUL) and left lower lobe (LLL) severity score for predicting ICU admission or death in patients with COVID-19 pneumonia. The estimates of the area under the ROC curves and their 95% CIs are shown.
COVID-19, coronavirus disease 2019; ROC, receiver-operating characteristic; CI, confidence interval.
All six subgroup CSSs were highly correlated with each other and the strongest Pearson correlation coefficients were found between total CSS and left lower lobe CSS and the weakest ones were between the right middle lobe and left lower lobe CSS (Table 3).
Table 3. Pearson correlation coefficients between severity score subgroups.
| Variables | Total CSS | Right upper lobe CSS | Right middle lobe CSS | Right lower lobe CSS | Left upper lobe CSS | Left lower lobe CSS |
|---|---|---|---|---|---|---|
| Total CSS | 1 | 0.839a | 0.789a | 0.762a | 0.861a | 0.772a |
| Right upper lobe CSS | 1 | 0.587a | 0.550a | 0.721a | 0.524a | |
| Right middle lobe CSS | 1 | 0.509a | 0.567a | 0.443a | ||
| Right lower lobe CSS | 1 | 0.721a | 0.516a | |||
| Left upper lobe CSS | 1 | 0.673a |
aP <0.001.
CSS, computed tomography CT severity score.
3. Logistic regression analysis
To determine the independent associations of poor prognosis (short-term outcomes) a forward stepwise binary logistic regression was performed to test nine variables: age, gender, hypertension, respiratory rate, O2 saturation, diabetes, chronic respiratory disease, cardiovascular disease, and chronic kidney disease. Table 4 shows the results of the age-adjusted and multivariate analysis. Older patients were significantly more frequent in the poor prognosis group (P <0.001). Of radiologic findings, in the age-adjusted model, CSS ≥ 12.5 (OR [95% CI] 4.49 [1.96, 10.29], P <0.001) could predict poor prognosis. All variables with a P <0.1 in the age-adjusted analysis were entered into a multivariate model to independently predict outcome measures and measure their association. Patients with poor prognosis were more likely than those of good prognosis to be older (OR [95% CI] 1.04 [1.01, 1.06], per year increase, P <0.01), be men (OR [95% CI] 2.16 [1.01, 4.66], P <0.05), have lower O2 saturation (OR [95% CI] 0.94 [0.89, 0.99], P <0.05) and to have higher total CSS (OR [95% CI] 3.93 [1.66, 9.29], P <0.001). Total CSS ≥12.5 was a stronger odds ratio to predict poor prognosis. When in multivariate analysis CSS considered as continues variable the association remained statistically significant (OR [95% CI] 1.24 [1.05, 1.46], P <0.05). Age-adjusted CSS was inversely correlated with O2 saturation (r = −0.408, P <0.001) but no correlation with respiratory rate (r = 0.115, P >0.05).
Table 4. Multivariate analysis of factors related to ICU admission and mortality (stepwise binary logistic regression model), significant adjusted odds ratios (95% CI).
| Variables | Age-adjusted odds ratio (95% CI) | Multivariate adjusted odds ratio (95% CI)a | |
|---|---|---|---|
| Gender | |||
| Women | 1.00 | 1.00 | |
| Men | 1.60 (0.71, 3.61) | 2. 16 (1.01, 4.66)b | |
| CT severity score | |||
| <12.5 | 1.00 | 1.00 | |
| ≥12.5 | 4.49 (1.96, 10.29)c | 3.93 (1.66, 9.29)c | |
| Hypertension | |||
| No | 1.00 | - | |
| Yes | 0.68 (0.29, 1.55) | - | |
| Diabetes mellitus | |||
| No | 1.00 | - | |
| Yes | 0.67 (0.27, 1.69) | - | |
| Cardiovascular disease | |||
| No | 1.00 | - | |
| Yes | 1.95 (0.82, 4.62) | - | |
| Chronic respiratory disease | |||
| No | 1.00 | - | |
| Yes | 0.65 (0.16, 2.68) | - | |
| Chronic Kidney disease | |||
| No | 1.00 | - | |
| Yes | 1.54 (0.44, 5.36) | - | |
| Age (year) | 1.06 (1.03, 1.09)c | 1.04 (1.01, 1.06)d | |
| CT severity score (continues variable) | 1.19 (1.10, 1.30)c | 1.24 (1.05, 1.46)b | |
| O2 saturation (continues variable) | 0.93 (0.88, 0.98)d | 0.94 (0.89, 0.99)b | |
| Respiratory rate (continues variable) | 1.05 (0.98, 1.13) | - | |
aOdds ratio (with 95% CI) calculated using binary logistic regression.
bP <0.05, cP <0.01, dP <0.001.
