Abstract
Background
Extensive‐stage small cell lung cancer (ES‐SCLC) is deemed as a fatal malignancy with a poor prognosis. Although immunotherapy has gradually played an important role in the treatment of ES‐SCLC since 2018, ES‐SCLC treatment data and patient outcome before 2018, when chemotherapy served as a fundamental therapeutic strategy, is still meaningful as a summary of the situation regarding previous medical treatment and is a baseline for comparative data. In addition, the prognostic factors of ES‐SCLC have failed to reach a consensus until now. Therefore, this study aimed to evaluate survival and identify the prognostic factors in an ES‐SCLC population.
Methods
We retrospectively collected the detailed medical records of 358 patients with ES‐SCLC from January 1, 2011 to December 31, 2018 in a Chinese top‐level cancer hospital. The prognostic factors were evaluated by Cox univariate and multivariate analysis.
Results
The median overall survival (OS) of ES‐SCLC patients (N = 358) was 14.0 months, the one‐ and two‐year OS rates were 56.2% and 21.7%, respectively. Moreover, we identified two demographic characters (age ≥ 70, smoking index ≥ 400), one tumor burden factor (bone multimetastasis), two tumor biomarkers (cyfra211, CA125) and two laboratory indexes (decreased Na, PLR < 76) as independent prognostic factors for OS in this patient population. Progression‐free survival (PFS) data of 238 patients was obtained for further analysis, and the median PFS was 6.2 months, and six‐month and one‐year PFS rates were 51.7% and 14.3%, respectively. Elevated cyfra211, decreased Hb and Na were identified as independent prognostic factors for PFS.
Conclusions
This study provides real‐world evidence of the survival and prognosis of ES‐SCLC patients which will enable better evaluation and clinical decision‐making in the future.
Keywords: extensive‐stage, prognosis, small cell lung cancer, survival
This study provides real‐world evidence of survival and prognosis of untreated extensive stage small cell lung cancer (ES‐SCLC). We retrospectively collected the detailed medical records of 358 patients with ES‐SCLC from 2011 to 2018 in a Chinese top‐level cancer hospital. The median overall survival (OS) of all patients was 14.0 months, and the one‐ and two‐year OS rates were 56.2% and 21.7%, respectively. The median progression‐free survival (PFS) of 238 patients was 6.2 months, and the six‐month and one‐year PFS rates were 51.7% and 14.3%, respectively. Moreover, several independent prognostic factors for OS and PFS of ES‐SCLC were identified.

INTRODUCTION
Lung cancer remains the leading cause of cancer death worldwide according to GLOBOCAN 2020 statistics. 1 Small cell lung cancer (SCLC) is characterized as one of the most lethal and aggressive types which accounts for around 15% of all cases of lung cancer. The Veterans Administration Lung Study Group (VALSG) system and the eighth edition of American Joint Committee on Cancer (AJCC eighth) TNM classification are the two most widely used staging systems and are commonly utilized together in the clinical staging of patients with SCLC. The VALSG system simply classifies SCLC patients into limited stage (LS‐SCLC) and extensive stage (ES‐SCLC), 2 whereas the AJCC eighth TNM classification demonstrates more detailed information of primary tumor, lymph nodal involvement and distant metastatic status.
The prognosis and treatment strategies of limited and extensive stage SCLC differ. Chemotherapy is the fundamental treatment strategy for patients with ES‐SCLC. Four to six cycles of platinum plus etoposide remained as the standard first‐line treatment of ES‐SCLC for decades. The median overall survival (OS) of ES‐SCLC has previously been reported to be 8–13 months. 3 , 4 Most recently, the additional adoption of immunotherapeutic agents atezolizumab or durvalumab in the first‐line treatment of ES‐SCLC has facilitated mild survival improvement of 2–3 months. Although immunology has gradually played a role in the treatment of ES‐SCLC since 2018, ES‐SCLC treatment data and patient outcome before 2018, when chemotherapy served as a fundamental therapeutic strategy, is still meaningful as a summary of the situation regarding previous medical treatment and is a baseline for comparative data. In addition, taking into account the fact that even patients at an extensive stage of disease can have dramatically different survival outcomes, efforts on univariate and multivariate analysis of various factors have been performed to explore their potential relationship with survival of SCLC patients, such as age, TNM stage, distant metastatic status, tumor markers, and inflammatory factors. However, to date, no consensus has been reached. In this study, survival information, detailed demographic, clinical and laboratory characters in a cohort of 358 patients with ES‐SCLC in a single oncology center in China are summarized in order to evaluate survival and identify potential prognostic factors.
METHODS
Cohort study and data collection
In this retrospective study, ES‐SCLC patients who were initially treated at the Department of Medical Oncology in Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College (CAMS&PUMC) between January 1, 2011 and December 31, 2018 were enrolled. The definition of ES‐SCLC was based on the National Comprehensive Cancer Network (NCCN) guidelines (version 3. 2020): “Those in stage IV (TxNxM1) or T3–4 due to multiple lung nodules that are too extensive or have tumor/nodal volume that is too large to be encompassed in a tolerable radiation plan”, based on which a few phase IIIB/IIIC (having contralateral supraclavicular lymph nodes metastasis) and all phase IV patients were enrolled in our study. The inclusion criteria were as follows: (i) pathological or cytological diagnosis of SCLC, (ii) a confirmed staging of extensive stage, (iii) diagnosis confirmed from January 1, 2011 to December 31, 2018, and (iv) having access to complete medical records and pretreatment laboratory and radiological data. Staging at diagnosis included a computed tomography (CT) scan of the chest and abdomen, a whole body bone scan, and magnetic resonance imaging (MRI) of the brain. The need for informed patient consent was waived because of the retrospective nature of the study.
