Abstract
Background
There have been recent proposals to categorize healthcare-associated infections (HCAIs) separately from community-acquired infections (CAIs). The aim of this study was to compare the antibiotic resistance of pathogens causing CAIs, HCAIs, and hospital-acquired infections (HAIs) in Korea, and to investigate the need for different empirical antibiotics therapy for CAIs and HCAIs.
Materials and Methods
This prospective study was conducted in a university hospital between March and December 2019. Inpatients who underwent a bacterial culture within 2 days of hospitalization, with a Enterobacteriaceae strain identified at the infection site and available antibiotic susceptibility results, were included in the analysis. Infections were classified as CAIs, HCAIs or HAIs, depending on the source.
Results
Of the 146 patients included in the analysis, the prevalence of fluoroquinolone-resistant Enterobacteriaceae was 18.8%, 38.5%, and 55.0%; the prevalence of pathogens showing third-generation cephalosporins resistance was 8.3%, 50.0%, and 60.0%; and the prevalence of pathogens showing piperacillin-tazobactam resistance was 8.3%, 7.7%, 15.0% in the CAIs, HCAIs, and HAIs groups, respectively. The prevalence of extended-spectrum beta-lactamase-positive pathogens was 6.3%, 47.3%, and 55.0% in the CAIs, HCAIs, and HAIs group, respectively, with no significant difference between the HCAIs and HAIs groups. Resistance patterns of the HCAIs group more closely resembled those of the HAIs group than those of the CAIs group.
Conclusion
The pathogens isolated from patients with HCAIs showed resistance patterns that were more similar to those of patients with HAIs than those with CAIs. Thus, CAIs and HCAIs should be distinguished from each other when selecting antibiotic agents.
Keywords: Community-acquired infection, Healthcare-associated infection, Hospital-acquired infection, Enterobacteriaceae
Introduction
In 2018, the Korean Global Antimicrobial Resistance Surveillance System (Kor-GLASS) research team reported the emergence of antibiotic-resistant hospital-acquired and community-acquired pathogens in Korea [1]. In particular, the prevalence of hospital-acquired and community-acquired urinary tract infection (UTI)-causing Escherichia coli resistant to ciprofloxacin was 59.5% and 42.8%, respectively, and the prevalence of E. coli resistant to cefotaxime was 46.1% and 30.0%, respectively. Similarly, Fasguba et al. [2] reported a higher rate of antibiotic resistance among hospital-acquired pathogens than community-acquired pathogens. However, these results are questionable because the prevalence of antibiotic resistance among community-acquired pathogens was higher than that observed in actual clinical settings. Furthermore, Kor-GLASS reported the prevalence of hospital-acquired and community-acquired Streptococcus pneumoniae isolates resistant to sulfamethoxazole-trimethoprim to be 20.0% and 30.4%, respectively, and the prevalence of S. pneumoniae resistant to erythromycin to be 60.0% and 78.3%, respectively. In this case, the higher prevalence of antibiotic resistance among community-acquired pathogens compared to that among hospital-acquired pathogens indicates that hospital-acquired infections (HAIs) and community-acquired infections (CAIs) have not been appropriately differentiated.
Infections can be divided into either CAIs or HAIs depending on the route of infection acquisition. Kor-GLASS defines HAIs as infections caused by hospital-acquired pathogens two or more calendar days after hospitalization, including a previous hospital stay at a different hospital in the case of hospital transfer. However, trends in medical service usage are changing. For instance, the numbers of long-term care facilities and procedures or operations performed at outpatient clinics without hospitalization have been increasing. In response to these changes, antibiotic susceptibility patterns of pathogens have also changed. Accordingly, several researchers have highlighted the need to create a new category specific to “healthcare-associated infections” (HCAIs)” distinguishable from CAIs [3,4]. Toward this end, the aim of the present study was to differentiate HCAIs from CAIs, and to compare the prevalence and patterns of antibiotic resistance of pathogens causing HCAIs with those of hospital-acquired and community-acquired pathogens. The findings of this study can provide further insight into whether the inclusion of HCAIs in CAIs is appropriate from a practical perspective, such as for the selection of empirical antibiotics.