ICU; intensive care unit; CI, confidence interval; CT, computed tomography.
Discussion
Hospitalized patients with COVID-19 may eventually need admission to ICU. This study showed the ability of CSS to predict ICU admission and mortality in 192 hospitalized patients with COVID-19 pneumonia, RT-PCR positive, and typical CT imaging findings, further emphasizing the utility of CSS in predicting adverse outcomes. A CSS was equal or greater than 12.5 had a sensitivity of 66.7% and specificity of 73.0% for ICU care and mortality. CSS ≥12.5 in patients with COVID-19 pneumonia predicts admission to ICU with an OR (95% CI) of 3.93 (1.66, 9.29). Age, gender, and lower O2 saturation are weaker ICU admission risk predictor than CSS. Similar to our findings, Khosravi et al. [18] and Abbasi B et al. [19] have shown that CSS was well associated with ICU admission, intubation, and mortality. Yang et al. [20] also designated a similar study with 102 patients using different CT-scoring methods that also giving proof for quantitative CT evaluation as an accurate predictive scale of clinical severity. A study of chest CT findings in relation to COVID-19 clinical conditions by Zhao et al. [9] was done using another method of CT scoring, which demonstrated a significantly higher extent of pulmonary parenchymal involvement in severe and fatal types of the disease than those with mild and common types.
CSS was significantly higher in patients with severe symptoms than those who presented with mild symptoms at the time of admission. Such a quantitative evaluation has been applied in relation to clinical classification by Li et al. [21], and the same results have yielded.
Consistent with prior studies [22], the present study found similarly increasing the ICU admission and mortality with increasing age, decreasing O2 saturation, and in men in the patients with COVID-19 pneumonia.
Similar to previous studies [19,23,24,25], most of our patients had bilateral pulmonary involvement, with GGO, consolidation, or both being the most frequent abnormality. The most frequently involved anatomical location in this study were lower zones. The distribution of the lesions was not shown to be associated with a poor prognosis.
Finally, to compare the performance of CT findings to diagnose the short-term outcome with clinical information at admissions, such as O2 saturation and respiratory rate and co-morbidities several logistics regression models were implemented, which showed the superiority of CSS over other clinical information and co-morbidities.
Our findings highlight the substantial role of non-contrast chest CT imaging and CSS in the presence of typical features for COVID-19 pneumonia as a reliable predictor of clinical severity and patient’s outcome. Our results suggested that more emphasis should be placed on the value of serial chest CT scans in early diagnosis and evaluation of disease progression. Patients with higher CSS may benefit from early ICU admission and can help inpatient discharging in the emergency departments, especially in settings with limited ICU beds.
We used a semi-quantitative scoring method previously used by others [10,15,18,19] to score the degree of involvement. However, there is little data available on the association of ICU care and mortality outcomes (which have higher cost and burden for the healthcare system) with initial CT findings in COVID-19 pneumonia. Other scoring systems have also been proposed to predict the prognosis of patients with COVID-19 pneumonia. Pan et al. proposed a scoring system based on the number of lobes and percentages of involvement in each lobe [26]. Colombi et al. [27] used a software-based calculation of aerated lung volume to predict ICU admission or death. Ufuk et al. [28] used the area of pectoralis major muscles in CT scans to predict the severity of the COVID-19 pneumonia outcomes. Yuan et al. [29] have proposed a scoring system based on the type of abnormality and the area involved by each type with reliable results in predicting mortality. However, the semi-quantitative scoring method used in this study can be easily implemented in clinical practice.
Our study has some limitations. This study was a single-center study with a relatively small number of cases and a small number of patients with severe symptoms. Therefore, other studies including multicenter prospective studies with larger populations with a higher number of individuals with severe symptoms, employing consecutive RT-PCR assays and follow-up genomic kinetic assessment will be more generalizable and add value to our achievements in a more comprehensive study. Because there was no information about the body weight and height of patients we cannot evaluate whether the increased overweight /obesity risk was mediated by ICU care and mortality. Due to RT-PCR kit shortages in the initial days of the COVID-19 epidemic in Iran, RT-PCR testing was not repeated in RT-PCR negative patients and we excluded these patients from the study. Also, nasopharyngeal sampling errors due to the anxiety and overly protective measures in the first few weeks had increased the rate of false-negative RT-PCR results. While the design of the study may limit its generalizability to other populations, these findings are meaningful in that they are specifically applicable to urban Iranian patients with COVID-19 pneumonia.
