Abstract
Background
Sinonasal teratocarcinosarcoma (SNTCS) is a rare malignancy of the anterior skull base with only 127 cases described in the English literature. Given the rarity of this tumor, new cases and analysis of published reports may assist in future management of SNTCS.
Objectives
1) Describe findings from a systematic review of all available literature for malignant SNTCS including the clinical presentation, treatment modalities and outcomes. 2) Present two new cases of this rare anterior skull base tumor. 3) Compare treatment outcomes with respect to recurrence and mortality.
Methods
A systematic review of all English literature available in 2 comprehensive databases was conducted by two independent reviewers using PRISMA guidelines. 85 publications were identified. Each case was reviewed for demographics, treatment and survival, and aggregate treatment outcomes were compared using Kaplan-Meier analysis.
Results
A total of 64 articles meeting inclusion criteria were reported in the literature between 1977-2018. This represented a total of 127 patients, with a strong male predominance (83%) and mean age of 50 years (range 10–82). Mean follow-up was 21 months. Recurrence rate was 38%, with mean survival at 2 years of 55%. Almost all patients underwent surgery as a primary treatment modality (90%). The majority of cases were treated with multimodal therapy, with 55% receiving surgery and radiation and 20% receiving surgery with adjuvant chemoradiation. Kaplan-Meier analysis demonstrated a significant survival advantage for patients treated with combined therapy compared to surgery alone (p < 0.001) but did not show differences in recurrence (p = 0.085).
Conclusion
Two-year survival rates for SNTCS are 55%. Multimodality treatment outcomes appear to be superior to surgery alone based on the published data of this rare skull base tumor, although heterogeneity of treatment methods and reporting bias limits the generalizability of these findings.
Keywords: anterior skull base, sarcoma, sinonasal teratocarcino sinus malignancy, systematic review, teratomasarcoma
Introduction
Sinonasal teratocarcinosarcoma (SNTCS) is a unique and aggressive malignancy of the anterior skull base. 1 Typically arising in the paranasal or ethmoid sinuses, this neoplasm poses substantial diagnostic challenges given its heterogeneous composition. 2 SNTCS tumors are diverse in nature and are composed of epithelial, neuroepithelial and mesenchymal tissue. 3 In order to establish the diagnosis of SNTCS a tumor must possess malignant epithelial components as well as two or more malignant mesenchymal fragments—i.e. fibroblasts, cartilage, bone, and/or smooth muscle. 4 Given this heterogeneity, biopsies can be misleading and predisposed to misdiagnosis, resulting in a delay in treatment. 3
The first sinonasal teratocarcinosarcoma was documented in 1966 as a malignant teratoma of the ethmoid sinus. 5 Descriptions and eventual naming of SNTCS were established by Shanmugaratnum and Heffner in 1983 and 1984, respectively. 6 , 7 To date, only 127 cases have been reported in the English literature.
While rare, the aggressive nature of this malignancy demands a high index of suspicion. In this study we describe our findings from the largest systematic review of all available literature on SNTCS. Additionally, in order to determine potential significant survival advantages based on treatment type, we compared reported outcomes across three modalities of treatment: surgery alone, surgery and adjuvant radiotherapy, and surgery with adjuvant chemo-radiation therapy.
Methods
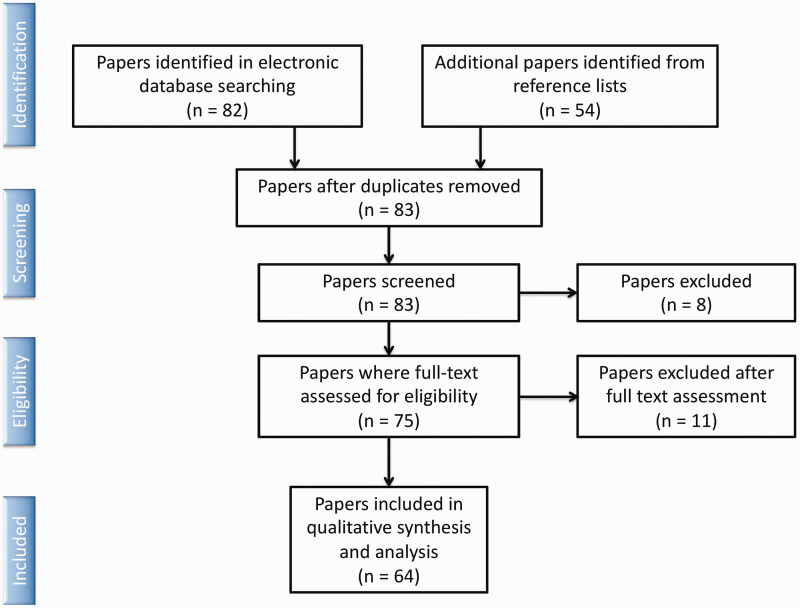
This systematic review and analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Inclusion criteria included papers with full English language text that provided descriptions of individual patient data, including description of disease and treatment course. Exclusion criteria included: non-English literature manuscripts, cases not involving the sinonasal pathways, cases that, upon further review, were without pathologic confirmation of teratocarcinosarcoma. The initial search used two comprehensive online databases: MEDLINE/PubMed and Google Scholar. Searches were conducted independently by two separate reviewers using key words: “sinonasal teratocarcinosarcoma,” “teratocarcinosarcoma,” “teratocarcinoma,” “malignant teratoma,” and “sinonasal teratoma.” Duplicates described in separate articles were removed. Articles were assessed for a primary outcome of disease-free survival and secondary outcomes of recurrence rate and treatment modality utilized. Additional data reviewed included demographics, tumor location, and clinical presentation. A total of 62 original articles and two review articles meeting inclusion criteria were reported in the literature from 1977–2018 and included in the study, representing a total of 127 unique patients (Figure 1).1–64 In addition to these reported cases, we also present two new cases of SNTCS treated at our institution with review of imaging, pathology and treatment outcome.
Figure 1.