Materials and Methods
1. Study design
This prospective study was conducted at a single institution in Korea with 655 wards from March to December 2019. The subjects of this study were patients who underwent a bacterial culture test within 2 days of hospitalization among all other patients admitted to the hospital due to fever. A list of newly admitted patients was obtained from all wards during the study period and the admission records of patients who were prescribed a bacterial culture test within 2 days after admission were reviewed. A single researcher directly examined the patients for any infection and obtained their consent forms. Infections were diagnosed according to the criteria developed by the Centers for Disease Control and Prevention/National Healthcare Safety Network Surveillance Definitions for Specific Types of Infections (2019) [5].
2. Sample collection and bacterial culture test
A medical team in one of the general wards, including the intensive care unit (ICU) and emergency department, collected blood samples from patients who were prescribed to obtain a bacterial culture from the clinical department. The bacterial culture tests were performed at the Department of Laboratory Medicine of the same institution and data were collected digitally. In cases where multiple bacterial cultures were performed for the same patient, only the results of the first sample were used in the analysis. In cases where two or more bacterial strains were identified in a sample obtained from an infection site, the bacterial strain identified from the blood sample was considered the causative agent of the infection.
Blood cultures were performed with at least two types of aerobes and anaerobes. Blood samples were incubated at 36.5 - 37°C for 7 days. For urine culture tests, a mid-stream urine sample was collected and incubated until there were at least 105 colony-forming unit per milliliter of bacteria. For the sputum culture test, expectorated sputum, bronchoalveolar lavage, or tracheal aspirates were collected from the lower respiratory tract. Bacteria identified in the lower respiratory tract samples, with >25 leukocytes and <10 epithelial cells visualized by Gram staining and observed under a microscope at low magnification, were included in the analysis. For bile and ascitic fluid culture tests, bile and ascitic fluids were collected in blood culture bottles and subjected to the same incubation process described above for the blood culture test. For the wound culture test, exudates from the wound site were collected using a sterilized cotton swab, inoculated on a blood agar plate with fluid thioglycolate medium, and incubated at 37°C for 72 h.
Bacteria were identified using the Vitek-2 system (BioMérieux Vitek, Hazelwood, MO, USA). Enterobacteriaceae were tested for extended-spectrum beta-lactamase (ESBL) production using Vitek-2 cards according to the manufacturer’s instructions.
3. Patient classification
Patients with identified bacterial strains were classified into the CAI, HCAI, and HAI groups. The HAI group included patients who had been at the hospital for at least 48 h prior to the infection [6]. The HCAI group included a set of patients who did not meet the criterion for inclusion in the HAI group and satisfied the following conditions: (1) received intravenous treatment in the last 30 days [7,8,9]; (2) received wound treatment in the last 30 days [7,8,10,11]; (3) hospitalization within 30 days [12,13]; (4) hospital treatment (outpatient) within the last 30 days [14]; (5) underwent dialysis in the last 30 days [8,10,12,15,16]; (6) stayed in a hospital for at least 2 days within the last 90 days [7,8,10,12,16,17]; (7) transferred from a care home or long-term care facility [7,8,11,13,16,17]; (8) immunosuppressed, including human immunodeficiency virus (HIV)-positive patients [10,15,18,19], patients with active or metastatic cancer [10,15,16,19] patients who received radiotherapy or chemotherapy within 6 months after hospitalization for treatment of malignant tumors [10,15,19], patients with a history of solid organ or bone marrow transplantation [15,19], and patients on immunosuppressants (prednisolone at 10 mg/day or other substitutes for over 30 days) [10,15,18,19]; (9) underwent an invasive procedure or operation in the last 6 months [11,12]; (10) family members with multidrug-resistant bacterial infection [7,8]; and (11) underwent antibiotic therapy in the last month [8,10]. Patients who did not meet the aforementioned conditions for classification in either the HAI or HCAI group were included in the CAI group.
4. Bacterial strain classification
Enterobacteriaceae identified in the isolates from the patients were included for antibacterial susceptibility and ESBL production analysis. A bacterial strain was deemed to be susceptible to fluoroquinolones or carbapenems if it was susceptible to all antibiotics belonging to these classes. Similarly, a bacterial strain was deemed to be resistant to an entire class of antibiotics if it was resistant to at least one antibiotic of that class. Intermediate antibiotic resistance was considered an indicator of resistance.
