Abstract
Background
It has recently been suggested that concomitant medication may affect the clinical outcome of patients treated with immune checkpoint inhibitors (ICIs). However, only a few studies on the impact of concomitant medication on immune‐related adverse events (irAEs) have previously been reported. Here, we aimed to determine the impact of concomitant medication on the efficacy and safety of ICIs.
Methods
We retrospectively analyzed the data of 300 patients treated with nivolumab or pembrolizumab for advanced non‐small cell lung cancer (NSCLC) between January 2016 and July 2018. Multivariate logistic regression analysis was used to assess the effect of concomitant medication on treatment response or irAEs. A multivariate Cox proportional hazards model was used to evaluate concomitant medication‐related factors associated with time‐to‐treatment failure or overall survival (OS).
Results
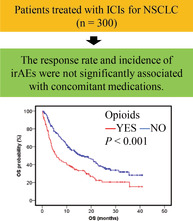
A total of 70 patients responded to treatment and 137 experienced irAEs. The response rate and incidence of irAEs in patients treated with ICIs were not significantly associated with concomitant medication. Multivariate analysis showed that the use of opioids was an independent factor (time‐to‐treatment failure: hazard ratio 1.39, p = 0.021, OS: hazard ratio 1.54, p = 0.007).
Conclusions
The efficacy and safety of nivolumab or pembrolizumab in the treatment of patients with advanced NSCLC were not significantly influenced by concomitant medication. However, opioid usage might be associated with shorter OS in patients treated with these ICIs. Further mechanistic investigations should explore whether these associations are purely prognostic or contribute to ICI resistance.
Keywords: concomitant medication, immune checkpoint inhibitors, immune‐related adverse events, non‐small cell lung cancer, opioids
Concomitant medication had no significant effect on the efficacy and safety of immune checkpoint inhibitors. Opioid use was associated with shorter overall survival and further study of this possible effect is required. This study is the first to demonstrate the impact of angiotensin receptor blockers or vitamin D on ICI treatment in patients with non‐small cell lung cancer.
INTRODUCTION
Lung cancer has the highest mortality rate among all cancer types worldwide. 1 Treatment for advanced non‐small cell lung cancer (NSCLC) is selected based on the expression of the driver oncogene, programmed death‐ligand 1 (PD‐L1), and other factors. 2 Immune checkpoint inhibitors (ICIs), such as nivolumab, pembrolizumab, and atezolizumab, which block the programmed cell death protein 1 (PD‐1)/PD‐L1 pathway in the immune system, have been approved for use in patients with advanced NSCLC. ICIs restore the antitumor activity of T cells and have received attention as an alternative treatment strategy to chemotherapy. However, only about 20% of patients respond to ICI monotherapy. 3 The efficacy of ICIs has been reported to be influenced by various factors, such as PD‐L1 expression, history of smoking, Eastern Cooperative Oncology Group Performance Status (ECOG PS), 4 and radiotherapy. 5 , 6 , 7 Furthermore, it has been reported that concomitant medication may affect the efficacy of ICIs. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17
In this regard, immunomodulatory effects have been reported for medications that are used in the treatment of common diseases, such as hyperlipidemia, diabetes, and hypertension. 10 The concurrent use of statins has been associated with an improved response and longer time‐to‐treatment failure (TTF) in patients treated with nivolumab for advanced NSCLC. 11 In patients treated with ICIs for metastatic malignant melanoma and advanced NSCLC, progression‐free survival (PFS) and overall survival (OS) tend to be longer in those who used metformin concomitantly, without an increase in adverse events. 16 , 17 In vivo, fibrates, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, and angiotensin receptor blockers (ARBs) have been shown to have a synergistic influence on the antitumor effect of ICIs. 18 , 19 , 20 In addition, the activities of the gut microbiota have been linked to the efficacy of ICIs. 21 It has been reported that the concomitant use of medications such as antibiotics and proton pump inhibitors (PPIs), may influence the outcome of treatment with ICIs, by affecting gut microbiota. 12
Regarding drug safety, it is known that immune‐related adverse events (irAEs) mimicking autoimmune disorders, are caused by treatment with ICIs. 22 Moreover, irAEs result from the activation of the immune system outside of the tumor microenvironment, which can occur in any organ. 23 It has also been reported that the occurrence of irAEs during treatment with ICIs is associated with a high therapeutic effect. 24 However, only the impact of the concomitant use of metformin on the occurrence of irAEs has been previously studied. 16 , 17 The purpose of this study was to clarify the impact of concomitant medication on the clinical outcomes of ICI treated patients with NSCLC.
METHODS
Patient data collection
We retrospectively collected the data of 304 patients treated with nivolumab or pembrolizumab for advanced NSCLC at the National Cancer Center Hospital East, between January 2016 and July 2018, from their medical records. The study protocol was approved by the Ethics Committee of the National Cancer Center (Approval No. 2018‐348). The ethics committee waived the requirement for informed consent due to the retrospective study design. As this study was a retrospective analysis of de‐identified data, written informed consent was not required. The following clinical factors were examined at the beginning of nivolumab or pembrolizumab therapy: age, sex, ECOG PS, histological types, epidermal growth factor receptor mutation (EGFR), PD‐L1 expression, line of chemotherapy, smoking status, history of radiation, and use of concomitant medications. The concomitant medications investigated included statins, fibrates, DPP‐4 inhibitors, metformin, ARBs, corticosteroids, antibiotics, probiotics, PPIs, nonsteroidal anti‐inflammatory drugs (NSAIDs), opioids, laxatives, and vitamin D. Medications used only as required, for example, painkillers and laxatives, were not included. Clinical follow‐up including physical examination, chest radiography, and routine laboratory tests were performed at least every four weeks. Computed tomography was performed at regular intervals according to local standards. The overall response was determined as stated by the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. 25 In this study, irAEs were identified as adverse events associated with nivolumab or pembrolizumab use and were graded based on the American Society of Clinical Practice guidelines 24 or the Common Terminology Criteria for Adverse Events v4.0. 26
Statistical analysis
To evaluate the efficacy of nivolumab or pembrolizumab, patients were divided into responders and nonresponders. Responders were defined as patients who achieved a complete response (CR) or partial response (PR), as stated by the RECIST v1.1. 25 Comparison of categorical variables between responders and nonresponders was performed using the chi‐squared or Fisher's exact test, where appropriate. Categorical variables between the groups with irAEs and without irAEs were compared using the chi‐squared or Fisher's exact test, where appropriate. Multivariate logistic regression analysis was used to assess the factors affecting response or the occurrence of irAEs. Factors included in the multivariate analysis were those with p‐values <0.2 in the univariate analysis.
