Abstract
Background
The occurrence of Graves’ disease and Hashimoto thyroiditis after coronavirus disease 2019 (COVID-19) raised concerns that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may trigger thyroid autoimmunity. We aimed to address the current uncertainties regarding incident thyroid dysfunction and autoimmunity among COVID-19 survivors.
Methods
We included consecutive adult COVID-19 patients without known thyroid disorders, who were admitted to Queen Mary Hospital from July 21 to September 21, 2020 and had serum levels of thyroid-stimulating hormone, free thyroxine, free triiodothyronine (fT3), and anti-thyroid antibodies measured both on admission and at 3 months.
Results
In total, 122 patients were included. Among 20 patients with abnormal thyroid function tests (TFTs) on admission (mostly low fT3), 15 recovered. Among 102 patients with initial normal TFTs, two had new-onset abnormalities that could represent different phases of thyroiditis. Among 104 patients whose anti-thyroid antibody titers were reassessed, we observed increases in anti-thyroid peroxidase (TPO) (P<0.001) and anti-thyroglobulin (P<0.001), but not anti-thyroid stimulating hormone receptor titers (P=0.486). Of 82 patients with negative anti-TPO findings at baseline, 16 had a significant interval increase in anti-TPO titer by >12 U, and four became anti-TPO-positive. Worse baseline clinical severity (P=0.018), elevated C-reactive protein during hospitalization (P=0.033), and higher baseline anti-TPO titer (P=0.005) were associated with a significant increase in anti-TPO titer.
Conclusion
Most patients with thyroid dysfunction on admission recovered during convalescence. Abnormal TFTs suggestive of thyroiditis occurred during convalescence, but infrequently. Importantly, our novel observation of an increase in anti-thyroid antibody titers post-COVID-19 warrants further follow-up for incident thyroid dysfunction among COVID-19 survivors.
Keywords: COVID-19, SARS-CoV-2, Thyroid function tests, Thyroiditis, Euthyroid sick syndromes, Thyroid gland
INTRODUCTION
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected millions of people worldwide [1]. The angiotensin-converting enzyme 2 (ACE2), which is the functional receptor for SARS-CoV-2, is expressed in many endocrine organs, including the thyroid [2]. Therefore, SARS-CoV-2-related thyroiditis could occur concurrently with COVID-19, or weeks after resolution of COVID-19 [3,4], suggesting that SARS-CoV-2 could affect the thyroid, directly (through a direct viral effect) or indirectly (through immune dysregulation) [4]. Of note, some patients who experienced SARS-CoV-2-related thyroiditis were observed to evolve into the subclinical hypothyroid phase, around 3 months after the diagnosis of COVID-19 [5]. Furthermore, the occurrence of Graves’ disease and Hashimoto thyroiditis after COVID-19 raised concerns that SARS-CoV-2 may trigger thyroid autoimmunity [6,7]. Indeed, Graves’ disease and Hashimoto thyroiditis have been reported to occur a few months after subacute thyroiditis, which is generally considered to be of viral origin, suggesting that viral infection may trigger autoimmune thyroid disorders [8,9]. These thyroid disorders are all clinically relevant, as they require timely initiation of anti-thyroid drugs or thyroxine replacement in addition to symptomatic management.
Existing studies that reported the results of reassessment thyroid function tests (TFTs) mainly focused on patients with initial TFT abnormalities [10–12]. Uncertainties remain regarding incident thyroid dysfunction and autoimmunity among COVID-19 survivors. We conducted a prospective study of thyroid function and autoimmunity among COVID-19 patients and previously reported the baseline findings of 191 of them [13]. Here, we report their thyroid function and autoimmunity upon follow-up 3 months later.
METHODS
Consecutive patients aged ≥18 years admitted to Queen Mary Hospital for COVID-19 from July 21 to September 21, 2020 were prospectively recruited. The presence of SARS-CoV-2 was confirmed in all patients by reverse-transcription polymerase chain reaction from a nasopharyngeal swab and/or deep throat saliva sample, using the LightMix SarbecoV E-gene assay (TIB Molbiol, Berlin, Germany), which targets the envelope protein (E) gene of SARS-CoV-2. Patients were excluded if they (1) had a history of thyroid disorders; (2) were on anti-thyroid drugs and/or thyroid hormone replacement; and (3) were on medications with a potential impact on thyroid function including a systemic steroid, amiodarone, heparin, and dopamine.
