Abstract
Accumulating evidence suggests that distinct aspects of successful navigation—path integration, spatial-knowledge acquisition, and navigation strategies—change with advanced age. Yet few studies have established whether navigation deficits emerge early in the aging process (prior to age 65) or whether early age-related deficits vary by sex. Here, we probed healthy young adults (ages 18–28) and midlife adults (ages 43–61) on three essential aspects of navigation. We found, first, that path-integration ability shows negligible effects of sex or age. Second, robust sex differences in spatial-knowledge acquisition are observed not only in young adulthood but also, although with diminished effect, at midlife. Third, by midlife, men and women show decreased ability to acquire spatial knowledge and increased reliance on taking habitual paths. Together, our findings indicate that age-related changes in navigation ability and strategy are evident as early as midlife and that path-integration ability is spared, to some extent, in the transition from youth to middle age.
Keywords: cognitive aging, virtual reality, path integration, wayfinding
Spatial navigation refers to processes by which we update our position and orientation in space, learn the layout of new places, and plan routes to goal locations in known environments. Successful navigation is a complex behavior, requiring the integration of multiple perceptual cues, memory, and executive processes (Chrastil, 2013; Hegarty et al., 2006; Wolbers & Hegarty, 2010). Despite the importance of navigation to our daily lives, there are large individual and sex differences in navigation ability (Hegarty et al., 2002, 2006; Ishikawa & Montello, 2006; Nazareth et al., 2019; Weisberg & Newcombe, 2016), and deficits in navigational abilities are apparent in older adult populations (ages 65 and older; Harris & Wolbers, 2012; Lester et al., 2017; Merhav & Wolbers, 2019; Zhong & Moffat, 2016). However, little is known about when these changes emerge during the aging process or whether sex differences evident in younger populations persist with age. This represents a critical gap in our understanding of the aging brain.
Spatial navigation has emerged as a promising behavioral marker for detecting individuals at risk for dementia. Young adults with a heightened genetic risk for Alzheimer’s disease have poorer navigation performance and altered neural activity during navigation, decades before the onset of disease symptoms (Coughlan, Coutrot, et al., 2018; Coughlan, Laczó, et al., 2018; Kunz et al., 2015). Approximately two thirds of Alzheimer’s disease patients are women (Hebert et al., 2013), suggesting that sex plays a role in disease risk. An emerging consensus is that the sweeping neuroendocrine changes that occur during the midlife transition to menopause may be a sex-specific risk factor for Alzheimer’s disease (Mielke et al., 2014). Notably, outside of the hypothalamus, brain regions that are particularly sensitive to changes in sex steroid hormones include the hippocampus (Hara et al., 2012), entorhinal cortex (Taylor et al., 2020), and prefrontal cortex (Jacobs & Goldstein, 2018)—key regions within the brain’s navigational circuitry (Lester et al., 2017; Moffat, 2009; Zhong & Moffat, 2016). Together, these findings suggest that taking into account sex differences in age-related changes in navigational ability could be critical for the early detection of individuals at risk for neurodegenerative disease.
In the present study, we probed healthy young (ages 18–28) and midlife (ages 43–61) men and women on three essential aspects of navigation: path integration, spatial-knowledge acquisition, and navigational strategy (Fig. 1). Studying the healthy aging brain allows us to gain a comprehensive understanding of when navigational deficits emerge in the normative aging process in men and women.
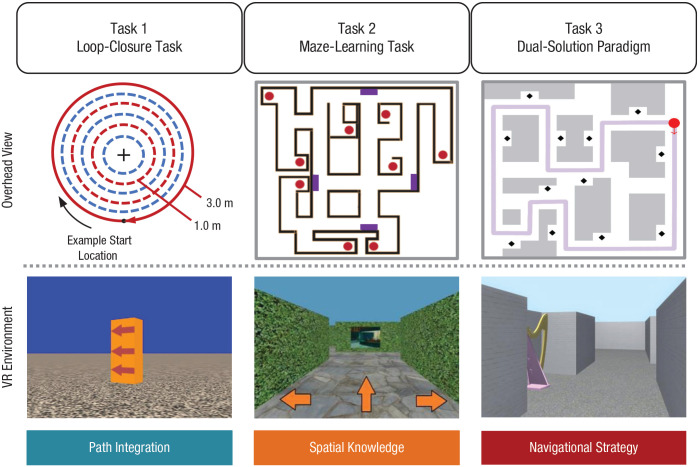
Fig. 1.
Three virtual reality (VR) tasks that probe unique aspects of navigation ability. Task 1, the loop-closure task, is a probe of path integration. Participants walk in a circle while tracking their start location, and they indicate when they think they have returned to the start. Beginning at the orange pole (visible in the screenshot from the task), participants completed loops at a 1.0-m, 2.0-m, and 3.0-m radius. Task 2, the maze-learning task, tests spatial-knowledge acquisition. Participants begin by freely exploring a novel maze environment and then, during the test phase, are asked to navigate to target locations without feedback. In the overhead view, purple rectangles indicate paintings that served as landmarks to guide and orient participants, and red dots indicate targets. Task 3, the dual-solution paradigm, probes navigational strategy. During the learning phase, participants are first guided on a fixed route (indicated by the light purple line). During the test phase, they are free to generate a novel shortcut to reach a target location or proceed along the learned path.
Path integration is the updating of one’s position and orientation during self-motion without external landmarks and relies on internal senses of self-motion from proprioceptive and vestibular systems, as well as visual information from optic flow (Loomis et al., 1993; Mittelstaedt & Mittelstaedt, 1980). Older adults (ages 65 or older) are impaired on path integration (Allen et al., 2004; Harris & Wolbers, 2012; Stangl et al., 2018), but it is unknown if these deficits are present earlier in the aging process. Further, it is unclear whether sex differences in path integration exist at any point across the life span (Coughlan, Coutrot, et al., 2018; Coutrot et al., 2019).
Here, we tested path-integration ability with a walking virtual reality (VR) task (Chrastil et al., 2019). The deficits in path integration observed in older adults (Allen et al., 2004; Harris & Wolbers, 2012; Stangl et al., 2018) were based on studies that provided only proprioception or only vision (e.g., using desktop virtual environments or blindfolded walking). In contrast, our task provides participants with both cues. On the basis of previous research, we might expect age-related deficits to emerge as early as midlife. However, age-related deficits in path integration might be spared when consistent cues from vision and proprioception are available. Sex differences are prevalent in the ability to form survey knowledge (Chrastil & Warren, 2013; Moffat et al., 1998; Nazareth et al., 2019; Waller, 2000), which is presumed to rely on path integration, so we might expect to observe a sex difference in path integration. However, to date, no direct test of sex differences in path integration has been performed using proprioceptive and visual cues, so this represents a novel aspect of our study.
Statement of Relevance.
