Abstract
Background
Patients with early lung cancer are the best candidates for surgical resection. However, those patients with high grade patterns (micropapillary or solid) do not have a good prognosis, even if they have been diagnosed with stage I lung adenocarcinoma. A new modified grading system has been introduced and this study aimed to identify the prognostic role of the new grading system in patients with stage IA lung adenocarcinoma.
Methods
Patients with pathological stage IA lung adenocarcinoma, according to the eighth TNM classification who underwent curative resection, were reviewed. The pathological data of stage IA adenocarcinoma was reviewed 1 (grade 1: lepidic predominant with no or less than 20% of high grade patterns, grade 2: acinar or papillary predominant with no or less than 20% of high grade patterns, grade 3: any tumor with 20% or more of high grade patterns). Prognostic factors were analyzed for disease‐free interval (DFI) and overall survival (OS) using Cox proportional models.
Results
The medical records of 429 patients with stage IA lung adenocarcinoma were reviewed. DFI (p < 0.001) and OS (p < 0.001) were significantly lower in patients diagnosed with grade 3 compared with grade 1 and grade 2. Multivariate analysis showed that smoking (p = 0.013), value of SUVmax (p = 0.005), lymphovascular invasion (p = 0.004) and grade 3 (p = 0.008) were significant prognostic factors for DFI.
Conclusions
The proportion of high grade patterns showed a different prognosis, even if curative resection had been performed for stage IA adenocarcinoma. This new grading system is more simple and useful in the prediction of a prognosis in patients with stage IA lung adenocarcinoma.
Keywords: adenocarcinoma, histological subtype, lung cancer, prognosis, stage IA
A cutoff value for classification in medical science has been widely used. However, invasive lung adenocarcinoma was classified by the predominant pattern. It was sometimes conflicting for predicting prognosis. Recently, a new classification using a cutoff value has been proposed. In the present study, we investigated the prognostic impact of the new grading system in stage IA lung adenocarcinoma.
INTRODUCTION
Lung cancer is the leading cause of cancer‐related death worldwide. 1 Surgical resection is the most curative treatment for stage I non‐small cell lung cancer (NSCLC). 2 Adenocarcinoma is the most common histological type of lung cancer. 3 For better management, a subtype for the histological classification of invasive lung adenocarcinoma was proposed by the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society (IASLC/ATS/ERS) in 2011. 4 This classification was based on six histological types (acinar, papillary, micropapillary, lepidic, solid and variants) and divided into three groups. Low grade is lepidic predominant, intermediate is acinar or papillary predominant and high grade is micropapillary (MPP) or solid predominant. 5 In this classification, the term “predominant” is used for all categories because invasive lung adenocarcinomas present heterogeneous histological patterns. Numerous studies have been carried out to show different prognoses according to the histological subtypes of invasive lung adenocarcinoma. The high grade patterns showed a worse prognosis than other types. 6 , 7 Furthermore, another study demonstrated that the minor subtype of MPP or solid patterns showed worse prognosis, earlier recurrence and more lymph node metastasis. 8 However, in this classification, additional prognostic factors including variant subtypes were not considered and a cutoff value was not proposed for prognostic factor. A new modified grading system has recently been introduced to supplement the histological classification. 5 In this modified grading system, a cutoff value for each grade has been proposed. The high risk group (grade 3) is any tumor with 20% or more of high grade patterns. In this study, we evaluated the prognosis using this new grading system in stage IA lung adenocarcinoma.