Each patient had blood tests upon admission before the initiation of treatment for COVID-19. Serum thyroid-stimulating hormone (TSH), free thyroxine (fT4), and free triiodothyronine (fT3) levels were measured with immunoassays. Basic hematology and biochemistry panels, and inflammatory markers (C-reactive protein [CRP] and erythrocyte sedimentation rate) were measured. The upper limit of normal for CRP was 0.76 mg/dL. Demographics, comorbidities, and COVID-19-related symptoms were recorded. COVID-19 severity was classified according to the ‘Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition)’ [14]. Clinical outcomes were recorded. Follow-up visits were arranged around 3 months from admission to reassess TFTs and anti-thyroid antibodies. Patients who had TFTs reassessed were included in the current study.
Serum TSH was measured with an ADVIA Centaur TSH3-Ultra assay (Siemens Healthcare Diagnostics Inc., Erlangen, Germany). Serum fT4 was measured with an ADVIA Centaur FT4 assay (Siemens Healthcare Diagnostics Inc.). Serum fT3 levels were measured with an ADVIA Centaur FT3 assay (Siemens Healthcare Diagnostics Inc.). The reference ranges for TSH, fT4, and fT3 were 0.35–4.8 mIU/L, 12–23 pmol/L, and 3.2–6.5 pmol/L, respectively. Anti-thyroid peroxidase antibody (TPO) titers were measured with a QUANTA Lite TPO enzyme-linked immunosorbent assay (ELISA, Inova Diagnostics, San Diego, CA, USA). Positive anti-TPO was defined as a measurement of >100 U. Regarding the overall precision of the anti-TPO titer, in the normal range (i.e., 0 to 100 U), the standard deviation (SD) was 6 U, with a coefficient of variation (CV) of 11.1%; in the moderate positive range, the SD was 28.3 U with a CV of 9.6%; and in the strong positive range, the SD was 44.4 U with a CV of 5.4% [15]. Hence, in the evaluation of patients with negative baseline anti-TPO, we defined a significant increase of anti-TPO titer to be >2 times the SD (i.e., >12 U) on reassessment, compared with that during hospitalization. Anti-thyroglobulin antibody (anti-Tg) titers were measured with a QUANTA Lite Thyroid T ELISA kit (Inova Diagnostics). Positive anti-Tg was defined as a measurement of >100 U. The precision of the anti-Tg assay in the normal range was not reported. Anti-TSH receptor antibody (anti-TSHR) titer was measured with the anti-TSH receptor (TRAb) Fast ELISA (IgG) test kit (EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany), using porcine TSHR. Anti-TSHR was considered positive if the measurement was >1 IU/L.
The study followed the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 13-265). All participants provided informed consent.
Data were presented as mean±SD, median with interquartile range (IQR), or number with percentage as appropriate. Between-group comparisons were performed with the t test and Mann-Whitney U test for continuous variables, and the chi-square or Fisher exact test for categorical variables, as appropriate. Data not normally distributed were logarithmically transformed before analyses. Two-sided P values <0.05 were considered to indicate statistical significance. All statistical analyses were performed with SPSS version 26 (IBM Corp., Armonk, NY, USA).
RESULTS
In total, 122 COVID-19 survivors who had reassessment TFTs at a median interval of 90 days (IQR, 81 to 96) were included in the analysis. Their baseline characteristics are shown in Table 1. Their median age was 58 years (IQR, 44 to 63), and 60 patients (49.2%) were men. The most common comorbidities were hypertension (n=29, 23.8%) and diabetes (n=19, 15.6%), and 18.1% of patients were smokers. At baseline, 99 (81.1%), 19 (15.6%), and four (3.3%) patients had mild, moderate, and severe COVID-19, respectively. None was critically ill on admission; that is, all patients were initially admitted to the non-intensive care unit. Ninety-one patients (74.6%) received interferon beta-1b [16], while 15 patients (12.3%) required dexamethasone [17]. The median length of stay was 8 days (IQR, 6 to 11).
Table 1.