Spatial navigation has emerged as a promising behavioral marker for detecting individuals at risk for dementia. However, few studies have established whether navigation deficits emerge early in the aging process (prior to age 65) in healthy individuals or whether age-related changes vary between men and women. This represents a critical gap in our understanding of the aging brain. In this study, we used immersive virtual technology to study three distinct aspects of successful navigation—path integration, wayfinding ability, and navigation strategy—to elucidate the cognitive changes that occur between young adulthood and midlife. Although path integration has typically been used as an early marker for dementia, our findings suggest that wayfinding and navigation strategy are more sensitive to the earliest stages of the aging process. Age-related deficits were evident by the middle decade of life and most pronounced in men. Understanding the trajectories of healthy aging—and how they differ for men and women—will pave the way for developing targeted behavioral markers for dementia.
Spatial-knowledge acquisition (sometimes referred to as cognitive mapping) is the ability to acquire spatial information, such as inferring how paths connect and where items are located in the broader environment (Golledge, 1999). Sex differences in spatial-knowledge acquisition from both route learning and free exploration have been observed in young adults (Chrastil & Warren, 2015; Coluccia & Louse, 2004; Hegarty et al., 2006; Montello et al., 1999). However, few studies have focused on spatial-knowledge acquisition from free exploration as opposed to route learning (e.g., Wiener et al., 2013) in older adults. Zhong and Moffat (2016) allowed participants to discover the correct route to a goal location, finding both sex and aging effects across young, midlife, and older populations. However, the effects of aging on the ability to learn a spatial layout from unrestricted exploration are still unknown.
We tested spatial-knowledge acquisition using a desktop VR maze task (Chrastil & Warren, 2015). Critically, participants were free to explore the maze, a novel paradigm for testing aging populations. We expect to see sex differences in spatial-knowledge acquisition for young adults (cf. Chrastil & Warren, 2015). However, although age effects have been observed in route learning in adults age 65 and older (Harris et al., 2012; Wiener et al., 2013; Zhong & Moffat, 2016), it is unclear whether unrestricted learning also leads to deficits in older adults. We also examined unrestricted exploration behavior to disambiguate whether deficits arise from a failure to fully explore a novel spatial environment or from a failure to consolidate exposure into spatial knowledge. Because the neuroendocrine changes during menopause affect the circuitry for acquiring spatial knowledge (Jacobs & Goldstein, 2018), we predicted that a male advantage would persist with age.
The term navigational strategies refers to the nature of the paths individuals select to navigate to a goal location in a known environment. Sex differences in navigation strategy have been observed in young adults (Boone et al., 2018): Women are more likely to follow well-learned (habitual) routes using cues from landmarks (an egocentric, response-based strategy), whereas men more often use their knowledge of the spatial layout of an environment to infer a shortcut to a goal location (an allocentric, place-based strategy). Older adults, compared with younger adults, are more likely to follow established routes than take novel shortcuts (Harris & Wolbers, 2014) and are less flexible at switching between response- and place-based strategies (Harris et al., 2012; Wiener et al., 2013). Despite accumulating evidence of navigation deficits in elderly populations, navigational strategies have yet to be examined earlier in the aging process—another critical gap in our understanding of the aging brain.
We had participants learn a set route in a desktop VR environment (Marchette et al., 2011) and then probed their navigational strategies by examining the paths they took when asked to navigate to target locations in the maze. This study is the first to investigate sex differences in strategy preference in midlife adults. On the basis of previous research with older adults, we hypothesized that midlife adults would show an increased reliance on habitual paths relative to young adults and that men would display a greater preference for taking novel shortcuts compared with women.
In sum, we probed three essential aspects of spatial navigation to determine whether behavioral deficits are detectable in the early stages of the aging process. Characterizing these changes in the healthy aging brain, and how they differ for men and women, is critical to future work establishing the earliest behavioral signs of Alzheimer’s disease and other dementias.
Method
Participants
One hundred fifty-one adults from the University of California, Santa Barbara, and the greater Santa Barbara community participated in the study. These consisted of 85 young adults (39 women; age range 18–28 years, M = 19.81, SD = 1.87) and 66 midlife adults (40 women; age range 43–61 years, M = 51.14, SD = 4.07). Participants gave written informed consent to take part in the study, which was approved by the University of California, Santa Barbara Human Subjects Committee. Young adults were compensated financially ($12 per hour) or through course credit. Midlife participants were compensated financially ($30 per hour to account for the additional costs of traveling from off campus).
Because of technical issues with the equipment and simulator-induced motion sickness, some participants could not complete all three tasks or were excluded from data analysis because of insufficient trials. Thus, final sample sizes are reported for each analysis and reported in the caption of each corresponding figure. A power analysis was performed for sample-size estimation on the basis of our 2 (age group) × 2 (sex) design. To detect a medium to large effect size (f = .30; Boone et al., 2018; Chrastil & Warren, 2015; Harris & Wolbers, 2012) using Cohen’s (1988) criteria with an α of .05 and 80% power, we needed a sample size of 90 participants. Thus, we felt that our sample was sufficiently powered to test effects of age and sex.
Task procedures
Participants completed three VR-based navigation tasks to assess path integration, spatial knowledge, and navigational strategy, respectively (Fig. 1).
Loop-closure task
The loop-closure task is an immersive, walking VR paradigm that assesses path-integration ability (Fig. 1, Task 1). A detailed description of the method has been reported previously (Chrastil et al., 2019). Briefly, participants saw a bare desert landscape devoid of landmarks or other orienting cues. Visual (optic flow), proprioceptive, and vestibular information was available to participants. An orange pole served as the start location for each trial, but it was not visible after the trial commenced. Participants walked along a circular loop while an experimenter guided them to ensure they stayed on the circumference of the circle. Participants clicked a button on a wireless remote to indicate when they thought they had returned to the start location. Participants completed 10 trials for each of three radii: 1.0 m, 2.0 m, and 3.0 m. Performance feedback was not provided, and there was no time limit to complete each trial.
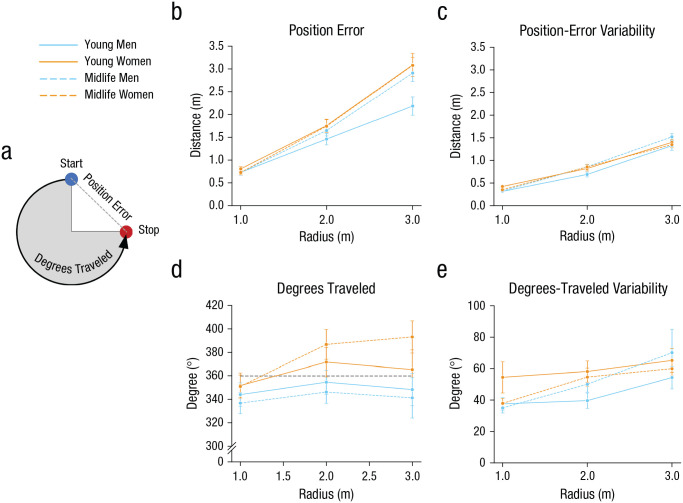
The primary dependent variables were position error and total degrees traveled (Fig. 2a). Position error is defined as the straight-line distance between the actual starting location for each trial and the location that the participant indicated was the start. Total degrees traveled represents the number of degrees traveled around the circular path (which can be greater than, less than, or equal to 360°). Variability of position error and degrees traveled, defined as the within-subjects standard deviation across the 10 trials at each radius, was also assessed. Variability across trials could indicate how well the participants were able to integrate the cues on a consistent basis; high variability could indicate less certainty in the integration (Chrastil et al., 2019). A mixed-model analysis of variance (ANOVA) was conducted for the four outcome measures with a 2 (age group: midlife, young) × 2 (sex: women, men) × 3 (radius size: 1.0 m, 2.0 m, 3.0 m) design. Corrections for sphericity were made where appropriate using the Greenhouse-Geisser correction.
