Abstract
Backround
Data on Dengue virus (DENV) infection prevalence, geographic distribution and risk factors are necessary to direct appropriate utilization of existing and emerging control strategies. This study aimed to determine the pooled prevalence, risk factors of DENV infection and the circulating serotypes within Nigeria from January 1, 2009 to December 31, 2020.
Materials and methods
Twenty-one studies out of 2,215 available articles were eligible and included for this systematic review. Relevant articles were searched, screened and included in this study according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) criteria. The risk of bias in primary studies was assessed by Cochrane's method. Heterogeneity of pooled prevalence was calculated using the chi-square test on Cochrane's Q statistic, which was quantified by I-square values. The random-effects analyses of proportions were used to determine the pooled prevalence of DENV antibodies, antigen and RNA from eligible studies.
Results
Of these, 3 studies reported co-circulation of all the 4 serotypes, while 2 separately reported co-circulation of DENV-1 &2 and DENV-1 to -3. All the antibody-based studies had significantly high heterogeneity (I2 >90%, P <0.05), while the NS1 and PCR-based studies had low heterogeneity (I2 <25%, P >0.05). The pooled prevalence of DENV IgM, IgG, RNA, NS1 and neutralizing antibodies were 16.8%, 34.7%, 7.7%, 7.7% and 0.7%, respectively. South-east Nigeria had the highest pooled DENV-IgG seropositivity, 77.1%. Marital status, gender, educational level and occupation status, the proximity of residence to refuse dumpsite, frequent use of trousers and long sleeve shirts were significantly associated with DENV IgG seropositivity (P <0.05).
Conclusion
Based on these findings, it can be inferred that Nigeria is hyperendemic for Dengue fever and needs concerted efforts to control its spread within and outside the country.
Keywords: DENV, Risk factor, Endemic, Dengue serotype, Systematic review, Nigeria
Introduction
Dengue fever is the most broadly spread mosquito-borne disease, transmitted by infected mosquitoes of Aedes species [1]. Classically, human Dengue virus (DENV) infection results from four serotypes (DENV-1-4) with 62 - 67% sequence homology [2]. The classification in the 4 serotypes was based on the immunological response of patients to primary DENV infection by one of the serotypes. Consequently, a primary infection of DEN protects against a secondary infection by a homologous DENV serotype but confers partial and transient protection against a heterologous DENV serotype [3].
Dengue fever is endemic in more than 100 countries with most cases reported from the Americas, South-east Asia and Western Pacific regions of World Health Organizaion [4]. In Africa, the first reported Dengue fever outbreaks occurred in Zanzibar (Tanzania) in 1,823 and 1870 [5]. Subsequently, several other African countries reported unconfirmed outbreaks of Dengue fever in the early 1900s [6]. Although many outbreaks aren't ever officially reported, between 1960 and 2017, more than 20 laboratory-confirmed Dengue fever epidemics were reported in more than 20 African countries [6,7].
In Nigeria, Dengue fever is endemic in almost all states and could be the leading cause of unclassified febrile illnesses [8]. Dengue fever has a mixed distribution among urban, and rural areas and was previously predominant reported in urban areas than in rural areas [9]. Surveillance for Dengue fever in Nigeria is subpar due to it is not a public health priority associated with a lack of public awareness of the virus and poor understanding by healthcare professionals evident in the misdiagnosis and underdiagnosis of the viral infection in many uncategorized febrile illnesses [10]. The Dengue disease burden may be grossly under-estimated in Nigeria [11]. A country is said to be hyperendemic for Dengue when all the four serotypes co-circulate at the same time [11].
Case detection, management, and vector control are the main strategies for the prevention and control of dengue virus transmission [12]. Information about Dengue disease burden, its prevalence, incidence and geographic distribution is necessary in decisions on appropriate utilization of existing and emerging prevention and control strategies. In cognizance of these, this study aimed to provide a systematic review on the pooled prevalence and estimate the risk factors of DENV infection in Nigeria. Furthermore, we reviewed the serotype distribution of DENV in circulation as well as the proportion of non-primary infections.
Meterials and Methods
1. Data sources and search strategy
Relevant articles were searched, screened and included in this study according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) criteria. Articles search through Web of Science, PubMed, Medline, EMBASE, Scopus, Google Scholar and Index Medicus for the Africa database using different combinations of the following keywords “Dengue virus”, “DENV Prevalence”, “Serological detection”, “Dengue Fever”, “DENV” and “Dengue combined with the names of Nigeria cities and states”. All databases were searched for only English-language full-text original articles published from January 1, 2010 to December 30, 2020. Multiple sources for article search were done to enhance the sensitivity of finding relevant articles.
2. Review selection
Studies identified through electronic and manual searches were listed in EndNote software (EndNote 20, Thomson Reuters, Toronto, Canada). After exclusion of duplicate citations, all authors separately reviewed titles and abstracts of selected articles. Relevant full-text studies obtained were assessed for eligibility and risk of bias. All original articles from peer-reviewed scientific journals with a cross-sectional or survey design that estimated the prevalence of DENV infection in humans and animals were considered potentially eligible for inclusion in this review. Relevant studies whose full-text was not available were sought through contacting the corresponding author through email. All studies outside of Nigeria were excluded. Any discordant from data interpretation between the authors were resolved through collective discussion.
3. Literature search strategy
In the study, we utilized the PICOS questions as a guideline that typically related to reviews of intervention effectiveness. So, the PICOS focused search strategy structure used in this study, focused on (P) which referred to population or patient, (I) to intervention or exposure, (C) refers to comparison, (O) refers to the outcome and (S) to study design. We used DENV infection to denote P, anti-DENV IgM, anti- DENV IgG, DENV NS1, DENV Neutralizing antibodies or DENV RNA positive to denote I, no DENV infection, DENV seronegative or DENV RNA negative to denote C and fever, febrile illness, DENV disease to denote O and cross-sectional study denotes S.
4. Assessment of study bias
The risk of bias in primary studies was assessed by Cochrane's method. The sample size of every study was included as one of the criteria for determining the risk of bias, as described by Humphrey et al [7]. DENV prevalence studies were evaluated in 3 domains, these include; sampling technique, the participation level of the subjects and DENV test method. Studies were categorized as low risk of bias if they used random sampling techniques, ˂80% involvement of respondents and the use of either virus neutralization test or NAAT for the determination of DENV prevalence from the overall study population [13].
Studies that did not provide information for the three aforementioned domains were classified as having an unclear risk of bias. When a study does not have one of the domains, it was considered a moderate risk of bias. Furthermore, if two domains were not obtainable, such studies were classified high risk of bias. The use of random sampling technique was only applicable for studies on the general population because there will be significantly high bias in selecting participants with acute fever infection from healthcare facilities. Studies on humans were considered to have high precision if their sample sizes were greater than 100 [14].
Our secondary outcomes of interest were the following: (1) proportion of primary and secondary infections among the laboratory-confirmed dengue patients. This classification was made based on the information about dengue serology provided included articles. Primary Dengue infection was defined as an acute infection, as indicated by qualitative detection of NS1 antigen, and/or IgM or HI antibodies or reverse transcription-polymerase chain reaction (RT-PCR) positivity and absence of IgG antibodies against dengue virus. A case of acute infection as defined above, in presence of IgG antibodies, was considered as secondary DENV infection (2) distribution of predominant and co-circulating dengue virus serotypes.