Flow diagram of studies identified by search and reference lists, studies excluded due to no cases of sinonasal teratocarcinosarcoma or no full English-language text, and studies included in final analysis.
Statistical analysis was performed using Minitab (Minitab LLC, State College, PA) and R (R Foundation for Statistical Computing, Vienna, Austria). Descriptive analyses included prevalence by age, sex, geographic location, follow up, treatment choice, and recurrence/mortality outcomes. Data from studies reporting individual case details were then pooled to perform a Kaplan-Meier survival analysis of overall survival and time to recurrence stratified by treatment choice. Time to survival was also assessed across three time periods (1968–1999, 2000–2009, 2010–2018). Pairwise testing between survival curves was then performed using the log-rank test to assess the relationship between treatment choice and outcome. A log-rank test was also used to assess the relationship between publication time period and survival to assess treatment outcome trends over time. An alpha level of 0.05 was prespecified as a threshold for statistical significance.
Case Reviews
Case 1
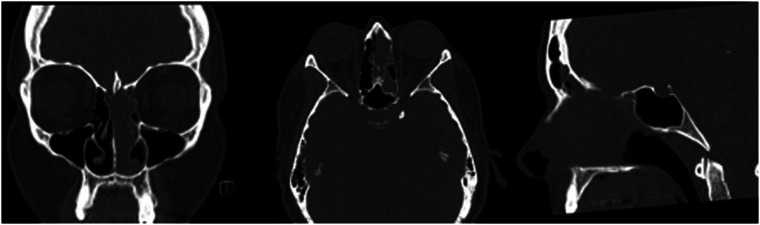
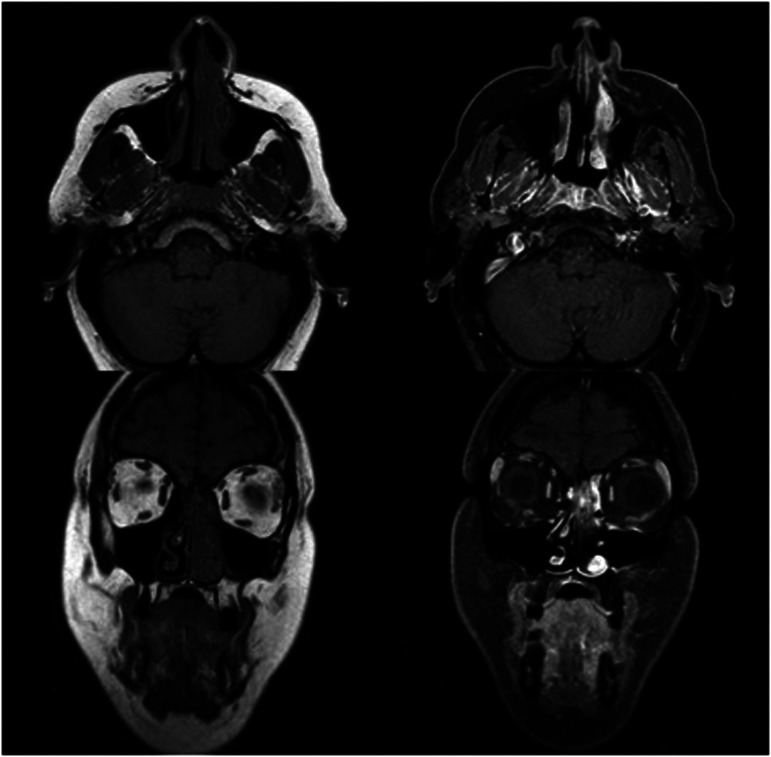
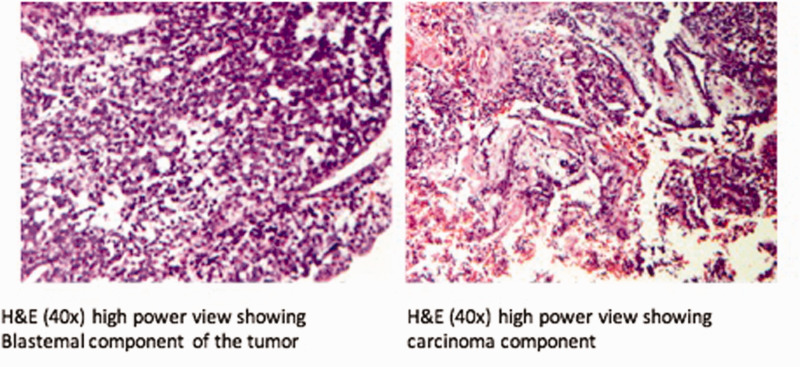
44-year-old female presented 3 weeks of facial pain, nasal obstruction and blurry vision. Non-contrast sinus CT scan demonstrated left sided nasal mass with extension into ethmoid and frontal sinuses (Figure 2). Magnetic resonance imaging (MRI) confirmed presence of a locally destructive 3.5 cm soft tissue mass with which involved the cribriform plate and with focal extension into the anterior cranial fossa (Figure 3). Biopsy of this mass confirmed SNTCS and subsequent anterior endoscopic skull base resection was performed (Figure 4). Surgical resection involved a superior septectomy connecting and widening frontal sinuses toward the cribriform plate. A posterior septectomy connected the sphenoid sinuses. The tumor was present on the left nasal cavity and was resected up towards the skull base. The anterior skull base was subsequently drilled to expose tumor and dura. At this point, several areas of the cribriform plate were removed to expose normal dura around the tumor, which was then incised and resected along with the residual tumor. Circumferential margins from the mucosa of the skull base, sphenoid sinus, frontal sinus, and nasal septum were all negative for malignancy. Skull base reconstruction utilized a multilayer technique with Biodesign inlay (Cook Medical, Bloomington, IN) and nasal septal flap overlay.
Figure 2.
CT sinus demonstrating soft tissue sinonasal mass in case 1.
Figure 3.
MRI with T2 hyperintense, enhancing 3.5 cm SNTCS involving the cribiform plateand anterior cranial fossa in case 1.