Fig. 2.
Position error and degrees traveled in the loop-closure task. Position error (a) is the straight-line distance between the actual starting location for each trial and the location that the participant indicated was the start. The blue dot indicates an example starting location along the loop, and the red dot indicates an example stopping location. Position error (represented by the gray dashed line between the blue and red dots) was measured in meters. Degrees traveled (represented by the black line on the circumference of the circle) indicates the total distance, in degrees, traversed by the participant. The arrow indicates the walking direction, which alternated between clockwise and counterclockwise. On the right, the graphs in the top row show position error (b) and position-error variability (c) as a function of the radius size of the loop, age group, and participant sex. The graphs in the bottom row show degrees traveled (d) and degrees-traveled variability (e) as a function of the radius size of the loop, age group, and participant sex. In (d), the gray dotted line at 360° represents the ideal degrees participants should travel. Error bars show standard errors of the mean. Analyses for this task were run with 27 young men, 25 young women, 23 midlife men, and 33 midlife women.
Maze-learning task
The maze-learning task assesses an individual’s ability to acquire spatial knowledge from free, unrestricted exploration of a virtual environment. Details of the task have been reported previously (Chrastil & Warren, 2014, 2015); one exception to the previous study is that, in this case, the maze-learning task was conducted with a desktop VR setup. Briefly, the virtual maze environment consists of hallways with tall, vertical hedges and nine target objects. During an initial exploration phase, participants were given two 8-min sessions to freely navigate the unfamiliar virtual environment with the goal of finding the nine target objects (indicated as red dots in the overhead view of the maze depicted in Fig. 1, Task 2). Paintings served as landmarks to guide and orient participants (indicated as purple rectangles in the overhead view depicted in Fig. 1, Task 2). Participants used the computer keyboard to indicate at each intersection whether they wanted to move left, right, or straight. Translational movement was fixed at 1.0 virtual meters per second, and rotation speed was fixed at 90° per second. During the test phase, participants completed 24 trials so we could assess their spatial knowledge of the environment. Each trial began with the participant located at one object in the maze and given the instruction to navigate to another object within a 45-s trial period. The objects were replaced with red spheres during the test phase to minimize feedback and landmark cues during navigation.
The main dependent variable was wayfinding success (a measure of spatial-knowledge acquisition based on free exploration), defined as the proportion of trials in which the participant reached the correct target object in time during the test phase. This variable was compared with chance (i.e., 1/9 potential objects in the maze = 11.11%). We also measured the total number of moves—key presses made to navigate the environment during the exploration phase—as a measure of how extensively participants explored the maze, to determine how exploration relates to test performance. A 2 (age group: midlife, young) × 2 (sex: women, men) between-subjects ANOVA was conducted to assess group differences in each of these variables. Finally, a Pearson’s correlation assessed the relationship between the number of moves made during the exploration phase and navigation success in the test phase. An analysis of covariance (ANCOVA) examined the effects of age and sex on navigation success after controlling for the number of moves made by participants during maze exploration.
Dual-solution paradigm
The dual-solution paradigm assesses individual differences in navigational strategy (i.e., the nature of the route individuals select to navigate to a goal location). Procedures were identical to those used by Boone et al. (2018). Participants first familiarized themselves with the navigation controls in an open desktop VR training maze prior to the task. The maze in the dual-solution paradigm consisted of an environment with 12 landmarks (shown as black diamonds in the overhead view depicted in Fig. 1, Task 3). Unlike in the maze-learning task, in which participants were free to explore the environment during the initial learning phase, all participants in the dual-solution paradigm followed a set route, giving them the same learning experience. Specifically, they were guided along the same route through the maze five times (depicted as a purple path starting at the red dot shown in the overhead view in Fig. 1, Task 3). Then, during the test phase, participants were placed at one object in the maze and instructed to navigate to another object. All objects were visible throughout the test phase. Participants had 40 s to find each target object, and they completed a total of 20 trials. Trials were coded to determine whether participants took the learned route or a novel shortcut when navigating to the target object. Trials were chosen so the shortest route to the target was at least 25% shorter than the learned route; shortcuts were on average 51% shorter. Participants had to take the shortest possible route for it to count as a shortcut (for additional details on trial coding, see the Supplemental Material available online and Boone et al., 2018, 2019).
Two measures of interest were calculated: wayfinding success (a measure of spatial-knowledge acquisition based on route experience), defined as the proportion of trials on which the participant reached the target object within the time limit, and the solution index, defined as the number of shortcuts (the shortest possible route) divided by the number of successful trials (a measure of strategy). A 2 (age group: midlife, young) × 2 (sex: women, men) between-subjects ANOVA was performed on each dependent variable. Heat maps were generated to provide a qualitative assessment of participants’ routes in the virtual environment by extracting their location every 100 ms per trial.
Results
Loop-closure task
Fifty-two young adults (25 women, 27 men) and 56 midlife adults (33 women, 23 men) were included in the data analysis. Participants’ data were removed if insufficient trials (n < 5 trials) were completed at a given radius, resulting in the removal of 105 data points for radius 1.0 m, 108 for radius 2.0 m, and 90 for radius 3.0 m (see the Supplemental Material).
We first tested the prediction that there would be age and sex differences in path-integration accuracy. Position error (Fig. 2a) did not differ significantly as a function of age, F(1, 85) = 0.92, p = .341, η p 2 = .01, 95% confidence interval (CI) for η p 2 = [0.00, .09], or sex, F(1, 85) = 2.84, p = .096, η p 2 = .03, 95% CI for η p 2 = [.00, .13]. As expected, position error increased with radius size, F(2, 170) = 328.57, p < .001, η p 2 = .80, 95% CI for η p 2 = [.74, .83] (see Fig. 2b), suggesting that path integration is more difficult with longer distances. Although there was a significant Age × Radius Size interaction, F(2, 170) = 4.15, p = .003, η p 2 = .05, 95% CI for η p 2 = [.00, .11], and Sex × Radius Size interaction, F(2, 170) = 7.13, p = .003, η p 2 = .08, 95% CI for η p 2 = [.01, .16], post hoc testing found no significant effects of sex or age at any radius size (all ps > .060, Bonferroni corrected). Further, no Age × Sex interaction, F(1, 85) = 1.21, p = .274, η p 2 = .01, 95% CI for η p 2 = [.00, .10], or three-way interaction, F(2, 170) = 2.68, p = .086, η p 2 = .03, 95% CI for η p 2 = [.00, .09], was observed.