5. Data extraction and curation
Data were extracted from the selected studies into an excel sheet. Data were extracted based on first author name, year of publication, state/city, sample size, participants' age, sex and other available sociodemographic variables of participants and DENV prevalence by assay type. Data cleaning was done to identify and correct errors during collection to minimize their effects on the results.
6. Statistical analysis
The crude prevalence of DENV infection was calculated based on crude numerators and denominators provided by all eligible studies. Heterogeneity of pooled prevalence was calculated using the chi-square test on Cochrane's Q statistic, which was quantified by I-squares values, assuming I-square values of 25, 50 and 75% respectively representing low, medium and high heterogeneity. Medcalc software Version 2019.19.0.7 (Ostend, Belgium) was used for all statistical analysis. P-values less than 0.05 at 95% confidence interval (CI) were considered statistically significant.
Results
1. Study selection and characteristics
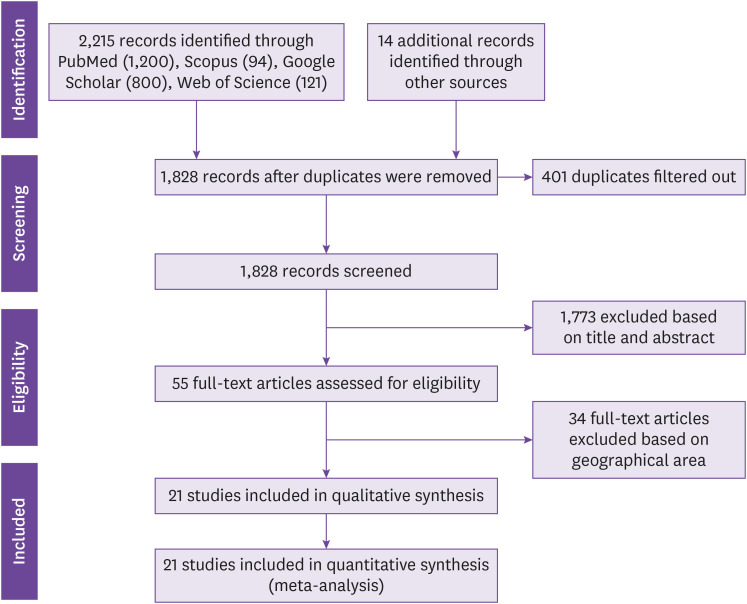
Our search yielded a total of 2,227 citations and 53 full articles were reviewed. Data were abstracted from 21 articles after applying exclusion criteria (Fig. 1). The studies were 21 eligible studies conducted from 2009 to 2020 [8,11,15,16,17,18,19,20,21,23,24,25,26,27,28,29,30,31]. Three out of the 21 studies reported co-circulation of all the 4 serotypes, while 2 studies separately reported co-circulation of DENV-1 & 2 and DENV-1 to 3 (Table 1). About 28.6%, 28.6% and 42.8% of the eligible studies had high, low and intermediate risk of biases, respectively.
Figure 1. PRISMA flow diagram of search strategy for inclusion of published studies.
PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Table 1. Study characteristics included in this systemic review.
| Reference | Gender of Study population; period of data collection; location | Sample size | Study design | Mean age (SD) or age range (years) | Method of detection | Dengue serotype detected | No. of Dengue cases alone/prevalence (%) | No. of Dengue co-infection cases/prevalence (%) | Overall dengue prevalence | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Mustapha et al. [15] | Recruitment of male and female participants in Gwagwalada, FCT, Abuja. | 178 | Cross sectional | ≤10 - >50 | Malaria: microscopy | NAa | 56/126 (44.4) | 23/52 (44.2) | IgG = 79/178 (44.4) | Intermediate |
| Dengue: ELISA-IgG | ||||||||||
| Ugwu et al. [16] | Recruitment of male and female participants in Jos, Plateau State. | 118 | Cross sectional | 1 - 60 | Dengue: ELISA-IgG, ELISA-IgM, ELISA-IgM/IgG | DENV 1 - 4 | Male = 8/42 (19.0) [IgM] | NAb | IgM = 13/118 (11.0) | Intermediate |
| Male = 9/42 (21.4) [IgG] | ||||||||||
| Male = 1/42 (2.4) [IgM/IgG] | IgG = 17/118 (14.4) | |||||||||
| Female = 5/76 (6.6) [IgM] | ||||||||||
| Female = 8/76 (10.5) [IgG] | IgM/IgG = 3/118 (2.5) | |||||||||
| Female = 2/76 (2.6) [IgM/IgG] | ||||||||||
| Sule et al. [17] | Recruitment of male and female participants in Osun State | 89 | Institutional-based, retrospective | 15 - 33 (19.7 ± 2.9) |
Dengue: ELISA-IgG, ELISA-IgM | DENV 1 - 4 | Male = 10/43 (23.3) [IgM] | NAb | IgM = 37/89 (41.6) | High |
| Male = 11/43 (25.6) [IgG] | ||||||||||
| Female = 27/46 (58.7) [IgM] | IgG = 30/89 (33.7) | |||||||||
| Female = 19/46 (41.3) [IgG] | ||||||||||
| Idoko et al. [18] | Recruitment of male and female participants in Kaduna State | 340 | Cross sectional | NAa | Malaria: microscopy | NAa | 4/182 (2.2) | 2/158 (1.3) | IgM = 6/334 (1.8) | Intermediate |
| Dengue: ELISA-IgM | ||||||||||
| Oladipo et al. [19] | Recruitment of male and female participants in Ogbomoso, Oyo State | 93 | Cross sectional | 37.6 ± 0.67 | Dengue: ELISA-IgM | NAa | Male = 7/37 (18.9) | NAb | IgM = 16/93 (17.2) | High |
| Female = 9/56 (16.1) | ||||||||||
| Suchi et al. [20] | Recruitment of male and female participants in Karu, Nasarawa State between February - July, 2017. | 400 | Cross sectional | ≤20 - ≥30 | Malaria: microscopy | NAa | 2/380 (0.5) | 10/20 (50) | IgM = 12/400 (3.0) | Low |
| Dengue: ELISA-IgM, ELISA-IgG, ELISA-NS1 | NS1 = 12/400 (3.0) | |||||||||
| IgG = 0/400 (0.0) | ||||||||||
| Mahmoud et al. [21] | Recruitment of male blood donors and pregnant female participants in Dutse, Jigawa State. | 391 | Cross sectional | 11 - 50 | Dengue: ELISA-IgG, ELISA-IgM, ELISA-IgM/IgG | NAa | Male = 35/233 (15) [IgM] | NAb | IgM = 58/391 (14.8) | Intermediate |
| Male = 38/223 (17) [IgG] | ||||||||||
| Male = 21/223 (9.4) [IgM/IgG] | IgG = 48/391 (12.3) | |||||||||
| Female = 23/118 (19.5) [IgM] | ||||||||||
| Female = 10/118 (8.5) [IgG] | IgM/IgG = 23/391 (5.9) | |||||||||
| Female = 2/118 (1.7) [IgM/IgG] | ||||||||||
| Oladipo et al. [22] | Recruitment of male and female participants from urban and rural areas of Oyo State. | 93 | Cross sectional | 1 - 75 | Dengue: ELISA-IgM, West Nile: ELISA-IgM, Yellow fever: ELISA-IgM. | NAa | NAc | NAc | IgM = 16/97 (16.5) | High |