Baseline Clinical Characteristics of 122 Patients Who Had Thyroid Function Reassessed
| Characteristic | Value |
|---|---|
| Age, yr | 58 (44–63) |
|
| |
| Male sex | 60 (49.2) |
|
| |
| Smoking | 19/105 (18.1) |
|
| |
| Drinking | 22/102 (21.6) |
|
| |
| Abnormal TFTs on admission | 20 (16.4) |
|
| |
| Baseline anti-TPO positivity | 25 (20.5) |
|
| |
| Baseline anti-Tg positivity | 13 (10.7) |
|
| |
| Baseline COVID-19 severity | |
| Mild | 99 (81.1) |
| Moderate | 19 (15.6) |
| Severe | 4 (3.3) |
|
| |
| Baseline CRP, mg/dL | 0.69 (0.31–2.21) |
|
| |
| Baseline ESR, mm/hr | 40 (24–65) |
|
| |
| Comorbidities | |
| Hypertension | 29 (23.8) |
| Diabetes mellitus | 19 (15.6) |
| CAD or heart failure | 4 (3.3) |
| Stroke or TIA | 3 (2.5) |
| Malignancy | 5 (4.1) |
| Pulmonary disease | 3 (2.5) |
|
| |
| Clinical course | |
| Length of hospitalization, day | 8 (6–11) |
| Interferon beta-1b treatment | 91 (74.6) |
| Dexamethasone requirement | 15 (12.3) |
| Oxygen requirement | 12 (9.8) |
| Intensive care unit admission | 2 (1.6) |
Values are expressed as median (interquartile range) or number (%).
TFT, thyroid function test; TPO, thyroid peroxidase antibody; Tg, thyroglobulin; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; CAD, coronary artery disease; TIA, transient ischemic attack.
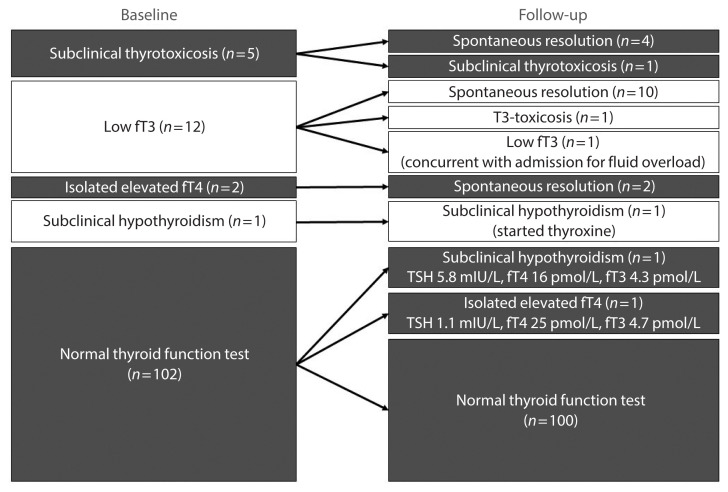
Fig. 1 shows the overview of the changes in TFT status in all 122 patients included in this study.
Fig. 1.
Changes in thyroid function status in all 122 patients included. fT3, free triiodothyronine; fT4, free thyroxine; TSH, thyroid-stimulating hormone.
Table 2 summarizes the reassessment findings of the 20 patients with abnormal TFTs on admission. The first five patients (number 1 to 5) had low TSH levels on admission, and four of these patients recovered spontaneously, in keeping with thyroiditis. Patient number 5 had persistent low TSH and positive anti-TSHR, and his fT3 and fT4 levels were suggestive of subclinical thyrotoxicosis. The next 14 patients (number 6 to 19) had low fT3 and/or high fT4 levels on admission, suggestive of non-thyroidal illness syndrome (NTIS). All had resolution of their thyroid dysfunction, except for patient number 7 and 10. Patient number 7 had T3-toxicosis upon reassessment, with persistently positive anti-TPO and anti-Tg but negative anti-TSHR, suggestive of either painless thyroiditis or Graves’ disease with negative anti-TSHR [18]. Patient number 10 had both low fT4 and fT3 levels on reassessment, suggestive of NTIS, as he was admitted for fluid overload and clinically ill at the time of reassessment. The remaining patient (number 20) likely had pre-existing Hashimoto thyroiditis diagnosed upon admission for COVID-19, requiring thyroxine replacement.
Table 2.