Variability in position error showed no main effects of age, F(1, 85) = 0.45, p = .502, η p 2 = .005, 95% CI for η p 2 = [.00, .07], or sex, F(1, 85) = 0.08, p = .779, η p 2 < .001, 95% CI for η p 2 = [.00, .05]. As shown in Figure 2c, there was a main effect of radius size, F(2, 170) = 452.01, p < .001, η p 2 = .84, 95% CI for η p 2 = [.80, .87], with increasing radius size leading to increased variability in participants’ estimates. There were no significant interactions for this variable (all ps > .133).
Degrees traveled around the circular loop path did not differ significantly as a function of age, F(1, 85) = 0.61, p = .437, η p 2 = .01, 95% CI for η p 2 = [.00, .08]. However, there was a significant main effect of sex, F(1, 85) = 5.82, p = .018, η p 2 = .06, 95% CI = [.00, .18], as shown in Figure 2d; women (M = 370.90°, SD = 68.21°, 95% CI = [360.58°, 381.21°]) tended to overshoot their estimate of the starting location, but men (M = 345.55°, SD = 53.75°, 95% CI = [336.49°, 354.62°]) tended to undershoot. A main effect of radius size, F(2, 170) = 9.33, p < .001, η p 2 = .10, 95% CI for η p 2 = [.03, .18], indicated that degrees traveled increased as the size of the radius increased. There were no significant interactions among these variables (all ps > .144).
Variability in degrees traveled also showed no significant main effects of age, F(1, 85) = 0.01, p = .929, η p 2 < .001, 95% CI for η p 2 = [.00, .03], or sex, F(1, 85) = 1.67, p = .200, η p 2 = .02, 95% CI for η p 2 = [.00, .11], but there was a main effect of radius size, F(2, 170) = 9.29, p < .001, η p 2 = .10, 95% CI for η p 2 = [.03, .18]. Variability increased as the size of the radius increased. None of the interactions for variability in degrees traveled were significant (all ps > .083; see Fig. 2e).
In summary, position error was similar across young and middle-aged men and women, whereas the analysis of degrees traveled showed that women tended to overshoot and men tended to undershoot. Overall, performance accuracy for the loop-closure task suggests negligible effects of age and sex on path-integration ability.
Maze-learning task
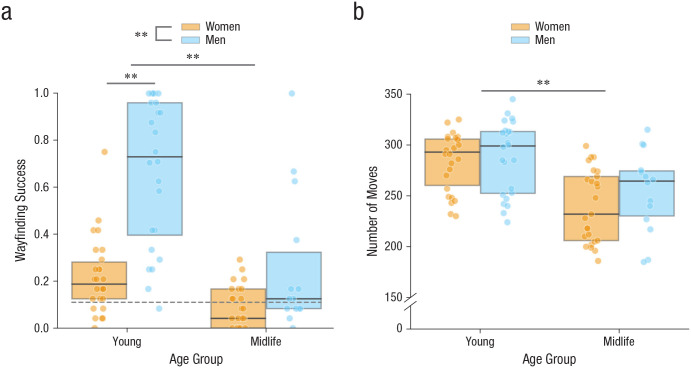
Fifty young adults (26 women, 24 men) and 39 midlife adults (25 women, 14 men) were included in the data analysis. First, we tested whether participants performed above chance on the measure of wayfinding success (one-tailed t tests). Young men (M = .67, SD = .31, 95% CI = [.54, .80]), t(23) = 8.72, p < .001, d = 1.78, 95% CI for d = [1.12, 2.42], and young women (M = .22, SD = .16, 95% CI = [.15, .29]), t(25) = 3.38, p = .001, d = 0.66, 95% CI for d = [0.23, 1.08], performed significantly above chance, whereas midlife men (M = .26, SD = .30, 95% CI = [.09, .43]), t(13) = 1.86, p = .043, d = 0.50, 95% CI for d = [−0.07, 1.04], and midlife women (M = .09, SD = .09, 95% CI = [.05, .13]), t(24) = −1.27, p = .892, d = −0.25, 95% CI for d = [−0.65, 0.15], performed close to or at chance.
Next, we tested the prediction that there would be age and sex differences in wayfinding success during the test phase. There was a significant main effect of age, F(1, 85) = 30.37, p < .001, η p 2 = .26, 95% CI for η p 2 = [.12, .40], with better performance by younger adults (M = .43, SD = .33, 95% CI = [.34, .53]) than midlife adults (M = .15, SD = .21, 95% CI = [.08, .22]), and a significant effect of sex, F(1, 85) = 39.86, p < .001, η p 2 = .32, 95% CI for η p 2 = [.16, .45], with men (M = .52, SD = .36, 95% CI = [.40, .64]) having more wayfinding success than women (M = .16, SD = .15, 95% CI = [.11, .20]). There was also a significant Age × Sex interaction, F(1, 85) = 8.04, p = .006, η p 2 = .09, 95% CI for η p 2 = [.01, .21]. An analysis of simple effects revealed significant effects of age on both sexes—men: F(1, 36) = 15.65, p < .001, η p 2 = .30, d = −1.33, 95% CI for d = [−2.08, −0.58]; women: F(1, 49) = 12.49, p = .002, η p 2 = .20, d = −0.99, 95% CI for d = [−1.59, −0.39]. An analysis of simple effects also indicated significant effects of sex at both ages, with substantially larger effects for young adults, F(1, 48) = 41.25, p < .001, η p 2 = .46, d = −1.82, 95% CI for d = [−2.49, 1.14], than for midlife adults, F(1, 37) = 7.18, p = .022, η p 2 = .16, d = −0.89, 95% CI for d = [−1.60, −0.19]. Although sex differences were lower in midlife adults compared with younger adults, it should be noted that performance was generally very poor among midlife adults, so this could partially reflect a floor effect.
During the initial maze-exploration phase, participants made on average 268.08 moves (SE = 39.94, 95% CI = [259.67, 276.49]) to explore the maze. Across all participants, a significant Pearson’s correlation test, r(87) = .34, p = .001, 95% CI for r = [.14, .51], indicated that wayfinding success was related to how much participants explored the maze. As shown in Figure 3b, a 2 (age group) × 2 (sex) ANOVA indicated a main effect of age, F(1, 85) = 26.99, p < .001, η p 2 = .24, 95% CI for η p 2 = [.10, .38], with younger adults (M = 286.14, SD = 31.91, 95% CI = [277.07, 295.21]) making more moves than midlife adults (M = 244.92, SD = 37.43, 95% CI = [232.79, 257.06]). For number of moves made during exploration, there was no main effect of sex, F(1, 85) = 1.51, p = .223, η p 2 = .02, 95% CI for η p 2 = [.00, .11]: Men (M = 275.66, SD = 40.34, 95% CI = [262.40, 288.92]) and women (M = 262.43, SD = 39.07, 95% CI = [251.44, 273.42]) performed similarly. There was no Age × Sex interaction for this variable, F(1, 85) = 0.57, p = .453, η p 2 = .01, 95% CI for η p 2 = [.00, .08].
Fig. 3.
Spatial-knowledge acquisition in the maze-learning task. Wayfinding success (a) and number of moves made during exploration (b) are shown separately for each age group and sex. In each boxplot, the horizontal line indicates the median, and the top and bottom edges of the box mark the 75th and 25th percentiles, respectively. Colored dots represent individual data points. Asterisks represent significant differences between age groups and sexes (**p < .001). The grey dashed line at 0.11 represents chance level (i.e., 1/9 potential objects in the maze = 11.11%). Analyses for this task were run with 24 young men, 26 young women, 14 midlife men, and 25 midlife women.