| Kolawole et al. [23] | Recruitment of male and female participants from Illorin, Kwara State. | 176 | Cross sectional | 0 - >70 | Malaria: RNeasy mini kits | DENV 1 - 4 | IgM = 76/171 (44.4) | IgM = 5/5 (100) | IgM = 81/176 (46.0) | Low |
| Dengue: ELISA-IgM, ELISA-IgG, RT-PCR | IgG = NAa | IgG = NAa | IgG = 48/176 (27.3) | |||||||
| RNA = 11/176 (6.3) | ||||||||||
| Dawurung et al. [24] | Recruitment of male and female participants from Jos, Plateau State. | 182 | Cross sectional | 0 - 70 | Malaria: microscopy, Typhoid: widal, Dengue: ELISA-NS1 antigen. | NAa | 2/96 (2.1) | 2/86 (2.3) | NS1 = 4/182 (2.2) | Low |
| Nasir et al. [11] | Recruitment of male and female participants in Gwagwalada, FCT, Abuja from May - August, 2016. | 171 | Hospital-based cross-sectional | 1 - ≥49 | Dengue: ELISA-NS1 & ELISA-IgG. | DENV 1 - 4 | Male = NAa [NS1] | NAb | NS1 = 3/171 (1.8) | Intermediate |
| Male = 28/72 (38.9) [IgG] | ||||||||||
| Female = NAa [NS1] | IgG = 62/171 (36.3) | |||||||||
| Female = 46/99 (46.5) [IgG] | NS1/IgG = 12/171 (7.0) | |||||||||
| Onoja et al. [25] | Recruitment of male and female participants in Cross River State. | 17 | Cross-sectional | 13 - 80 | Dengue: RT-PCR | DENV 1 - 2 | NAa | NAb | RNA = 3/17 (17.6) | Intermediate |
| Hamisu et al. [26] | Recruitment of male and female participants in Maiduguri, Borno State from January - May, 2016. | 91 | Cross-sectional | 1 - ≥60 | Dengue: ELISA-NS1 & ELISA-IgM. | NAa | Male = 2/18 (11.1) [NS1] | NAb | NS1 = 9/91 (9.9) | Intermediate |
| Male = 4/18 (22.2) [IgM] | IgM = 34/91 (37.4) | |||||||||
| Female = 7/73 (9.6) [NS1] | NS1/IgM = 3/91 (3.3) | |||||||||
| Female = 30/73 (41.1) [IgM] | ||||||||||
| Baba et al. [27] | Recruitment of participants in FCT-Abuja, Gombe, Kano, Calabar and Maiduguri, Borno State from June 2001 - July, 2002. | 1948 | Cross-sectional | NAa | WNV: RT-PCR, PRNT-neutralizing Ab; Dengue: ELISA-IgG, ELISA-IgM, PRNT-neutralizing Ab. | DENV 1 - 4 | 11/1,946 (0.6) | 2/2 (100) | IgM = 13/1,948 (0.7) | Low |
| IgG = 979/1,948 | ||||||||||
| Neutralizing Ab = 13/1,948 | ||||||||||
| Adesina & Adeniji [28] | Recruitment of male and female participants in Ile-Ife. | 179 | Cross-sectional | <1 - 90 | Malaria: microscopy | NAa | 9/44 (20.5) | 37/135 (27.4) | IgM = 46/179 (25.7) | Intermediate |
| Dengue: ELISA-IgM | ||||||||||
| Abdulaziz et al. [29] | Recruitment of male and female participants in Kano in November 2017. | 424 | Hospital-based, cross-sectional | <10 - ≥50 | Malaria: RDT | NAa | 332/343 (96.8) | 67/81 (82.7) | IgM = 399/424 (94.1) | Intermediate |
| Dengue: ELISA-IgM | ||||||||||
| Otu et al. [8] | Recruitment of male and female participants in Calabar, Cross River State between January 4 – August 24 2017. | 420 | Cross-sectional | 34 (1 - 99) | Malaria: microscopy | NAa | 16/202 (7.9) | 8/218 (3.7) | IgM/IgG = 24/420 (5.7) | Intermediate |
| Dengue: RDT-IgM/IgG | ||||||||||
| Chukwuma et al. [30] | Recruitment of male and female participants in Nnewi, Anambra State. | 96 | Cross-sectional | 0 - 5 | Malaria: microscopy | NAa | 3/17 (17.6) | 71/79 (89.9) | IgM = 74/96 (77.1) | High |
| Dengue: ELISA-IgM | ||||||||||
| Onyedibe et al. [31] | Recruitment of participants in Jos, Plateau State and Maiduguri, Borno State from March-August, 2014. | 529 | Cross-sectional | <18 - 57 | Malaria: microscopy, Dengue: ELISA-NS1/IgM, ELISA-IgM/IgG, ELISA-IgM & ELISA-IgG. | NAa | 83/412 (20.1) | 28/117 (23.9) | IgG = 23/529 (4.3) | Low |
| IgM = 29/529 (5.4) | ||||||||||
| NS1 = 7/529 (1.3) | ||||||||||
| IgM/IgG = 39/529 (7.3) | ||||||||||
| IgM/NS1 = 13/529 (2.5) | ||||||||||
| Oyero & Ayukekbong [32] | Recruitment of male and female participants in Ibadan, Oyo State from January - April, 2013. | 188 | Cross sectional | 4 - 82 (31) | Malaria: microscopy, Dengue: ELISA-NS1, ELISA-IgM & ELISA-IgG. | NAa | IgG = 94/144 (65.3) | IgG = 44/44 (100) | IgG = 138/188 (73) | Low |
| NS1 = 48/144 (33) | NS1 = 19/44 (43) | NS1 = 67/188 (35) | ||||||||
| Ayolabi et al. [33] | Recruitment of male and female participants in Lagos, Lagos State from April - August, 2018. | 130 | Cross sectional | ≤60 | Dengue: RT-PCR/Sequencing | DENV 1 - 3 | NAa | NAb | RNA = 11/130 (8.5) | Low |
anot available, bnot applicable, caccessible.
SD, standard deviation; FCT, federal capital territory; ELISA: enzyme linked immunosrobent assay; IgG, immunoglulin G; NA: nucleic acid; DENV, Dengue virus; IgM, immunoiglobulin M; NS1, non-structural protein-1; RNA, ribonucleic acid; RT-PCR, reverse transcriptase-polymerase chain reaction; WNV, West Nile virus; PRNT, plaque reduction neutralization test; RDT, rapid diagnostic test.
A total of 6,210 participants from 21 studies across Nigeria were recruited to participate in the several studies included in the systematic review. Based on sample size across each geopolitical zones of Nigeria, North-central had the highest sample size included in the study (1,752), followed by North-central (1,251), North-west (1,422), South-west (1,171), North-east (1,015), South-south (754) and South-east (96) (Table 2).