TTF was defined as the period from the date of nivolumab or pembrolizumab treatment initiation to the date of treatment discontinuation because of disease progression, death, or severe adverse events. OS was defined as the period from the date of nivolumab or pembrolizumab treatment initiation to the date of patient's death, irrespective of the cause. TTF and OS were censored on the day of data cutoff (i.e., May 31, 2019), and were estimated using Kaplan–Meier curves with a two‐sided log‐rank test. A multivariate Cox proportional hazards model was used to evaluate factors with p‐values <0.2.
Two‐sided p‐values <0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences v22.0 (IBM Corp.).
RESULTS
Patient characteristics
In total, 304 patients were treated with nivolumab (3 mg/kg each cycle) or pembrolizumab (200 mg/bodyweight each cycle) for advanced NSCLC. Four patients with incomplete clinical data were excluded, and the data of the remaining 300 patients were analyzed. The patient characteristics are summarized in Table 1. Among these patients, 189 (63.0%) had adenocarcinoma, 54 (18.0%) had ECOG PS ≥2, and 250 (83.3%) were current or former smokers. The PD‐L1 expression status of 163 patients (54.3%) was unknown. The median line of chemotherapy was 2 (range: 1–11 lines). Medications used concomitantly at the beginning of nivolumab or pembrolizumab therapy are detailed in Table 1 and Table S1. In all, 254 patients (84.7%) used concomitant medications. The most frequently used concomitant medications included PPIs in 163 patients (54.3%), NSAIDs in 140 patients (46.7%), opioids in 114 patients (38.0%), and laxatives in 101 patients (33.7%).
TABLE 1.
Patient characteristics and concomitant medications
| Factors | n = 300 | % |
|---|---|---|
| Age, years (median [range]) | 65 (31–82) | |
| Sex | ||
| Male | 226 | 75.3 |
| Female | 74 | 24.7 |
| ECOG PS | ||
| 0/1 | 65/181 | 82.0 |
| 2/3/4 | 51/2/1 | 18.0 |
| Histological types | ||
| Adenocarcinoma | 189 | 63.0 |
| Squamous cell carcinoma | 74 | 24.7 |
| Others | 37 | 12.3 |
| EGFR mutation | ||
| Mutant | 36 | 12.0 |
| PD‐L1 expression | ||
| ≧50% | 67 | 22.3 |
| 1–49% | 47 | 15.7 |
| <1% | 23 | 7.7 |
| Unknown | 163 | 54.3 |
| PD‐L1 monotherapy | ||
| Nivolumab | 203 | 67.7 |
| Pembrolizumab | 97 | 32.3 |
| Lines of chemotherapy | 2 (1–11) | |
| 1 | 40 | 13.3 |
| 2 | 122 | 40.7 |
| 3/4/5/6/7/9/11 | 64/35/27/7/3/1/1 | 46.0 |
| Smoking status | ||
| Current or former | 250 | 83.3 |
| Never | 50 | 16.7 |
| History of radiotherapy | ||
| Yes | 156 | 52.0 |
| Concomitant medications | n | % |
| Statins | 26 | 8.7 |
| Fibrates | 3 | 1.0 |
| DPP‐4 inhibitors | 22 | 7.3 |
| Metformin | 8 | 2.7 |
| ARBs | 40 | 13.3 |
| Corticosteroids | 12 | 4.0 |
| Antibiotics | 14 | 4.7 |
| Probiotics | 14 | 4.7 |
| NSAIDs | 140 | 46.7 |
| PPIs | 163 | 54.3 |
| Opioids | 114 | 38.0 |
| Laxatives | 101 | 33.7 |
| Vitamin D | 58 | 19.3 |
Abbreviations: ARBs, angiotensin receptor blockers; DPP‐4, dipeptidyl peptidase‐4; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; NSAIDs, nonsteroidal anti‐inflammatory drugs; PD‐L1, programmed death‐ligand 1; PPIs, proton pump inhibitors.
Efficacy
Of the 300 patients, five achieved CR, 65 achieved PR, 65 achieved stable disease (SD), and 119 developed progressive disease (PD) according to RECIST v1.1. 25 Response was not evaluated in 46 patients due to early death or failure to follow‐up. The overall response rate of patients treated with nivolumab or pembrolizumab was 23.3% (95% confidence interval [CI]: 18.9–28.4%). In the univariate analysis, there was a significant difference in the response rate with respect to the use of laxatives (odds ratio [OR] 0.51; 95% CI: 0.27–0.94, p = 0.029). Nonsquamous histology (OR 3.17; 95% CI: 1.43–7.05, p = 0.005) and first‐ or second‐lines of chemotherapy (first‐line OR 3.91; 95% CI: 1.71–8.94, p = 0.001, second‐line OR 1.97; 95% CI: 1.03–3.77, p = 0.040) were independently associated with better responses (Table 2).
TABLE 2.