Figure 4.
H&E histology showing blastemal (A) and carcinomatous (B) components of SNTCS from case 1.
The patient then underwent adjuvant concurrent chemoradiation with carboplatin and etoposide as chemotherapeutic agents. Radiotherapy was performed using intensity-modulated radiation therapy of 60 Gray (Gy) in 30 fractions. Initial surveillance consisted of an MRI every six months. After one year with no signs of disease, the interval between scans was increased to one year. The patient has no evidence of disease on endoscopic exam or surveillance imaging 2 years following completion of therapy.
Case 2
60-year old male with past medical history of two plasmacytomas: one of the right nasal cavity for which he received 46 Gy of radiation when he was 43 years of age and a second in the right neck which was excised and treated with adjuvant 45 Gy radiotherapy at age 59. The patient subsequently developed symptoms of nasal obstruction for which he underwent endoscopic resection of a presumed nasal polyp at an outside facility with final pathology consistent with teratocarcinosarcoma and was referred to our center for definitive treatment.
He subsequently underwent right endoscopic medial maxillectomy with negative margins on final pathology. No adjuvant treatment was given as the patient had previously received two courses of radiotherapy and was not considered a candidate for further radiation therapy. Post-operative cancer surveillance included semiannual endoscopic exams for six years postoperatively and annually thereafter. The patient also underwent MRI imaging at two and three years postoperatively with no evidence of disease. Further imaging was deferred in favor of clinical exam due to patient preference. He remains disease free nine years postoperatively with no evidence of disease.
Results
A total of 62 original articles and two review articles meeting inclusion criteria were reported in the literature between 1977–2019 and included in the study (Table 1). Fourteen cases were case series while 49 were individual case reports. This represented a total of 127 patients. This patient population had a strong male predominance (83%). The mean patient age was 50 years (range 10–82). The vast majority of patients (90%) underwent surgery as the primary treatment modality, typically followed with secondary adjuvant therapy. The majority of cases were treated with multimodal therapy, notably with surgery and radiation (55%), while surgery with adjuvant chemo-RT was also commonly used (20%; Table 1). More studies describing SNTCS have been published in the United States than any other nation (34%) followed by India (23%) and Japan (11%). Mean follow-up across all studies was 21 months. Mean time to recurrence was 19.5 months. Recurrence rate was 38%, with mean survival at 2 years of 55%.
Table 1.
List and Description of Papers Reviewed.
| Author | Year | Article Type | Number of Novel Patients | Included in K-M Analysis | Time to Recurrence (Months, avg if >1) | Follow-up Duration (Months, avg if >1) |
|---|---|---|---|---|---|---|
| Abt | 1970 | Case Report | 1 | Yes | 1 | 72 |
| Agrawal N | 2012 | Case Report | 1 | Yes | – | 45 |
| Batsakis JG | 1995 | Review | – | No | – | – |
| Bhalla V | 2016 | Case Series | 2 | No | – | – |
| Budrukkar A | 2010 | Case Series | 22 | No | – | 34 |
| Carrizo F | 2006 | Case Series | 2 | Yes | 24 | 30 |
| Chakraborty S | 2016 | Case Report | 1 | No | – | 0 |
| Chao KK | 2004 | Case Report | 1 | Yes | – | 6 |
| Devgan BK | 1978 | Case Report | 1 | No | 0 | N/A |
| Dicke TE | 1970 | Case Report | 1 | Yes | 3 | 14 |
| Endo H | 2001 | Case Report | 1 | Yes | 54 | 84 |
| Fatima SS | 2013 | Case Series | 6 | Yes | – | 24 |
| Fernandez PL | 1995 | Case Report | 1 | Yes | 7 | 7 |
| Foong YC | 2017 | Case Report | 1 | Yes | 24 | 24 |
| Fukuoka K | 2000 | Case Report | 1 | Yes | 30 | 30 |
| Heffner DK | 1984 | Case Series | 15 | No | – | 33 |
| Jin W | 2018 | Case Report | 1 | Yes | 6 | 7 |
| Joshi A | 2014 | Case Series | 2 | Yes | 3 | 3 |
| Joshi A | 2015 | Case Report | 1 | Yes | 5 | 72 |
| Kane SV | 2009 | Case Report | 1 | No | – | 10 |
| Kim JH | 2011 | Case Report | 1 | Yes | 2 | 18 |
| Krishna KK | 2007 | Case Report | 1 | Yes | 6 | 12 |
| Kurmi DJ | 2017 | Case Report | 1 | Yes | 6 | 6 |