Table 2. Summary of prevalence study characteristics of Dengue virus infection in Nigeria.
| Features | Categories | Number of studies | Number of participants | Number of Dengue cases (%) | P-value | Chi-square |
|---|---|---|---|---|---|---|
| Geo-political zone | North-east | 4a, b | 1,015 | 93 (9.2) | <0.0001 | 626.1 |
| North-west | 4a | 1,422 | 534 (37.6) | |||
| North-central | 8a, b | 1,752 | 413 (23.5) | |||
| South-west | 7a | 1,171 | 402 (34.3) | |||
| South-east | 1 | 96 | 74 (77.1) | |||
| South-south | 3a | 754 | 30 (3.9) | |||
| Laboratory protocol for Dengue detection | RDT | 1 | 420 | 24 (5.7) | ND | ND |
| PCR | 3 | 323 | 25 (7.7) | |||
| ELISA | 17 | 5,465 | 1,303 (23.8) | |||
| PRNT | 1 | 1,948 | 13 (0.67) |
aIncluded a study with study areas from six different geographical regions.
bIncluded a study with study areas from two different geographical regions.
RDT, rapid diagnostic test; ND, not done; PCR, polymerase chain reaction; ELISA, enzyme linked immunosorbent assay; PRNT, plaque reduction neutralization test.
2. Summary of prevalence study characteristics of Dengue virus infection in Nigeria
The prevalence of DENV ranged from 3.9% to 77.1% among the analyzed studies (Table 2). Stratified analysis showed a wide variation in the prevalence of DENV based on the region of study and laboratory protocol for Dengue detection. DENV prevalence was significantly highest in South-east of Nigeria 74 (77.1%), followed by North-west 534 (37.6%), South-west 402 (34.3%), North-central 413 (23.5%), North-east 93 (9.2%), while the least was South-south 30 (3.9%) (P <0.0001) (Table 2). Among all the eligible studies, the prevalence of DENV was estimated as 1,303 (23.8%), 25 (7.7%), 24(5.7%) and 13 (0.67%) by enzyme linked immunosorbent assay (ELISA), RT-PCR, rapid diagnistic test (RDT) and plaque reduction neutralization test (PRNT), respectively (Table 2).
3. Pooled prevalence of Dengue virus infection in Nigeria
The prevalence of DENV infection varied depending on the clinical presentation and biomarkers of infection (Table 3). The pooled IgM seroprevalence of DENV infection among the study participants was 16.8% (95% confidential interval [CI]: 15.8 - 17.9), IgG 34.7% (95% CI: 33.2 - 36.2) and IgM/IgG was 6.1% (95% CI: 4.9 - 7.5) (Table 3). The prevalence of DENV NS1, DENV IgM/NSI combined seropositivity, and DENV IgG/NS1 combined seropositivity among the study participants were 7.7% (95% CI: 6.3 - 9.4), 7.8% (95% CI: 4.6 - 12.4) and 7.0% (95% CI: 3.7 - 11.9), respectively (Table 3). The pooled prevalence of DENV RNA positivity was 7.7% (95% CI: 5.1 - 11.2) while, the prevalence of DENV neutralizing antibody was 0.7% (95% CI: 0.36 - 1.1). All the antibody-based studies had significantly high heterogeneity (I2 >90%, P <0.05), while the NS1 and PCR-based studies have low heterogeneity (I2 <25%, P >0.05).
Table 3. Pooled prevalence of Dengue virus infection in Nigeria.
| DENV biomarkers | Number of studies | Number of participants | Number of Dengue cases | Prevalence (%, 95% CI) | H | I2 | P-value |
|---|---|---|---|---|---|---|---|
| IgM seroprevalence | 14 | 4,965 | 834 | 16.8 (15.8 - 17.9) | 551 | 92.9 | <0.0001 |
| IgG seroprevalence | 10 | 4,107 | 1,424 | 34.7 (33.2 - 36.2) | 389 | 92.5 | <0.0001 |
| IgM/IgG seroprevalence | 4 | 1,458 | 89 | 6.1 (4.9 - 7.5) | 298 | 96.4 | <0.0001 |
| NS1 prevalence | 6 | 1,161 | 90 | 7.7 (6.3 - 9.4) | 3.09 | 19.3 | 0.19 |
| IgM/NS1 seroprevalence | 2 | 620 | 16 | 7.8 (4.6 - 12.4) | 1.53 | 20.1 | 0.13 |
| IgG/NS1 seroprevalence | 1 | 171 | 12 | 7.0 (3.7 - 11.9) | NA | NA | NA |
| Viral RNA prevalence | 3 | 323 | 25 | 7.7 (5.1 - 11.2) | 3.16 | 16.1 | 0.06 |
| Neutralizing antibody prevalence | 1 | 1,948 | 13 | 0.7 (0.36 - 1.1) | NA | NA | NA |
DENV, Dengue virus; CI, confidence interval; H, heterogeity index; IgM, immunoglobulin M; IgG, immunoglobulin G; NSI, non-structural protein-1; RNA, ribonucleic acid; NA, not available.
4. Pooled Socio-demographic risk factors of Dengue virus IgG seropositivity in Nigeria
Out of 12 eligible studies, 775 participants were ≤20 years of age, 881 participants 21 - 30 years and 836 participants ≥30 years. Those between 21 - 30 years had odds ratio (OR) of 0.66 (95% CI: 0.54 - 0.81) and was not significantly associated with pooled DENV IgG seroprevalence when compared with participants ≥30 years (P >0.05). Out of 13 eligible studies, there were 1,259 male and 1,284 pooled participants (Table 4). The males had OR of 0.83 (95% CI: 0.71 - 0.98) and represent a significant risk factor of DENV IgG pooled seroprevalence when compared with female participants (P = 0.0023). Based on the marital status of the 3 eligible studies, there were 295 married and 178 singles. Of this, the married participants had OR of 1.73 (95% CI: 1.15 - 2.60) and represent a significant risk factor of DENV IgG pooled seroprevalence when compared with the singles (P = 0.008). Based on employment status on the 7 eligible studies, there were 892 employed and 447 unemployed participants. The employed participants had an OR of 0.34 (95% CI: 0.27 - 0.44) and represent a significant risk factor of DENV IgG pooled seroprevalence when compared with the unemployed participants (P <0.0001) (Table 4).
Table 4. Pooled sociodemographic risk factors of Dengue virus seropositivity in Nigeria.