5. Data collection
Basic demographic and clinical information of the patients, including gender, hospital record number, age, diagnosis, Charlson comorbidity index, history of ICU admission, length of hospital stay, culture test type, antibiotic susceptibility of the identified bacteria, and antibiotic use, were collected by reviewing medical charts and case report forms.
6. Statistical analysis
The Kruskal-Wallis and Mann-Whitney U tests were used to compare clinical and demographic data of patients among the three groups. The Chi-square test and Fisher’s exact test were used to assess differences in antibiotic susceptibility. The level of statistical significance was set at P <0.05. All statistical analyses were performed using IBM SPSS version 20 (IBM SPSS Inc., Armonk, NY, USA).
7. Ethics statement
This study was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital and all researchers complied with research ethics (IRB no. 2019-01-029-002).
Results
1. Basic patient characteristics according to infection classification
Of the 691 patients who underwent a bacterial culture test within 2 days of admission, 567 were included in this study, after excluding 124 patients who refused to participate. Enterobacteriaceae were identified at the infection site in 146 out of the 567 patients, showing a positivity rate of 25.7%. These 146 isolates were included in the final analysis. Of these 146 patients, 48 (32.9%), 78 (53.4%), and 20 (13.7%) had CAIs, HCAIs, and HAIs, respectively.
Table 1 lists the baseline characteristics of the patients; no significant difference was found in the sex ratio (P = 0.89), age (P = 0.40), or rates of ICU admission (P = 0.87) among the three groups. Patients in the HAIs group had a significantly higher Charlson comorbidity index than those of the other two groups, which had similar Charlson comorbidity indices. Patients in the HAIs group had the longest hospital duration (P = 0.004), whereas the average hospital durations in the CAIs and HCAIs groups were not significantly different (P = 0.28). Therefore, the HCAIs and CAIs groups had similar baseline characteristics, which differed from those of the HAIs group.
Table 1. Clinical characteristics of patients whose causative pathogens were isolated.
| Characteristic | Community-acquired infections (n = 48) | Healthcare-associated infections (n = 78) | Hospital-acquired infections (n = 20) | P-value |
|---|---|---|---|---|
| Male, n (%) | 22 (45.8) | 33 (42.3) | 8 (40.0) | 0.890 |
| Age, mean ± SD | 69.1 ± 14.5 | 68.6 ± 13.8 | 73.0 ± 10.7 | 0.400 |
| Charlson comorbidity index, mean ± SD | 3.4 ± 2.0 | 3.9 ± 2.6 | 5.3 ± 2.0 | 0.007 |
| Intensive care unit admission, n (%) | 12 (25.0) | 23 (29.4) | 6 (30.0) | 0.870 |
| Duration of hospital stay (days), mean ± SD | 12.6 ± 11.0 | 16.4 ± 18.2 | 28.4 ± 37.7 | 0.004 |
SD, standard deviation.
Of the patients admitted for an HCAI caused by Enterobacteriaceae, 53.8% were treated at the hospital (outpatient) within 30 days, 44.9% underwent antibiotic therapy within 30 days, 37.2% stayed at a hospital for at least 2 days within 90 days, 32.1% were hospitalized within 30 days, 32.1% received intravenous treatment within 30 days, 17.9% underwent an invasive procedure or operation within 6 months, 10.3% were transferred from a care home or long-term care facility, 6.4% had received chemotherapy or radiation therapy due to a malignant tumor within 6 months, 5.1% had active or metastatic cancer, 1.3% used immunosuppressants, 0% had a history of solid organ or bone marrow transplantation, 0% were HIV-positive, 2.5% were on dialysis within 30 days, 1.3% received wound treatment at home within 30 days and 0% had a family member with multi-drug resistant bacterial infection (Table 2).