| Leelamma JP | 2018 | Case Report | 1 | No | – | – |
| Lim CCT | 2008 | Case Report | 1 | Yes | 7 | 8 |
| Liu JK | 2012 | Case Report | 1 | No | – | – |
| McKean EL | 2014 | Case Report | 1 | No | – | – |
| Misra P | 2014 | Review | – | No | – | – |
| Mohanty S | 2013 | Case Report | 1 | Yes | 12 | 12 |
| Mondal SK | 2012 | Case Report | 1 | Yes | 24 | 24 |
| Nitsche M | 2005 | Case Report | 1 | Yes | 36 | 36 |
| Ogawa T | 2000 | Case Report | 1 | Yes | 9 | 9 |
| Oka K | 2007 | Case Report | 1 | Yes | 12 | 12 |
| Pai SA | 1998 | Case Series | 4 | Yes | 18 | 15 |
| Palled S | 2015 | Case Report | 1 | Yes | 60 | 60 |
| Patchefsky A | 1968 | Case Report | 1 | Yes | 0.25 | 3 |
| Peng G | 2011 | Case Series | 2 | Yes | – | 25 |
| Petrovich Z | 1977 | Case Report | 1 | Yes | 60 | 60 |
| Prasad KC | 2003 | Case Report | 1 | Yes | 13 | 13 |
| Rotenberg | 2002 | Case Report | 1 | Yes | 6 | 6 |
| Sable M | 2017 | Case Report | 1 | No | – | 1 |
| Salem F | 2008 | Case Series | 3 | No | 12 | 12 |
| Seo E | 2017 | Case Report | 1 | No | – | – |
| Shanmugaratnam | 1983 | Case Series | 3 | Yes | 23 | 35 |
| Sharma HS | 1998 | Case Report | 1 | No | 9 | 12 |
| Shemen L | 1995 | Case Report | 1 | Yes | 60 | 60 |
| Shimazaki | 2000 | Case Report | 1 | No | – | 22 |
| Shorter C | 2010 | Case Report | 1 | Yes | 6 | 6 |
| Smith SL | 2008 | Case Series | 10 | Yes | – | 131 |
| Sobani ZA | 2012 | Case Report | 1 | Yes | 6 | 6 |
| Su YY | 2010 | Case Report | 1 | Yes | 18 | 43 |
| Szudek J | 2005 | Case Report | 1 | No | – | – |
| Takasaki K | 2006 | Case Report | 1 | Yes | 41 | 41 |
| Terasaka S | 1998 | Case Report | 1 | Yes | 31 | 31 |
| Thomas J | 2011 | Case Report | 1 | No | – | – |
| Tokunaga T | 2012 | Case Report | 1 | Yes | 24 | 24 |
| Vranic S | 2008 | Case Report | 1 | No | 8 | – |
| Wahid FI | 2012 | Case Report | 1 | No | – | – |
| Wang SY | 2007 | Case Series | 5 | No | 70 | – |
| Wassef SN | 2012 | Case Report | 1 | Yes | 48 | 48 |
| Wei S | 2008 | Case Report | 1 | Yes | 12 | 12 |
| Weinberg BD | 2014 | Case Report | 1 | Yes | 24 | 32 |
| Wellman M | 2002 | Case Report | 1 | No | 48 | 48 |
| Yang S | 2013 | Case Series | 2 | No | – | 24 |
“K-M” refers to Kaplan-Meier analysis. A “Yes” in this category demonstrates that some or all cases reported in this manuscript presented enough case information for inclusion survival analysis. Time to recurrence and follow-up duration were averaged if more than one case applied per article.
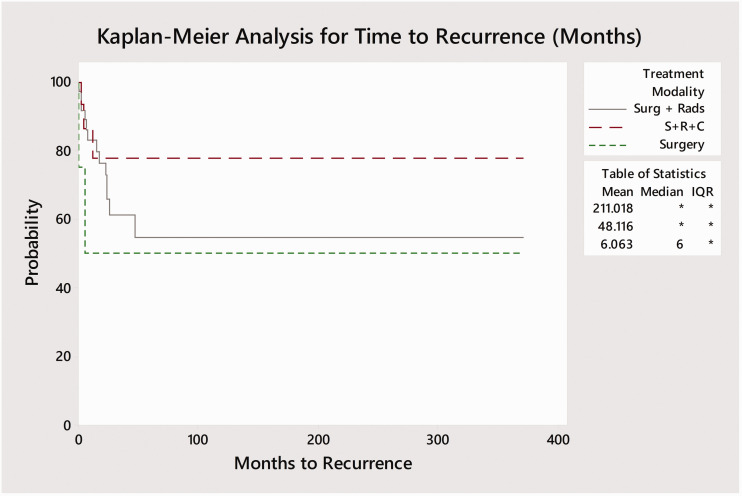
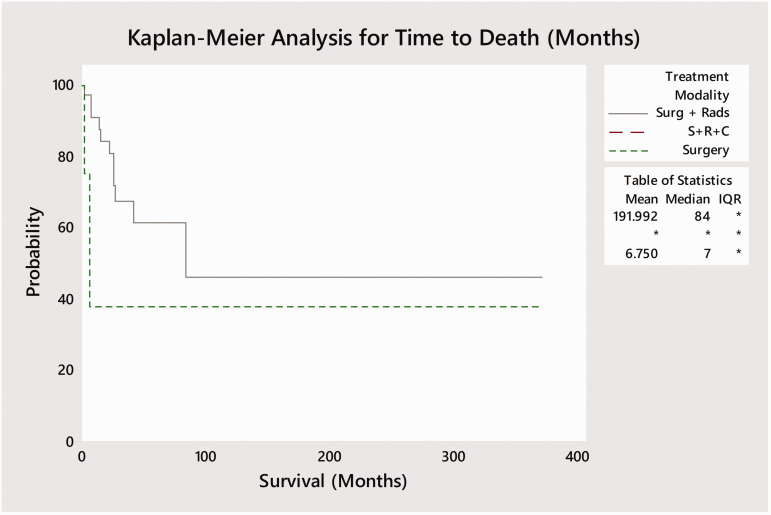
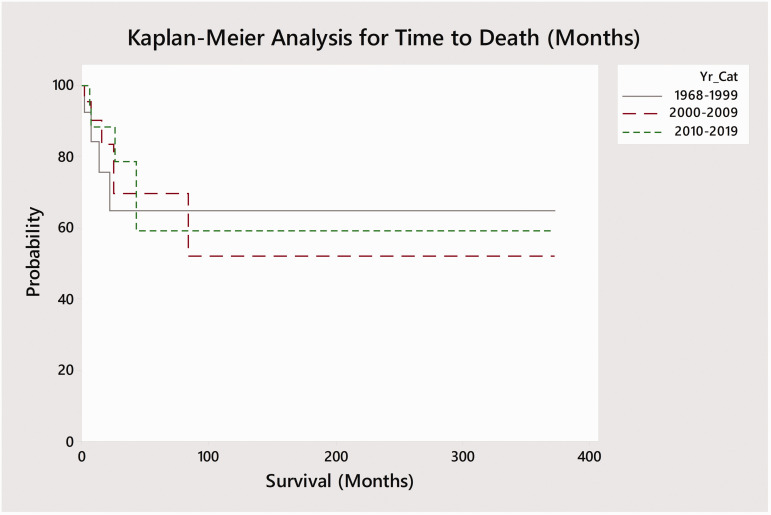
Of the 127 reported cases in the literature, full outcome data was only available for only 58 cases (Table 1). Surgery alone was performed in 4 cases while 36 cases received adjuvant radiotherapy following surgery and 16 cases received adjuvant radiotherapy and chemotherapy. One patient each received radiotherapy in isolation and surgery with adjuvant chemotherapy (without radiotherapy). Kaplan-Meier curves were plotted based on treatment modality – single (surgery), bimodality (surgery and radiation), and trimodality (surgery and adjuvant chemo-radiation) by time to recurrence and mortality from the end of treatment (Figures 5 and 6). No deaths occurred amongst the 16 trimodality cases while 13 of 36 (36.1%) bimodality cases and 2 of 4 (50%) surgery cases resulted in death. Log-rank tests demonstrated significant differences between time to mortality between the three main courses of treatment (p < 0.001; surgery, bimodality, and trimodality) but did not identify a significant difference in time to recurrence (p = 0.085). A pairwise log-rank test for time to death identified significant differences in survival between specific modes of treatment. Trimodality was found to be associated with significantly delayed time to death as compared to bimodality (p = 0.05) and surgery without adjuvant treatment (p = 0.004). Similarly, patients receiving bimodality were also found to have improved survival compared those receiving surgery alone (p = 0.004). A Kaplan-Meier survival plot was also performed to compare time to mortality across three time periods (1968–1999, 2000–2009, 2010–2018; Figure 7). A log-rank test found no significant difference in time to survival between these time points (p = 0.8).