| Variables | Categories | No. of pooled participants (no. of studies) | No. of pooled Dengue positive cases (%) | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| Age (years) | ≤20 | 915 (14) | 293 (32.0) | 1.18 (0.98 - 1.41) | 0.08 |
| 21 - 30 | 981 (14) | 309 (31.5) | 1.15 (0.96 - 1.38) | 0.128 | |
| >30 | 914 (14) | 365 (39.9) | 1.00 (reference) | ||
| Gender | Male | 1,395 (15) | 486 (34.8) | 0.79 (0.68 - 0.92) | 0.0023a |
| Female | 1,466 (15) | 584 (39.8) | 1.00 (reference) | ||
| Marital Status | Married | 295 (3) | 113 (38.7) | 1.73 (1.15 - 2.60) | 0.008a |
| Single | 178 (3) | 47 (26.4) | 1.00 (reference) | ||
| Residence location | Rural | 186 (4) | 64 (34.4) | 0.79 (0.54 - 1.14) | 0.201 |
| Urban | 347 (4) | 139 (40.1) | 1.00 (reference) | ||
| Level of education | Primary | 276 (4) | 172 (62.3) | 1.27 (0.95 - 1.77) | 0.101 |
| Secondary | 143 (4) | 56 (39.2) | 0.51 (0.34 - 0.74) | 0.005a | |
| Tertiary | 421 (4) | 236 (56.1) | 1.00 (reference) | ||
| No formal education | 111 (4) | 66 (59.5) | 1.149 (0.75 - 1.76) | 0.519 | |
| Employment status | Employed | 892 (7) | 363 (40.7) | 0.34 (0.27 - 0.44) | <0.0001a |
| Unemployed | 447 (7) | 298 (66.7) | 1.00 (reference) | ||
| Proximity of residence to refuse dumpsite | No | 396 (3) | 213 (53.8) | 0.51 (0.38 - 0.69) | <0.0001a |
| Yes | 361 (3) | 251 (69.5) | 1.00 (reference) | ||
| Proximity of residence to open gutters/ponds/stagnant water | No | 428 (2) | 298 (69.6) | 1.19 (0.81 - 1.74) | 0.377 |
| Yes | 164 (2) | 108 (65.9) | 1.00 (reference) | ||
| Frequently Opened household water containers | No | 306 (2) | 229 (74.8) | 4.32 (3.05 - 6.13) | <0.0001a |
| Yes | 287 (2) | 117 (40.8) | 1.00 (reference) | ||
| Use of mosquito-treated bed net | No | 303 (3) | 189 (62.4) | 1.02 (0.75 - 1.39) | 0.895 |
| Yes | 412 (3) | 255 (61.9) | 1.00 (reference) | ||
| Frequent wearing of trousers and long sleeve shirts | No | 177 (2) | 61 (34.5) | 2.22 (1.36 - 3.62) | 0.0024a |
| Yes | 172 (2) | 33 (19.2) | 1.00 (reference) | ||
| Use of indoor daily mosquito insecticide spray | No | 224 (3) | 104 (46.4) | 0.61 (0.45 - 0.84) | 0.0021a |
| Yes | 546 (3) | 320 (58.6) | 1.00 (reference) | ||
| Malaria status | Negative | 1,749 (11) | 613 (35.0) | 1.25 (1.06 - 1.47) | 0.009a |
| Positive | 1,013 (11) | 306 (30.2) | 1.00 (reference) |
aSignificant association (P <0.05).
OR, odds ratio; CI, confidence interval.
Based on malaria parasitemia status on the 10 eligible studies, there were 1,605 and 969 participants who had negative and positive malaria results, respectively. Those without malaria (negative) had an OR of 5.65 (95% 4.73 - 6.7) and represent a significant risk factor of DENV IgG pooled seroprevalence when compared with those with malaria positive results (P <0.0001) (Table 4).
Out of the 361 and 396 participants who reside and who do not reside in proximity to waste dumpsites from 3 eligible studies, those who do not have OR of 0.51 (95% CI: 0.38 - 0.69; P <0.0001). Out of the 287 and 306 participants who frequently opened household water containers and those who do not from 2 eligible studies, those who do not have an OR of 4.32 (95% CI: 3.05 - 6.13; P <0.0001). Out of the 172 and 177 participants who frequent wearing trousers and long sleeve shirts and those who do not from 2 eligible studies, those who do not have an OR of 2.22 (95% 1.36 - 3.62; P <0.0001) (Table 4).
Discussion
The mosquito-borne DENV is endemic in Nigeria and most parts of the tropics. However, the pattern of distribution and prevalence of DENV in all 6 geopolitical regions of Nigeria have not been sufficiently studied. To address this gap, the current study provided a systematic review of DENV infection prevalence studies conducted in Nigerian residents. To the best of our knowledge, this is the first systemic review of DENV infection in Nigeria that reported a relatively high prevalence as well as varied heterogeneity based on sampling technique, the participation level of the subjects and laboratory method for DENV investigation.
Epidemiological surveys could be done for 10 years span. In the light of this, previous studies [11,24,33,34] have recommended a thorough and consistent method for the surveillance of DENV and other arboviruses to determine periods of epidemics and make proper decisions on how to design and implement appropriate public healthcare needs at community levels. Periodic reports of data on DENV infection prevalence and epidemics have been advocated to be integrated as part of the surveillance system to prevent further or control transmission [34].
1. Spatial distribution of DENV infection across geographical zones of Nigeria
Out of 36 states and the federal capital of Nigeria (Abuja), only 13 have had studies conducted for the seroprevalence and molecular analysis of DENV infection in febrile patients and other populations. Despite the non-availability of data from the other 23 states, it could be suggested that DENV might have been transmitted within these areas as well, especially when considerations are made for the constant inter-state migration, movements and transportation by Nigerians. Human transportation has been reported to be another contributing factor in the expansion of the ecological niche of DENV vectors in Nigeria [35]. The eggs of Aedes aegypti was reported to have been favourably transported from South-east Asia through Europe to Africa due to economic ties between the countries of these continents [36].
This speculation confirms the hypothesis that DENV has dispersed across geopolitical zones of Nigeria, affecting all areas, especially in urban, semi-urban and rural regions that are close to endemic regions of the virus [37]. This argument could further be valid when we consider the routes of DENV transmission, which is common to those of other mosquito-borne viruses such as Chikungunya and yellow fever whose mechanical vectors are present all year round [37]. The interstate transmission of DENV may be facilitated rapidly due to the wide range of mechanical vectors and reservoirs involved [37].
The spatial differences in DENV seroprevalence were analyzed by subgrouping DENV studies based on geopolitical zones where the studies were conducted. A significant association was observed between the pooled DENV seroprevalence and the geopolitical regions of Nigeria. This present study revealed heterogeneity in DENV prevalence across the geopolitical zones. The seroprevalence of DENV IgG was greatest in South-east Nigeria (77.1%), followed by North-west (37.6%), South-west (34.3%), Northcentral (23.5%), and least in South-southern Nigeria (3.9%). Similar geographical variation and heterogeneity of DENV seroprevalence were documented in a meta-analysis study conducted in Sudan, where viral prevalence was at its peak in Central (43%), Western (36%), Northern (24%) and Eastern (23%) zones of Sudan [38]. The relatively high prevalence of DENV in South-east and South-west regions of Nigeria could be due to the proximity of localities to the dense rainforest in such areas which provide enabling breed sites for DENV vectors [11]. Whereas, the low prevalence in South-south zone also known as the riverine areas of Nigeria could be due to the relatively low forest cover in these areas. The primary host of DENV virus (i.e., non-human primates [NHP]) are abundantly found in the forests. So, the absence or low forest presence and NHP in this zone could explained lesser or no sylvan-to-urban spillover of Aedes aegypti and DENV [39].
Western and Central parts of Africa have been reported to have the worst hits of DENV infection owing to the poor infrastructural amenities in urban settings, especially the deficient healthcare systems. This, together with environmental and climatic factors facilitate the transmission of DENV across vectors and humans [39].