Data collected in this study included age, gender, body mass index (BMI), smoking index (the number of cigarettes smoked per day * years of tobacco smoking) (<400 vs. ≥400), the Eastern Cooperative Oncology Group (ECOG) performance status (PS) (0–1 vs. 2–4), weight loss, detailed TNM classification, detailed metastatic information (ipsilateral or contralateral lung, pleural, brain, liver, bone, adrenal glands, pancreas, malignant pleural or pericardial effusions, superior vena cava syndrome, and pelvic lymph node), treatment modality (no treatment, chemotherapy regimens, chemotherapy cycles, chest radiation, prophylactic cranial irradiation [PCI]), date of first treatment, progression and death. Metastatic site was defined as metastases of distant organs or lung/ pleura/malignant effusions, excluding lymph node metastasis. Laboratory data were collected from hematological tests which had been performed within a week before any anticancer treatment, including serum sodium (Na), blood albumin (ALB), lactic dehydrogenase (LDH), serum creatinine (Cr), hemoglobin (Hb), absolute lymphocyte count (ALC), absolute neutrophil count (ANC), platelet (PLT), neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), neuron specific enolase (NSE), progastrin‐releasing peptide (proGRP), cytokeratin 19 fragment (cyfra21‐1), carcinoembryonic antigen (CEA), and carbohydrate antigen‐125 (CA125).
Follow‐up
All patients were actively followed up until December 31, 2020. The follow‐up information was obtained by telephone call or directly from the electronic medical record system documents. The primary endpoints were overall survival (OS) and progression‐free survival (PFS). OS was defined as the time from the start of any treatment until the date of death of any cause or last follow‐up day. Patients who had not died at the last follow‐up were defined as alive, and those who were lost to follow‐up were defined as censored. PFS was defined as the time from the start of any treatment until progression. Those patients in whom disease progression information had been lost were deleted when we undertook a separate analysis of PFS.
Data analysis
Cox proportional hazard models were used to evaluate prognostic factors for OS and PFS by univariate and multivariate analysis. Continuous variables were transformed into binary variables by their optimal cutoff values which were determined by the “surv_cutpoint” function of the “survminer” R package. The optimal cutoff value of age for prognosis of OS was 70, NLR of 4 and PLR of 76, respectively. All statistical analyses were performed by R 4.0.3 and SPSS 26.0 software, factors with p‐values < 0.05 at univariate Cox analysis were adopted into multivariate Cox regression to perform independent prognostic analysis, and p‐values <0.05 were considered statistically significant.
RESULTS
Summary of patient basic characteristics and survival
A total of 358 patients with ES‐SCLC treated in the Cancer Hospital of CAMS&PUMC from January 1, 2011 to December 31, 2018 met the inclusion criteria and were included in the final analysis. The median patient age was 60 years old (interquartile range [IQR] 53–66); males and females accounted for 79.9% and 20.1% of all patients, respectively; 9.8% of patients had an ECOG PS score of ≥2. Among them, 11 (3.1%) patients were phase IIIB, 22 (6.1%) were phase IIIC, 105 (29.3%) were phase IVA and 220 (61.5%) were phase IVB. A total of 94 (26.3%) patients had liver metastasis (including oligo and multiple metastasis), 82 (22.9%) patients had liver multimetastasis, 106 (29.6%) had bone metastasis, 81 (22.6%) had bone multimetastasis, 57 (17.3%) had brain metastasis, 45 (12.6%) had brain multimetastasis, 51 (14.2%) had adrenal gland metastasis, 16 (4.5%) had adrenal gland multimetastasis, three (0.8%) had pancreatic metastasis, 70 (19.6%) had ipsilateral pulmonary metastasis, 35 (11.2%) had bilateral pulmonary metastasis, 48 (13.4%) had pleural metastasis, 61 (17.0%) had malignant effusion metastasis, 44 (12.3%) had pelvic lymph node metastasis, and 38 (10.6%) had superior vena cava obstruction syndrome (SVCOS). In total, 300 patients (83.8%) received ≥4 cycles of chemotherapy, and 58 patients (16.2%) received 0–4 cycles. Among those who received ≥4 cycles of chemotherapy, regimens included 44.7% of etoposide plus cisplatin (EP), 33.0% of etoposide plus carboplatin (CE), 4.4% of other platinum‐based chemotherapy and 1.7% of other nonplatinum‐based chemotherapy; 149 patients (41.6%) received chest radiation and 40 patients (11.1%) received PCI. Until the last follow‐up on December 31, 2020, the median OS of 460 ES‐SCLC patients was 14.0 months (95% confidence interval [CI]: 12.4–15.5 ), the one‐ and two‐year OS rates were 56.2% (95% CI: 51.3%–61.6%) and 21.7% (95% CI: 17.7%–26.5%), respectively. PFS data of 238 patients was obtained for further survival analysis. The median PFS was 6.2 months (95% CI: 5.9–6.8 ), and the six‐month and one‐year PFS rates were 51.7% (95% CI: 45.7%–58.4%) and 14.3% (95% CI: 10.5%–19.5%), respectively. Figure 1 shows the median OS and PFS of this cohort.
FIGURE 1.