Figure 5.
Kaplan-Meier plot with recurrence events for each cohort based on modality of treatment vs time (months). Log-Rank test was not significant between treatment modalities (p = 0.085). Time to recurrence was defined as the time from the end of treatment to recurrence.
Figure 6.
Kaplan-Meier survival analysis based on modality of treatment vs. months to death for cases were survival data was available. Log-Rank test was significant between treatment modalities (p < 0.001). Survival was defined as the time from the end of treatment to death. Note that no deaths were reported in patients who underwent trimodality with surgery followed by adjuvant radiotherapy and chemotherapy.
Figure 7.
Kaplan-Meier survival analysis based on era and months to death for cases where survival data was available. Log-rank test did not identify a significant difference between time to survival by era (p = 0.8). Survival was defined as the time from the end of treatment to death.
Discussion
Sinonasal teratocarcinosarcoma is an exceptionally rare and aggressive disease with a poor prognosis. Due to its rarity and the heterogeneous nature of the neoplasm itself, there is vast variation in disease presentation and management. The neoplasm itself features both teratoma and carcinosarcoma components, including epithelial and mesenchymal tissue (Figure 4). 29 The epithelial tissue may be highly variable with both benign and malignant squamous and glandular components and may include columnar and/or cuboidal cells with or without cilia. Mesenchymal components may appear as various muscle types, cartilage, and/or bone tissue. Additionally, many cases include poorly differentiated neuroepithelial tissue with neural rosettes. 4 Given this large histologic heterogeneity, small biopsy samples are often inadequate for diagnostic purposes. Diagnosis may be assisted by the appearance of “fetal-appearing” squamous epithelium with large nucleoli and the absence of germ cell components, however this finding is not universal. 7 , 34 Immunohistochemistry often demonstrates expression of CD99, vimentin, and neuron-specific enolase, while STNCS cells are typically negative for leukocyte common antigen, beta-HCG, and neurofilament protein. 4 , 34
Despite its rarity, SNTCS is an aggressive tumor with a mean 2-year survival rate of 55% and a recurrence rate of 38%. Both patients treated at our institution show no evidence of disease at 2 and 9 years post-operatively, respectively. Interestingly, our second patient had extensive radiation therapy to the ipsilateral head and neck area prior to identification of the SNTCS tumor. It is unclear if this represents an inciting event correlating to his disease etiology, however this is feasible given known associations of radiation induced skull base sarcomas. 65
SNTCS patients most often present with signs and symptoms of nasal obstruction, as seen in the two patients described in this study, with possible tumor origination from a pluripotential progenitor cells. 10 While primarily a disease of middle age adults, SNTCS has been observed in patients 10–82 years of age. Interestingly, the literature demonstrates a male predilection for SNTCS, with men accounting for over 80% of patients in this review. The reasons for this are still unknown and may reflect gender disparities in access to care around the world leading to publication bias or a fundamental biological process that favors oncogenesis in men.
Only 85 prior studies have examined SNTCS. These have varied considerably from case reports to literature reviews with most focusing on basic science rather than clinical management. 46 Despite the limited sample, certain treatment trends are identifiable. Bimodality consisting of surgery with adjuvant radiation appears to be the most commonly selected treatment, used in 62% of cases for which treatment data is available, while trimodality with surgery, adjuvant radiotherapy, and adjuvant chemotherapy was the second most common treatment of choice, used in 28% of the cases. Using a pairwise log-rank test, both bimodality and trimodality were found to be associated with significantly longer time to death as compared to surgery alone. Interestingly, trimodality was also associated with significantly delayed time to death as compared to bimodality, despite being used less frequently. While not statistically significant, the Kaplan-Meier curves also demonstrate possible differences between treatment strategies with regard to time to recurrence, with multimodal therapy appearing to have a lower proportion of patients with post-treatment recurrence compared to surgery alone. Future meta-analyses with larger cohorts may be sufficiently powered to detect if this is a true difference. Time to survival, meanwhile, has not appeared to change over time, as demonstrated by a Kaplan-Meier analysis with a log-rank test comparing survival across different eras (p = 0.8), suggesting that treatment outcomes have been relatively stable over analysis period.