Patients with Abnormal Thyroid Function Test on Admission Who Had a Reassessment after Discharge (n=20)
| Patient no. | On admission | COVID-19 treatment | Reassessment | Remarks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| TSH, mIU/La | fT4, pmol/La | fT3, pmol/La | Anti-TPO, Ub | Anti-Tg, Ub | Anti-TSHR, IU/Lb | Days | TSH, mIU/La | fT4, pmol/La | fT3, pmol/La | Anti-TPO, Ub | Anti-Tg, Ub | Anti-TSHR, IU/Lb | |||
| 1 | <0.01c | 22 | 5.2 | 627.76c | 4.12 | 9.9c | IFN+RIB | 88 | 0.99 | 13 | 4.4 | 525.95c | 5.12 | 4.2c | Spontaneous resolution |
|
| |||||||||||||||
| 2 | 0.06c | 16 | 3.8 | 17.01 | 4.98 | 0.9 | IFN+RIB | 27 | 0.70 | 21 | 3.7 | NA | NA | NA | Spontaneous resolution |
|
| |||||||||||||||
| 3 | 0.24c | 17 | 3.2 | 10.25 | 18.52 | 0.5 | IFN+RIB | 99 | 0.86 | 20 | 4.3 | 17.79 | 19.69 | 1.0 | Spontaneous resolution |
|
| |||||||||||||||
| 4 | 0.26c | 22 | 3.4 | 10.08 | 3.80 | 0.8 | IFN+RIB | 92 | 0.31c | 23 | 5.1 | 10.04 | 5.74 | 1.5c | Resolved to normal 3 weeks later |
|
| |||||||||||||||
| 5 | 0.31c | 20 | 5.4 | 25.34 | 6.77 | 1.2c | IFN+RIB | 95 | 0.12c | 17 | 5.5 | 27.92 | 7.05 | 1.3c | Thyroid ultrasonography: unremarkable echogenicity and vascularity |
|
| |||||||||||||||
| 6 | 0.53 | 20 | 2.8c | 33.13 | 4.19 | 0.6 | IFN+RIB | 89 | 0.64 | 18 | 3.9 | 32.99 | 6.33 | 1.0 | |
|
| |||||||||||||||
| 7 | 0.55 | 16 | 2.9c | 151.02c | 2,957.1c | NA | IFN+RIB+DEX | 102 | 0.02c | 21 | 7.2 | 100.12c | 2,793.9c | 0.8 | Pending thyroid ultrasonography and nuclear scan |
|
| |||||||||||||||
| 8 | 0.56 | 17 | 2.9c | 27.05 | 3.73 | 0.1 | IFN+RIB+DEX | 111 | 3.4 | 20 | 4.9 | 21.12 | 6.31 | 1.2c | |
|
| |||||||||||||||
| 9 | 0.60 | 16 | 3.0c | 113.31c | 4.10 | 1.2c | IFN+RIB | 92 | 1.3 | 19 | 5.1 | 145.85c | 6.62 | 0.8 | |
|
| |||||||||||||||
| 10 | 0.83 | 15 | 2.3c | 13.91 | 3.63 | 0.4 | IFN+RIB+DEX | 52 | 1.4 | 11c | 2.1c | NA | NA | NA | Admitted for fluid overload on reassessment |
|
| |||||||||||||||
| 11 | 1.0 | 14 | 2.9c | 28.98 | 9.26 | 0.9 | IFN+RIB+DEX | 97 | 2.3 | 14 | 4.8 | 55.33 | 12.15 | 0.6 | |
|
| |||||||||||||||
| 12 | 1.1 | 24 | 3.0c | 18.48 | 11.32 | 1.2c | None | 81 | 2.6 | 20 | 3.5 | 19.22 | 11.99 | 1.4c | |
|
| |||||||||||||||
| 13 | 1.2 | 14 | 2.8c | 51.56 | 9.67 | 1.0 | IFN+RIB+DEX | 74 | 2.1 | 19 | 5.2 | 50.67 | 10.51 | 1.1c | |
|
| |||||||||||||||
| 14 | 1.6 | 14 | 3.1c | 65.48 | 26.26 | 0.2 | None | 15 | 1.3 | 18 | 4.7 | NA | NA | NA | |
|
| |||||||||||||||
| 15 | 1.7 | 15 | 3.1c | 48.13 | 4.33 | 0.7 | IFN+RIB | 47 | 1.3 | 14 | 3.9 | NA | NA | NA | |
|
| |||||||||||||||
| 16 | 1.8 | 13 | 2.8c | 1,579.9c | 16.33 | 0.3 | IFN+RIB | 90 | 1.4 | 13 | 4.2 | 977.35c | 11.65 | 0.6 | |
|
| |||||||||||||||
| 17 | 1.9 | 14 | 2.9 | 19.83 | 4.48 | 0.8 | IFN+RIB | 63 | 2.9 | 13 | 4.1 | 25.88 | 10.00 | 1.5 | |
|
| |||||||||||||||
| 18 | 2.6 | 24c | 4.0 | 32.44 | 257.5c | 0.6 | IFN+RIB | 21 | 1.8 | 17 | 4.4 | NA | NA | NA | |
|
| |||||||||||||||
| 19 | 2.8 | 24c | 4.4 | 42.46 | 4.12 | 1.3c | IFN+RIB | 93 | 2.1 | 22 | 4.2 | 60.84 | 6.34 | 0.9 | |
|
| |||||||||||||||
| 20 | 11c | 12 | 3.9 | 18,719c | 177.1c | NA | IFN+RIB | 47 | 10c | 12 | NA | NA | NA | NA | Started thyroxine replacement |
TSH, thyroid-stimulating hormone; fT4, free thyroxine; fT3, free triiodothyronine; TPO, thyroid peroxidase; Tg, thyroglobulin; TSHR, TSH receptor antibody; COVID-19, coronavirus disease 2019; IFN, interferon beta-1b; RIB, ribavirin; NA, not available; DEX, dexamethasone.