Because of this difference in moves across age groups, we conducted a two-way ANCOVA on wayfinding success to test whether the differences in moves made between age groups during the exploration phase affected performance in the test phase. After controlling for moves made, we found that the main effects of age, F(1, 84) = 18.51, p < .001, η p 2 = .18, 95% CI for η p 2 = [.05, .32], and sex, F(1, 84) = 37.52, p < .001, η p 2 = .31, 95% CI for η p 2 = [.15, .44], remained significant, as did the two-way interaction between age and sex, F(1, 84) = 8.46, p = .005, η p 2 = .09, 95% CI for η p 2 = [.01, .22]. An analysis of simple effects of age remained significant for men, F(1, 35) = 11.30, p = .008, η p 2 = .24, d = −1.81, 95% CI for d = [−2.61, −1.00], but not women, F(1, 48) = 4.86, p = .128, η p 2 = .09, d = −0.35, 95% CI for d = [−0.92, 0.21]. The simple effect of sex remained statistically significant for young adults, F(1, 47) = 40.57, p < .001, η p 2 = .46, d = −1.62, 95% CI for d = [−2.27, −0.96], but not for midlife adults, F(1, 36) = 5.46, p = .100, η p 2 = .13, d = −0.72, 95% CI for d = [−1.42, −0.02], as shown in Figure 3a.
In summary, men showed a steep age-related deficit in wayfinding success. The sex difference favoring men in young adulthood was eliminated by midlife.
Dual-solution paradigm
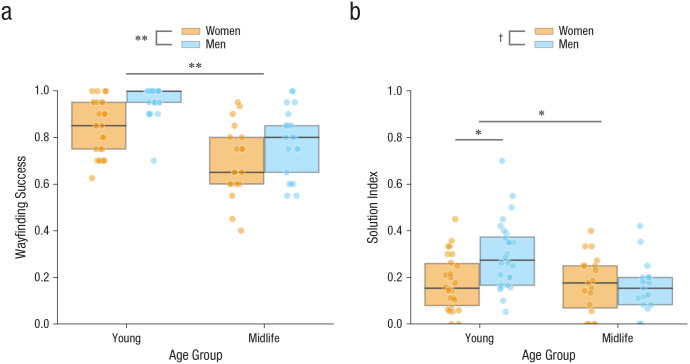
Fifty-four young adults (26 women, 28 men) and 40 midlife adults (19 women, 21 men) were included in the data analysis. We first tested the prediction that young adults would outperform midlife adults on wayfinding success. We observed a main effect of age, F(1, 90) = 43.04, p < .001, η p 2 = .32, 95% CI for η p 2 = [.17, .45], with younger participants (M = .91, SD = .11, 95% CI = [.88, .93]) outperforming midlife participants (M = .74, SD = .15, 95% CI = [.69, .79]), and a main effect of sex, F(1, 90) = 15.95, p < .001, η p 2 = .15, 95% CI for η p 2 = [.04, .28], with men (M = .88, SD = .14, 95% CI = [.84, .92]) outperforming women (M = .78, SD = .15, 95% CI = [.74, .83]; see Fig. 4a), but with no Age × Sex interaction, F(1, 90) = 0.38, p = .537, η p 2 = .004, 95% CI for η p 2 = [.00, .07].
Fig. 4.
Performance in the dual-solution paradigm. Wayfinding success (a) and the proportion of shortcuts taken on successful trials (solution index; b) are shown separately for each age group and sex. In each boxplot, the horizontal line indicates the median, and the top and bottom edges of the box mark the 75th and 25th percentiles, respectively. Colored dots represent individual data points. Asterisks represent significant differences between conditions and groups (*p < .01, **p < .001), and the dagger represents marginal significance (p < .10). Analyses for this task were run with 28 young men, 26 young women, 21 midlife men, and 19 midlife women.
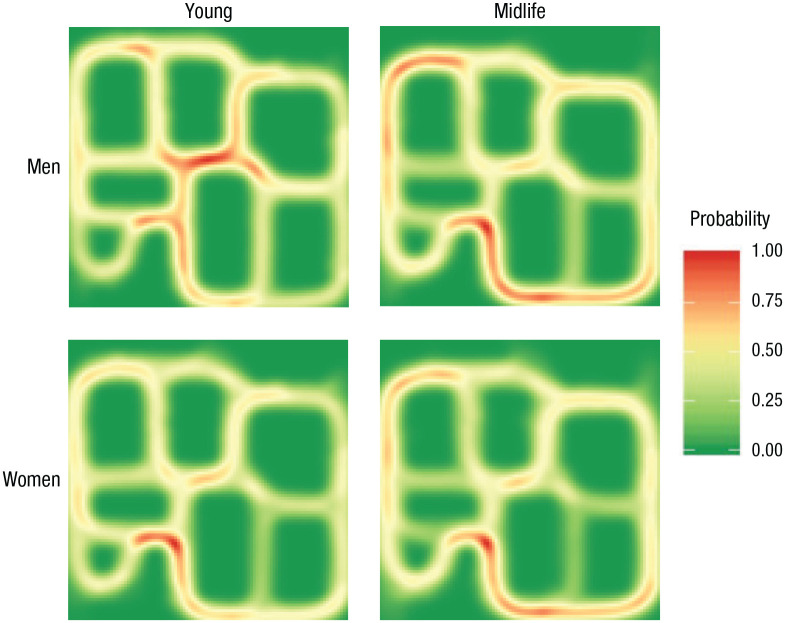
We then tested the prediction that younger adults would take more shortcuts on successful trials (i.e., have a higher solution index) than midlife adults. We observed a main effect of age, F(1, 90) = 7.83, p = .006, η p 2 = .08, 95% CI for η p 2 = [.01, .20], with younger adults (M = .24, SD = .15, 95% CI = [.20, .28]) taking more shortcuts than midlife adults (M = .16, SD = .11, 95% CI = [.13, .20]). We observed a marginally significant main effect of sex, F(1, 90) = 3.64, p = .06, η p 2 = .04, 95% CI for η p 2 = [.00, .14], with men (M = .23, SD = .15, 95% CI = [.19, .28]) tending to take more shortcuts than women (M = .17, SD = .12, 95% CI = [.14, .21]). However, there was a significant Age × Sex interaction, F(1, 90) = 6.59, p = .012, η p 2 = .07, 95% CI for η p 2 = [.00, .18] (see Fig. 4b). An analysis of simple effects revealed that the effect of age was significant for men, F(1, 47) = 14.00, p < .001, η p 2 = .23, d = −1.08, 95% CI for d = [−1.70, −0.46], but not for women, F(1, 43) = 0.03, p = 1.00, η p 2 < .001, d = −0.05, 95% CI for d = [−0.66, 0.56]. Simple-effects analysis also indicated significant effects of sex for young adults, F(1, 52) = 10.47, p = .004, η p 2 = .17, d = −0.88, 95% CI for d = [−1.45, −0.31], but not for midlife adults, F(1, 38) = 0.23, p = 1.00, η p 2 = .01, d = 0.15, 95% CI for d = [−0.49, 0.79]. Heat maps were generated to qualitatively visualize the routes people chose to reach a target (Fig. 5), and they indicated that young men were more likely to take a shortcut through the center of the maze, whereas young women, midlife men, and midlife women favored the learned route along the periphery.