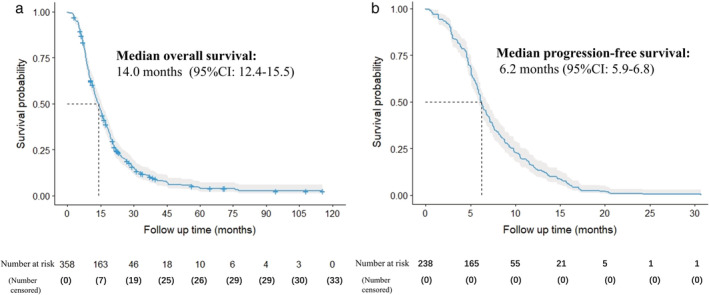
Overall survival (a) and progression‐free survival (b)
Prognostic analysis of overall survival for ES‐SCLC
A total of 19 factors before treatment were found to have a negative association with OS on univariate analysis (Table 1): age ≥ 70, sex as male, smoking index ≥ 400, weight loss, phase (IVB/IVA/IIIC/IIIB), metastatic sites ≥ 2, liver multimetastasis, bone multimetastasis, brain multimetastasis, pleural metastasis, elevated tumor biomarkers (NSE, Cyfra211, CA125), low pretreatment Hb/Na/ALB, high pretreatment LDH, PLR < 76 and NLR ≥ 4.
TABLE 1.
Univariate analysis of pretreatment prognostic factors for overall survival (OS) and progression‐free survival (PFS)
| Factors | OS | PFS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | Median (months) | HR | p‐value | N (%) | Median (months) | HR | p‐value | ||
| DC | Age (years ): | 1.78 | 0.0002 | 1.52 | 0.06 | ||||
| ≥70 | 49 (13.7%) | 9.7 | 23 (9.7%) | 4.8 | |||||
| <70 | 309 (86.3%) | 15.2 | 215 (90.3%) | 6.3 | |||||
| Gender: | 1.32 | 0.049 | 1.27 | 0.14 | |||||
| Male | 286 (79.9%) | 13.3 | 191 (80.3%) | 6.1 | |||||
| Female | 72 (20.1%) | 17.0 | 47 (20.9%) | 7.5 | |||||
| Performance status: | 1.37 | 0.095 | 1.06 | 0.79 | |||||
| 2 ~ 4 | 35 (9.8%) | 9.3 | 24 (10.1%) | 6.25 | |||||
| 0 ~ 1 | 321 (90.2%) | 14.5 | 213 (89.9%) | 6.2 | |||||
| BMI: | 1.07 | 0.22 | 1.05 | 0.47 | |||||
| Normal (18.5–23.9) | 150 (42.9%) | 14.8 | 99 (41.6%) | 5.9 | |||||
| Low (<18.5) | 10 (2.8%) | 10.5 | 7 (2.9%) | 8.4 | |||||
| High (>23.9) | 190 (54.3%) | 14.2 | 126 (52.9%) | 6.1 | |||||
| Smoking index: | 1.28 | 0.04 | 1.03 | 0.85 | |||||
| ≥400 | 236 (66.5%) | 13.4 | 157 (66.5%) | 6.2 | |||||
| <400 | 119 (33.5%) | 14.4 | 79 (33.5%) | 6.1 | |||||
| Smoking status: | 1.07 | 0.62 | 1.03 | 0.85 | |||||
| Current or former smoker | 274 (77.0%) | 13.6 | 184 (78.0%) | 6.15 | |||||
| Never smoker | 82 (23.0%) | 14.2 | 52 (22.0%) | 6.45 | |||||
| Weight loss: | 1.51 | 0.0008 | 1.29 | 0.07 | |||||
| Yes | 99 (27.7%) | 12.0 | 71 (29.8%) | 5.7 | |||||
| No | 259 (72.3%) | 14.8 | 167 (70.2%) | 6.4 | |||||
| CC | Histology: | 1.49 | 0.34 | 0.79 | 0.60 | ||||
| Pure | 350 (98%) | 14.2 | 233 (2.1%) | 6.2 | |||||
| Compound | 7 (2%) | 14.8 | 5 (97.9%) | 5.6 | |||||
| TNM stage: | 1.39 | <0.0001 | 1.12 | 0.21 | |||||
| IIIB | 11 (3.1%) | 23.4 | 4 (1.7%) | 8.1 | |||||
| IIIC | 22 (6.1%) | 17.2 | 19 (8.0%) | 6.4 | |||||
| IVA | 105 (29.3%) | 16.4 | 68 (28.6%) | 7.1 | |||||
| IVB | 220 (61.5%) | 11.5 | 147 (61.8%) | 6.1 | |||||
| IVB vs. non‐IVB | 1.55 | 0.0002 | 1.17 | 0.24 | |||||
| IVB | 220 (61.5%) | 11.8 | 147 (61.8%) | 6.6 | |||||
| IIIB–IVA | 138 (38.5%) | 17.0 | 91 (38.2%) | 6.1 | |||||
| Metastatic sites | 1.39 | 0.006 | 1.10 | 0.51 | |||||
| <1 | 243 (67.9%) | 14.5 | 160 (67.2%) | 6.45 | |||||
| ≥2 | 115 (32.1%) | 12.1 | 78 (32.8%) | 5.80 | |||||
| Liver metastasis | 1.39 | 0.008 | 1.16 | 0.31 | |||||
| Yes | 94 (26.3%) | 10.7 | 68 (28.6%) | 6.05 | |||||
| No | 264 (73.7%) | 15.0 | 170 (71.4%) | 6.40 | |||||
| Liver multimetastasis | 1.50 | 0.002 | 1.14 | 0.39 | |||||
| Yes | 82 (22.9%) | 9.9 | 58 (24.4%) | 6.05 | |||||