METHODS
This retrospective study was approved by the Institutional Review Board of the Catholic Medical Center (Republic of Korea) (XC20RADI0019). We reviewed the electronic medical records (EMRs) of patients who underwent curative resection for NSCLC from January 2010 to December 2015 at Seoul and Bucheon St. Mary's Hospital. A total of 1245 patients underwent surgical resection of NSCLC with a curative aim. In these patients, we reviewed the EMRs of patients with pathological stage IA adenocarcinoma according to the eighth edition of the tumor‐node‐metastasis (TNM) classification of lung cancer. First, the patients with nonadenocarcinoma, wedge resection and perioperative death were excluded. On the pathological reports, the patients with pathological entire tumor size>3 cm, lymph node metastasis, visceral pleural invasion or incomplete resection (R1) were excluded. Second, the cases with adenocarcinoma in situ and minimally invasive adenocarcinoma were excluded. The patients with the pathological reports that did not include the histological subtypes were excluded. Finally, we reviewed the EMRs and imaging studies. The patients with multifocal ground‐glass opacities (GGOs), synchronous or metachronous lung cancer, and those who received neoadjuvant chemotherapy were excluded. The subjects of the analysis were 429 patients with pathological stage IA invasive adenocarcinoma.
Patient characteristics were reviewed including age, sex, underlying disease, smoking history, other previous malignancies and surgical procedure. Preoperative assessments included chest computed tomography (CT), positron emission tomography‐CT (PET‐CT), brain magnetic resonance imaging (MRI), bone scanning and bronchoscopy. The imaging data of the patients were obtained including tumor location, presence of GGOs (pure or part‐solid nodule), maximum standardized uptake value (SUVmax) and clinical stage. Pathological results were also collected for analysis including tumor size, grade, predominant pattern, number of lymph node (LN) dissections, and lymphovascular invasion (LVI).
The patients were divided into three groups according to the new modified grading system (grade 1: lepidic predominant with no or less than 20% of high grade patterns; grade 2: acinar or papillary predominant with no or less than 20% of high grade patterns and grade 3: any tumor with 20% or more of high grade patterns).
All patients were followed until recurrence and death or loss of follow‐up (F/U). Overall survival (OS) was defined as the interval from the date of surgery to the date of death. Recurrence was defined as local or extrathoracic metastasis based on clinical and pathological evidence, and the disease‐free interval (DFI) was obtained (from date of surgery to the date of recurrence or death).
Statistical analysis
All statistical analyses were carried out using SPSS version 18 (SPSS Inc.). Continuous variables were compared using the Kruskal‐Wallis test, and categorical variables were compared using the chi‐square test and Fisher's exact test.
DFI and OS were estimated by the Kaplan–Meier method and log‐rank test. Prognostic factors associated with recurrence and survival were determined using the Cox proportional hazards model after checking the proportionality assumption. Variables with p‐values less than 0.05 in the univariate analysis were included in the multivariate analysis.
RESULTS
According to the new grading system, the number of grade 1 and 2 patients were 191 (44.5%) and 200 cases (46.6%), respectively. Grade 3 patients included 38 cases (8.9%).
The characteristics of the three groups with pathological stage IA adenocarcinoma are shown in Table 1. The three groups were significantly different in terms of age (p = 0.013), smoking history (p = 0.005), carcinoembryonic antigen (CEA) (p < 0.001), and maximum standardized uptake on PET‐CT (SUVmax) (p < 0.001). The value of CEA and SUVmax tended to be higher in grade 3 patients. The three groups were not significantly different with regard to clinical stage and tumor location. For GGO features (pure or part‐solid nodule), there was a significant difference (p < 0.001). The GGO lesions were more prominent in patients with grade 1 compared with grades 2 and 3. For the surgical procedure, lobectomy was conducted more in grade 3 patients (p = 0.046).
TABLE 1.