2. Laboratory protocols used by eligible studies and cross-reactivity of flaviviruses
Cross-reactions remain one of the major obstacles in serological investigations for DENV and other flaviviruses. Viral neutralization assays, considered as the reference standard serologic test for DENV [37], was performed in approximately 14% of the general population involved in the seroprevalence studies in this review. However, the main challenge associated with the use of plaque reduction neutralization test (PRNT) is its reduced sensitivity for serological investigations, as it can only measure immunoglobulins at levels that can neutralize flaviviruses; hence, its use is restricted in weakly-exposed individuals [37].
Methodologically, most of the serological methods used in the eligible studies utilized the enzyme immunoassay (EIA) protocols to detect DENV antibodies. Although this protocol is convenient to use, sensitive and easily accessible, its reliability in DENV seroepidemiology is potentially uncertain especially in regions where the prevalence of co-circulation of similar viruses that share significant nucleotide homology with DENV. For instance, West Nile virus (WNV) is observed to be endemic in Nigeria based on its relatively high prevalence of 14.3% [37]. Besides, vaccine-derived, as well as antibodies against yellow fever following infection, can cross-react with anti-DENV in Yellow fever Virus (YFV) endemic regions such as Nigeria [37].
The detection rate DENV infection of ELISA-based NS1 and RT-PCR assays had similar values (7.7%). The NS1 assay has severally been favourable comparable results in detecting DENV infection than RT-PCR. This could be due to the maximum sensitivity of NS1 antigen detection by ELISA by 1st and 2nd days of fever, while that of real-time RT-PCR in three days post-onset of fever [11]. This simple and readily available (in point-of-care test format) can be utilized by resource-limited settings for the serodiagnosis of DENV infection at the community levels [11].
3. Pooled seroprevalence of DENV infection in Nigeria
Analysis of study groups based on location and laboratory protocol performed to investigate DENV infection were done to determine accurate estimates of the DENV prevalence. Variation between these laboratory protocols was investigated, DENV IgG was used to assess past exposure to infection, while DENV IgM to assess active or acute infection [11]. The present systematic review on DENV infection studies revealed an overall pooled DENV-IgG prevalence of 32.8% (95% CI: 31.4 - 34.3) which was significantly higher compared to DENV-IgM (16.8% [95%CI: 15.8 - 17.9]) and DENV-RNA (7.3% [95% CI: 4.0 - 11.8%]). These indicative of the transmission of DENV to be most likely to be both endemic and epidemic. These findings are similar to those reported in a systematic review of DENV prevalence conducted in Sudan which documented significantly lower DENV-IgM prevalence 22% (95% CI: 13 - 31%) compared to that of DENV-IgG (38% [95% CI: 26 - 51%]) [38], Middle East and North Africa with DENV-IgG prevalence ranging from 0 - 61% [7] and in an African-based systematic study which also reported a much higher prevalence of DENV-IgG (24.8% [95% CI: 13.8 - 37.8%]) compared to DENV-IgM (10.8% [95% CI: 3.8 - 20.6%]) and DENV-RNA (8.4% [95% CI: 3.7 - 14.4%]) [40].
DENV RNA is detectable in infected individuals few days after infection till the sixth day post-onset of clinical symptoms such as fever and headache [37]. DENV IgM is measurable from third-day post symptom onset till the third month, while IgG is detectable from day 10 post-onset of symptoms and can remain detectable for months and years [11]. However, a secondary acute DENV infection for 2 or more times can result in an immune response that generates IgG at a rapid rate [11].
From the present systematic review, it was not surprising that the proportion (40.6% [2,006/4,938]) of febrile participants had a significantly higher prevalence of DENV IgG compared to that (36.7% [212/577]) of their healthy counterparts. Most of the febrile participants complained of non-specific symptoms (especially headache and fever) had either serological or molecular evidence of DENV infection. Hence, DENV investigations should be considered for one of the differentials tests for fever and headache. Since these symptoms are not specifically associated with DENV, so individuals infected with DENV can be misdiagnosed with other febrile illnesses, especially in localities that are endemic with other Flaviviruses (e.g., WNV, YFV) [37]. A similar finding of DENV prevalence was reported as being higher in febrile individuals compared to those that are healthy in a meta-analysis and systematic review which involved studies collated from Central, Eastern, Northern, Southern and Western regions of Africa, although the proportions between the study groups were not significant [6].
Based on the non-specific presentations of DENV febrile illnesses, it is recommended that the use of RT-PCR for DENV testing is crucial for differential diagnosis. Pairing the serologic and genomic outcomes of DENV diagnosis can enhance the rate of detecting DENV. However, out of the 21 studies included in this review, a study used a combined protocol of serological and RT-PCR assays for the detection of DENV infection [23], while a second study confirmed the outcome of its ELISA results using PRNT [27].
In consolidation of the serological detection of DENV, it is essential to adopt the antigen-based enzyme immunoassays (EIAs) which include the recombinant non-structural protein 1 (NS1) antigen especially in localities where other genetically related flaviviruses co-circulate. Our systematic review reported a pooled DENV-NS1, DENV-IgM/NS1 and DENV-IgG/NS1 seroprevalence rates of 2.4% (95% CI: 1.5 - 3.5), 7.8% (95% CI: 4.6 - 12.4) and 7.0% (95% CI: 3.7 - 11.9) respectively in febrile cases. This assay has been used to improve the diagnostic performance of serologic markers of DENV and it is significant in the differential diagnosis of flavivirus infections by targeting unique receptors during the active, acute or infective stage of DENV infection.
4. Pooled risk factors for anti-DENV IgG seropositivity
Several sociodemographic and ecologic factors in Nigeria promote the spread of the DENV. In the present study, sociodemographic factors including marital status (P = 0.008), gender (P = 0.026), educational level (P = 0.005) and occupation status of participants (P <0.0001) were significantly associated with pooled DENV seropositivity. Of note, the pooled DENV IgG seroprevalence in this study was relatively higher in those >30 years of age. This may be indicative of the relative protection in older participants. The increase in DENV antibodies with age from adulthood suggests that infection occurs from middle to older age. Also, those ≤20 years of age may be involved in more domestic (indoor activities) which increased their susceptibility to bitten by mosquitoes carrying DENV unlike their counterparts between 21 - 30 years of age who could be involved in more outdoor activities [11].
Despite the ubiquitous nature of the mechanical vector, its overall population density is greater in regions close to dense rain forest. This could explain why most of the eligible studies with higher DENV prevalence were from the South-east, South-west zone and other neighbouring states. Furthermore, enzootic transmission, high humidity, temperature and rainfall patterns, which vary across geopolitical regions have been implicated in influencing the population density of Aedes mosquitoes and DENV infections. The influence of the level of literacy on the seroprevalence of anti-DENV could be due to the roles of public enlightenment and education in improving people knowledge and awareness about DENV infection. Hence, increasing the frequency of seeking medical assistance/healthcare which likely minimizes the risk of contracting DENV.
On the other hand, the proximity of residence to refuse dumpsite (P <0.0001), frequently opened household water containers (P <0.0001), the frequent wearing of trousers and long sleeve shirts (P = 0.0024), use of indoor mosquito insecticide spray daily (P = 0.0021) and malaria status (P = 0.0024) were significant risk factors of DENV seropositivity in humans. In the same light, previous studies have associated these factors especially the use of open water vessels with increased breeding sites of A. aegypti [11,28].