As with any individual patient data, survival analysis of rare disease based on case reports, this study is not without limitations. Historical trends in treatment are dependent on methods used in previous studies, and selection bias is inevitable as only a limited subset of cases may have been reported as a result of rare presentation or outcomes. Additionally, given the retrospective nature of this study, we were unable to control for functional status, comorbidities, or differences within management strategies. Length of follow-up varied considerably between studies as well, with the average duration of follow up exceeding average time to recurrence by 1.5 months (21 to 19.5 months). Consequently, studies with shorter length of follow-up may not have captured later recurrence. While a formal analysis of heterogeneity was unable to be performed, the variety of cases across time, age, and location suggests the cases were fairly heterogeneous. Furthermore, cases where surgery was performed without adjuvant therapy may reflect patient inability to tolerate further treatment, which may have contributed to the poor results in this cohort. The relatively few numbers of surgery only patients further limits direct comparison against other treatment modalities and generalization of this data should be undertaken with caution. Nevertheless, this study represents the largest and most complete systematic review of the literature regarding SNTCS to date, and further demonstrates the need for comprehensive multi-institutional databases to study outcomes of rare skull base pathologies.
Conclusion
Sinonasal teratocarcinosarcoma (SNTCS) is a rare and frequently misdiagnosed sinonasal neoplasm. Two-year survival rates for this aggressive skull base tumor appear to be 55% with a recurrence rate of 38%. Bimodality with surgery and adjuvant radiotherapy is the most common treatment modality utilized, followed by trimodality. While trimodality may be more effective than bimodality, both forms of adjuvant therapy appear more effective than surgery alone in managing SNTCS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Douglas J. Totten https://orcid.org/0000-0002-0665-9653
References
- 1.Carrizo F, Pineda-Daboin K, Neto AG.Luna MA. Pharyngeal teratocarcinosarcoma: review of the literature and report of two cases. Ann Diagn Pathol. 2006; 10(6):339–342. [DOI] [PubMed] [Google Scholar]
- 2.Fernández PL, Cardesa A, Alós L.Pinto J, Traserra J. Sinonasal teratocarcinosarcoma: an unusual neoplasm. Pathol Res Pract. 1995; 191(2):166–171. [DOI] [PubMed] [Google Scholar]
- 3.Sable M, Kakkar A, Garg K.Suri V. Sinonasal teratocarcinosarcoma: an underdiagnosed entity posing diagnostic challenges. Turk Neurosurg. 2017; 27(3):468–471. [DOI] [PubMed] [Google Scholar]
- 4.Kurmi DJ, Mittal RS, Sharma A.Gandhi A, Singhvi S. Sinonasal teratocarcinosarcoma involving nasal cavity, nasopharynx, and all paranasal sinuses with bilateral orbital and intracranial extension: a rare case report. Asian J Neurosurg. 2017; 12(2):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patchefsky A, Sundmaker W, Marden PA. Malignant teratoma of the ethmoid sinus. Report of a case A. Cancer. 1968; 21(4):714–721. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugaratnam K, Kunaratnam N, Chia KB.Chiang GS, Sinniah R. Teratoid carcinosarcoma of the paranasal sinuses. Pathology. 1983; 15(4):413–419. [DOI] [PubMed] [Google Scholar]
- 7.Heffner DK, Hyams VJ. Teratocarcinosarcoma (malignant teratoma?) of the nasal cavity and paranasal sinuses: a clinicopathologic study of 20 cases. Cancer. 1984; 53(10):2140–2154. [DOI] [PubMed] [Google Scholar]
- 8.Abt AT. Malignant teratoma of the paranasal sinuses. Arch Pathol Lab Med. 1970; 90(2):76–180. [PubMed] [Google Scholar]
- 9.Agrawal NC, Chintagumpala M, Hicks J, Eldin K.Paulino AC. Sinonasal teratocarcinosarcoma in an adolescent male. J Pediatr Hematol/Oncol. 2012; 24(7):304–307. [DOI] [PubMed] [Google Scholar]
- 10.Batsakis JG, El-Naggar AK, Luna MA. Teratomas of the head and neck with emphasis on malignancy. Ann Otol Rhinol Laryngol. 1995; 104(6):496–500. [DOI] [PubMed] [Google Scholar]
- 11.Bhalla VC, Chowdhury N, Alvi S, Chamoun R.Beahm DD. Two cases of sinonasal teratocarcinosarcoma: confounders, treatment, and review of the literature. J Neurol Surg Part B Skull Base. 2016; 77:P049. [Google Scholar]
- 12.Birkeland AB, Yanik M, Scott MV, et al. Pathogenetic analysis of sinonasal teratocarcinosarcomas reveal actionable β-catenin overexpression and a β-catenin mutation. J Neurol Surg B Skull Base. 2017; 78(4):346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty S, Chowdhury AR, Bandyopadhyay G. Sinonasal teratocarcinosarcoma: case report of an unusual neoplasm. J Oral Maxillofac Pathol. 2016; 20(1):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao KK, Eng TY, Barnes J.Dahiha R. Sinonasal teratocarcinosarcoma. Am J Clin Oncol. 2004; 27(1):29–32. [DOI] [PubMed] [Google Scholar]