Baseline patient characteristics in stage IA lung adenocarcinoma
| Grade 1 (n = 191) | Grade 2 (n = 200) | Grade 3 (n = 38) | p‐value | |
|---|---|---|---|---|
| Age | 60.6 (35–80) | 63.7 (25–82) | 67.8 (36–82) | 0.013 |
| Male | 76 (39.8) | 88 (44) | 23 (60.5) | 0.062 |
| Smoking | 39 (20.4) | 57 (28.5) | 17 (44.7) | 0.005 |
| CEA | 1.39 (0.05–10.48) | 1.44 (0.22–9) | 1.67 (0.5–14.19) | <0.001 |
| SUVmax | 1.47 (0–8.9) | 2.24 (0–14.6) | 5.3 (1–17.6) | <0.001 |
| Clinical stage IA | 169 (88.5) | 174 (87) | 30 (79) | 0.281 |
| Clinical stage IB | 21 (11) | 24 (12) | 7 (18.4) | 0.439 |
| Clinical stage II, III | 1 (0.5) | 2 (1) | 1 (2.6) | 0.462 |
| Tumor location | ||||
| RUL | 36 (18.8) | 50 (25) | 6 (15.8) | 0.225 |
| RML | 15 (7.9) | 15 (7.5) | 4 (10.5) | 0.817 |
| RLL | 64 (33.5) | 70 (35) | 11 (28.9) | 0.765 |
| LUL | 42 (22) | 44 (22) | 9 (23.7) | 0.972 |
| LLL | 34 (17.8) | 21 (10.5) | 8 (21.1) | 0.064 |
| GGO | 160 (83.8) | 114 (57) | 13 (34.2) | <0.001 |
| Lobectomy | 163 (85.3) | 182 (91) | 37 (97.4) | 0.046 |
| Segmentectomy | 27 (14.1) | 17 (8.5) | 1 (2.6) | 0.049 |
| Bilobectomy | 1 (0.5) | 1 (0.5) | 0 (0) | 0.906 |
| VATS | 164 (85.9) | 163 (81.5) | 35 (92.1) | 0.192 |
Note: Data are presented as the median (minimum‐maximum) or frequencies and percentages as appropriate.
Abbreviations: CEA, carcinoembryonic antigen; Grade 1, lepidic predominant with no or less than 20% of high grade patterns; Grade 2, acinar or papillary predominant with no or less than 20% of high grade patterns; Grade 3, any tumor with 20% or more of high grade patterns; GGO, ground glass opacity; LLL, left lower lobe; LUL, left upper lobe, RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; SUVmax, maximum standardized uptake value; VATS, video‐assisted thoracoscopic surgery.
The pathological data are presented in Table 2. In pathological stage IA adenocarcinoma, the median entire tumor size was 1.8 cm (range 0.3–3) in 429 patients. Entire tumor size was greater in grade 3 (p < 0.001). With regard to differentiation, there was a significant difference in the three groups. Well differentiated was the most common in grade 1 patients (p < 0.001). Poorly differentiated was the most common in grade 3 (p < 0.001). Comparing the three groups, there was no significant difference in resected margins (p = 0.585) and the number of dissected lymph nodes (p = 0.334).
TABLE 2.
Pathological characteristics in stage IA lung adenocarcinoma
| Grade 1 (n = 191) | Grade 2 (n = 200) | Grade 3 (n = 38) | p‑‐value | |
|---|---|---|---|---|
| Entire tumor size | 1.58 (0.6–3) | 1.86 (0.3–3) | 2.06 (0.7–3) | <0.001 |
| Differentiation | ||||
| Well | 172 (90.1) | 75 (37.5) | 2 (5.3) | <0.001 |
| Moderate | 18 (9.4) | 121 (60.5) | 21 (55.3) | <0.001 |
| Poorly | 1 (0.5) | 4 (2) | 15 (39.4) | <0.001 |
| Predominant type | ||||
| Acinar | 0 (0) | 171 (85.5) | 13 (34.2) | <0.001 |
| Papillary | 0 (0) | 29 (14.5) | 5 (13.2) | <0.001 |
| Lepidic | 191 (100) | 0 (0) | 4 (10.5) | <0.001 |
| MPP | 0 (0) | 0 (0) | 2 (5.3) | <0.001 |
| Solid | 0 (0) | 0 (0) | 14 (36.8) | <0.001 |
| Margin | 3.66 (0.2–8.5) | 3.53 (0.1–10) | 3.15 (0.5–7) | 0.585 |