Fig. 5.
Heat maps illustrating the routes participants took to reach a target in the dual-solution paradigm. The most-favored routes are indicated in red. Results are shown separately for men and women in both the young and midlife adult groups. Analyses for this task were run with 28 young men, 26 young women, 21 midlife men, and 19 midlife women.
In summary, men showed an age-related shift in wayfinding strategies, whereas both young and midlife women tended to take learned routes.
Discussion
The present study tested the effects of age and sex on three core aspects of spatial navigation in young and midlife adults: path integration, spatial-knowledge acquisition, and navigational strategy. Building on previous chronological-aging studies (Driscoll et al., 2005; Zhong & Moffat, 2016), we found that some age-related differences in spatial navigation are evident by midlife. Although path-integration ability was largely preserved with age in the loop-closure task, pronounced age-related differences were observed in the ability to acquire spatial knowledge in the maze-learning task and in the selection of a navigational strategy in the dual-solution paradigm. No major sex difference was observed for path integration, but sex differences were found for acquiring spatial knowledge and navigation strategy. Overall, sex differences present in young adults tended to be reduced with age.
Previous findings indicate poor path integration in adults 65 years or older (Adamo et al., 2012; Coughlan, Coutrot, et al., 2018; Harris & Wolbers, 2012), but we saw no such change in midlife adults, suggesting that age-related changes arise later in the aging process. In previous studies, older adults had access to a single cue (vision or proprioception), whereas our task provided participants with multiple cues. Future studies should examine whether reduced performance is evident in midlife when performance is constrained to a single cue. Although there was a sex difference in degrees traveled, with women tending to overshoot and men tending to undershoot, this difference did not affect the overall position error. The tendency to overshoot could be a cautionary measure by women to ensure they reach the start (e.g., Gagnon et al., 2016). In sum, we found little evidence for sex or aging effects in path integration.
Sex differences were evident in measures of spatial-knowledge acquisition from both unrestricted free exploration (maze-learning task) and route-based learning (dual-solution paradigm). In the maze-learning task, midlife adults did not explore as much as younger adults. After accounting for differing numbers of exploration moves, we found that sex differences remained robust in young adults and diminished in midlife adults. Floor effects were present in the maze-learning task for midlife adults, making sex differences less detectible. It is possible that age-related changes in the brain hinder the ability for midlife adults to create a comprehensive cognitive map, which in turn makes sex differences in performance harder to detect. In the dual-solution paradigm, wayfinding success indicates how well participants learned the environment from a route. Despite equal exposure to the route, midlife adults were less successful than younger adults, consistent with previous studies on learning from routes (Harris & Wolbers, 2014; Wiener et al., 2013; Zhong & Moffat, 2016). Together, these findings suggest that spatial learning is impaired as early as midlife. These data are consistent with findings from an earlier study demonstrating age-related performance decrements in a virtual Morris water-maze task by midlife (Driscoll et al., 2005).
Navigation strategies in the dual-solution paradigm indicated that young men took more shortcuts than young women, a finding that echoes previous results by Boone et al. (2018, 2019). Our study provides the first evidence that age-related differences in navigation strategies are evident by midlife, with midlife adults using fewer shortcuts than younger adults. This result is consistent with reports that older adults use more habitual routes when navigating (Harris et al., 2012; Lester et al., 2017; Wiener et al., 2013) and suggests that strategies have already shifted by midlife. In addition, we found that the sex difference observed in young adults did not persist in midlife adults. Further, the heat maps indicated that although young men were more likely to take shortcuts through the middle of the maze, all other groups relied on the learned route to navigate within the maze. Thus, the major change with age was a reduction of place-based strategies in men.
Several limitations should be considered when interpreting these findings. First, it is possible that older adults have less experience with using computer gaming controls (such as those used in the dual-solution paradigm) compared with younger adults, and this could contribute to navigation inefficiency in the desktop virtual environments. However, age-related differences also existed for the maze-learning task, which requires the use of only a single button press at each intersection. This indicates that poorer performance for midlife adults is unlikely to be due to computer experience alone and is likely to be related to age-related changes in participants’ brains that deterred successful acquisition of spatial information from the environment. A direct assessment of participants’ gaming experience and experience in virtual environments was not acquired, so this issue cannot be fully resolved.
Second, the midlife period is characterized by significant neuroendocrine changes in women. Our sample included midlife women, spanning the spectrum from late premenopausal to early postmenopausal. Although the study was not powered to assess performance by reproductive stage or endocrine status in the current sample, this will be a major focus of our future research. Further, young adult women were tested independently of menstrual-cycle stage. Given accumulating evidence that menstrual-cycle stage and sex-hormone concentrations impact spatial cognition (Courvoisier et al., 2013; Hussain et al., 2016) and aspects of navigation (Korol et al., 2004), future studies should clarify the extent to which these relationships hold across measures of path integration, wayfinding, and navigation strategy.
Finally, the largest age effects were observed in men, with a steep decline in wayfinding success (maze-learning task) and a shift toward taking habitual routes (dual-solution paradigm). It is possible that these unexpected effects have a neuroendocrine basis. Testosterone production in men begins to diminish when they are in their early 30s and gradually declines throughout the adult life span (Feldman et al., 2002). Testosterone loss influences cognitive and brain function in aging men, including visual and verbal memory and spatial cognition (Moffat, 2005). Driscoll and colleagues (2005) found that the male spatial advantage in a virtual Morris water-maze task is related to circulating testosterone. Thus, the role of testosterone should be considered as a factor in future studies of navigation and aging.
In sum, we examined signatures of early aging in three navigational tasks, opening up new avenues for understanding healthy aging. The differing patterns of age and sex across our three navigational tasks suggest that different aspects of navigation could tap into separate brain systems. Although path integration has typically been used as an early marker for dementia (Coughlan, Laczó, et al., 2018; Kunz et al., 2015), our findings suggest that spatial-knowledge acquisition and strategy use are more sensitive to the earliest stages of the aging process. Understanding the trajectories of healthy aging—and how they differ for men and women—will help pave the way for developing behavioral and neural markers for dementia.
Supplemental Material
Supplemental material, sj-docx-1-pss-10.1177_0956797620979185 for Age-Related Changes in Spatial Navigation Are Evident by Midlife and Differ by Sex by Shuying Yu, Alexander P. Boone, Chuanxiuyue He, Rie C. Davis, Mary Hegarty, Elizabeth R. Chrastil and Emily G. Jacobs in Psychological Science
Acknowledgments
A. P. Boone is now at the School of Psychological Science, Oregon State University. We thank Mustafa Shakir, Julia Woods, Chloe Lopez, and Aidan Galati for assistance with data collection and coding. The views and conclusions in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the U.S. government. The U.S. government is authorized to reproduce and distribute reprints for government purposes notwithstanding any copyright notation herein.