Univariate and multivariate analyses of variable factors of response
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | RR (%) | OR | 95% CI | p‐value | OR | 95% CI | p‐value | |
| Age, years | ||||||||
| ≧70 | 21.8 | 0.76 | 0.43–1.34 | 0.334 | ||||
| <70 | 27.0 | |||||||
| Sex | ||||||||
| Male | 23.9 | |||||||
| Female | 21.6 | 0.88 | 0.47–1.65 | 0.688 | ||||
| ECOG PS | ||||||||
| 0 or 1 | 24.0 | 1.23 | 0.60–2.54 | 0.570 | ||||
| 2, 3, or 4 | 20.4 | |||||||
| Histology | ||||||||
| Squamous | 12.2 | 0.38 | 0.18–0.80 | 0.009 | 3.17 | 1.43–7.05 | 0.005 | |
| Nonsquamous | 27.0 | |||||||
| EGFR mutation | ||||||||
| Yes | 13.9 | 0.49 | 0.18–1.32 | 0.153 | 2.08 | 0.74–5.90 | 0.167 | |
| No | 24.6 | |||||||
| Line of chemotherapy | ||||||||
| 1 | 45.0 | 0.001 | 3.91 | 1.71–8.94 | 0.001 | |||
| 2 | 24.6 | 1.97 | 1.03–3.77 | 0.040 | ||||
| ≧3 | 15.9 | |||||||
| Smoking status | ||||||||
| Current or former | 23.6 | 1.10 | 0.53–2.27 | 0.807 | ||||
| Never | 22.0 | |||||||
| History of radiotherapy | ||||||||
| Yes | 20.5 | 0.72 | 0.42–1.23 | 0.229 | ||||
| No | 26.4 | |||||||
| Use of statins | ||||||||
| Yes | 11.5 | 0.40 | 0.12–1.39 | 0.137 | 3.00 | 0.82–10.92 | 0.096 | |
| No | 24.5 | |||||||
| Use of fibrates | ||||||||
| Yes | 33.3 | 1.65 | 0.15–18.5 | 0.551 a | ||||
| No | 23.2 | |||||||
| Use of DPP‐4 | ||||||||
| Yes | 31.8 | 1.59 | 0.62–4.08 | 0.328 | ||||
| No | 22.7 | |||||||
| Use of metformin | ||||||||
| Yes | 37.5 | 2.02 | 0.47–8.65 | 0.395 a | ||||
| No | 22.9 | |||||||
| Use of ARBs | ||||||||
| Yes | 25.0 | 1.11 | 0.51–2.40 | 0.789 | ||||
| No | 23.1 | |||||||
| Use of corticosteroids | ||||||||
| Yes | 16.7 | 0.65 | 0.14–3.03 | 0.739 a | ||||
| No | 23.6 | |||||||
| Use of antibiotics | ||||||||
| Yes | 21.4 | 0.89 | 0.24–3.29 | 1.000 a | ||||
| No | 23.4 | |||||||
| Use of probiotics | ||||||||
| Yes | 21.4 | 0.89 | 0.24–3.29 | 1.000 a | ||||
| No | 23.4 | |||||||
| Use of NSAIDs | ||||||||
| Yes | 18.6 | 0.60 | 0.35–1.04 | 0.068 | 1.59 | 0.82–10.92 | 0.172 | |
| No | 27.5 | |||||||
| Use of PPIs | ||||||||
| Yes | 19.6 | 0.64 | 0.37–1.09 | 0.098 | 0.97 | 0.50–1.88 | 0.936 | |
| No | 27.7 | |||||||
| Use of opioids | ||||||||
| Yes | 17.5 | 0.58 | 0.32–1.04 | 0.063 | 1.62 | 0.83–3.17 | 0.162 | |
| No | 26.9 | |||||||
| Use of laxatives | ||||||||
| Yes | 15.8 | 0.51 | 0.27–0.94 | 0.029 | 1.29 | 0.63–2.64 | 0.482 | |
| No | 27.1 | |||||||
| Use of vitamin D | ||||||||
| Yes | 17.2 | 0.63 | 0.30–1.33 | 0.222 | ||||
| No | 24.8 | |||||||
Abbreviations: ARBs, angiotensin receptor blockers; CI, confidence interval; DPP‐4, dipeptidyl peptidase‐4; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; NSAIDs, nonsteroidal anti‐inflammatory drugs; OR, odds ratio; PPIs, proton pump inhibitors; RR, response rate.
Fisher's exact test.
The median TTF and OS in all patients treated with nivolumab or pembrolizumab were 2.5 months (95% CI: 1.8–3.2) and 11.7 months (95% CI: 9.0–14.4), respectively. The TTF and OS, with respect to the use of concomitant medications, are presented in Figures 1 and 2. The median OS was shorter in patients who used NSAIDs (8.8 vs. 15.9 months, p = 0.014). The median TTF and OS were shorter in patients who used PPIs (1.9 vs. 3.5 months, p = 0.018; 7.9 vs. 19.6 months, p < 0.001, respectively). The median TTF and OS were shorter in patients who used opioids (1.8 vs. 3.5 months, p = 0.003; 5.7 vs. 15.9 months, p < 0.001, respectively). The median TTF and OS were shorter in patients who used laxatives (1.6 vs. 3.2 months, p = 0.023; 5.6 vs. 17.3 months, p = 0.001, respectively). Multivariate analysis for TTF showed that higher ECOG PS (hazard ratio [HR] 0.67; 95% CI: 0.46–0.96, p = 0.027), later lines of chemotherapy (first line HR 0.65; 95% CI: 0.42–0.99, p = 0.043), and opioid use (HR 1.39; 95% CI: 1.05–1.85, p = 0.021) were independently associated with shorter TTF. Multivariate analysis for OS showed that higher ECOG PS (HR 0.51; 95% CI: 0.36–0.74, p < 0.001), squamous histology (HR 1.52; 95% CI: 1.10–2.10, p = 0.012), and the use of opioids (HR 1.54; 95% CI: 1.12–2.11, p = 0.007) were independently associated with shorter OS (Table 3).
FIGURE 1.