| LVI | 17 (8.9) | 42 (21) | 19 (50) | <0.001 |
| Number of dissected LN | 10.1 (0–53) | 11.5 (0–49) | 11.5 (1–38) | 0.334 |
| MLND | 118 (61.8) | 139 (69.5) | 32 (84.2) | 0.018 |
| MLNS | 68 (35.6) | 56 (28) | 6 (15.8) | 0.037 |
| pStage IA1 | 35 (18.3) | 20 (10) | 5 (13.1) | 0.059 |
| pStage IA2 | 110 (57.6) | 112 (56) | 15 (39.5) | 0.117 |
| pStage IA3 | 46 (24.1) | 68 (34) | 18 (47.4) | 0.0071 |
| Adjuvant treatment | 4 (2.1) | 3 (1.5) | 0 (0) | 0.636 |
| Recurrence | 2 (1) | 26 (13) | 13 (34.2) | <0.001 |
| Death | 5 (2.6) | 21 (10.5) | 6 (15.8) | <0.011 |
Note: Data are presented as the medians (minimum–maximum) or frequencies and percentages as appropriate.
Abbreviations: Grade 1, lepidic predominant with no or less than 20% of high grade patterns; Grade 2, acinar or papillary predominant with no or less than 20% of high grade patterns; Grade 3, any tumor with 20% or more of high grade patterns; LN, lymph node; LVI, lymphovascular invasion; MLND, mediastinal lymph node dissection; MLNS, mediastinal lymph node sampling.
Mediastinal LN evaluation was conducted in 419 patients (97.7%). In some cases, the surgeon decided to omit the mediastinal LN evaluation if the lesion was pure GGO or a GGO dominant lesion. According to the guidelines of complete lung resection, 9 mediastinal lymph node dissection (MLND) was conducted in 289 patients (67.4%) and mediastinal lymph node sampling (MLNS) in 130 patients (30.3%). The three groups were significantly different in terms of mediastinal LN evaluation. MLND was conducted more in grade 3 patients (p = 0.018).
LVI was identified in 85 patients (19.8%). LVI was more common in grade 3 patients (p < 0.001). For the pathological stage, 60 patients (14%) were IA1, 237 patients (55.2%) were IA2, and 132 patients (30.8%) were IA3. IA3 was the most common in grade 3 patients (p = 0.007).
Adjuvant chemotherapy was conducted in seven patients (1.6%). During the F/U period, 41 patients (9.6%) experienced recurrence after surgery and 32 patients (7.5%) died. Median DFI was 60 months (range 1–120) and OS was 70 months (range 1–125).
For the treatment after recurrence, systemic chemotherapy was conducted in 11 patients. Chemoradiation therapy was carried out in 12 patients and tyrosine kinase inhibitors (TKIs) were administered in 10 patients. Others were loss to F/U or denied treatment.
Survival curve analysis was conducted for DFI and OS according to the pathological stage (Figure 1). However, there was no significant difference for DFI (p = 0.430) and OS (p = 0.918).
FIGURE 1.
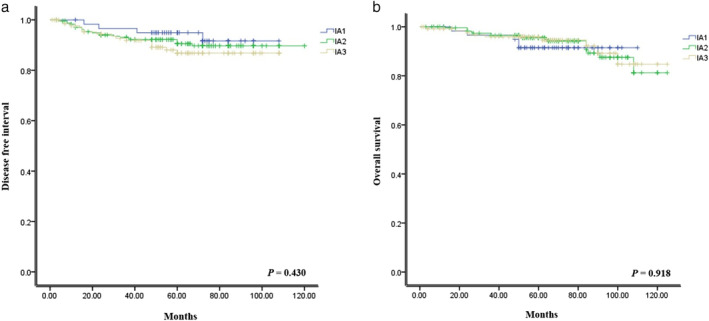
Survival curves for (a) disease free interval and (b) overall survival in stage IA lung adenocarcinoma according to the pathological stage
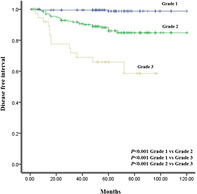
Kaplan–Meier curves for DFI and OS were obtained according to the new grading system (Figure 2). There was a significant difference between the three grades for DFI, respectively (p < 0.001). There was a significant difference between grades 1 and 2, and 3 (p = 0.001, p < 0.001). However, there was no difference between grades 2 and 3 for OS (p = 0.128).