- 15.Dicke TE, Gates GA. Malignant teratoma of the paranasal sinuses: report of a case. Arch Otolaryngol. 1970; 91(4):391–394. [DOI] [PubMed] [Google Scholar]
- 16.Endo H, Hirose T, Kuwamura KI.Sano, T. Sinonasal teratocarcinosarcoma. Pathol Int. 2001; 51(2):107–112. [DOI] [PubMed] [Google Scholar]
- 17.Fatima SS, Minhas K, Din NU.Fatima S, Ahmed A, Ahmad Z. Sinonasal teratocarcinosarcoma: a clinicopathologic and immunohistochemical study of 6 cases. Ann Diagn Pathol. 2013; 17(4):313–318. [DOI] [PubMed] [Google Scholar]
- 18.Foong YC, Murdolo V, Naiman N.Hepner L, Awad R. Sinonasal teratocarcinosarcoma: a case report. J Med Case Rep. 2017; 11(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuoka K, Hirokawa M, Shimizu M, et al. Teratocarcinosarcoma of the nasal cavity report of a case showing favorable prognosis. APMIS. 2000; 108(9): 553–557. [DOI] [PubMed] [Google Scholar]
- 20.Jin WT, Teng V, Zhao P, Zhang W.Li X, Li Y. Sinonasal teratocarcinosarcoma masquerading as an olfactory neuroblastoma. Int J Clin Exp Pathol. 2018; 11(2):910–915. [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi AD, Manickam DR, Noronha V, et al. Recurrent sinonasal teratocarcinosarcoma with intracranial extension: case report. Indian J Cancer. 2014; 51(3):398–400. [DOI] [PubMed] [Google Scholar]
- 22.Joshi AN, Sharma M, Dhumal S, et al. Neoadjuvant chemotherapy in advanced sinonasal teratocarcinosarcoma with intracranial extension: Report of two cases with literature review. J Cancer Res Ther. 2015; 11(4):1003–1005. [DOI] [PubMed] [Google Scholar]
- 23.Kane SV, Karpate AA, Bal M.Juvekar SL, Pai PS. Chemotherapy-Induced neuronal maturation in sinonasal teratocarcinosarcoma—a unique observation. Head Neck Pathol. 2009; 3(1):31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J-H, Maeng Y-H, Lee J-S.Jung S, Lim SC, Lee MC. Sinonasal teratocarcinosarcoma with rhabdoid features. Pathol Int. 2011; 61(12):762–767. [DOI] [PubMed] [Google Scholar]
- 25.Krishna Kumar K, Sundararajan I, Rangachari V.Sumathi V. Sinonasal teratocarcinosarcoma—a case report. Indian J Otolaryngol Head Neck Surg. 2007; 59(2):148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leelamma JP, Mohan BP, Srinivasan A. Sinonasal teratocarcinosarcoma—a rare tumour not so rarely misdiagnosed. Iran J Pathol. 2018; 13(1):85–88. [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JK, Eloy JA. Modified one-piece extended transbasal approach for resection of giant anterior skull base sinonasal teratocarcinosarcoma. J Neurosurg. 2012; 32 Suppl: E4. [PubMed] [Google Scholar]
- 28.McKean EB, Burgin S, Sullivan SE, Nor J.Brenner C. Targetable pathway genome sequencing for a sinonasal teratocarcinosarcoma and xenograft chemotherapeutic testing for personalized medicine. J Neurol Surg Part B: Skull Base. 2015; 75:A196. [Google Scholar]
- 29.Misra P, Husain Q, Svider PF.Sanghvi S, Liu JK, Eloy JA. Management of sinonasal teratocarcinosarcoma: a systematic review. Am J Otolaryngol. 2014; 35(1):5–11. [DOI] [PubMed] [Google Scholar]
- 30.Mondal SK, Mandal PK, Guha A.Roy S. Sinonasal teratocarcinosarcoma of the ethmoid and paranasal sinus: a rare neoplasm. J Res Med Sci. 2012; 17(6):575–577. [PMC free article] [PubMed] [Google Scholar]
- 31.Nitsche M, Hermann RM, Christiansen H.Berger J, Pradier O. Rationale for individualized therapy in sinonasal teratocarcinosarcoma (SNTC): case report. Onkolagie. 2005; 28(12):653–656. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa T, Ikeda K, Watanabe M, et al. A case report of sinonasal teratocarcinosarcoma. Tohoku J Exp Med. 2000; 190(1):51–59. [DOI] [PubMed] [Google Scholar]
- 33.Oka K, Kanayama R, Fukunaga M, et al. Nasal teratocarcinosarcoma—a case report. Pathol Res Pract. 2007; 203(7):549–553. [DOI] [PubMed] [Google Scholar]
- 34.Pai SA, Naresh KN, Masih K.Ramarao C, Borges AM. Teratocarcinosarcoma of the paranasal sinuses: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998; 29(7):718–722. [DOI] [PubMed] [Google Scholar]
- 35.Palled S, Patil S, Kumar R.Reddy SP. Sinonasal teratocarcinosarcoma (TCS): tackle aggressively. J Radiother Pract. 2015; 14(4):438–441. [Google Scholar]
- 36.Peng G, Ke Y, Wang T.Feng Y, Li Y, Wu G. Intensity-modulated radiotherapy for sinonasal teratocarcinosarcoma. J Huazhong Univ Sci Technol Med Sci. 2011; 31(6):857–860. [DOI] [PubMed] [Google Scholar]
- 37.Petrovich Z, Wollman J, Acquarelli M.Barton R. Malignant teratoma of the nasal cavity. J Surg Oncol. 1977; 9(1):21–28. [DOI] [PubMed] [Google Scholar]
- 38.Prasad KC, Pai RR, Padmanabhan K, Chawla S. Teratocarcinosarcoma of the nose, paranasal sinuses and nasopharynx. J Laryngol Otol. 2003; 117(4):321–324. [DOI] [PubMed] [Google Scholar]
- 39.Rotenberg BE-H, Lodha A.MacCormick A, Ngan BY, Forte V. Nasopharyngeal teratocarcinosarcoma. Int J Pediatr Otorhinolaryngol. 2002; 62(2):159–164. [DOI] [PubMed] [Google Scholar]
- 40.Salem F, Rosenblum MK, Jhanwar SC.Kancherla P, Ghossein RA, Carlson DL. Teratocarcinosarcoma of the nasal cavity and paranasal sinuses: report of 3 cases with assessment for chromosome 12p status. Hum Pathol. 2008; 39(4):605–609. [DOI] [PubMed] [Google Scholar]