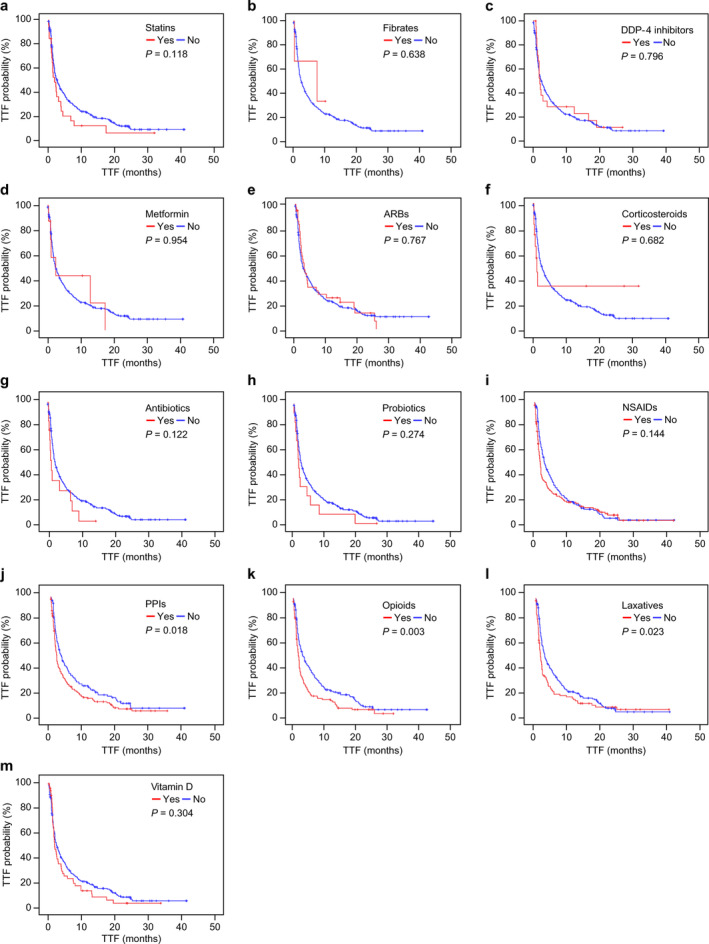
Kaplan–Meier curves of time‐to‐treatment failure (TTF) with or without concomitant medication. TTF was not significantly different with (red lines) or without (blue lines) each concomitant medication. (a) TTF with or without statins (median TTF: 1.9 vs. 2.8 months, log‐rank p = 0.118); (b) TTF with or without fibrates (median TTF: 7.5 vs. 2.5 months, log‐rank p = 0.638); (c) TTF with or without dipeptidyl peptidase‐4 (DPP‐4) inhibitors (median TTF: 2.1 vs. 2.6 months, log‐rank p = 0.796); (d) TTF with or without metformin (median TTF: 2.5 vs. 2.5 months, log‐rank p = 0.954); (e) TTF with or without angiotensin receptor blockers (ARBs) (median TTF: 2.8 vs. 2.4 months, log‐rank p = 0.767); (f) TTF with or without corticosteroids (median TTF: 1.2 vs. 2.6 months, log‐rank p = 0.682); (g) TTF with or without antibiotics (median TTF: 1.2 vs. 2.6 months, log‐rank p = 0.122); (h) TTF with or without probiotics (median TTF: 1.5 vs. 2.6 months, log‐rank p = 0.274); (i) TTF with or without nonsteroidal anti‐inflammatory drugs (NSAIDs) (median TTF: 1.8 vs. 3.3 months, log‐rank p = 0.144); (j) TTF with or without proton pump inhibitors (PPIs) (median TTF: 1.9 vs. 3.5 months, log‐rank p = 0.018); (k) TTF with or without opioids (median TTF: 1.8 vs. 3.5 months, log‐rank p = 0.003); (l) TTF with or without laxatives (median TTF: 1.6 vs. 3.2 months, log‐rank p = 0.023); (m) TTF with or without vitamin D (median TTF: 1.9 vs. 2.8 months, log‐rank p = 0.304)
FIGURE 2.
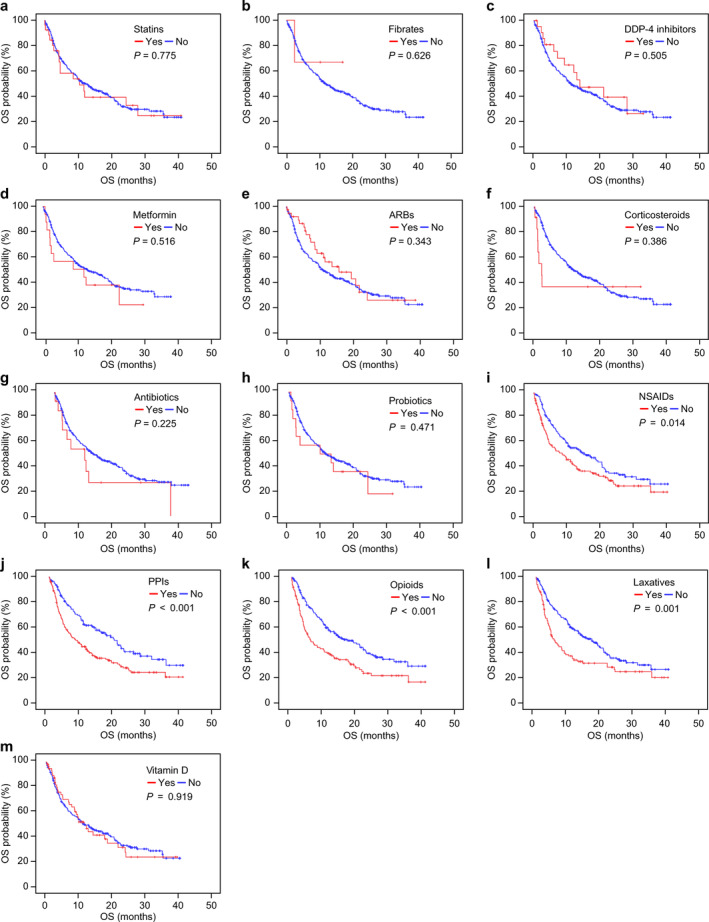
Kaplan–Meier curves of overall survival (OS) with or without concomitant medication. OS was not significantly different with (red lines) or without (blue lines) each concomitant medication. (a) OS with or without statins (median OS: 10.5 vs. 11.7 months, log‐rank p = 0.775); (b) OS with or without fibrates (median OS: No data vs. 11.7 months, log‐rank p = 0.626); (c) OS with or without dipeptidyl peptidase‐4 (DPP‐4) inhibitors (median OS: 13.8 vs. 11.3 months, log‐rank p = 0.505); (d) OS with or without metformin (median OS: 12.9 vs. 11.7 months, log‐rank p = 0.516); (e) OS with or without angiotensin receptor blockers (ARBs) (median OS: 15.9 vs. 10.9 months, log‐rank p = 0.343); (f) OS with or without corticosteroids (median OS: 2.3 vs. 11.8 months, log‐rank p = 0.386); (g) OS with or without antibiotics (median OS: 9.3 vs. 12.0 months, log‐rank p = 0.225); (h) OS with or without probiotics (median OS: 9.7 vs. 11.7 months, log‐rank p = 0.471); (i) OS with or without nonsteroidal anti‐inflammatory drugs (NSAIDs) (median OS: 8.8 vs. 15.9 months, log‐rank p = 0.014); (j) OS with or without proton pump inhibitors (PPIs) (median OS: 7.9 vs. 19.6 months, log‐rank p < 0.001); (k) OS with or without opioids (median OS: 5.7 vs. 15.9 months, log‐rank p < 0.001); (l) OS with or without laxatives (median OS: 5.6 vs. 17.3 months, log‐rank p = 0.001); (m) OS with or without vitamin D (median OS: 11.7 vs. 11.3 months, log‐rank p = 0.919)
TABLE 3.