FIGURE 2.
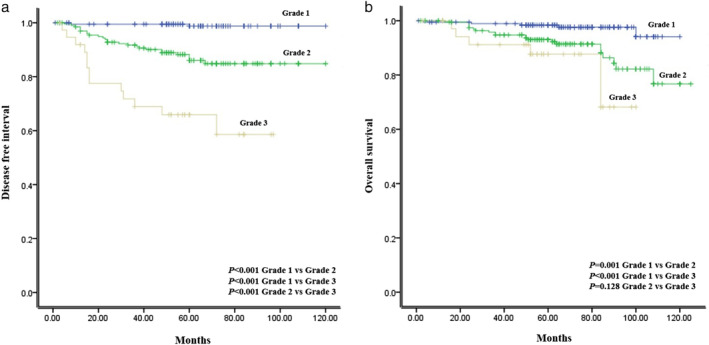
Survival curves for (a) disease free interval and (b) overall survival in stage IA lung adenocarcinoma according to the new grading system
In the analysis of prognostic factors related to recurrence in stage IA adenocarcinoma (Table 3), smoking (p = 0.013), SUVmax (p = 0.005), grade 3 (p = 0.008), and LVI (p = 0.004) were associated with recurrence by multivariate analysis.
TABLE 3.
Cox proportional analysis for disease‐free interval in stage IA lung adenocarcinoma
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p‐value | OR | 95% CI | p‐value | |
| Male | 1.969 | 1.168–3.321 | 0.011 | 1.039 | 0.512–2.109 | 0.915 |
| Smoking | 2.817 | 1.680–4.724 | <0.001 | 2.382 | 1.197–4.742 | 0.013 |
| CEA | 1.206 | 1.097–1.325 | <0.001 | 1.046 | 0.922–1.187 | 0.483 |
| SUVmax | 1.211 | 1.142–1.285 | <0.001 | 1.123 | 1.035–1.219 | 0.005 |
| Tumor size | 1.633 | 1.051–2.539 | 0.029 | 0.909 | 0.542–1.524 | 0.717 |
| Poorly differentiated | 4.309 | 2.039–9.109 | <0.001 | 0.718 | 0.267–1.932 | 0.512 |
| Grade 3 | 4.940 | 2.736–8.920 | <0.001 | 2.726 | 1.302–5.707 | 0.008 |
| LVI | 4.143 | 2.462–6.970 | <0.001 | 2.406 | 1.326–4.368 | 0.004 |
Abbreviations: CEA, carcinoembryonic antigen; Grade 3, any tumor with 20% or more of high grade patterns; LVI, lymphovascular invasion; OR, overall response; SUVmax, maximum standardized uptake value.
In the analysis for OS in stage IA adenocarcinoma (Table 4), smoking (p = 0.040), and SUVmax (p = 0.044) were associated with survival by multivariate analysis.
TABLE 4.