Reference ranges: TSH 0.35–4.8 mIU/L, fT4 12–23 pmol/L, fT3 3.2–6.5 pmol/L;
Positive anti-TPO defined as >100 U, positive anti-Tg defined as >100 U, positive anti-TSHR defined as >1 IU/L;
Values out of the reference ranges.
For the 102 patients with normal TFTs on admission, two (2.0%) had new-onset abnormal TFTs. These TFT changes were compatible with different phases of thyroiditis: one had mildly elevated TSH (5.8 mIU/L), with normal fT4 (16 pmol/L) and fT3 (4.3 pmol/L); the other had mildly raised fT4 (25 pmol/L), with normal TSH (1.1 mIU/L) and fT3 (4.7 pmol/L). In the latter case, assay variability could not be completely excluded.
Of the 122 patients included in this study, 104 (85.2%) had anti-thyroid antibody titers reassessed. In these 104 patients, we observed an increase in anti-TPO titer upon reassessment (baseline 28.32 U [IQR, 14.01 to 67.44] vs. reassessment 35.04 U [IQR, 18.76 to 99.05], P<0.001). We further studied the change in anti-TPO titer according to interferon exposure. Among the 82 patients exposed to interferon, a significant increase in the anti-TPO titer was observed (baseline 27.66 U [IQR, 13.92 to 63.63] vs. reassessment 35.04 U [IQR, 18.88 to 102.07], P<0.001). A similar trend was also observed among the 22 patients not exposed to interferon (baseline 30.49 U [IQR, 15.58 to 90.73] vs. reassessment 35.48 U [IQR, 16.00 to 99.72], P=0.088). Among the 82 patients negative for anti-TPO at baseline who had anti-TPO reassessed, 16 (19.5%) had an interval increase in anti-TPO titer by >12 U, of whom four patients became positive for anti-TPO (Table 3). Factors associated with a significant increase in anti-TPO titer included worse baseline clinical severity (P=0.018), elevated CRP during hospitalization (P=0.033), and a higher baseline anti-TPO titer (P=0.005) (Table 4). Of note, there was no difference in the proportion of patients treated with interferon beta-1b or dexamethasone between those with and without a significant increase in anti-TPO titer. For the 22 patients with positive anti-TPO findings at baseline, only one patient became negative for anti-TPO (the anti-TPO titer went from 124.88 U at baseline to 95.82 U on reassessment).
Table 3.
Thyroid Function and Antibody Profile of Patients with New-Onset Anti-Thyroid Peroxidase Antibody Positivity
| Patient no. | On admission | COVID-19 treatment | Reassessment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| TSH, mIU/La | fT4, pmol/La | fT3, pmol/La | Anti-TPO, Ub | Anti-Tg, Ub | Anti-TSHR, IU/Lb | Days | TSH, mIU/La | fT4, pmol/La | fT3, pmol/La | Anti-TPO, Ub | Anti-Tg, Ub | Anti-TSHR, IU/Lb | ||
| 21 | 1.2 | 17 | 3.5 | 6.85 | 4.90 | 1.6c | IFN+RIB | 93 | 2.8 | 20 | 5.0 | 141.24c | 8.31 | 1.2c |
|
| ||||||||||||||
| 22 | 1.3 | 18 | NA | 45.45 | 4.31 | 1.0 | IFN+RIB | 89 | 1.7 | 17 | 5.3 | 148.21c | 6.58 | 1.4c |
|
| ||||||||||||||
| 23 | 2.2 | 16 | 4.6 | 97.06 | 4.84 | 0.2 | IFN+RIB | 90 | 1.7 | 20 | 5.6 | 122.37c | 7.31 | 1.2c |
|
| ||||||||||||||
| 24 | 1.1 | 17 | 4.4 | 99.85 | 6.62 | 0.7 | IFN+RIB | 105 | 0.47 | 19 | 5.2 | 128.51c | 9.67 | 0.7 |
TSH, thyroid-stimulating hormone; fT4, free thyroxine; fT3, free triiodothyronine; TPO, thyroid peroxidase; Tg, thyroglobulin; TSHR, TSH receptor antibody; COVID-19, coronavirus disease 2019; IFN, interferon beta-1b; RIB, ribavirin.