Table 2. Distribution of factors defining HCAIs in 78 patients with Enterobacteriaceae infection (duplicates allowed).
| HCAIs defining factors | n (%) | |
|---|---|---|
| Hospital treatment (outpatient) within 30 days | 42 (53.8) | |
| Antibiotic therapy within 30 days | 35 (44.9) | |
| Stayed at a hospital for at least 2 days (at least 3 hospital days) within 90 days | 29 (37.2) | |
| Hospitalization within 30 days | 25 (32.1) | |
| Received an intravenous treatment within 30 days | 25 (32.1) | |
| Underwent an invasive procedure or surgery within 6 months | 14 (17.9) | |
| Transfer from a care home or long-term care facility | 8 (10.3) | |
| Immunocompromised status | ||
| Chemotherapy or radiation therapy for a malignant tumor within 6 months after hospitalization | 5 (6.4) | |
| Active or metastatic cancer | 4 (5.1) | |
| Immunosuppressant | 1 (1.3) | |
| History of solid organ or bone marrow transplantation | 0 (0.0) | |
| HIV-positive patient | 0 (0.0) | |
| Dialysis within 30 days | 2 (2.5) | |
| Received a wound treatment within 30 days | 1 (1.3) | |
| A family member has multidrug-resistant bacterial infection | 0 (0.0) | |
HCAIs, healthcare-associated infections; HIV, human immunodeficiency virus.
The disease categories caused by identified Enterobacteriaceae were UTIs in 99 cases (67.8%), intraabdominal infections in 21 cases (14.4%), pneumonia in 18 cases (12.3%), and skin and soft tissue infections in 8 cases (5.5%).
2. Causative bacteria and antibiotic susceptibility
The Enterobacteriaceae identified through culturing included E. coli (100 strains, 68.5%), Klebsiella spp. (28 strains, 19.2%), Proteus mirabilis (8 strains, 5.5%), Citrobacter spp. (5 strains, 3.4%), Enterobacter cloacae (2 strains, 1.4%), Serratia marcescens (2 strains, 1.4%), and Haemophilus influenzae (1 strain, 0.7%).
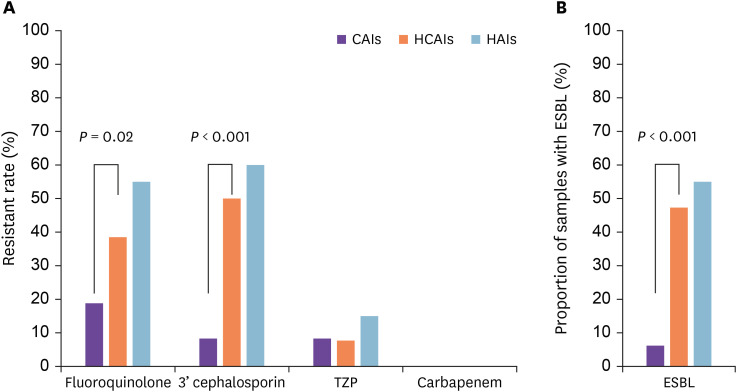
Among the Enterobacteriaceae identified as the causal pathogen, the antibiotic resistance rates against fluoroquinolones were 18.8%, 38.5%, and 55.0% in the CAIs, HCAIs, and HAIs groups, respectively. The difference between the HCAIs and HAIs groups was not significant (P = 0.18), whereas a significantly higher fluoroquinolones resistance rate was observed in the HCAIs group than in the CAIs group (P = 0.02). The antibiotic resistance rates of these strains against third-generation cephalosporins were 8.3%, 50.0%, and 60.0% in the CAIs, HCAIs, and HAIs groups, respectively; again, the HCAIs and HAIs groups did not differ significantly (P = 0.29), whereas the difference between the HCAIs and CAIs groups was significant (P <0.001) (Fig. 1A and Table 3).
Figure 1. Antibiotic resistance (A) and ESBL production (B) of Enterobacteriaceae based on different routes of infection acquisition.
ESBL, extended-spectrum beta-lactamases; CAIs, community-acquired infections; HCAIs, healthcare-associated infections; HAIs, hospital-acquired infections; 3’ cephalosporin, third-generation cephalosporin; TZP, piperacillin-tazobactam.
Table 3. Antibiotic resistance rates of Enterobacteriaceae based on different routes of infection acquisition.
| Antibiotic | Resistance, n (%) | P-values among the three groups | |||||
|---|---|---|---|---|---|---|---|
| CAIs (n = 48) | HCAIs (n = 78) | HAIs (n = 20) | CAIs vs. HCAIs vs. HAIs | CAIs vs. HCAIs | CAIs vs. HAIs | HCAIs vs. HAIs | |
| Fluoroquinolone | 9 (18.8) | 30 (38.5) | 11 (55.0) | 0.008 | 0.020 | 0.003 | 0.180 |
| Third-generation cephalosporin | 4 (8.3) | 39 (50.0) | 12 (60.0) | <0.001 | <0.001 | <0.001 | 0.290 |
| Piperacillin-tazobactam | 4 (8.3) | 6 (7.7) | 3 (15.0) | 0.550 | 0.570 | 0.330 | 0.260 |
| Carbapenem | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - | - | - |
The statistical significance of the comparison of antibiotic resistance between CAIs, HCAIs, and HAIs was tested by chi-square test. Fisher’s exact test was used when the number of samples in either group was less than 5.
