Abstract
Objectives:
In pediatric Crohn’s disease, infliximab trough concentrations after standard weight-based induction therapy are commonly below 7 μg/mL. Clinical treatment outcomes are associated with post-induction infliximab trough concentration. Markers of inflammation are associated with low infliximab concentrations during maintenance dosing. We sought to determine if early markers of disease activity are associated with inadequate post-induction infliximab trough concentrations in pediatric Crohn’s disease.
Methods:
We performed a retrospective single-center case-control study of pediatric Crohn’s disease patients to assess the association between baseline and week-2 biomarkers (albumin, C-reactive protein, and erythrocyte sedimentation rate) and inadequate post-induction infliximab trough concentration (<7 μg/mL) in patients treated with standard 5mg/kg dosing. Baseline and week-2 biomarker values were coded as dichotomous variables at clinically useful thresholds. Univariable logistic regression was used to calculate odds ratios of developing an inadequate infliximab trough concentration for each threshold, as well as thresholds in combination.
Results:
Fifty-five patients were evaluated. Early biomarker thresholds significantly associated with inadequate post-induction infliximab trough concentrations included baseline C-reactive protein >1mg/dL (odds ratio [OR] 4.58; 95% confidence interval [CI] 1.24–17.01), both baseline C-reactive protein >0.5 mg/dL and albumin <3.5 g/dL (OR 8.31; 95% CI 1.99–34.63), and week-2 C-reactive protein >0.5 mg/dL or albumin <3.5 mg/dL or erythrocyte sedimentation rate >25mm/hour (OR 11.08; 95% CI 2.14–57.22).
Conclusions:
Routine baseline and week-2 markers of disease activity at clinically useful thresholds were associated with inadequate post-induction infliximab trough concentration in pediatric Crohn’s disease patients receiving standard weight-based induction dosing.
Keywords: albumin, C-reactive protein, erythrocyte sedimentation rate, therapeutic drug monitoring
The rate of clinical response to infliximab (IFX) treatment after 54 weeks of standard weight-based dosing is only 63.5% in pediatric Crohn’s disease (CD) (1). Standard weight-based IFX induction dosing for the treatment of CD in children and adults is 5 mg/kg/dose administered at 0, 2, and 6 weeks, with maintenance dosing continued every 8 weeks (1,2). The American Gastroenterological Association recommends a target serum IFX concentration of ≥5 μg/mL for adult inflammatory bowel disease (IBD) patients during maintenance therapy (3). Pediatric guidelines agree with this target and recommend therapeutic drug monitoring at the end of induction dosing, before the fourth dose (14 weeks after start of therapy) (4). Importantly, post-induction IFX trough concentrations have been shown to predict clinical and biochemical remission at week 52 in pediatric CD patients (5). A post-induction trough concentration target of 5 μg/mL may be too low; in a prospective study of adults with CD, the only factor independently associated with primary nonresponse at week 14 was an IFX trough below 7 μg/mL. In this study, IFX trough below 7 μg/mL also was correlated with immunogenicity and nonremission at week 54 (6). Notably, a recent observational study demonstrated low post-induction IFX trough concentrations in children (median 2.1 μg/mL; interquartile range [IQR] 1.1–4.3) (7).
Biomarkers of disease activity, such as hypoalbuminemia and increased serum C-reactive protein (CRP) are associated with low IFX trough concentrations and loss of therapeutic response during maintenance therapy (8–11). Pharmacokinetic modeling in pediatric CD demonstrates that standard IFX maintenance dosing in children often results in inadequate drug exposure, especially when albumin concentrations are low (12). In a large pediatric study evaluating 228 children on maintenance therapy, increased body weight, low serum albumin, high erythrocyte sedimentation rate (ESR) and antibodies to IFX (ATI) predicted an inadequate maintenance IFX trough (13).
Given pharmacokinetic variability of IFX, standard weight-based dosing does not guarantee adequate exposure post-induction. The aim of our study was to determine if routinely ordered baseline or week 2 serum markers of disease activity (albumin, CRP, and ESR) were associated with inadequate (<7 μg/ml) post-induction trough concentrations in a cohort of pediatric CD patients receiving standard 5 mg/kg dosing.
METHODS
Study Design
We performed a retrospective single-center case-control study of pediatric CD patients initiating IFX to assess the association between baseline and week-2 serum markers of disease activity (albumin, CRP, and ESR) and inadequate post-induction IFX trough concentration. Patients treated with IFX therapy between January 1, 2013 and June 20, 2019 at Children’s National Hospital were identified using a divisional infusion database that included 371 IBD patients who initiated IFX during that time.