Reference ranges: TSH 0.35–4.8 mIU/L, fT4 12–23 pmol/L, fT3 3.2–6.5 pmol/L;
Positive anti-TPO defined as >100 U, positive anti-Tg defined as >100 U, positive anti-TSHR defined as >1 IU/L;
Values out of the reference ranges.
Table 4.
Comparison of the Clinical Features of Patients Negative for Anti-TPO at Baseline According to Whether They Had a Significant Increase in the Anti-TPO Titer
| Variable | Significant increase in anti-TPO titer | No significant increase in anti-TPO titer | P value |
|---|---|---|---|
| Number | 16 | 66 | - |
|
| |||
| Baseline characteristics | |||
| Age, yr | 61 (49–65) | 55 (41–63) | 0.429 |
| Male sex | 9 (56.3) | 30 (45.5) | 0.436 |
| Smoking | 2/12 (16.7) | 8/61 (13.1) | 0.665 |
| Drinking | 4/11 (36.4) | 11/60 (18.3) | 0.228 |
| TSH, mIU/L | 1.1 (0.9–1.8) | 1.1 (0.8–1.5) | 0.631 |
| fT4, pmol/L | 18 (17–19) | 18 (17–20) | 0.554 |
| fT3, pmol/L | 3.9 (3.6–4.4) | 4.1 (3.6–4.5) | 0.676 |
| Baseline anti-TPO titer, U | 43 (22–54) | 18 (10–33) | 0.005a |
| COVID-19 severity | 0.018a | ||
| Mild | 11 (68.8) | 55 (83.3) | |
| Moderate | 2 (12.5) | 11 (16.7) | |
| Severe | 3 (18.8) | 0 | |
|
| |||
| Clinical course | |||
| Elevated CRP during hospitalization | 15 (93.8) | 44 (66.7) | 0.033a |
| Peak ESR during hospitalization, mm/hr | 68 (41–96) | 56 (35–93) | 0.564 |
| Length of hospitalization, day | 8 (6–11) | 8 (6–11) | 0.874 |
| Interferon beta-1b treatment | 14 (87.5) | 51 (77.3) | 0.503 |
| Dexamethasone requirement | 2 (12.5) | 7 (10.6) | 0.999 |
| Oxygen requirement | 2 (12.5) | 6 (9.1) | 0.650 |
| Intensive care unit admission | 1 (6.3) | 1 (1.5) | 0.354 |
Values are expressed as median (interquartile range) or number (%).
TPO, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone; fT4, free thyroxine; fT3, free triiodothyronine; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Values with statistical significance.
We also observed an increase in anti-Tg titers upon reassessment (baseline 6.62 U [IQR, 4.89 to 15.57] vs. reassessment 8.71 U [IQR, 6.65 to 15.44], P<0.001). Among the 82 interferon users, anti-Tg titers increased upon reassessment (baseline 6.70 U [IQR, 4.90 to 18.37] vs. reassessment 8.93 U [IQR, 6.61 to 16.93], P<0.001). Again, a similar change was seen among the 22 patients not exposed to interferon (baseline 6.60 U [IQR, 4.61 to 9.54] vs. reassessment 7.80 U [IQR, 6.59 to 11.92], P=0.002). We did not observe incident positivity for anti-Tg, and all patients positive for anti-Tg at baseline remained positive upon reassessment. There was no significant change in the anti-TSHR titer upon reassessment (baseline 1.0 IU/L [IQR, 0.8 to 1.2] vs. reassessment 1.0 IU/L [IQR, 0.8 to 1.3], P=0.486).