CAIs, community-acquired infections; HCAIs, healthcare-associated infections; HAIs, hospital-acquired infections.
Of the 51 patients harboring ESBL-positive bacterial strains, 3 (6.3%), 37 (47.3%), and 11 (55.0%) had CAIs, HCAIs, and HAIs, respectively. A significantly higher proportion of ESBL-positive bacterial strains was observed in the HCAIs group than in the CAIs group (P <0.001; Fig. 1B). None of these ESBL-positive bacterial strains showed resistance to carbapenem. Among the ESBL-positive pathogens, the prevalence of antibiotic resistance to ciprofloxacin, gentamicin, ampicillin-sulbactam, sulfamethoxazole-trimethoprim, and piperacillin-tazobactam (TZP) was 72.5%, 60.8%, 58.8%, 51.0%, and 17.7%, respectively.
Among the defining factors of HCAIs, the response rate of hospital treatment within 30 days (outpatient) was the highest. Of the 78 patients classified as having HCAIs, 20 (25.6%) were included in the HCAIs group based on this factor alone. The third-generation cephalosporins resistance rate of these 20 causal pathogens was 55% (11 cases), which was much higher than the 8.3% of the CAIs group (P <0.001), and the ESBL-positive rate was also 55% (11 cases), which was also significantly higher than the 7.7% rate of the CAIs group (P <0.001). The resistance rate to fluoroquinolone was 35% (7 cases), which was not significantly different from the rate of 18.8% of the CAIs group (P = 0.13), and the resistance rate to TZP was 5% (1 case), which was not significantly different from the rate of 8.3% of the CAIs group (P = 0.54). None of these strains showed resistance to carbapenem.
DISCUSSION
In this study, we found a significant difference in antibiotic resistance against fluoroquinolones and third-generation cephalosporin in Enterobacteriaceae from the HCAIs and CAIs groups. This finding highlights the need to review existing guidelines regarding UTIs, which accounted for the majority of infections caused by Enterobacteriaceae in this study. The 2018 clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections [20] excludes patients with diabetes, those who are immunosuppressed, or patients with chronic comorbidities from their definition of patients with community-acquired UTIs. However, in addition to the aforementioned patients, other patients in the HCAIs group harbored pathogens with a distinct antibiotic susceptibility profile from that exhibited in the CAIs group. As HCAIs are included in CAIs, the current guidelines, which recommend fluoroquinolones or third-generation cephalosporins as empirical antibiotics for the treatment of patients with community-acquired UTIs, should consider distinguishing between CAIs and HCAIs in future revisions. In addition, it is necessary to identify and analyze each agent causing endemic HCAIs. The antibiotic resistance rate of community-acquired UTI pathogens against fluoroquinolones has been reported to be 30 - 40% [20], which is higher than that observed in this study (19.2%). Thus, the antibiotic resistance rate of community-acquired pathogens against fluoroquinolones could be lower if HCAIs are categorized separately from CAIs.
In the present analysis of 78 patients with healthcare-associated Enterobacteriaceae infections, 33.3% harbored strains that were resistant to fluoroquinolones and third-generation cephalosporins. Of the 30 patients with pathogens resistant to fluoroquinolones, 26 (86.7%) were also resistant to third-generation cephalosporins. Of the 39 patients with pathogens resistant to third-generation cephalosporins, 26 (66.7%) were also resistant to fluoroquinolones. Thus, third-generation cephalosporins and fluoroquinolones cannot substitute one another for treating patients with healthcare-associated infections and should not be used concomitantly.