Multivariate Cox proportional hazards model for time‐to‐treatment failure (TTF) and overall survival (OS)
| TTF | OS | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | p‐value | HR | 95% CI | p‐value |
| ECOG PS | ||||||
| 0 or 1 | 0.67 | 0.46–0.96 | 0.027 | 0.51 | 0.36–0.74 | <0.001 |
| 2, 3 or 4 | ||||||
| Histology | ||||||
| Squamous | 1.52 | 1.10–2.10 | 0.012 | |||
| Nonsquamous | ||||||
| EGFR mutation | ||||||
| Yes | ||||||
| No | 1.20 | 0.80–1.78 | 0.376 | |||
| Line of chemotherapy | ||||||
| 1 | 0.65 | 0.42–0.99 | 0.043 | 0.68 | 0.41–1.12 | 0.129 |
| 2 | 0.77 | 0.58–1.02 | 0.066 | 0.77 | 0.56–1.05 | 0.092 |
| ≧3 | ||||||
| Use of statins | ||||||
| Yes | 1.53 | 0.99–2.37 | 0.057 | |||
| No | ||||||
| Use of antibiotics | ||||||
| Yes | 1.47 | 0.80–2.70 | 0.210 | |||
| No | ||||||
| Use of NSAIDs | ||||||
| Yes | 1.01 | 0.76–1.35 | 0.930 | 1.08 | 0.78–1.50 | 0.627 |
| No | ||||||
| Use of PPIs | ||||||
| Yes | 1.17 | 0.87–1.57 | 0.299 | 1.36 | 0.96–1.91 | 0.081 |
| No | ||||||
| Use of opioids | ||||||
| Yes | 1.39 | 1.05–1.85 | 0.021 | 1.54 | 1.12–2.11 | 0.007 |
| No | ||||||
| Use of laxatives | ||||||
| Yes | 1.01 | 0.75–1.36 | 0.961 | 1.14 | 0.82–1.58 | 0.450 |
| No | ||||||
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; HR, hazard ratio; NSAIDs, nonsteroidal anti‐inflammatory drugs; PPIs, proton pump inhibitors.
Safety
The proportion of patients who experienced irAEs was 45.7% (n = 137), among whom 32 (10.7%) had two or more irAEs. The most frequent irAEs were skin toxicities (n = 63), including rash, pruritus, and erythema multiforme, pneumonitis (n = 31), and thyroid dysfunction (n = 27). A total of 38 patients (12.7%) experienced irAEs of grade 3 or higher (Table 4). In the univariate analysis, there was no significant difference in the incidence of irAEs with respect to the use of concomitant medications. All three patients who used fibrates experienced irAEs, but the OR could not be calculated. Therefore, the p‐value for the use of fibrates was <0.2, but it was not included in the multivariate analysis. In the multivariate analysis, only ECOG PS was an independent factor (OR 0.31; 95% CI: 0.16–0.62, p = 0.001) (Table 5).
TABLE 4.
Immune‐related adverse event (irAE)‐types in patients treated with nivolumab or pembrolizumab
| irAEs (n = 137) | All grade n | ≧grade 3 n |
|---|---|---|
| Pneumonitis | 31 | 13 |
| Colitis (including diarrhea) | 20 | 7 |
| Skin toxicities | 63 | 6 |
| Thyroid dysfunction | 27 | 0 |
| Adrenal insufficiency/hypophysitis | 10 | 8 |
| Diabetes | 3 | 3 |
| Musculoskeletal (e.g., myalgia and arthralgia) | 4 | 0 |
| Hepatitis | 5 | 1 |
| Renal toxicities | 1 | 0 |
| Other | 15 | 2 |
TABLE 5.
Univariate and multivariate analyses of variable factors of immune‐related adverse events (irAEs)
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | irAE (%) | OR | 95% CI | p‐value | OR | 95% CI | p‐value |
| Age, years | |||||||
| ≧70 | 51.7 | ||||||
| <70 | 43.1 | 0.71 | 0.43–1.17 | 0.174 | 1.42 | 0.85–2.39 | 0.184 |
| Sex | |||||||
| Male | 49.1 | ||||||
| Female | 35.1 | 0.56 | 0.33–0.97 | 0.036 | 1.70 | 0.83–3.47 | 0.148 |
| ECOG PS | |||||||
| 0 or 1 | 50.4 | 3.21 | 1.64–6.28 | <0.001 | 0.31 | 0.16–0.62 | 0.001 |
| 2, 3, or 4 | 24.1 | ||||||
| Histology | |||||||
| Squamous | 43.2 | 0.88 | 0.52–1.49 | 0.630 | |||
| Nonsquamous | 46.5 | ||||||
| EGFR mutation | |||||||
| Yes | 41.7 | 0.83 | 0.41–1.68 | 0.608 | |||
| No | 46.2 | ||||||
| Line of chemotherapy | |||||||
| 1 | 50.0 | ||||||
| 2 | 48.4 | ||||||
| ≧3 | 42.0 | 0.498 | |||||
| Smoking status | |||||||
| Current or former | 47.6 | 1.62 | 0.86–3.03 | 0.133 | 0.98 | 0.43–2.26 | 0.967 |
| Never | 36.0 | ||||||
| History of radiotherapy | |||||||
| Yes | 47.4 | 1.16 | 0.74–1.83 | 0.522 | |||
| No | 43.8 | ||||||
| Use of statins | |||||||
| Yes | 46.2 | 1.02 | 0.46–2.29 | 0.958 | |||
| No | 45.6 | ||||||
| Use of fibrates | |||||||
| Yes | 100 | 0.094 a | |||||
| No | 45.1 | ||||||
| Use of DPP‐4 | |||||||
| Yes | 40.9 | 0.81 | 0.34–1.96 | 0.642 | |||
| No | 46.0 | ||||||
| Use of metformin | |||||||
| Yes | 62.5 | 2.02 | 0.47–8.61 | 0.476 a | |||
| No | 45.2 | ||||||
| Use of ARBs | |||||||
| Yes | 42.5 | 0.86 | 0.44–1.69 | 0.666 | |||
| No | 46.2 | ||||||
| Use of corticosteroids | |||||||
| Yes | 33.3 | 0.58 | 0.17–1.98 | 0.381 | |||
| No | 46.2 | ||||||
| Use of antibiotics | |||||||
| Yes | 50.0 | 1.20 | 0.41–3.51 | 0.739 | |||
| No | 45.5 | ||||||
| Use of probiotics | |||||||
| Yes | 28.6 | 0.46 | 0.14–1.50 | 0.188 | 2.05 | 0.61–6.88 | 0.244 |
| No | 46.5 | ||||||
| Use of NSAIDs | |||||||
| Yes | 45.0 | 0.95 | 0.60–1.50 | 0.828 | |||
| No | 46.3 | ||||||
| Use of PPIs | |||||||
| Yes | 42.9 | 0.79 | 0.50–1.24 | 0.302 | |||
| No | 48.9 | ||||||
| Use of opioids | |||||||
| Yes | 42.1 | 0.79 | 0.50–1.27 | 0.332 | |||
| No | 47.8 | ||||||
| Use of laxatives | |||||||
| Yes | 47.5 | 1.12 | 0.69–1.81 | 0.645 | |||
| No | 44.7 | ||||||
| Use of vitamin D | |||||||
| Yes | 44.8 | 0.96 | 0.54–1.71 | 0.886 | |||
| No | 45.9 | ||||||
Abbreviations: ARBs, angiotensin receptor blockers; CI, confidence interval; DPP‐4, dipeptidyl peptidase‐4; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; NSAIDs, nonsteroidal anti‐inflammatory drugs; OR, odds ratio; PPIs, proton pump inhibitors.