The high DENV prevalence observed in this review may indicate the poor level of public health interventions to effectively control vector transmission. This can be further explained by the weak healthcare systems in middle to low-income countries such as Nigeria which leads to the inability of sustaining efficient and durable interventions. The high rate of urbanization in Nigeria can equally explain the high prevalence of DENV. The burden of DENV infection has been reported to be associated with the rising rate of urbanization, coupled with poor infrastructure in tropical and subtropical areas [10]. The mechanism behind the association between the rise in DENV infection prevalence in recent time could be due to the increased vector population and breeding sites as a result of urbanization and deforestation. The exponential increase of these breeding sites is favoured by climatic as well as environmental patterns of Nigeria that encourage the development of the mosquito's life cycle, hence favouring enhanced DENV transmission. Indeed, A. aegypti thrive better in warm and humid climatic conditions which typically exist in most parts of Nigeria. This favours the survival of the mosquito eggs compared to the cold and temperate climatic regions which encourages hibernation while suppressing conservation and development.
5. Limitations of study
Several limitations were associated with this review. First, most eligible studies lacked comprehensive data and there was no conformity in documented studies due to significant heterogeneity in the determination of DENV prevalence across selected studies. The heterogeneity may be as a result of diverse geopolitical regions, study population, dissimilar sample size, study design and choice of laboratory protocol. To overcome this limitation, there is the need for nationwide serological surveillance of DENV infection using culture-based microneutralization assay (gold standard) to simultenously determine the prevalence of DENV infection by geopolitical zones. This laboratory technique excludes the possibility of cross-reaction with other flaviviruses.
Moreover, our study could not adequately study the influence of climatic and environmental factors that influence the breeding sites of DENV vectors because these factors were not considered by the eligible studies in this systematic review. Second, all studies but two research works that utilized ELISA to detect anti-DENV IgM and anti-DENV IgG did not confirm positive cases using either RT-PCR or PRNT as reference protocols.
In conclusion, the findings from our study reveal the endemicity of DENV infection in Nigerian residents which are driven by various sociodemographic and ecologic factors. This necessitates the need for the adoption of the One Health strategic approach to effectively and efficiently halt the Aedes mosquito population and spread of DENV. Also, there is a need to constitute and sustain the DENV and other arboviruses national surveillance system, re-train healthcare providers on accurate DEN diagnosis and support more studies to generate comprehensive data on DENV phylogenetics in all hosts within its transmission cycles. Our findings further indicate the need to encourage adequate measures of controlling DENV and its vectors to minimize infection within-country and possible international transmission.
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: INA, AUE.
- Formal analysis: AUE, INA.
- Investigation: INA, AOE, AUE, CMU, AD, BNE, MABM, JON.
- Methodology: AUE, INA, BIS, YU, AUE, MSA, HY, SO AMG.
- Supervision: INA, AUE.
- Writing - original draft: AUE, INA, AOE, AMG, BIS, YU, CMU, AD, BNE, MABM, AUE, MSA, HY, SO.
- Writing - review & editing: AUE, INA, AMG, AOE, BIS, YU, MS, CMU, AD, BNE, MABM, AUE, MSA, HY, SO.
References
- 1.Powell JR. Mosquito-borne human viral diseases: Why Aedes aegypti? Am J Trop Med Hyg. 2018;98:1563–1565. doi: 10.4269/ajtmh.17-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katzelnick LC, Fonville JM, Gromowski GD, Bustos Arriaga J, Green A, James SL, Lau L, Montoya M, Wang C, VanBlargan LA, Russell CA, Thu HM, Pierson TC, Buchy P, Aaskov JG, Muñoz-Jordán JL, Vasilakis N, Gibbons RV, Tesh RB, Osterhaus AD, Fouchier RA, Durbin A, Simmons CP, Holmes EC, Harris E, Whitehead SS, Smith DJ. Dengue viruses cluster antigenically but not as discrete serotypes. Science. 2015;349:1338–1343. doi: 10.1126/science.aac5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reich NG, Shrestha S, King AA, Rohani P, Lessler J, Kalayanarooj S, Yoon IK, Gibbons RV, Burke DS, Cummings DA. Interactions between serotypes of dengue highlight epidemiological impact of cross-immunity. J R Soc Interface. 2013;10:20130414. doi: 10.1098/rsif.2013.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) Dengue and severe dengue. [Accessed 26 August 2020]. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- 5.Kuno G. A re-examination of the history of etiologic confusion between Dengue and Chikungunya. PLoS Negl Trop Dis. 2015;9:e0004101. doi: 10.1371/journal.pntd.0004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simo FBN, Bigna JJ, Kenmoe S, Ndangang MS, Temfack E, Moundipa PF, Demanou M. Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies. Sci Rep. 2019;9:13626. doi: 10.1038/s41598-019-50135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphrey JM, Cleton NB, Reusken CB, Glesby MJ, Koopmans MP, Abu-Raddad LJ. Dengue in the Middle East and North Africa: a systematic review. PLoS Negl Trop Dis. 2016;10:e0005194. doi: 10.1371/journal.pntd.0005194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otu AA, Udoh UA, Ita OI, Hicks JP, Egbe WO, Walley J. A cross-sectional survey on the seroprevalence of dengue fever in febrile patients attending health facilities in Cross River State, Nigeria. PLoS One. 2019;14:e0215143. doi: 10.1371/journal.pone.0215143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otu A, Ebenso B, Etokidem A, Chukwuekezie O. Dengue fever - an update review and implications for Nigeria, and similar countries. Afr Health Sci. 2019;19:2000–2007. doi: 10.4314/ahs.v19i2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17:1349–1354. doi: 10.3201/eid1708.101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasir IA, Agbede OO, Dangana A, Baba M, Haruna AS. Dengue virus non-structural Protein-1 expression and associated risk factors among febrile Patients attending University of Abuja Teaching Hospital, Nigeria. Virus Res. 2017;230:7–12. doi: 10.1016/j.virusres.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Rather IA, Parray HA, Lone JB, Paek WK, Lim J, Bajpai VK, Park YH. Prevention and control strategies to counter dengue virus infection. Front Cell Infect Microbiol. 2017;7:336. doi: 10.3389/fcimb.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebman KA, Stoddard ST, Morrison AC, Rocha C, Minnick S, Sihuincha M, Russell KL, Olson JG, Blair PJ, Watts DM, Kochel T, Scott TW. Spatial dimensions of dengue virus transmission across interepidemic and epidemic periods in Iquitos, Peru (1999-2003) PLoS Negl Trop Dis. 2012;6:e1472. doi: 10.1371/journal.pntd.0001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatcher EL, Zhdanov SA, Bao Y, Blinkova O, Nawrocki EP, Ostapchuck Y, Schäffer AA, Brister JR. Virus Variation Resource - improved response to emergent viral outbreaks. Nucleic Acids Res. 2017;45:D482–90. doi: 10.1093/nar/gkw1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustapha JO, Emeribe AU, Nasir IA. Survey of malaria and anti-dengue virus IgG among febrile HIV-infected patients attending a tertiary hospital in Abuja, Nigeria. HIV AIDS (Auckl) 2017;9:145–151. doi: 10.2147/HIV.S134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingsley UB, Tabitha VS, Lohya N, Joseph AOA. Dengue Virus Antibodies in Patients Presenting with Pyrexia attending Jos University Teaching Hospital, Jos, Nigeria. Saudi J Pathol Microbiol. 2018;3:47–55. [Google Scholar]