Cox proportional analysis for overall survival in stage IA lung adenocarcinoma
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p‐value | OR | 95% CI | p‐value | |
| Age | 1.050 | 1.008–1.093 | 0.018 | 1.035 | 0.994–1.077 | 0.096 |
| Male | 2.509 | 1.209–5.205 | 0.014 | 1.180 | 0.454–3.066 | 0.735 |
| Smoking | 3.274 | 1.635–6.556 | 0.001 | 2.638 | 1.043–6.670 | 0.040 |
| CEA | 1.160 | 1.010–1.332 | 0.035 | 0.972 | 0.794–1.189 | 0.780 |
| SUVmax | 1.221 | 1.125–1.324 | <0.001 | 1.119 | 1.003–1.247 | 0.044 |
| Poorly differentiated | 5.231 | 2.002–13.668 | 0.001 | 1.459 | 0.370–5.763 | 0.590 |
| Grade 3 | 3.326 | 1.356–8.158 | 0.009 | 1.292 | 0.390–4.281 | 0.675 |
| LVI | 3.344 | 1.647–6.786 | 0.001 | 1.961 | 0.875–4.397 | 0.102 |
Abbreviations: CEA, carcinoembryonic antigen; Grade 3, any tumor with 20% or more of high grade patterns; LVI, lymphovascular invasion; OR, overall response; SUVmax, maximum standardized uptake value.
DISCUSSION
Lung cancer is one of the cancers with the worst prognosis. Non‐small cell lung cancer (NSCLC) accounts for more than 80% of all lung cancers. 10 Adenocarcinoma is the most common histological type of NSCLC, accounting for almost half of all lung cancers. 11 Curative resection is the most definitive treatment for patients with stage I lung adenocarcinoma, although the prognosis after curative resection remains unsatisfactory. The five‐year survival rate is approximately 80% for pathological stage I NSCLC. TNM staging is a widely used system that predicts prognosis and determines treatment policies. The TNM staging classification for lung cancer was updated by the IASLC group in 2017. However, its prognostic accuracy remains limited.
A new classification for invasive lung adenocarcinoma based on the predominant histological pattern was introduced in 2011, including low grade (lepidic predominant), intermediate (acinar or papillary predominant) and high grade (MPP or solid predominant). 5 These classifications are correlated with prognosis. Numerous retrospective studies and meta‐analyses have indicated that high grade patterns (solid or MPP predominant subtypes) have shown a worse prognosis than other types. These subtypes are associated with more LN metastasis, greater tumor sizes and higher SUVmax values. Ujiie et al. indicated that the solid predominant histological subtype is an independent prognostic factor for early recurrence after curative resection in stage I lung adenocarcinoma patients. 7 Adenocarcinoma subtypes also influence lymph node metastasis in small‐sized lung cancer. 12 Patients with solid histological subtypes less than 1 cm have been found to have significantly more lymph node metastases. The MPP and solid histological subtypes also showed higher SUVmax values than the other subtypes, reflecting malignant potential and prognosis. 13 For pathological evaluation according to different histological subtypes, solid subtypes are associated with atypia, mitotic activity, necrosis and LVI. 14 Lung adenocarcinoma is heterogeneous. Most adenocarcinomas present with a mixture of histological subtypes. 3 It is well known that the lepidic predominant subtype shows a more favorable prognosis than the other subtypes, and many studies have indicated that high grade patterns are not usually mixed with the lepidic subtype, including in our study. 15 Many studies have also tried to identify the genetic features according to the histological subtypes in lung adenocarcinoma. In the solid subtype, EGFR mutations are less frequent, but ALK and KRAS mutations have been found to be more common. 16 , 17 , 18 This means that the effects of tyrosine kinase inhibitors (TKIs) are not satisfactory, resulting in a worse prognosis. In recent studies, immunotherapy has emerged as a treatment for advanced NSCLC. A previous study has reported that programmed cell death 1 ligand (PD‐L1) expression in cancer cells could avoid immune reactions with a worse prognosis. 19 Dong et al. investigated the association between PD‐L1 and the solid subtype. They found that PD‐L1 expression was increased in the solid predominant type. 20 Further studies are needed to determine whether immunotherapy is an effective treatment for solid subtype adenocarcinoma. Minor solid or MPP subtype are also considered prognostic factors. 21 , 22 Minor subtype indicates the subtype occupies no less than 5% but is not predominant. Another study also showed that the small proportion of MPP had a prognostic factor even though the proportion was lower than 5% which is disregarded according to the current classification 23 so this classification using histological subtypes according to the predominant patterns is a little complex, conflicting and not effective to predict prognosis in invasive lung adenocarcinoma because quantified criteria have not been proposed. Furthermore, the proportion of high grade patterns were significantly lower in stage IA invasive adenocarcinoma. In our study, there were only 16 cases (3.8%) of MPP or solid predominant subtypes and the sample size was too small to accurately predict a prognosis. However, the new proposal grading system is more simple and intuitive in predicting a prognosis. In particular, a cutoff of 20% for high grade patterns as a key role for prognosis in invasive adenocarcinoma has been proposed.