Fisher's exact test.
DISCUSSION
In the present study, the impact of concomitant medication use on the efficacy and safety of immune checkpoint inhibitors was evaluated. The overall response rate, median TTF, and OS found in this study were consistent with those reported in previous studies. 3 , 6 , 7 , 11 , 15 , 27 In our study, patients with NSCLC of nonsquamous histology had a significantly higher response rate than those with NSCLC of squamous histology. The overall response rate for the first line was significantly higher than that for second or later lines. These results are consistent with the findings of previous studies. 28 , 29 In our study, the TTF and OS of patients treated with ICIs as the first or second line tended to be better than those treated with ICIs as the third line or higher, which is in agreement with a previous report. 29 Consistent with existing reports, nonsquamous histology was independently associated with better OS than squamous histology. 5 ECOG PS was independently associated with the shortening of TTF or OS, which is consistent with existing reports. 5 , 6 , 30
The proportion of patients who experienced irAEs in the present study was similar to that reported in a previous study. 24 However, in contrast to previous studies, the incidence of irAEs was significantly lower in patients with an ECOG PS of 2 or higher. 31 , 32 This may be explained by the fact that the median TTF (1.0 month) (95% CI: 0.8–1.2) was significantly shorter in patients with an ECOG PS of 2 or higher. It has been previously reported that there was no difference in the incidence of irAEs with or without metformin intake, 16 , 17 and our study revealed that use of other concomitant medications had no significant effect on irAEs. The expression of IFN‐γ mRNA was previously shown to be upregulated in vivo following bezafibrate administration plus PD‐1 blockage 33 ; thus, the incidence of irAEs might increase with fibrate use. In our study, all three patients who used fibrates experienced irAEs, but a larger sample size should be used in the future to examine the relationship between the intake of fibrates and irAEs more thoroughly.
To our knowledge, this is the first study to demonstrate no association between ARBs or vitamin D and the efficacy and safety of nivolumab or pembrolizumab in patients with NSCLC. Notably, for the first time, we found that there was no significant difference in the incidence of irAEs in patients treated with nivolumab or pembrolizumab for NSCLC with or without the use of concomitant medications, excluding metformin. However, our study had several limitations; this was a retrospective study conducted at a single center. Patient adherence and the duration of use of medications with immunomodulatory effects, as well as patient history of noncancerous diseases, were unknown. A small number of patients were treated with concomitant medications. Other factors that may affect the efficacy of ICIs, such as site of metastasis 34 and PD‐L1 expression, 35 were not considered. In addition, irAEs were not evaluated based on whether steroids were needed.
Although the use of opioids was an independent factor for TTF and OS, data from this retrospective analysis indicate that the clinical outcomes of patients treated with nivolumab or pembrolizumab for NSCLC were not significantly different with or without concomitant medication. The results obtained for each concomitant medication are discussed below.
Statin use was found to be associated with better responses or longer TTF in patients treated with nivolumab for advanced NSCLC. 11 In this study, the use of statins was limited to 10 cases. However, in another study in which statins were used concomitantly in 13.8% or 26.5% of patients, there were no significant differences in response, PFS, and OS. 15 , 36 It has been reported that the depletion of membrane cholesterol may lead to immunosuppression 37 ; however, in other studies including this study, cholesterol levels were unknown. In the future, the influence of statins and cholesterol status on the efficacy of ICIs in a larger number of patients, should be evaluated.
As in this study, the impact of metformin on the efficacy of ICIs has only been assessed in relatively small groups of patients. 15 , 16 , 17 , 36 The results of clinical trials currently in progress, which consider the concomitant use of metformin and nivolumab, are expected in the future. 38 , 39
Although the number of patients who used fibrates in this study was small, fibrate use had no significant effect on the efficacy of ICIs, as previously reported. 11 , 15
Similar to the findings of a previous study, 11 there was no significant difference in the efficacy of ICIs with or without concomitant use of DPP‐4 inhibitors. The in vivo synergistic effects of DPP‐4 inhibitors were observed previously in combination with cytotoxic T‐lymphocyte‐associated protein 4 inhibitors, but not with PD‐1 inhibitors. 19 Therefore, the impact of DPP‐4 inhibitors may be different in patients treated with PD‐L1 inhibitors.