In summary, our findings revealed that CT images and CSS can predict ICU admission and mortality in patients with COVID-19 pneumonia. Clinicians and radiologists can use CSS as a measure of disease severity and an indicator of poor prognosis, including the need for mechanical ventilation. Also, our findings showed the superiority of CT findings over other clinical information and co-morbidities in predicting the short-term outcome. Further efforts are needed to predict the value and reliability of CSS using large prospective studies.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support from the Avicenna Center of Excellence (ACE).
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: MJ, SH, AS.
- Data curation: SH, AH, RS, MME, NK, MD.
- Formal analysis: MJ, MM, NB.
- Funding acquisition: SH.
- Investigation: MJ, SH, AS.
- Methodology: MJ, SH, AH.
- Project administration: SH, AS.
- Resources: SH.
- Software: MJ.
- Supervision: SH.
- Validation: MJ.
- Visualization: MJ, SH, AS, RS, MME, NK, MD.
- Writing - original draft: MJ.
- Writing - review & editing: MJ, SH, AS.
References
- 1.Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguière AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Müller S, Rickerts V, Stürmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 2.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO coronavirus (COVID-19) Dashboard. 2020. [Accessed 26 June 2020]. Available at: https://covid19.who.int/
- 5.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, Dai J, Sun Q, Zhao F, Qu J, Yan F. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:E115–7. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venugopal VK, Mahajan V, Rajan S, Agarwal V, Rajan R, Syed S, Mahajan H. A systematic meta-analysis of CT features of COVID-19: lessons from radiology. medRxiv. 2020 [Google Scholar]
- 9.Ma C, Jiawei G, Hou P, Zhang L, Bai Y, Guo Z, Wu H, Zhang B, Li P, Zhao X. Incidence, clinical characteristics and prognostic factor in patients with COVID-19: a systematic review and meta-analysis. medRxiv. 2020 [Google Scholar]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahdavi A, Khalili N, Davarpanah AH, Faghihi T, Mahdavi A, Haseli S, Sabri A, Kahkouee S, Kazemi MA, Mehrian P, Falahati F, Bakhshayeshkaram M, Taheri MS. Radiologic management of COVID-19: preliminary experience of the Iranian Society of Radiology COVID-19 Consultant Group (ISRCC) Iran J Radiol. 2020;17:e102324 [Google Scholar]
- 12.World Health Organization (WHO) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. Available at: https://apps.who.int/iris/handle/10665/331446.
- 13.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, Henry TS, Kanne JP, Kligerman S, Ko JP, Litt H. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - secondary publication. J Thorac Imaging. 2020;35:219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203:782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, Shi H, Zhou M. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296:E55–64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou S, Wang Y, Zhu T, Xia L. CT Features of Coronavirus Disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 17.Ooi GC, Khong PL, Müller NL, Yiu WC, Zhou LJ, Ho JC, Lam B, Nicolaou S, Tsang KW. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230:836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 18.Yang R, Li X, Liu H, Zhen Y, Zhang X, Xiong Q, Luo Y, Gao C, Zeng W. Chest CT severity score: an imaging tool for Assessing Severe COVID-19. Radiol Cardiothorac Imaging. 2020;2:e200047. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khosravi B, Aghaghazvini L, Sorouri M, Naybandi Atashi S, Abdollahi M, Mojtabavi H, Khodabakhshi M, Motamedi F, Azizi F, Rajabi Z, Kasaeian A, Sima AR, Davarpanah AH, Radmard AR. Predictive value of initial CT scan for various adverse outcomes in patients with COVID-19 pneumonia. Heart Lung. 2021;50:13–20. doi: 10.1016/j.hrtlng.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbasi B, Akhavan R, Ghamari Khameneh A, Zandi B, Farrokh D, Pezeshki Rad M, Feyzi Laein A, Darvish A, Bijan B. Evaluation of the relationship between inpatient COVID-19 mortality and chest CT severity score. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.09.056. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y, Liu X, Huang M, Liao Y, Li S. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30:4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanne JP. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295:16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Lei P, Zeng B, Li Z, Yu P, Fan B, Wang C, Li Z, Zhou J, Hu S, Liu H. Coronavirus disease (COVID-19): spectrum of CT findings and temporal progression of the disease. Acad Radiol. 2020;27:603–608. doi: 10.1016/j.acra.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong Y, Sun D, Liu Y, Fan Y, Zhao L, Li X, Zhu W. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol. 2020;55:332–339. doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colombi D, Bodini FC, Petrini M, Maffi G, Morelli N, Milanese G, Silva M, Sverzellati N, Michieletti E. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296:E86–96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ufuk F, Demirci M, Sagtas E, Akbudak IH, Ugurlu E, Sari T. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur J Radiol. 2020;131:109271. doi: 10.1016/j.ejrad.2020.109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15:e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]