- 17.Sule WF, Fadamita TO, Lawal OA, Adebimpe WO, Opaleye OO, Oluwyelu DO. Probable primary and secondary dengue viral infections and associated host factors among university undergraduates in Osun State, Nigeria. Alexandria J Med. 2019;55:25–30. [Google Scholar]
- 18.Idoko MO, Ado SA, Umoh VJ. Prevalence of Dengue virus and Malaria in patients with Febrile complaints in Kaduna Metropolis, Nigeria. Br Microbiol Res J. 2015;8:343–347. [Google Scholar]
- 19.Oladipo EK, Amanetu C, Gbadero TA, Oloke JK. Detectable anti-dengue virus IgM antibodies among healthy individuals in Ogbomoso, Oyo State, Nigeria. Am J Infect Dis. 2014;10:64–67. [Google Scholar]
- 20.Suchi NK, Mohammed HI, Ademola AO, Rinmecit PG. Parallel and concurrent infection of Dengue virus and Plasmodium falciparum among patients with febrile illnesses attending Bingham University Health Centre, Karu, Nigeria. Int J Trop Dis Health. 2020;41:45–51. [Google Scholar]
- 21.Mahmoud MA, Gwarzo MY, Sarkinfada F. Dengue virus immunoglobulinaemia among pregnant women and blood donors in Nigeria: need for integration into disease management policy. J Health Commun. 2018;3:40. [Google Scholar]
- 22.Oladipo EK, Awoyelu EH, Oloke JK. Yellow fever, Dengue fever and West Nile viruses co-circulation in Ogbomoso. IJMDC. 2018;2:50–54. [Google Scholar]
- 23.Kolawole OM, Seriki AA, Irekeola AA, Bello KE, Adeyemi OO. Dengue virus and malaria concurrent infection among febrile subjects within Ilorin metropolis, Nigeria. J Med Virol. 2017;89:1347–1353. doi: 10.1002/jmv.24788. [DOI] [PubMed] [Google Scholar]
- 24.Dawurung JS, Baba MM, Stephen G, Jonas SC, Bukbuk DN, Dawurung CJ. Serological evidence of acute dengue virus infection among febrile patients attending Plateau State Specialist Hospital Jos, Nigeria. Rep Opinion. 2010;2:71–76. [Google Scholar]
- 25.Onoja AB, George UE, Gadzama IS. Co-circulation of dengue virus 1 and 2 several years after single serotype detection in Cross River State, Nigeria. Sokoto J Vet Sci. 2020;18:167–170. [Google Scholar]
- 26.Hamisu TM, El-Yuguda AD, Abubakar MB, Shettima YM, Maina MM, Zanna MY, Baba SS, Andrew A, Terhemen IC. Prevalence of Dengue virus infection among febrile outpatients attending University of Maiduguri Teaching Hospital in Borno State, Nigeria. IOSR-JDMS. 2017;16:155–159. [Google Scholar]
- 27.Baba M, Saron MF, Vorndam Vance VA, Adeniji JA, Diop O, Olaleye D. Dengue virus infections in patients suspected of malaria/typhoid in Nigeria. J Am Sci. 2009;5:129–134. [Google Scholar]
- 28.Adesina OA, Adeniji JA. Incidence of Dengue virus infections in febrile episodes in Ile-Ife, Nigeria. Afr J Infect Dis. 2016;10:21–24. [Google Scholar]
- 29.Abdulaziz MM, Ibrahim AI, Ado M, Ameh C, Umeokonkwo C, Sufyan MB, Balogun MS, Ahmed SA. Prevalence and factors associated with dengue fever among febrile patients attending secondary health facilities in Kano metropolis, Nigeria. Afr J Clin Exp Microbiol. 2020;21:340–348. [Google Scholar]
- 30.Chukwuma GO, Audu JS, Chukwuma OM, Manafa PO, Ebugosi RS, Akulue JC, Aneke JC, Ahaneku GI, Nchinda GW, Esimone CO. Seroprevalence of dengue virus among children with febrile illness in Nnewi, Nigeria. J Med Res. 2018;4:24–30. [Google Scholar]
- 31.Onyedibe K, Dawurung J, Iroezindu M, Shehu N, Okolo M, Shobowale E, Afolaranmi T, Dahal S, Maktep Y, Pama P, Isa S, Egah D. A cross sectional study of dengue virus infection in febrile patients presumptively diagnosed of malaria in Maiduguri and Jos plateau, Nigeria. Malawi Med J. 2018;30:276–282. doi: 10.4314/mmj.v30i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed A, Ali Y, Elmagboul B, Mohamed O, Elduma A, Bashab H, Mahamoud A, Khogali H, Elaagip A, Higazi T. Dengue fever in the Darfur Area, Western Sudan. Emerg Infect Dis. 2019;25:2126. doi: 10.3201/eid2511.181766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oyero OG, Ayukekbong JA. High dengue NS1 antigenemia in febrile patients in Ibadan, Nigeria. Virus Res. 2014;191:59–61. doi: 10.1016/j.virusres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Ayolabi CI, Olusola BA, Ibemgbo SA, Okonkwo GO. Detection of Dengue viruses among febrile patients in Lagos, Nigeria and phylogenetics of circulating Dengue serotypes in Africa. Infect Genet Evol. 2019;75:103947. doi: 10.1016/j.meegid.2019.103947. [DOI] [PubMed] [Google Scholar]
- 35.Lutomiah J, Barrera R, Makio A, Mutisya J, Koka H, Owaka S, Koskei E, Nyunja A, Eyase F, Coldren R, Sang R. Dengue Outbreak in Mombasa City, Kenya, 2013-2014: Entomologic Investigations. PLoS Negl Trop Dis. 2016;10:e0004981. doi: 10.1371/journal.pntd.0004981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Althouse BM, Hanley KA, Diallo M, Sall AA, Ba Y, Faye O, Diallo D, Watts DM, Weaver SC, Cummings DA. Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in Senegal. Am J Trop Med Hyg. 2015;92:88–97. doi: 10.4269/ajtmh.13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oderinde BS, Mora-Cárdenas E, Carletti T, Baba MM, Marcello A. Prevalence of locally undetected acute infections of Flaviviruses in North-Eastern Nigeria. Virus Res. 2020;286:198060. doi: 10.1016/j.virusres.2020.198060. [DOI] [PubMed] [Google Scholar]
- 38.Elduma AH, LaBeaud AD. A Plante J, Plante KS, Ahmed A. High seroprevalence of Dengue virus infection in Sudan: systematic review and meta-analysis. Trop Med Infect Dis. 2020;5:120. doi: 10.3390/tropicalmed5030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young KI, Mundis S, Widen SG, Wood TG, Tesh RB, Cardosa J, Vasilakis N, Perera D, Hanley KA. Abundance and distribution of sylvatic dengue virus vectors in three different land cover types in Sarawak, Malaysian Borneo. Parasit Vectors. 2017;10:406. doi: 10.1186/s13071-017-2341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simo FBN, Bigna JJ, Kenmoe S, Ndangang MS, Temfack E, Moundipa PF, Demanou M. Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies. Sci Rep. 2019;9:13626. doi: 10.1038/s41598-019-50135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]