ARBs previously showed in vivo synergistic effects with PD‐L1 antibody treatment by reducing the production of various immunosuppressive cytokines. 20 However, in this study, it was clarified for the first time that the use of ARBs does not affect the efficacy of ICIs in patients with NSCLC. The impact of ARBs on the tumor microenvironment in mouse colorectal cancer models has been previously reported. 20 , 40 The impact of ARBs on the immune response may differ depending on the cancer type. Furthermore, the ARB used in mouse models was valsartan at a dose of 15 mg/kg, 20 which is higher than the normal dose in humans. This difference in dose may have affected the results of the mouse study.
Use of corticosteroids at a dose ≥10 mg prednisone‐equivalent was previously associated with poorer outcomes in patients treated with PD‐1 inhibitors for NSCLC. 9 , 36 The impact of corticosteroids on the efficacy of ICIs was probably not observed in our study because the corticosteroid dose used was less than 10 mg prednisone‐equivalent in most patients.
The overall response was significantly different in patients who had used antibiotics within 30 days prior to the start of treatment with ICIs, than in patients who had used antibiotics concurrently with ICIs, as previously reported. 8 , 36 In contrast, PFS and OS were previously lower in patients with higher ratios of “days under antibiotics/days under ICIs”. 13 The duration of antibiotic use may have influenced the results of our study.
Although the number of patients who used probiotics in this study was small, as previously reported, probiotic use had no significant effect on the efficacy of ICIs. 15
The overall response and OS in patients treated with ICIs for NSCLC were not significantly different from those obtained in a previous study. 15 However, unlike in our study, the PFS in patients who used NSAIDs one month before and after starting treatment with nivolumab was significantly longer in the multivariate analysis of the previous study. 15 These contradictory findings may be due to the fact that NSAIDs were only used in our study during ICI initiation. In addition, the use of opioids was not considered in the previous study. A total of 70 patients (50.0%) concomitantly used NSAIDs and opioids, and this might have led to bias in the results of our study.
In previous studies, the OS of patients who used PPIs was significantly shorter in the ICI group in the chemotherapy group. 12 , 36 A total of 79 patients (48.5%) used PPIs concomitantly with opioids, and this might have led to bias in the results of our study, as opioid use was not considered in previous studies. The effects of PPIs on the biological processes of the gut microbiota as well as on the efficacy of ICIs should be clarified through further research.
Opioids affect immune cells via direct interactions with immune cells expressing opioid receptors or via indirect immunosuppressive effects, for example, through the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis. 41 In this study, the overall response was not significantly different with or without the use of opioids, but TTF and OS were significantly shortened in patients who had used opioids, as previously reported. 13 Opioids are used for the treatment of pain associated with disease progression, and as such, the shortened TTF or OS of patients using opioids may reflect this progression. Further research is needed to determine whether use of opioids is purely a poor prognostic factor or a factor that directly affects the efficacy of ICIs.
Disease control rates have been reported to be previously lower in NSCLC patients with stool abnormalities than in those without stool abnormalities. 27 In future, the effects of laxatives as well as bowel movements on ICI treatment outcomes should be examined. In addition, 65 patients (64.4%) used laxatives concomitantly with opioids, which was an independent factor for TTF and OS in our study.
Vitamin D deficiency has been associated with poor survival in melanoma patients. The impact of vitamin D supplementation in clinical trials remains controversial. Recently, it was suggested that vitamin D works synergistically with ICIs due to its immunomodulatory effects and associated upregulation of PD‐L1 expression. 14 In our study, for the first time, the efficacy of ICIs in patients with NSCLC was found not to be significantly different with or without the use of vitamin D. However, vitamin D plasma levels were not measured in our study. Therefore, in future, the impact of vitamin D on ICI treatment as well as vitamin D plasma levels should be examined.
In conclusion, in this study, the efficacy and safety of nivolumab or pembrolizumab in the treatment of advanced NSCLC were not significantly different with or without concomitant medication. Our results suggest that use of opioids might be associated with shorter OS in patients treated with ICIs. Whether use of opioids is purely prognostic or contributes to resistance to ICIs remains unclear. The impact of concomitant medications on the clinical outcomes of ICI treatment should be clarified through further studies, by elucidating the underlying biological mechanisms.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Table S1 Concomitant medications
ACKNOWLEDGMENTS
The authors thank all patients and coinvestigators for supporting this study, the National Cancer Center for statistical advice, and Editage (www.editage.com) for English language editing.
Miura K, Sano Y, Niho S, et al. Impact of concomitant medication on clinical outcomes in patients with advanced non‐small cell lung cancer treated with immune checkpoint inhibitors: A retrospective study. Thorac Cancer. 2021;12:1983–1994. 10.1111/1759-7714.14001
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2. Akamatsu H, Ninomiya K, Kenmotsu H, Daga H, Goto Y, Kozuki T, et al. The Japanese Lung Cancer Society Guideline for non‐small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD‐1 and PD‐L1 in cancer treatment. Semin Oncol. 2015;42:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, ET MF, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 5. Califano R, Lal R, Lewanski C, Nicolson MC, Ottensmeier CH, Popat S, et al. Patient selection for anti‐PD‐1/PD‐L1 therapy in advanced non‐small cell lung cancer: implications for clinical practice. Future Oncol. 2018;14:2415–31. [DOI] [PubMed] [Google Scholar]
- 6. Garde‐Noguera J, Martin‐Martorell P, De Julián M, Perez‐Altozano J, Salvador‐Coloma C, García‐Sanchez J, et al. Predictive and prognostic clinical and pathological factors of nivolumab efficacy in non‐small‐cell lung cancer patients. Clin Transl Oncol. 2018;20:1072–9. [DOI] [PubMed] [Google Scholar]
- 7. Yamaguchi O, Kaira K, Hashimoto K, Mouri A, Miura Y, Shiono A, et al. Radiotherapy is an independent prognostic marker of favorable prognosis in non‐small cell lung cancer patients after treatment with the immune checkpoint inhibitor, nivolumab. Thorac Cancer. 2019;10:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non‐small cell lung cancer. J Clin Oncol. 2018;36:2872–8. [DOI] [PubMed] [Google Scholar]