Footnotes
ORCID iD: Emily G. Jacobs  https://orcid.org/0000-0003-0001-5096
https://orcid.org/0000-0003-0001-5096
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797620979185
Transparency
Action Editor: M. Natasha Rajah
Editor: Patricia J. Bauer
Author Contributions
M. Hegarty, E. R. Chrastil, and E. G. Jacobs contributed equally to this work. M. Hegarty, E. R. Chrastil, and E. G. Jacobs developed the study concept. S. Yu and R. C. Davis ran the experiments. The data-analysis concept was developed by S. Yu, A. P. Boone, C. He, M. Hegarty, E. R. Chrastil, and E. G. Jacobs. S. Yu analyzed the data. S. Yu, M. Hegarty, E. R. Chrastil, and E. G. Jacobs wrote the manuscript. A. P. Boone, C. He, and R. C. Davis edited the manuscript. All the authors approved the final manuscript for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported by grants from the California Nanosystems Institute (to E. G. Jacobs, E. R. Chrastil, and M. Hegarty), the Brain and Behavior Research Foundation (to E. G. Jacobs), and the U.S. Army Research Office. The work was accomplished under Cooperative Agreement W911-NF-19-2-0026 for the Institute for Collaborative Biotechnologies (funding to E. R. Chrastil); the University of California, Santa Barbara Academic Senate (funding to E. G. Jacobs, E. R. Chrastil, and M. Hegarty); and the Hellman Fellows Fund (funding to E. G. Jacobs).
Open Practices: Data and materials for this study have not been made publicly available, and the design and analysis plans were not preregistered.
References
- Adamo D. E., Briceño E. M., Sindone J. A., Alexander N. B., Moffat S. (2012). Age differences in virtual environment and real world path integration. Frontiers in Aging Neuroscience, 4, Article 26. 10.3389/fnagi.2012.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. L., Kirasic K. C., Rashotte M. A., Haun D. B. M. (2004). Aging and path integration skill: Kinesthetic and vestibular contributions to wayfinding. Perception & Psychophysics, 66(1), 170–179. 10.3758/BF03194870 [DOI] [PubMed] [Google Scholar]
- Boone A. P., Gong X., Hegarty M. (2018). Sex differences in navigation strategy and efficiency. Memory & Cognition, 46(6), 909–922. 10.3758/s13421-018-0811-y [DOI] [PubMed] [Google Scholar]
- Boone A. P., Maghen B., Hegarty M. (2019). Instructions matter: Individual differences in navigation strategy and ability. Memory & Cognition, 47(7), 1401–1414. 10.3758/s13421-019-00941-5 [DOI] [PubMed] [Google Scholar]
- Chrastil E. R. (2013). Neural evidence supports a novel framework for spatial navigation. Psychonomic Bulletin & Review, 20(2), 208–227. 10.3758/s13423-012-0351-6 [DOI] [PubMed] [Google Scholar]
- Chrastil E. R., Nicora G. L., Huang A. (2019). Vision and proprioception make equal contributions to path integration in a novel homing task. Cognition, 192, Article 103998. 10.1016/j.cognition.2019.06.010 [DOI] [PubMed] [Google Scholar]
- Chrastil E. R., Warren W. H. (2013). Active and passive spatial learning in human navigation: Acquisition of survey knowledge. Journal of Experimental Psychology: Learning, Memory, and Cognition, 39(5), 1520–1537. 10.1037/a0032382 [DOI] [PubMed] [Google Scholar]
- Chrastil E. R., Warren W. H. (2014). From cognitive maps to cognitive graphs. PLOS ONE, 9(11), Article e112544. 10.1371/journal.pone.0112544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrastil E. R., Warren W. H. (2015). Active and passive spatial learning in human navigation: Acquisition of graph knowledge. Journal of Experimental Psychology: Learning, Memory, and Cognition, 41(4), 1162–1178. 10.1037/xlm0000082 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Erlbaum. [Google Scholar]
- Coluccia E., Louse G. (2004). Gender differences in spatial orientation: A review. Journal of Environmental Psychology, 24(3), 329–340. 10.1016/j.jenvp.2004.08.006 [DOI] [Google Scholar]
- Coughlan G., Coutrot A., Khondoker M., Minihane A.-M., Spiers H. J., Hornberger M. (2018). Impact of sex and APOE status on spatial navigation in pre-symptomatic Alzheimer’s disease. BioRxiv. 10.1101/287722 [DOI]
- Coughlan G., Laczó J., Hort J., Minihane A.-M., Hornberger M. (2018). Spatial navigation deficits—overlooked cognitive marker for preclinical Alzheimer disease? Nature Reviews Neurology, 14(8), 496–506. 10.1038/s41582-018-0031-x [DOI] [PubMed] [Google Scholar]
- Courvoisier D. S., Renaud O., Geiser C., Paschke K., Gaudy K., Jordan K. (2013). Sex hormones and mental rotation: An intensive longitudinal investigation. Hormones and Behavior, 63(2), 345–351. 10.1016/j.yhbeh.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Coutrot A., Schmidt S., Coutrot L., Pittman J., Hong L., Wiener J. M., Hölscher C., Dalton R. C., Hornberger M., Spiers H. J. (2019). Virtual navigation tested on a mobile app is predictive of real-world wayfinding navigation performance. PLOS ONE, 14(3), Article e0213272. 10.1371/journal.pone.0213272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I., Hamilton D. A., Yeo R. A., Brooks W. M., Sutherland R. J. (2005). Virtual navigation in humans: The impact of age, sex, and hormones on place learning. Hormones and Behavior, 47(3), 326–335. 10.1016/j.yhbeh.2004.11.013 [DOI] [PubMed] [Google Scholar]
- Feldman H. A., Longcope C., Derby C. A., Johannes C. B., Araujo A. B., Coviello A. D., Bremner W. J., McKinlay J. B. (2002). Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts Male Aging Study. The Journal of Clinical Endocrinology & Metabolism, 87(2), 589–598. 10.1210/jcem.87.2.8201 [DOI] [PubMed] [Google Scholar]
- Gagnon K. T., Cashdan E. A., Stefanucci J. K., Creem-Regehr S. H. (2016). Sex differences in exploration behavior and the relationship to harm avoidance. Human Nature, 27(1), 82–97. 10.1007/s12110-015-9248-1 [DOI] [PubMed] [Google Scholar]
- Golledge R. G. (1999). Wayfinding behavior: Cognitive mapping and other spatial processes. Johns Hopkins University Press. [Google Scholar]
- Hara Y., Park C. S., Janssen W. G. M., Roberts M. T., Morrison J. H., Rapp P. R. (2012). Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiology of Aging, 33(2), 421.e17–421.e28. 10.1016/j.neurobiolaging.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. A., Wiener J. M., Wolbers T. (2012). Aging specifically impairs switching to an allocentric navigational strategy. Frontiers in Aging Neuroscience, 4, Article 29. 10.3389/fnagi.2012.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. A., Wolbers T. (2012). Ageing effects on path integration and landmark navigation. Hippocampus, 22(8), 1770–1780. 10.1002/hipo.22011 [DOI] [PubMed] [Google Scholar]
- Harris M. A., Wolbers T. (2014). How age-related strategy switching deficits affect wayfinding in complex environments. Neurobiology of Aging, 35(5), 1095–1102. 10.1016/j.neurobiolaging.2013.10.086 [DOI] [PubMed] [Google Scholar]