- 41.Seo E, Yang N. P03.23 sinonasal teratocarcinosarcoma with intracranial extension: case report and literature review. Neuro-Oncology. 2017; 19(suppl_3):iii38–iii39. [Google Scholar]
- 42.Sharma HS, Abdullah JM, Othman NH.Muhamad M. Teratocarcinosarcoma of the nasal cavity and ethmoid. J Laryngol Otol. 1998; 112(7):682–686. [DOI] [PubMed] [Google Scholar]
- 43.Shemen L, Galantich P, Murali R. Malignant teratocarcinosarcoma of the sphenoid sinus. J Otolaryngol Head Neck Surg. 1995; 112(3):496–500. [DOI] [PubMed] [Google Scholar]
- 44.Shimazaki H, Aida S, Tamai S.Miyazawa T, Nakanobou M. Sinonasal teratocarcinosarcoma: ultrastructural and immunohistochemical evidence of neuroectodermal origin. Ultrastruct Pathol. 2000; 24(2):115–122. [DOI] [PubMed] [Google Scholar]
- 45.Shorter C, Nourbakhsh A, Dean M.Thomas-Ogunniyi J, Lian T, Guthikonda B. Intracerebral metastasis of a sinonasal teratocarcinosarcoma: a case report. Skull Base. 2010; 20(5):393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SL, Hessel AC, Luna MA.Malpica A, Rosenthal DI, El-Naggar AK. Sinonasal teratocarcinosarcoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2008; 134(6):592–595. [DOI] [PubMed] [Google Scholar]
- 47.Sobani ZAA, Junaid M, Salahuddin I. Sinonasal teratocarcinosarcoma. J Pak Med Assoc. 2012; 62(6):633–635. [PubMed] [Google Scholar]
- 48.Su Y-Y, Friedman M, Huang C-C.Wilson M, Lin HC. Sinonasal teratocarcinosarcoma. Am J Otolaryngol. 2010; 31(4):300–303. [DOI] [PubMed] [Google Scholar]
- 49.Szudek J, Bullock M, Taylor SM. Sinonasal teratocarcinosarcoma involving the cavernous sinus. J Otolaryngol. 2005; 34(4):286–288. [DOI] [PubMed] [Google Scholar]
- 50.Takasaki K, Sakihama N, Takahashi H. A case with sinonasal teratocarcinosarcoma in the nasal cavity and ethmoid sinus. Eur Arch Otorhinolaryngol. 2006; 263(6):586–591. [DOI] [PubMed] [Google Scholar]
- 51.Tchoyoson LC, Thiagarajan A, Sim CS, Khoo ML.Shakespeare TP, Ng I. Craniospinal dissemination in teratocarcinosarcoma. J Neurosurg. 2008; 109(2):321–324. [DOI] [PubMed] [Google Scholar]
- 52.Terasaka SMM, Whiting DM, Fukushima T.Espejo EJ, Nathan G. Prolonged survival in a patient with sinonasal teratocarcinosarcoma with cranial extension. Case report. J Neurosurg. 1998; 88(4):753–756. [DOI] [PubMed] [Google Scholar]
- 53.Thomas J, Adegboyega P, Iloabachie K.Mooring JW, Lian T. Sinonasal teratocarcinosarcoma with yolk sac elements: a neoplasm of somatic or germ cell origin? Ann Diagn Pathol. 2011; 15(2):135–139. [DOI] [PubMed] [Google Scholar]
- 54.Tokunaga T, Sunaga H, Kimura Y.Tsuda G, Fujieda S. A case of sinonasal teratocarcinosarcoma treated with surgery and post-operative intensity-modulated radiotherapy (IMRT). Auris Nasus Larynx. 2012; 39(6):641–645. [DOI] [PubMed] [Google Scholar]
- 55.Vranic S, Caughron SK, Djuricic S, et al. Hamartomas, teratomas and teratocarcinosarcomas of the head and neck: report of 3 new cases with clinico-pathologic correlation, cytogenetic analysis, and review of the literature. BMC Ear Nose Throat Disord. 2008; 8(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wahid FJ, Khan Q, Khan IA. Sinonasal teratocarcinosarcoma. J Coll Phys Surg Pak. 2012; 22(5):335–337. [PubMed] [Google Scholar]
- 57.Wang SZ, Li SM, Lin L, et al. Sinonasal teratocarcinosarcoma: a clinical, radiologic and pathologic study of 5 cases. Zhonghua Bing Li Xue Za Zhi. 2007; 36(8):534–538. [PubMed] [Google Scholar]
- 58.Wassef SN, Kapur P, Barnett SL.Meyers LL. Sinonasal teratocarcinosarcoma with intracranial extension: case report and literature review. Ear Nose Throat J. 2012; 91(12):536–539. [DOI] [PubMed] [Google Scholar]
- 59.Wei S, Carroll W, Lazenby A.Bell W, Lopez R, Said-Al-Naief N. Sinonasal teratocarcinosarcoma: report of a case with review of literature and treatment outcome. Ann Diagn Pathol. 2008; 12(6):415–425. [DOI] [PubMed] [Google Scholar]
- 60.Weinberg B, Newell K, Wang F. A case of a beta-human chorionic gonadotropin secreting sinonasal teratocarcinosarcoma. J Neurol Surg Rep. 2014; 75(1):e103–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wellman M, Kerr PD, Battistuzzi S.Cristante L. Paranasal sinus teratocarcinosaroma with intradural extension. J Otolaryngol. 2002; 31(3):173–176. [DOI] [PubMed] [Google Scholar]
- 62.Yang SS, Sun R, Liang J, Zhou Z.Zhou J, Rui J. Sinonasal teratocarcinosarcoma: a clinical and pathological analysis. Int J Surg Pathol. 2013; 21(1):37–43. [DOI] [PubMed] [Google Scholar]
- 63.Devgan BD, Gross CW. Teratocarcinoma of the ethmoid sinus: review of literature plus a new case report. Otolaryngol Head Neck Surg. 1978; 86(5):689–695. [DOI] [PubMed] [Google Scholar]
- 64.Budrukkar A, Agarwal JP, Kane S, et al. Management and clinical outcome of sinonasal teratocarcinosarcoma: single institution experience. J Laryngol Otol. 2010; 124(7):739–743. [DOI] [PubMed] [Google Scholar]
- 65.Thiagarajan A, Iyer NG. Radiation-induced sarcomas of the head and neck. World J Clin Oncol. 2014; 5(5):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]