Patients were included in the study if they had a diagnosis of CD, were greater than 1 and less than 18 years old at the time of starting IFX, were antitumor-necrosis-factor-α (anti-TNFα)-naïve, and underwent standard IFX induction therapy of 5 mg/kg/dose (rounded to nearest 100 mg) at standard dose intervals (0, 2, and 6 weeks). Inclusion required a post-induction IFX concentration drawn just before the fourth dose (week 14±2 weeks). All IFX trough concentrations and ATI were measured via drug-tolerant liquid chromatography-tandem mass spectrometry assays (Mayo Clinic Laboratory or Prometheus Laboratories). The Mayo Clinic assay measures ATI when IFX concentrations are below 5.1 μg/mL. The Prometheus Laboratories assay reports ATI regardless of serum IFX concentration.
Patients were excluded if they received greater than standard 5 mg/kg dosing, were prescribed an escalated induction dosing regimen, were missing all baseline or week-2 laboratory testing in the Electronic Medical Record, did not have an IFX trough drawn before the fourth dose, required surgery before the fourth dose, had a diagnosis of ulcerative colitis or IBD unclassified, or were not anti-TNFα-naive. Using these strict enrollment criteria, our study population represented a cohort of pediatric CD patients who were prescribed standard IFX induction therapy at the decision of their physician, and/or at payer-mandate.
For patients who met inclusion criteria, the following information at time of first IFX dose was recorded: demographics (age, sex, race), nutritional data (weight, height, BMI), CD phenotype and distribution (according to Montreal classification), and concomitant CD medications. Laboratory markers of disease activity (albumin, CRP, ESR) closest to and within 1 week of first IFX dose, as well as at time of week 2 dose, were recorded. All laboratory studies for markers of disease activity were run at the Children’s National Hospital clinical laboratory.
The exposures of interest included serum markers of disease activity (albumin, CRP, and ESR) at the time of drug initiation (baseline) and at second infusion (2 weeks from drug initiation). The primary outcome of interest was inadequate post-induction IFX trough concentration, defined as <7 μg/mL, with a sensitivity analysis examining a post-induction IFX trough <5 μg/mL. We divided patients into “cases” and “controls” based on these thresholds—with cases defined as patients with a post-induction IFX trough less than 7 μg/mL (considered “inadequate”) and controls defined as patients with a post-induction IFX trough greater than or equal to 7 μg/mL (considered “adequate”).
Continuous values of markers of disease activity at baseline and week 2 were converted to dichotomous variables as follows: CRP >0.5 mg/dL, >1.0 mg/dL and >2.0 mg/dL, albumin <3 g/dL and <3.5 g/dL, and ESR >25 mm/hour. Biomarkers were chosen because of their frequent utilization instandard clinical practice, and the range of values was selected based upon prevalence among CD patients (8,9,11). Optimal cut-point values based on receiver-operator curves were not calculated because of our study’s case-control study design and the selection bias this would present. Combinations of these dichotomous variables were analyzed (ie, CRP >1 mg/dL and albumin <3.5 g/dL). Because of similar means for ESR at baseline between groups, thresholds involving ESR were not used for baseline labs; however, given the significantly different means for week 2 ESR, a threshold of 25 mm/hour both in isolation and combination was used at week 2. Given the overall improvement in biomarkers between baseline and week 2, including overall decrease in CRP and ESR, and increase in albumin, not all thresholds were used in combination for week 2 data as most patients did not meet the more severe thresholds, such as CRP >1 mg/dL, CRP >2 mg/dL, or albumin <3.0 g/dL. In addition, combinations of subjects meeting at least 1 criterion (ie, CRP >0.5 mg/dL or Albumin <3.5 g/dL or ESR >25 mm/hour) were used for week-2 data in order to increase the sensitivity of our thresholds and the proportion of patients meeting criteria. Otherwise, identical analyses were implemented for baseline and week-2 data.