Prognostic factor analysis is important in stage IA lung adenocarcinoma. Surgical resection is the most curative treatment option, and there is no need for adjuvant treatment in most cases. Furthermore, the number of patients with stage IA adenocarcinoma has been increasing. If one of the distinctive prognostic factor represents significantly different results within the same stage, adjuvant treatment or a revision of the staging system might be needed. There was no significant difference for DFI according to the TNM staging system in our study. However, there was a significant difference for DFI according to the new grading system.
A cutoff value has been widely used as a guideline. However, a cutoff value was not used in the classification of invasive lung adenocarcinoma because of the heterogeneity. A new grading system with a cutoff value for invasive lung adenocarcinoma has recently been proposed. 5 The results from this study are analogous to those from our study.
However, our study indicated that grade 3 was not a prognostic factor for OS by multivariate analysis. We speculated that this was because there were many noncancer‐related deaths in stage IA adenocarcinoma. Thirty‐two patients died during the F/U period, but 17 (53.1%) were not cancer related. Another reason was that there are many adjuvant treatment modalities for lung adenocarcinoma that increase survival.
There were several limitations in this study. First, it was a retrospective, nonrandomized design with a relatively small sample size for analyzing DFI and OS. There were many noncancer‐related deaths in stage IA adenocarcinoma. Furthermore, we were unable to determine whether grade 3 serves as a prognostic factor for OS. Further large‐scale studies will be needed to determine the prognostic role of the new grading system in stage IA adenocarcinoma. Moreover, this study was not from multiple centers; thus, selection bias may be inevitable.
In conclusion, grade 3 indicates a significant prognostic impact on DFI. This new grading system is simple and useful in the prediction of prognosis in stage IA lung adenocarcinoma.
CONFLICT OF INTEREST
No conflict of interest has been declared by the authors.
ACKNOWLEDGMENTS
This manuscript has been edited by native English‐speaking experts of American Journal Experts.
Jeon HW, Kim Y‐D, Sim SB, Moon MH. Significant difference in recurrence according to the proportion of high grade patterns in stage IA lung adenocarcinoma. Thorac Cancer. 2021;12:1952–1958. 10.1111/1759-7714.13984
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2. Goya T, Asamura H, Yoshimura H, Kato H, Shimokata K, Tsuchiya R, et al. Prognosis of 6644 resected non‐small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50:227–34. [DOI] [PubMed] [Google Scholar]
- 3. Tsao MS, Marguet S, Le Teuff G, Lantuejoul S, Shepherd FA, Seymour L, et al. Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection. J Clin Oncol. 2015;33:3439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moreira AL, Ocampo PSS, Xia Y, Zhong H, Russell PA, Minami Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the international association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15:1599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JK, Kim JJ, Moon SW, Lee KY. Lymph node involvement according to lung adenocarcinoma subtypes: lymph node involvement is influenced by lung adenocarcinoma subtypes. J Thorac Dis. 2017;9:3903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ujiie H, Kadota K, Chaft JE, Buitrago D, Sima CS, Lee MC, et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol. 2015;33:2877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yanagawa N, Shiono S, Abiko M, Katahira M, Osakabe M, Ogata SY. The clinical impact of solid and micropapillary patterns in resected lung adenocarcinoma. J Thorac Oncol. 2016;11:1976–83. [DOI] [PubMed] [Google Scholar]