Less than 10% of the pathogens isolated from patients with Enterobacteriaceae in the CAIs group were ESBL-positive, whereas nearly 50% of those isolated from patients in the HCAIs and HAIs groups were ESBL-positive. Approximately 94.9% and 86.7% of the Enterobacteriaceae strains of healthcare-associated infections resistant to third-generation cephalosporins and fluoroquinolones, respectively, were ESBL-positive. Dalhoff et al. [21] reported that ESBL-positive strains have high resistance to fluoroquinolones, suggesting the inappropriate classification of HCAIs and CAIs as a single category. In the present study, none of the ESBL-positive bacteria showed resistance to carbapenem. Considering that carbapenem can be used as a first-line antibiotic for ESBL-positive bacterial infections with TZP as a substitute [22], and taking into account the antibiotic resistance of these strains, it may be more appropriate to empirically use TZP and carbapenems for patients with healthcare-associated infections instead of third-generation cephalosporin or fluoroquinolones.
This study has a few limitations. First, previous studies have reported that antibiotic exposure is associated with antibiotic resistance [23,24]. Indeed, a high proportion of patients in the HCAIs group had a history of medication, including antibiotics. This suggests that the HCAIs group harbored antibiotic-resistant pathogens more frequently than the CAIs group due to previous exposure to antibiotics. However, we did not assess the differences according to antibiotic types, frequency, period of use, or number of hospital stays. Whether these factors could be used to classify HCAIs remains to be determined.
Second, among the definition factors of HCAIs, the response rate of “hospital treatment within 30 days (outpatient)” was the highest. As this single factor of 24 patients included in the HCAIs group, third-generation cephalosporins and ESBL-positive expression were different from those of the CAIs group, but there was no significant difference in the resistance rate of the other antibiotic groups. Therefore, subgroup analysis of the definition factors of HCAIs will be needed in the future.
Third, a detailed analysis was planned for evaluating differences between each infection group for each HCAIs definition factor, but this analysis was not ultimately possible because of the small number of cases. If more cases can be collected in the future, it is likely that the antibiotic resistance rate of Enterobacteriaceae can be investigated according to acquisition type in various disease groups.
In summary, the Enterobacteriaceae pathogens isolated from patients with HCAIs showed resistance patterns that were more similar to those of patients with HAIs than those with CAIs. Thus, CAIs and HCAIs should be distinguished from each other when selecting antibiotic agents.
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: YKC, YBS.
- Data curation: EJB, JJP.
- Formal analysis: YKC, YBS.
- Investigation: YKC, EJB.
- Methodology: JJP, YBS.
- Project administration: JL, YBS.
- Resources: JL.
- Software: JL.
- Supervision: YBS.
- Validation: JJP, YBS.
- Visualization: YKC, JJP.
- Writing - original draft: YKC.
- Writing - review & editing: YBS.
References
- 1.Lee H, Yoon EJ, Kim D, Jeong SH, Won EJ, Shin JH, Kim SH, Shin JH, Shin KS, Kim YA, Uh Y, Yang JW, Kim IH, Park C, Lee KJ. Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: first one-year report from Kor-GLASS. Euro Surveill. 2018;23:1800047. doi: 10.2807/1560-7917.ES.2018.23.42.1800047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G. Ciprofloxacin resistance in community- and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2015;15:545. doi: 10.1186/s12879-015-1282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso T, Almeida M, Friedman ND, Aragão I, Costa-Pereira A, Sarmento AE, Azevedo L. Classification of healthcare-associated infection: a systematic review 10 years after the first proposal. BMC Med. 2014;12:40. doi: 10.1186/1741-7015-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 5.National Healthcare Safety Network (NHSN) National Healthcare Safety Network (NHSN) patient safety component manual. [Accessed 15 January 2019]. Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/pcsManual_current.pdf.