DISCUSSION
To date, our study is the largest reassessment cohort assessing both thyroid function and autoimmunity among COVID-19 survivors. Regarding TFTs suggestive of NTIS and thyroiditis, the great majority recovered during convalescence, except for two patients with pre-existing evidence of autoimmunity: one had persistent subclinical thyrotoxicosis, while the other developed T3 toxicosis. We found that abnormal TFTs suggestive of different phases of thyroiditis could occur during the convalescence period, but were observed in only 2% of the patients. A more informed assessment of the utility of TFT surveillance post-COVID-19 and risk factors of new-onset thyroid dysfunction will be made possible by studying a larger reassessment cohort with a longer follow-up.
Interestingly, we demonstrated an increase in anti-TPO and anti-Tg titers 3 months post-COVID-19. A major confounder in this study was that a high proportion of the patients were exposed to interferon beta-1b, which has been associated with incident thyroid dysfunction and autoantibodies in patients with multiple sclerosis [19]. However, in contrast to the treatment regimen for multiple sclerosis, interferon treatment lasted only for 1 to 2 weeks in our COVID-19 patients. As 70% of our patients received interferon, we analyzed the changes in anti-thyroid antibody titers according to interferon exposure. In separate analyses, we still observed a similar magnitude of increase in anti-TPO and anti-Tg titers among interferon users and non-users. Moreover, a similar prevalence of interferon treatment was found in patients with significant increases in the anti-TPO titer, compared to those without. Nonetheless, to clarify the role of SARS-CoV-2 in the induction of thyroid autoimmunity, it will be necessary to analyze a larger reassessment cohort of COVID-19 patients not exposed to interferon to provide a more definitive answer.
Several factors associated with a significant increase in the anti-TPO titer were identified: worse clinical severity, elevated CRP during hospitalization, and higher baseline anti-thyroid antibody titer. These findings extended the understanding from case reports of Graves’ disease and Hashimoto thyroiditis occurring after COVID-19 [6,7], supporting the proposal that the hyperinflammatory state in COVID-19 may trigger autoimmunity. Our findings may add to the existing list of autoantibodies and autoimmune conditions in neurological, hematological, and rheumatological systems occurring after COVID-19 [20]. As the occurrence of anti-TPO can precede thyroid dysfunction [21], further follow-up is warranted for potential subsequent thyroid dysfunction.
Whether our observations of the increase in anti-TPO and anti-Tg titers at 3 months post-COVID-19 represent a transient or persistent phenomenon requires longer follow-up for clarification. Nonetheless, our observations are consistent with the existing literature describing associations of thyroid autoimmunity with other viruses [22]. For instance, a higher prevalence of anti-TPO/Tg positivity has been reported among human T-lymphotropic virus type I (HTLV-I) carriers [23] and patients with chronic hepatitis C infection [24].
Taken together, for patients with abnormal TFTs or evidence of thyroid autoimmunity during COVID-19, reassessment is necessary to monitor whether the condition resolves or progresses during convalescence. However, current evidence suggests that routine reassessment TFT is not necessary among patients with initially normal TFTs. Nonetheless, as COVID-19 may have an impact on thyroid autoimmunity, especially among patients with more severe COVID-19 and significant inflammatory responses during the acute illness, clinicians may have to watch out for the subsequent development of thyroid autoimmunity.
The main strength of our study was the comprehensive reassessment of both thyroid function and anti-thyroid antibodies, showing an increase in anti-TPO and anti-Tg titers and incident anti-TPO positivity, despite the anti-TPO and anti-Tg assays being semi-quantitative. There are certain limitations in this study. Firstly, 70% of our patients received interferon beta-1b, potentially confounding the analysis of SARS-CoV-2-related incident thyroid dysfunction and autoimmunity. Secondly, most patients did not have thyroid imaging to assess the potential impact of SARS-CoV-2 on the thyroid beyond TFT and anti-thyroid antibodies.
In conclusion, we provided the novel observation of an increase in anti-thyroid antibody titers post-COVID-19, which warrants further follow-up for potential incident thyroid dysfunction among COVID-19 survivors.
Acknowledgments
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: D.T.W.L., K.S.L.L. Acquisition, analysis, or interpretation of data: D.T.W.L., C.H.L., W.S.C., A.C.H.L., A.R.T., C.H.Y.F., C.Y.L., E.K.H.L., K.S.L.L. Drafting the work or revising: D.T.W.L., C.H.L., W.S.C., A.C.H.L., K.K.W.T., K.C.B.T., Y.C.W., C.W.L., I.F.N.H., K.S.L.L. Final approval of the manuscript: D.T.W.L., C.H.L., W.S.C., A.C.H.L., A.R.T., C.H.Y.F., C.Y.L., E.K.H.L., K.K.W.T., K.C.B.T., Y.C.W., C.W.L., I.F.N.H., K.S.L.L.