| No | 276 (77.1%) | 15.2 | 180 (75.6%) | 6.40 | |||||
| Bone metastasis | 1.30 | 0.03 | 1.11 | 0.47 | |||||
| Yes | 106 (29.6%) | 11.9 | 68 (28.6%) | 5.6 | |||||
| No | 252 (70.4%) | 14.5 | 170 (71.4%) | 6.5 | |||||
| Bone multimetastasis | 1.64 | 0.0001 | 1.34 | 0.07 | |||||
| Yes | 81 (22.6%) | 9.7 | 51 (21.4%) | 5.4 | |||||
| No | 277 (77.4%) | 15.2 | 187 (78.6%) | 6.4 | |||||
| Brain metastasis | 1.19 | 0.24 | 0.78 | 0.14 | |||||
| Yes | 62 (17.3%) | 13.8 | 41 (17.2%) | 8.7 | |||||
| No | 296 (82.7%) | 14.0 | 197 (82.8%) | 6.1 | |||||
| Brain multimetastasis | 1.43 | 0.03 | 0.94 | 0.75 | |||||
| Yes | 45 (12.6%) | 10.6 | 26 (89.1%) | 6.3 | |||||
| No | 313 (87.4%) | 14.3 | 212 (10.9%) | 6.2 | |||||
| Adrenal gland metastasis | 0.89 | 0.46 | 0.77 | 0.14 | |||||
| Yes | 51 (14.2%) | 15.6 | 37 (15.5%) | 6.7 | |||||
| No | 307 (85.8%) | 13.5 | 201 (84.5%) | 6.1 | |||||
| Adrenal gland multimetastasis | 1.02 | 0.95 | 0.71 | 0.34 | |||||
| Yes | 16 (4.5%) | 13.7 | 8 (3.4%) | 10.9 | |||||
| No | 342 (95.5%) | 14.2 | 230 (96.6%) | 6.1 | |||||
| Pancreatic metastasis | 2.19 | 0.18 | 1.50 | 0.57 | |||||
| Yes | 3 (0.8%) | 8.7 | 2 (0.8%) | 5.8 | |||||
| No | 355 (99.2%) | 14.2 | 236 (99.2%) | 6.2 | |||||
| Ipsilateral lung metastasis | 0.91 | 0.51 | 1.01 | 0.96 | |||||
| Yes | 70 (19.6%) | 13.6 | 41 (17.2%) | 5.8 | |||||
| No | 288 (80.4%) | 14.2 | 197 (82.8%) | 6.3 | |||||
| Contralateral lung metastasis | 0.89 | 0.52 | 1.43 | 0.12 | |||||
| Yes | 40 (11.2%) | 15.1 | 22 (9.2%) | 5.5 | |||||
| No | 318 (88.8%) | 13.8 | 216 (90.8%) | 6.3 | |||||
| Pleural metastasis | 1.47 | 0.015 | 1.06 | 0.78 | |||||
| Yes | 48 (13.4%) | 10.3 | 29 (12.2%) | 5.9 | |||||
| No | 310 (86.6%) | 14.5 | 209 (87.8%) | 6.2 | |||||
| Malignant effusion | 1.23 | 0.16 | 1.12 | 0.50 | |||||
| Yes | 61 (17.0%) | 12.3 | 44 (18.5%) | 6.2 | |||||
| No | 297 (83.0%) | 14.2 | 194 (81.5%) | 6.2 | |||||
| Pelvic lymph node metastasis | 0.89 | 0.51 | 1.41 | 0.06 | |||||
| Yes | 44 (12.3%) | 18.3 | 34 (14.3%) | 5.5 | |||||
| No | 313 (87.7%) | 13.6 | 203 (85.7%) | 6.4 | |||||
| SVCOS | 1.27 | 0.18 | 1.14 | 0.55 | |||||
| Yes | 38 (10.6%) | 15.4 | 24 (10.1%) | 6.4 | |||||
| No | 320 (89.4%) | 14.0 | 214 (89.9%) | 6.2 | |||||
| LE | NSE | 1.19 | 0.02 | 1.15 | 0.1 | ||||
| Elevated (>16.3 ng/ml) | 278 (82.2%) | 13.2 | 184 (80.7%) | 6.1 | |||||
| Normal (≤16.3 ng/ml) | 60 (17.8%) | 17.4 | 44 (19.3%) | 7.4 | |||||
| proGRP | 1.04 | 0.75 | 0.97 | 0.81 | |||||
| Elevated (>63 pg/ml) | 165 (87.3%) | 15.2 | 130 (90.9%) | 6.3 | |||||
| Normal (≤63 pg/ml) | 24 (12.7%) | 17.0 | 13 (9.1%) | 3.6 | |||||
| CYRA21‐1 | 1.27 | <0.0001 | 1.26 | 0.0008 | |||||
| Elevated (>3.3 ng/ml) | 142 (42.4%) | 11.9 | 92 (40.7%) | 5.6 | |||||
| Normal (≤3.3 ng/ml) | 193 (57.6%) | 17.1 | 134 (59.3%) | 6.5 | |||||
| CEA | 1.07 | 0.23 | 1.06 | 0.40 | |||||
| Elevated (>5 ng/ml) | 140 (41.5%) | 13.6 | 94 (41.2%) | 6.3 | |||||
| Normal (≤5 ng/ml) | 197 (58.5%) | 14.3 | 134 (58.8%) | 6.1 | |||||
| CA125 | 1.25 | 0.0002 | 1.10 | 0.15 | |||||
| Elevated (>35 U/ml) | 130 (39.5%) | 12.0 | 88 (39.6%) | 5.9 | |||||
| Normal (≤35 U/ml) | 199 (60.5%) | 16.2 | 134 (60.4%) | 6.4 | |||||
| Na | 1.52 | 0.008 | 1.46 | 0.04 | |||||