- 9. Rami‐Porta R, Wittekind C, Goldstraw P, International Association for the Study of Lung Cancer (IASLC) staging committee . Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49:25–33. [DOI] [PubMed] [Google Scholar]
- 10. Kanitkar AA, Schwartz AG, George J, Soubani AO. Causes of death in long‐term survivors of non‐small cell lung cancer: a regional surveillance, epidemiology, and end results study. Ann Thorac Med. 2018;13:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J, Rosso S, Coebergh JWW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. [DOI] [PubMed] [Google Scholar]
- 12. Yu Y, Jian H, Shen L, Zhu L, Lu S. Lymph node involvement influenced by lung adenocarcinoma subtypes in tumor size ≤3 cm disease: a study of 2268 cases. Eur J Surg Oncol. 2016;42:1714–9. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura H, Saji H, Shinmyo T, Tagaya R, Kurimoto N, Koizumi H, et al. Close association of IASLC/ATS/ERS lung adenocarcinoma subtypes with glucose‐uptake in positron emission tomography. Lung Cancer. 2015;87:28–33. [DOI] [PubMed] [Google Scholar]
- 14. Mäkinen JM, Laitakari K, Johnson S, Mäkitaro R, Bloigu R, Pääkkö P, et al. Histological features of malignancy correlate with growth patterns and patient outcome in lung adenocarcinoma. Histopathology. 2017;71:425–36. [DOI] [PubMed] [Google Scholar]
- 15. Zhao ZR, Xi SY, Li W, Situ DR, Chen KM, Yang H, et al. Prognostic impact of pattern‐based grading system by the new IASLC/ATS/ERS classification in Asian patients with stage I lung adenocarcinoma. Lung Cancer. 2015;90:604–9. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Li J, Wang R, Li Y, Pan Y, Cai D, et al. The prognostic and predictive value of solid subtype in invasive lung adenocarcinoma. Sci Rep. 2014;24:7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Possidente L, Landriscina M, Patitucci G, Borgia L, Lalinga V, Vita G. ALK rearrangement in specific subtypes of lung adenocarcinoma: immunophenotype and morphological features. Med Oncol. 2017;34:76. [DOI] [PubMed] [Google Scholar]
- 18. Kadota K, Yeh YC, D'Angelo SP, Moreira AL, Kuk D, Sima CS, et al. Association between mutations and histologic patterns of mucin in lung adenocarcinoma: invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am J Surg Pathol. 2014;38:1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan ZK, Ye F, Wu X, An HX, Wu JX. Clinicopathological and prognostic significance of programmed cell death ligand 1 (PD‐L1) expression in patients with non‐small cell lung cancer: a meta‐analysis. J Thorac Dis. 2015;7:462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong ZY, Zhang C, Li YF, Su J, Xie Z, Liu SY, et al. Genetic and immune profiles of solid predominant lung adenocarcinoma reveal potential immunotherapeutic strategies. J Thorac Oncol. 2018;13:85–96. [DOI] [PubMed] [Google Scholar]
- 21. Chen T, Luo J, Gu H, Gu Y, Huang Q, Wang Y, et al. Impact of solid minor histologic subtype in postsurgical prognosis of stage I lung adenocarcinoma. Ann Thorac Surg. 2018;105:302–8. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Y, Wang R, Shen X, Pan Y, Cheng C, Li Y, et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma area predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol. 2016;23:2099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee G, Lee HY, Jeong JY, Han J, Cha MJ, Lee KS, et al. Clinical impact of minimal micropapillary pattern in invasive lung adenocarcinoma: prognostic significance and survival outcomes. Am J Surg Pathol. 2015;39:660–6. [DOI] [PubMed] [Google Scholar]