- Hebert L. E., Weuve J., Scherr P. A., Evans D. A. (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778–1783. 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty M., Montello D. R., Richardson A. E., Ishikawa T., Lovelace K. (2006). Spatial abilities at different scales: Individual differences in aptitude-test performance and spatial-layout learning. Intelligence, 34(2), 151–176. 10.1016/j.intell.2005.09.005 [DOI] [Google Scholar]
- Hegarty M., Richardson A. E., Montello D. R., Lovelace K., Subbiah I. (2002). Development of a self-report measure of environmental spatial ability. Intelligence, 30(5), 425–447. 10.1016/S0160-2896(02)00116-2 [DOI] [Google Scholar]
- Hussain D., Hanafi S., Konishi K., Brake W. G., Bohbot V. D. (2016). Modulation of spatial and response strategies by phase of the menstrual cycle in women tested in a virtual navigation task. Psychoneuroendocrinology, 70, 108–117. 10.1016/j.psyneuen.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Montello D. R. (2006). Spatial knowledge acquisition from direct experience in the environment: Individual differences in the development of metric knowledge and the integration of separately learned places. Cognitive Psychology, 52(2), 93–129. 10.1016/j.cogpsych.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Jacobs E. G., Goldstein J. M. (2018). The middle-aged brain: Biological sex and sex hormones shape memory circuitry. Current Opinion in Behavioral Sciences, 23, 84–91. 10.1016/j.cobeha.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol D. L., Malin E. L., Borden K. A., Busby R. A., Couper-Leo J. (2004). Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and Behavior, 45(5), 330–338. 10.1016/j.yhbeh.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Kunz L., Schröder T. N., Lee H., Montag C., Lachmann B., Sariyska R., Reuter M., Stirnberg R., Stöcker T., Messing-Floeter P. C., Fell J., Doeller C. F., Axmacher N. (2015). Reduced grid-cell–like representations in adults at genetic risk for Alzheimer’s disease. Science, 350(6259), 430–433. 10.1126/science.aac8128 [DOI] [PubMed] [Google Scholar]
- Lester A. W., Moffat S. D., Wiener J. M., Barnes C. A., Wolbers T. (2017). The aging navigational system. Neuron, 95(5), 1019–1035. 10.1016/j.neuron.2017.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis J. M., Klatzky R. L., Golledge R. G., Cicinelli J. G., Pellegrino J. W., Fry P. A. (1993). Nonvisual navigation by blind and sighted: Assessment of path integration ability. Journal of Experimental Psychology: General, 122(1), 73–91. 10.1037/0096-3445.122.1.73 [DOI] [PubMed] [Google Scholar]
- Marchette S. A., Bakker A., Shelton A. L. (2011). Cognitive mappers to creatures of habit: Differential engagement of place and response learning mechanisms predicts human navigational behavior. The Journal of Neuroscience, 31(43), 15264–15268. 10.1523/JNEUROSCI.3634-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhav M., Wolbers T. (2019). Aging and spatial cues influence the updating of navigational memories. Scientific Reports, 9, Article 11469. 10.1038/s41598-019-47971-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. M., Vemuri P., Rocca W. A. (2014). Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clinical Epidemiology, 6, 37–48. 10.2147/CLEP.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstaedt M.-L., Mittelstaedt H. (1980). Homing by path integration in a mammal. Naturwissenschaften, 67(11), 566–567. 10.1007/BF00450672 [DOI] [Google Scholar]
- Moffat S. D. (2005). Effects of testosterone on cognitive and brain aging in elderly men. Annals of the New York Academy of Sciences, 1055(1), 80–92. 10.1196/annals.1323.014 [DOI] [PubMed] [Google Scholar]
- Moffat S. D. (2009). Aging and spatial navigation: What do we know and where do we go? Neuropsychology Review, 19, Article 478. 10.1007/s11065-009-9120-3 [DOI] [PubMed] [Google Scholar]
- Moffat S. D., Hampson E., Hatzipantelis M. (1998). Navigation in a “virtual” maze: Sex differences and correlation with psychometric measures of spatial ability in humans. Evolution and Human Behavior, 19(2), 73–87. 10.1016/S1090-5138(97)00104-9 [DOI] [Google Scholar]
- Montello D. R., Lovelace K. L., Golledge R. G., Self C. M. (1999). Sex-related differences and similarities in geographic and environmental spatial abilities. Annals of the Association of American Geographers, 89(3), 515–534. 10.1111/0004-5608.00160 [DOI] [Google Scholar]
- Nazareth A., Huang X., Voyer D., Newcombe N. (2019). A meta-analysis of sex differences in human navigation skills. Psychonomic Bulletin & Review, 26(5), 1503–1528. 10.3758/s13423-019-01633-6 [DOI] [PubMed] [Google Scholar]
- Stangl M., Achtzehn J., Huber K., Dietrich C., Tempelmann C., Wolbers T. (2018). Compromised grid-cell-like representations in old age as a key mechanism to explain age-related navigational deficits. Current Biology, 28(7), 1108–1115.e6. 10.1016/j.cub.2018.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. M., Pritschet L., Olsen R. K., Layher E., Santander T., Grafton S. T., Jacobs E. G. (2020). Progesterone shapes medial temporal lobe volume across the human menstrual cycle. NeuroImage, 220, Article 117125. 10.1016/j.neuroimage.2020.117125 [DOI] [PubMed] [Google Scholar]
- Waller D. (2000). Individual differences in spatial learning from computer-simulated environments. Journal of Experimental Psychology: Applied, 6(4), 307–321. 10.1037/1076-898X.6.4.307 [DOI] [PubMed] [Google Scholar]
- Weisberg S. M., Newcombe N. S. (2016). How do (some) people make a cognitive map? Routes, places, and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 42(5), 768–785. 10.1037/xlm0000200 [DOI] [PubMed] [Google Scholar]
- Wiener J. M., de Condappa O., Harris M. A., Wolbers T. (2013). Maladaptive bias for extrahippocampal navigation strategies in aging humans. The Journal of Neuroscience, 33(14), 6012–6017. 10.1523/JNEUROSCI.0717-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T., Hegarty M. (2010). What determines our navigational abilities? Trends in Cognitive Sciences, 14(3), 138–146. 10.1016/j.tics.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Zhong J. Y., Moffat S. D. (2016). Age-related differences in associative learning of landmarks and heading directions in a virtual navigation task. Frontiers in Aging Neuroscience, 8, Article 122. 10.3389/fnagi.2016.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pss-10.1177_0956797620979185 for Age-Related Changes in Spatial Navigation Are Evident by Midlife and Differ by Sex by Shuying Yu, Alexander P. Boone, Chuanxiuyue He, Rie C. Davis, Mary Hegarty, Elizabeth R. Chrastil and Emily G. Jacobs in Psychological Science