| Decreased (≤137 ng/ml) | 54 (16.5%) | 11.0 | 37 (16.9%) | 6.0 | |||||
| Normal (137–147 ng/ml) | 274 (83.5%) | 14.5 | 182 (83.1%) | 6.3 | |||||
| LDH | 1.46 | 0.001 | 1.25 | 0.12 | |||||
| Elevated (>250 U/L) | 131 (38.8%) | 11.4 | 77 (35.5%) | 5.9 | |||||
| Normal (120–250 U/L) | 207 (61.2%) | 15.6 | 143 (64.5%) | 6.7 | |||||
| ALB | 1.58 | 0.03 | 1.73 | 0.03 | |||||
| Decreased (<40 g/L) | 28 (8.0%) | 9.2 | 17 (7.3%) | 4.8 | |||||
| Normal (40–55 g/L) | 323 (92.0%) | 14.5 | 215 (92.7%) | 6.3 | |||||
| Cr | 1.96 | 0.04 | 0.93 | 0.89 | |||||
| Elevated (>97 umol/L) | 10 (3.8%) | 8.65 | 5 (2.2%) | 4.4 | |||||
| Normal or decreased (≤97umol/L) | 336 (96.2%) | 14.3 | 223 (97.8%) | 6.2 | |||||
| NLR | 1.36 | 0.04 | 1.29 | 0.17 | |||||
| <4 | 302 (84.4%) | 14.2 | 33 (13.9%) | 5.8 | |||||
| ≥4 | 56 (15.6%) | 10.9 | 205 (86.1%) | 6.3 | |||||
| PLR | 1.62 | 0.007 | 0.76 | 0.20 | |||||
| <76 | 35 (9.8%) | 11.0 | 24 (10.1%) | 5.4 | |||||
| ≥76 | 323 (90.2%) | 14.3 | 214 (89.9%) | 6.4 | |||||
| Hb | 2.24 | <0.0001 | 2.16 | 0.001 | |||||
| Decreased (<115 g/L) | 32 (8.9%) | 8.9 | 20 (8.4%) | 4.2 | |||||
| Normal (115–150 g/L) | 326 (91.1%) | 14.5 | 218 (91.6%) | 6.4 | |||||
| PLT | 1.06 | 0.36 | 1.15 | 0.05 | |||||
| Normal (100–300 *109/L) | 253 (70.7%) | 13.8 | 160 (67.2%) | 6.4 | |||||
| Decreased (<100 *109/L) | 8 (2.2%) | 4.6 | 6 (2.5%) | 2.1 | |||||
| Elevated (>300 *109/L) | 97 (27.1%) | 15.4 | 72 (30.3%) | 6.1 | |||||
Abbreviations: ALB, blood albumin; BMI, body mass index; CA125, carbohydrate antigen‐125; CC, clinical characteristics; CEA, carcinoembryonic antigen; Cr, serum creatinine; cyfra21‐1, cytokeratin 19 fragment; DC, demographic characteristics; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; LDH, lactic dehydrogenase; LE, laboratory examination; Na, serum sodium; NLR, neutrophil‐to‐lymphocyte ratio; NSE, neuron specific enolase; PLR, platelet‐to‐lymphocyte ratio; PLT, platelet; proGRP, progastrin‐releasing peptide; PS, performance status.
For tumor burden, detailed metastatic information of organs involved in each patient was recorded. We found bone, liver and brain multimetastasis had better prognostic predictive values than their respective metastasis (regardless of oligo‐ or multiple metastasis), and in addition, TNM stage, metastatic sites ≥2 and pleural metastasis also showed statistical significance in univariate analysis. Metastasis of the ipsilateral/contralateral lung, malignant effusion, pancreas and adrenal gland metastasis failed to show any significant correlation.
On initial multivariate analysis, metastatic sites ≥ 2 were strangely turned into a protective factor (HR = 0.68, p = 0.03), which might have been caused by the collinearity between metastatic sites ≥2 and TNM phase, and therefore metastatic sites ≥ 2 were deleted in the final analysis. The multivariate analysis indicated that age ≥ 70 (HR 1.56, p = 0.03), smoking index ≥ 400 (HR 1.46, p = 0.02), bone multimetastasis (HR 1.72, p = 0.001), PLR <76 (HR 1.59, p = 0.03), elevated Cyfra211 (HR 1.21, p = 0.01), elevated CA125 (HR 1.18, p = 0.02), low pretreatment Na (HR 1.49, p = 0.03) were independent risk factors (Table 2).
TABLE 2.