- 6.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers JD, Taylor JK, Singanayagam A, Fleming GB, Akram AR, Mandal P, Choudhury G, Hill AT. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis. 2011;53:107–113. doi: 10.1093/cid/cir274. [DOI] [PubMed] [Google Scholar]
- 8.Guimarães C, Lares Santos C, Costa F, Barata F. Pneumonia associada aos cuidados de saúde versus pneumonia adquirida na comunidade: entidades diferentes, abordagens distintas. Rev Port Pneumol. 2011;17:168–171. doi: 10.1016/j.rppneu.2011.01.001. [Pneumonia associated with health care versus community acquired pneumonia: different entities, distinct approaches] [DOI] [PubMed] [Google Scholar]
- 9.Ha YE, Kang CI, Joo EJ, Park SY, Kang SJ, Wi YM, Chung DR, Peck KR, Lee NY, Song JH. Clinical implications of healthcare-associated infection in patients with community-onset acute pyelonephritis. Scand J Infect Dis. 2011;43:587–595. doi: 10.3109/00365548.2011.572907. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber MP, Chan CM, Shorr AF. Resistant pathogens in nonnosocomial pneumonia and respiratory failure: is it time to refine the definition of health-care-associated pneumonia? Chest. 2010;137:1283–1288. doi: 10.1378/chest.09-2434. [DOI] [PubMed] [Google Scholar]
- 11.Giannella M, Pinilla B, Capdevila JA, Martínez Alarcón J, Muñoz P, López Álvarez J, Bouza E, Estudio de Neumonía En Medicina Interna study Group from the Sociedad Española de Medicina Interna Pneumonia treated in the internal medicine department: focus on healthcare-associated pneumonia. Clin Microbiol Infect. 2012;18:786–794. doi: 10.1111/j.1469-0691.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 12.Siegman-Igra Y, Fourer B, Orni-Wasserlauf R, Golan Y, Noy A, Schwartz D, Giladi M. Reappraisal of community-acquired bacteremia: a proposal of a new classification for the spectrum of acquisition of bacteremia. Clin Infect Dis. 2002;34:1431–1439. doi: 10.1086/339809. [DOI] [PubMed] [Google Scholar]
- 13.Swenson BR, Metzger R, Hedrick TL, McElearney ST, Evans HL, Smith RL, Chong TW, Popovsky KA, Pruett TL, Sawyer RG. Choosing antibiotics for intra-abdominal infections: what do we mean by “high risk”? Surg Infect (Larchmt) 2009;10:29–39. doi: 10.1089/sur.2007.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matlow AG, Morris SK. Control of antibiotic-resistant bacteria in the office and clinic. CMAJ. 2009;180:1021–1024. doi: 10.1503/cmaj.071891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollef MH, Zilberberg MD, Shorr AF, Vo L, Schein J, Micek ST, Kim M. Epidemiology, microbiology and outcomes of healthcare-associated and community-acquired bacteremia: a multicenter cohort study. J Infect. 2011;62:130–135. doi: 10.1016/j.jinf.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Lenz R, Leal JR, Church DL, Gregson DB, Ross T, Laupland KB. The distinct category of healthcare associated bloodstream infections. BMC Infect Dis. 2012;12:85. doi: 10.1186/1471-2334-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu CL, Ku SC, Yang KY, Fang WF, Tu CY, Chen CW, Hsu KH, Fan WC, Lin MC, Chen W, Ou CY, Yu CJ. Antimicrobial drug-resistant microbes associated with hospitalized community-acquired and healthcare-associated pneumonia: a multi-center study in Taiwan. J Formos Med Assoc. 2013;112:31–40. doi: 10.1016/j.jfma.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: A distinct entity? Insights from a large U.S. database. Crit Care Med. 2006;34:2588–2595. doi: 10.1097/01.CCM.0000239121.09533.09. [DOI] [PubMed] [Google Scholar]
- 19.Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–3573. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang CI, Kim J, Park DW, Kim BN, Ha US, Lee SJ, Yeo JK, Min SK, Lee H, Wie SH. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother. 2018;50:67–100. doi: 10.3947/ic.2018.50.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalhoff A. Resistance surveillance studies: a multifaceted problem--the fluoroquinolone example. Infection. 2012;40:239–262. doi: 10.1007/s15010-012-0257-2. [DOI] [PubMed] [Google Scholar]
- 22.Seo YB, Lee J, Kim YK, Lee SS, Lee JA, Kim HY, Uh Y, Kim HS, Song W. Randomized controlled trial of piperacillin-tazobactam, cefepime and ertapenem for the treatment of urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli . BMC Infect Dis. 2017;17:404. doi: 10.1186/s12879-017-2502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, Guerin PJ, Piddock LJ. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387:176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 24.Kim HY, Lee SJ, Lee DS, Yoo JM, Choe HS. Microbiological characteristics of unresolved acute uncomplicated cystitis. Microb Drug Resist. 2016;22:387–391. doi: 10.1089/mdr.2015.0241. [DOI] [PubMed] [Google Scholar]