- 10. Matsushita M, Kawaguchi M. Immunomodulatory effects of drugs for effective cancer immunotherapy. J Oncol. 2018;2018:8653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omori M, Okuma Y, Hakozaki T, Hosomi Y. Statins improve survival in patients previously treated with nivolumab for advanced non‐small cell lung cancer: an observational study. Mol Clin Oncol. 2019;10:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chalabi M, Cardona A, Nagarkar DR, Scala AD, Gandara DR, Rittmeyer A, et al. Efficacy of chemotherapy and atezolizumab in patients with non‐small cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31:525–31. [DOI] [PubMed] [Google Scholar]
- 13. Iglesias‐Santamaría A. Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin Transl Oncol. 2020;22:1481–90. [DOI] [PubMed] [Google Scholar]
- 14. Stucci LS, D'Oronzo S, Tucci M, Macerollo A, Ribero S, Spagnolo F, et al. Vitamin D in melanoma: controversies and potential role in combination with immune check‐point inhibitors. Cancer Treat Rev. 2018;69:21–8. [DOI] [PubMed] [Google Scholar]
- 15. Svaton M, Zemanova M, Zemanova P, Kultan J, Fischer O, Skrickova J, et al. Impact of concomitant medication administered at the time of initiation of nivolumab therapy on outcome in non‐small cell lung cancer. Anticancer Res. 2020;40:2209–17. [DOI] [PubMed] [Google Scholar]
- 16. Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti‐PD‐1/anti‐CTLA‐4) in metastatic malignant melanoma. J Immunother Cancer. 2018;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Afzal MZ, Dragnev K, Sarwar T, Shirai K. Clinical outcomes in non‐small‐cell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors. Lung Cancer Manage. 2019;8:LMT11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, et al. Mitochondrial activation chemicals synergize with surface receptor PD‐1 blockade for T cell‐dependent antitumor activity. Proc Natl Acad Sci U S A. 2017;14:E761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barreira da Silva R, Laird ME, Yatim N, Fiette L, Ingersoll MA, Albert ML. Dipeptidyl peptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol. 2015;16:850–8. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura K, Yaguchi T, Ohmura G, Kobayashi A, Kawamura N, Iwata T, et al. Involvement of local renin‐angiotensin system in immunosuppression of tumor microenvironment. Cancer Sci. 2018;109:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Routy B, Le Chatelier E, Derosa L, CPM D, Alou MT, Dallière R, et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science. 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- 22. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mangan BL, McAlister RK, Balko JM, Johnson DB, Moslehi JJ, Gibson A, et al. Evolving insights into the mechanisms of toxicity associated with immune checkpoint inhibitor therapy. Br J Clin Pharmacol. 2020;86:1778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das S, Johnson DB. Immune‐related adverse events and anti‐tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 26. Institute NC . Common terminology criteria for adverse events (CTCAE) version 4.0. National Cancer Institute: Bethesda; 2015. [Google Scholar]
- 27. Katayama Y, Yamada T, Tanimura K, Yoshimura A, Takeda T, Chihara Y, et al. Impact of bowel movement condition on immune checkpoint inhibitor efficacy in patients with advanced non‐small cell lung cancer. Thorac Cancer. 2019;10:526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobayashi H, Omori S, Nakashima K, Wakuda K, Ono A, Kenmotsu H, et al. Response to the treatment immediately before nivolumab monotherapy may predict clinical response to nivolumab in patients with non‐small cell lung cancer. Int J Clin Oncol. 2017;22:690–7. [DOI] [PubMed] [Google Scholar]
- 29. Lang D, Huemer F, Rinnerthaler G, Horner A, Wass R, Brehm E, et al. Therapy line and associated predictors of response to PD‐1/PD‐L1 inhibitor monotherapy in advanced non‐small cell lung cancer: a retrospective bi‐centric cohort study. Target Oncol. 2019;14:707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Facchinetti F, Mazzaschi G, Barbieri F, Passiglia F, Mazzoni F, Berardi R, et al. First‐line pembrolizumab in advanced non‐small cell lung cancer patients with poor performance status. Eur J Cancer. 2020;130:155–67. [DOI] [PubMed] [Google Scholar]
- 31. Fujii T, Colen RR, Bilen MA, Hess KR, Hajjar J, Suarez‐Almazor ME, et al. Incidence of immune‐related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs. 2018;36:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akamatsu H, Murakami E, Oyanagi J, Shibaki R, Kaki T, Takase E, et al. Immune‐related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non‐small cell lung cancer. Oncologist. 2020;25:e679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan H, Xu B, Zhu N, Ren B. PGC‐1α activator‐induced fatty acid oxidation in tumor‐infiltrating CTLs enhances effects of PD‐1 blockade therapy in lung cancer. Tumori. 2020;106:55–63. [DOI] [PubMed] [Google Scholar]
- 34. Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, et al. Clinical characteristics of liver metastasis in nivolumab‐treated patients with non‐small cell lung cancer. Anticancer Res. 2018;38:4723–9. [DOI] [PubMed] [Google Scholar]
- 35. Igawa S, Sato Y, Ryuge S, Ichinoe M, Katono K, Hiyoshi Y, et al. Impact of PD‐L1 expression in patients with surgically resected non‐small cell lung cancer. Oncology. 2017;92:283–90. [DOI] [PubMed] [Google Scholar]
- 36. Cortellini A, Maio M, Nigro O, Leonetti A, Cortinovis DL, Aerts JG, et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non‐small cell lung cancer treated with first‐line pembrolizumab versus cytotoxic chemotherapy. J Immunother Cancer. 2021;9:e002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roy K, Ghosh M, Pal TK, Chakrabarti S, Roy S. Cholesterol lowering drugs may influence cellular immune response by altering MHC II function. J Lipid Res. 2013;54:3106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nivolumab and metformin hydrochloride in treating patients with stage III‐IV non‐small cell lung cancer that cannot be removed by surgery. Available from: https://clinicaltrials.gov/ct2/show/NCT03048500. Accessed October 24, 2020.
- 39. Kubo T, Ninomiya T, Hotta K, Kozuki T, Toyooka S, Okada H, et al. Study protocol: phase‐Ib trial of nivolumab combined with metformin for refractory/recurrent solid tumors. Clin Lung Cancer. 2018;19:e861–4. [DOI] [PubMed] [Google Scholar]
- 40. Fong W, To KKW . Drug repurposing to overcome resistance to various therapies for colorectal cancer. Cell Mol Life Sci. 2019;76:3383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boland JW, Pockley AG. Influence of opioids on immune function in patients with cancer pain: from bench to bedside. Br J Pharmacol. 2018;175:2726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Concomitant medications