Multivariate analysis of pretreatment prognostic factors for overall survival (OS)
| Factors | OS | ||
|---|---|---|---|
| HR | 95% CI | p‐value | |
| Age (≥70 vs. <70) | 1.56 | 1.05–2.33 | 0.03 |
| Sex (Male vs. Female) | 1.08 | 0.75–1.57 | 0.67 |
| Smoking index (≥400 vs. <400) | 1.46 | 1.07–2.00 | 0.02 |
| Phase (IVB/ IVA /IIIC /IIIB) | 1.15 | 0.94–1.41 | 0.18 |
| Weight loss (Yes vs. No) | 1.30 | 0.96–1.76 | 0.09 |
| Liver multimetastasis (Yes vs. No) | 0.99 | 0.70–1.41 | 0.97 |
| Bone multimetastasis (Yes vs. No) | 1.72 | 1.24–2.40 | 0.001 |
| Brain multimetastasis (Yes vs. No) | 1.17 | 0.75–1.83 | 0.48 |
| Pleural metastasis (Yes vs. No) | 1.07 | 0.72–1.60 | 0.73 |
| PLR (<76 vs. ≥76) | 1.59 | 1.04–2.44 | 0.03 |
| NLR (≥4 vs. <4) | 1.18 | 0.82–1.69 | 0.37 |
| NSE (elevated vs. normal) | 1.19 | 0.99–1.43 | 0.06 |
| Cyfra211 (elevated vs. normal) | 1.21 | 1.04–1.40 | 0.01 |
| CA125 (elevated vs. normal) | 1.18 | 1.02–1.36 | 0.02 |
| Hb (decreased vs. normal) | 1.53 | 0.91–2.56 | 0.11 |
| Na (decreased vs. normal) | 1.49 | 1.04–2.13 | 0.03 |
| LDH (elevated vs. normal) | 1.06 | 0.95–1.43 | 0.72 |
| ALB (decreased vs. normal) | 0.85 | 1.17–1.42 | 0.54 |
Abbreviations: ALB, blood albumin; CA125, carbohydrate antigen‐125; cyfra21‐1, cytokeratin 19 fragment; Hb, hemoglobin; LDH, lactic dehydrogenase; Na, serum sodium; NLR, neutrophil‐to‐lymphocyte ratio; NSE, neuron specific enolase; PLR, platelet‐to‐lymphocyte ratio.
Additionally, Table 3 demonstrates the key roles of treatments in prolonging survival (both OS and PFS) of SCLC. Chemotherapy, chest radiation and PCI were proven to be potent protective factors for ES‐SCLC again in this study, which is similar to that reported in other studies. The median survival of patients receiving < 4 cycles chemotherapy was much worse than those who received ≥ 4 cycles chemotherapy (OS: 6.3 vs. 15.5 months, HR = 2.94, p < 0.0001; PFS: 2.1 vs. 6.7 months, HR = 4.76, p < 0.0001), and subgroup analysis revealed patients who underwent platinum‐based chemotherapy could achieve better survival than those who underwent non‐platinum‐based chemotherapy, while there were no significant difference among EP/CE/other platinum‐based chemotherapies.
TABLE 3.
Treatment diagram of patient cohort
| N (%) | OS | HR | p‐value | N (%) | PFS | HR | p‐value | |
|---|---|---|---|---|---|---|---|---|
| <4 cycles of chemotherapy | 58 (16.2%) | 6.3 | 2.94 | <0.0001 | 26 (10.9%) | 2.1 | 4.76 | <0.0001 |
| ≥4 cycles of chemotherapy | 300 (83.8%) | 15.5 | 1 | 212 (89.1%) | 6.7 | |||
| EP | ‐ 160 (44.7%) | ‐ 16.6 | ‐ 1 | ‐ 117 (49.2%) | ‐ 7.1 | ‐ 1 | ||
| CE | ‐ 118 (33%) | ‐ 14.8 | ‐ 1.15 | ‐ 0.27 | ‐ 77 (32.4%) | ‐ 6.6 | ‐ 0.97 | ‐ 0.86 |
| Other platinum‐based chemotherapy | ‐ 16 (4.4%) | ‐ 14.6 | ‐ 1.62 | ‐ 0.07 | −14 (5.9%) | ‐ 5.9 | ‐ 1.64 | ‐ 0.08 |
| Non‐platinum‐based chemotherapy | ‐ 6 (1.7%) | ‐ 9.25 | ‐ 2.84 | ‐ 0.01 | ‐ 4 (1.7%) | ‐ 5.2 | ‐ 3.05 | ‐ 0.03 |
| Chest radiation | 149 (41.6%) | 17.3 | 0.54 | <0.0001 | 105(44.1%) | 8.4 | 0.51 | <0.0001 |
| No chest radiation | 209 (58.4%) | 11.0 | 1 | 133(55.9%) | 5.1 | 1 | ||
| PCI | 40 (11.2%) | 28.7 | 0.40 | <0.0001 | 29 (12.2%) | 10.5 | 0.47 | 0.0002 |
| No PCI | 309 (88.8%) | 12.7 | 1 | 206 (87.8%) | 5.9 | 1 |
Abbreviations: CE, etoposide plus carboplatin; EP, etoposide plus cisplatin; OS, overall survival; PCI, prophylactic cranial irradiation; PFS, progression‐free survival.
Prognostic analysis of progression‐free survival for ES‐SCLC
Four pretreatment laboratory indexes before treatment were found having a negative association with PFS on univariate analysis (Table 1): elevated tumor biomarker cyfra211, and decreased Hb/ALB/Na. The final multivariate analysis indicated that elevated cyfra211 (HR 1.22, p = 0.006), anemia (HR 1.92, p = 0.01), low Na (HR 1.46, p = 0.04) were independent negative pretreatment indicators for PFS of patients with ES‐SCLC (Table 4).
TABLE 4.
Multivariate analysis of pretreatment prognostic factors for progression‐free survival (PFS)
| Factors | PFS | ||
|---|---|---|---|
| HR | 95% CI | p‐value | |
| Cyfra21‐1 (elevated vs. normal) | 1.22 | 1.06–1.40 | 0.006 |
| Hb (decreased vs. normal) | 1.92 | 1.14–3.23 | 0.01 |
| Na (decreased vs. normal) | 1.46 | 1.01–2.11 | 0.04 |
| ALB (decreased vs. normal) | 1.41 | 0.84–2.37 | 0.19 |
Abbreviations: ALB, blood albumin; cyfra21‐1, cytokeratin 19 fragment; Hb, hemoglobin; Na, serum sodium.