REFERENCES
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–93. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Pal R, Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest. 2020;43:1027–31. doi: 10.1007/s40618-020-01276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brancatella A, Ricci D, Viola N, Sgro D, Santini F, Latrofa F. Subacute thyroiditis after SARS-COV-2 infection. J Clin Endocrinol Metab. 2020;105:dgaa276. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scappaticcio L, Pitoia F, Esposito K, Piccardo A, Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev Endocr Metab Disord. 2020;25:1–13. doi: 10.1007/s11154-020-09615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brancatella A, Ricci D, Cappellani D, Viola N, Sgro D, Santini F, et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection?: insights from a case series. J Clin Endocrinol Metab. 2020;105:dgaa537. doi: 10.1210/clinem/dgaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tee LY, Harjanto S, Rosario BH. COVID-19 complicated by Hashimoto’s thyroiditis. Singapore Med J. 2020 Jul 16; doi: 10.11622/smedj.2020106. [Epub]. [DOI] [PMC free article] [PubMed]
- 7.Mateu-Salat M, Urgell E, Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID-19. J Endocrinol Invest. 2020;43:1527–8. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakano Y, Kurihara H, Sasaki J. Graves’ disease following subacute thyroiditis. Tohoku J Exp Med. 2011;225:301–9. doi: 10.1620/tjem.225.301. [DOI] [PubMed] [Google Scholar]
- 9.Papi G, Ezzat S. Progression of subacute (de Quervain’s) thyroiditis into Hashimoto’s thyroiditis. Thyroid. 2004;14:477–8. doi: 10.1089/105072504323150840. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid. 2021;31:8–11. doi: 10.1089/thy.2020.0363. [DOI] [PubMed] [Google Scholar]
- 11.Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8:739–41. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoo B, Tan T, Clarke SA, Mills EG, Patel B, Modi M, et al. Thyroid function before, during, and after COVID-19. J Clin Endocrinol Metab. 2021;106:e803–11. doi: 10.1210/clinem/dgaa830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lui DT, Lee CH, Chow WS, Lee AC, Tam AR, Fong CH, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021;106:e926–35. doi: 10.1210/clinem/dgaa813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.China National Health Commission. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment (7th edition) [Internet] Chinese Society Cardiology; c2020. [cited 2021 May 10]. Available from: http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. [Google Scholar]
- 15.QUANTA Lite TPO for in vitro diagnostic use (Laboratory reference manual) San Diego: INOVA Diagnostics Inc; 2020. [Google Scholar]
- 16.Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tozzoli R, Bagnasco M, Giavarina D, Bizzaro N. TSH receptor autoantibody immunoassay in patients with Graves’ disease: improvement of diagnostic accuracy over different generations of methods: systematic review and meta-analysis. Autoimmun Rev. 2012;12:107–13. doi: 10.1016/j.autrev.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Caraccio N, Dardano A, Manfredonia F, Manca L, Pasquali L, Iudice A, et al. Long-term follow-up of 106 multiple sclerosis patients undergoing interferon-beta 1a or 1b therapy: predictive factors of thyroid disease development and duration. J Clin Endocrinol Metab. 2005;90:4133–7. doi: 10.1210/jc.2004-2326. [DOI] [PubMed] [Google Scholar]
- 20.Halpert G, Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev. 2020;19:102695. doi: 10.1016/j.autrev.2020.102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siriwardhane T, Krishna K, Ranganathan V, Jayaraman V, Wang T, Bei K, et al. Significance of anti-TPO as an early predictive marker in thyroid disease. Autoimmune Dis. 2019;2019:1684074. doi: 10.1155/2019/1684074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akamine H, Takasu N, Komiya I, Ishikawa K, Shinjyo T, Nakachi K, et al. Association of HTLV-I with autoimmune thyroiditis in patients with adult T-cell leukaemia (ATL) and in HTLV-I carriers. Clin Endocrinol (Oxf) 1996;45:461–6. doi: 10.1046/j.1365-2265.1996.741562.x. [DOI] [PubMed] [Google Scholar]
- 24.Antonelli A, Ferri C, Fallahi P, Ferrari SM, Ghinoi A, Rotondi M, et al. Thyroid disorders in chronic hepatitis C virus infection. Thyroid. 2006;16:563–72. doi: 10.1089/thy.2006.16.563. [DOI] [PubMed] [Google Scholar]