DISCUSSION
In this study, we retrospectively analyzed the basic characteristics, treatment modalities and survival of 358 patients with ES‐SCLC and investigated the potential prognostic indicators. This study focused on ES‐SCLC and excluded LS‐SCLC patients as treatment principles and survival differ in limited and extensive stage patients with SCLC. To the best of our knowledge, although a number of previous retrospective studies have discussed SCLC survival and prognostic factors, no study has previously collected such detailed demographic, clinical and laboratory data of purely ES‐SCLC patients. Moreover, a comprehensive prognostic analysis for both OS and PFS is presented in our study. Such “real world” data may contribute to a more profound understanding of this disease, providing the current medical treatment situation for ES‐SCLC in China, and offering a benchmark to the current standard therapeutic modality with the addition of immune‐agents together with future prospective studies.
In our prognostic analysis, we identified two demographic characters (age ≥ 70, smoking index ≥ 400), one tumor burden factor (bone multimetastasis), two tumor biomarkers (cyfra211, CA125) and two laboratory indexes (decreased Na and PLR < 76) as independent prognostic factors for OS in this patient population. Elevated cyfra211, decreased Hb and Na were also identified as independent prognostic factors for PFS.
Advancing age is deemed as the most important risk factor for developing cancer and has also been found to be a negative prognostic factor in many cancers. In our study, the optimal cutoff value of age was determined by the survminer package of R. We explored a range of age cutoffs (60/65/70) and determined that an age of 70 or higher is most strongly correlated with OS, whilst its correlation with PFS is insignificant. A previously published pooled analysis of 1303 patients with LS‐SCLC found elderly patients (age ≥ 70) had a worse OS (HR 1.38, 95% CI: 1.18–1.63) and PFS (HR 1.19, 95% CI: 1.03–1.39) and had more difficulty in tolerating therapy. 5
Smoking is deemed as the most important risk factor of tumorigenesis of SCLC. In this study, 77.0% of the patients were current or former smokers, and 66.5% of the patients had a smoking index of 400 or higher. Some retrospective studies and meta‐analysis have found smoking status is an independent prognostic factor, 6 , 7 whereas in this study no significant survival difference between smokers and non‐smokers was discovered (p = 0.62); however, patients with a smoking index ≥400 were found to be linked to a poorer OS (p = 0.04).
For tumor burden, detailed metastatic information of organs involved in each patient is recorded. In this study, bone multimetastasis was considered as an independent prognostic indicator for OS, whose value surpassed bone metastasis, any other organ metastasis, TNM stage and the number of metastatic sites. Andriani et al. also reported that bone metastasis was related to a reduced OS, in addition to the finding that patients with an early‐onset bone metastasis had a poorer OS than patients with a late‐onset bone metastasis. 8 While consensus has not yet been reached, different studies might select factors on behalf of tumor burdens. The study by Xie et al. found liver metastasis and metastatic sites >2 were prognostic indicators for ES‐SCLC. 9 Another retrospective study enrolled phase and laterality of lung infiltration in a prognostic prediction model of SCLC. 10
Two tumor biomarkers ‐ cyfra211 and CA125 ‐ are considered as independent prognostic factors for OS; moreover, cyfra211 has also shown an independent prognostic value in PFS prediction. The predictive value of cyfra211 and NSE has been proposed in the study by Pan et al. 11 To the best of our knowledge, our study is the first to have explored and found the prognostic predictive value of CA125 in SCLC. Another tumor biomarker, progastrin‐releasing peptide (ProGRP), is deemed as being equipped with high sensitivity and specificity in diagnosis of SCLC, and whilst its predictive function was not confirmed in our study, this might due to its late application in clinics and many patients in early years who were not tested for ProGRP.
Hyponatremia is often seen in SCLC, and is thought to be caused by one of the paraneoplastic syndromes—syndrome of inappropriate antidiuretic hormone secretion (SIADH). In this study, the incidence of hyponatremia was 16.5%, which is lower than the 44%–47% reported in other studies which specifically investigated the association between hyponatremia and SCLC prognosis, but hyponatremia was also found as a significant prognostic factor associated with poor prognosis. In addition, the severity of hyponatremia, as well as failure to normalize plasma sodium within the first two cycles of chemotherapy, have also been found to be potential negative prognostic indicators. 12 , 13 However, in another pooled study which analyzed the impact of hyponatremia on various cancer types including lymphoma, breast, colorectal, SCLC, and non‐small cell lung cancer, the prognostic value of hyponatremia only failed to be seen in SCLC (HR = 1.5, p = 0.19), which might be due to the limited number of SCLC patients enrolled in the study (n = 80). 14
Anemia conferred shorter PFS of ES‐SCLC in our study but failed to show a correlation with poor OS. Its prognostic value has been confirmed in lymphoma but few studies have investigated its association with SCLC survival.
Most tumors are infiltrated by inflammatory and immune cells. Although the role of inflammatory cells in the pathogenesis of SCLC has not yet been fully elucidated, high pretreatment PLR and NLR have been found to be connected with a shorter survival in SCLC. PLR >258 was a risk factor for SCLC in the study by Pan et al. 11 and PLR > 140 was a risk factor for LS‐SCLC in the study by Suzuki et al., 15 but these two cutoff values were meaningless in our data, and we found decreased PLR (PLR < 76) was an independent prognostic indicator (HR = 1.64, p = 0.007), which appears to be contradictory to that reported in previous studies. NLR ≥ 4 was meaningful in the univariate analysis while it failed to show statistical significance in the multivariate analysis.
The retrospective nature and system bias is the major limitation of this study since all data were collected based on clinical documents of medical systems and follow‐up information was largely based on telephone call dictations with patients’ families. In addition, some patients were excluded due to incomplete clinical records. Moreover, to date, no extensively used clinical prognostic models exist after much exploration, and more effort should be made on the level of molecular mechanism, such as four subtypes of SCLC stratified by RNA sequencing reported in the study by Gay et al. 16
In conclusion, ES‐SCLC is a fatal malignancy with poor survival and unsatisfying treatment strategies. This study provides real‐world evidence of the survival and prognosis of untreated ES‐SCLC patients which will enable better evaluation and clinical decision‐making in the future.
CONFLICT OF INTEREST
All authors approved the final manuscript and no conflict of interest is reported.
ACKNOWLEDGMENTS
This work was financially supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant no. 2016‐I2M‐1‐001) and China National Major Project for New Drug Innovation (2017ZX09304015).
Huang L‐L, Hu X‐S, Wang Y, et al. Survival and pretreatment prognostic factors for extensive‐stage small cell lung cancer: A comprehensive analysis of 358 patients. Thorac Cancer. 2021;12:1943–1951. 10.1111/1759-7714.13977
Funding information CAMS Innovation Fund for Medical Sciences (CIFMS), Grant/Award Number: 2016‐I2M‐1‐001; China National Major Project for New Drug Innovation, Grant/Award Number: 2017ZX09304015
Contributor Information
Yan Wang, Email: wangyanyifu@163.com.
Jun‐Ling Li, Email: lijunling@cicams.ac.cn.
Yuan‐Kai Shi, Email: syuankai@cicams.ac.cn.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram MD, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2. Shepherd FA, Ginsberg RJ, Haddad R, Feld R, Sagman U, Evans WK, et al. Importance of clinical staging in limited small‐cell lung cancer: a valuable system to separate prognostic subgroups. The University of Toronto Lung Oncology Group. J Clin Oncol. 1993;11(8):1592–7. [DOI] [PubMed] [Google Scholar]
- 3. Fiegl M, Pircher A, Waldthaler C, Gamerith G, Kocher F, Pall G, et al. Small steps of improvement in small‐cell lung cancer (SCLC) within two decades: a comprehensive analysis of 484 patients. Lung Cancer. 2014;84(2):168–74. [DOI] [PubMed] [Google Scholar]
- 4. Fukui T, Itabashi M, Ishihara M, Hiyoshi Y, Kasajima M, Igawa S, et al. Prognostic factors affecting the risk of thoracic progression in extensive‐stage small cell lung cancer. BMC Cancer. 2016;16:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stinchcombe TE, Fan W, Schild SE, Vokes EE, Bogart J, Le QT, et al. A pooled analysis of individual patient data from National Clinical Trials Network clinical trials of concurrent chemoradiotherapy for limited‐stage small cell lung cancer in elderly patients versus younger patients. Cancer. 2019;125(3):382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu X, Jiang T, Li W, Li X, Zhao C, Shi J, et al. Characterization of never‐smoking and its association with clinical outcomes in Chinese patients with small‐cell lung cancer. Lung Cancer. 2018;115:109–15. [DOI] [PubMed] [Google Scholar]
- 7. Torres‐Duran M, Ruano‐Ravina A, Kelsey KT, Parente‐Lamelas I, Provencio M, Leiro‐Fernández V, et al. Small cell lung cancer in never‐smokers. Eur Respir J. 2016;47(3):947–53. [DOI] [PubMed] [Google Scholar]
- 8. Charpidou A, Tsagouli S, Gkiozos I, Grapsa D, Moutsos M, Kiagia M, et al. Bone metastases in patients with small cell lung carcinoma: rate of development, early versus late onset, modality of treatment, and their impact on survival. A single‐institution retrospective cohort study. Clin Exp Metastasis. 2016;33(5):453–60. [DOI] [PubMed] [Google Scholar]
- 9. Xie D, Marks R, Zhang M, Jiang G, Jatoi A, Garces YI, et al. Nomograms predict overall survival for patients with small‐cell lung cancer incorporating pretreatment peripheral blood markers. J Thorac Oncol. 2015;10(8):1213–20. [DOI] [PubMed] [Google Scholar]
- 10. Wang S, Yang L, Ci B, Maclean M, Gerber DE, Xia G, et al. Development and validation of a nomogram prognostic model for SCLC patients. J Thorac Oncol. 2018;13(9):1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan H, Shi X, Xiao D, et al. Nomogram prediction for the survival of the patients with small cell lung cancer. J Thorac Dis. 2017;9(3):507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Y, Sun N, Sun P, et al. Clinical characteristics and prognosis of elderly small cell lung cancer patients complicated with hyponatremia: a retrospective analysis. Anticancer Res. 2017;37(8):4681–6. [DOI] [PubMed] [Google Scholar]
- 13. Hansen O, Sorensen P, Hansen KH. The occurrence of hyponatremia in SCLC and the influence on prognosis: a retrospective study of 453 patients treated in a single institution in a 10‐year period. Lung Cancer. 2010;68(1):111–4. [DOI] [PubMed] [Google Scholar]
- 14. Castillo JJ, Glezerman IG, Boklage SH, et al. The occurrence of hyponatremia and its importance as a prognostic factor in a cross‐section of cancer patients. BMC Cancer. 2016;16:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suzuki R, Wei X, Allen PK, et al. Prognostic significance of total lymphocyte count, neutrophil‐to‐lymphocyte ratio, and platelet‐to‐lymphocyte ratio in limited‐stage small‐cell lung cancer. Clin Lung Cancer. 2019;20(2):117–23. [DOI] [PubMed] [Google Scholar]
- 16. Gay CM, Stewart CA, Park EM, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39(3):346–60.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]