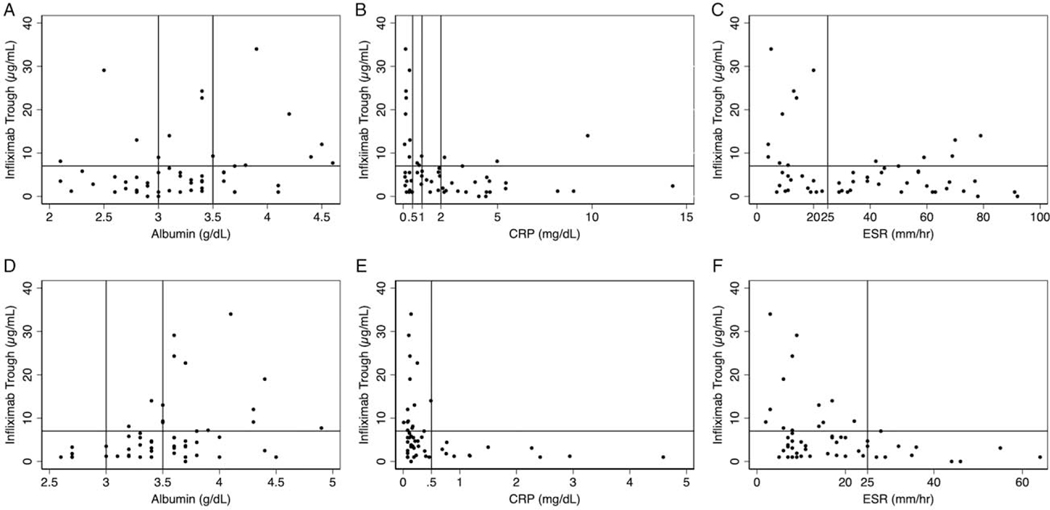
Baseline and week-2 values of markers of disease activity were plotted versus their corresponding infliximab trough concentration for each patient and represented graphically with demarcation of chosen clinical thresholds (Fig. 1).
FIGURE 1.
Baseline (A–C) and week 2 (D–F) serum albumin (A and D), CRP (B and E), and ESR (C and F) versus infliximab trough, with demarcation of thresholds used. CRP = C-reactive protein; ESR = erythrocyte sedimentation.
Statistical Considerations
Continuous variables were summarized as means and standard deviations. Categorical variables were summarized as counts and percentages. A Shapiro-Wilk test was used to assess normality of continuous variables. Cases and controls were compared using 2-sample unpaired t-test for continuous variables with normal distributions and Mann-Whitney U test for continuous variables with skewed distributions. Fisher exact test was used to compare categorical variables with 2 categories and chi-squared test was used for categorical variables with more than 2 categories. Changes in biomarker values from baseline to week-2 were analyzed using paired t-test. A 2-sided P value of less than 0.05 was considered statistically significant. Patients were excluded from a specific analysis if they were missing either baseline and/or week 2 laboratory markers of disease activity.
Univariable logistic regression was used to test the effects of baseline and week 2 albumin, CRP, or ESR on inadequate post-induction IFX trough. Odds ratios (OR) of developing an inadequate post-induction IFX trough were calculated. The association between baseline covariables and the primary outcome were assessed via univariable linear regression to assess for possible confounding and the need for multivariable regression analyses. Multicollinearity of serum markers of disease activity was assessed using univariable linear regression to test their association with one another.
Univariable logistic regression was used to calculate the odds of developing an inadequate post-induction IFX trough for each biomarker threshold in cases versus controls. For thresholds in which no subject meeting the laboratory threshold went on to have an adequate post-induction IFX trough, odds ratios could not be calculated and instead chi-squared P values of the association between post-induction IFX trough less than 7 μg/ml and meeting the laboratory threshold were calculated and reported.
Analyses were performed using SAS9.4 software (SAS Institute, NC).
Ethical Considerations
This study was approved by the Children’s National Hospital (Washington, DC) Institutional Review Board on March 13, 2017.
RESULTS
Fifty-five of the 371 patients examined for eligibility met inclusion criteria. Our stringent exclusion criteria resulted in a high exclusion rate (85.2%) because of factors including diagnosis of ulcerative colitis or IBD unclassified, prior anti-TNFα exposure, nonstandard dosing schedule, and unavailability of post-induction IFX trough or baseline laboratory markers. Demographic data, post-induction IFX trough concentration, and ATI are presented in Table 1. Demographics (age, sex, race), BMI, disease location, and phenotype, and the percentage of patients taking immunomodulators and systemic corticosteroids at time of IFX initiation were similar between groups. Forty of 55 subjects had a post-induction IFX trough <7 μg/mL (cases), and 15/55 had a post-induction IFX trough at least 7 μg/mL (controls). Only 1/40 cases and 0/15 controls had ATI detected at post-induction. The number of values available for analysis for each biomarker is presented in Table 2. Baseline ESR was missing in 1 patient. Week-2 albumin, CRP, and ESR were missing in 2, 2, and 3 patients, respectively. Nineteen patients had a baseline CRP below 0.5 (35%). Twenty-five patients had a baseline CRP below 1 (45%).
TABLE 1.
Cohort demographic and disease phenotype characteristics at time of first infliximab dose and post-induction infliximab concentration, stratified by infliximab trough <7 μg/mL
| Total N = 55 | IFX trough <7 μg/mL (‘‘inadequate’’) N = 40 | IFX trough >7 μg/mL (‘‘adequate’’) N = 15 | ||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | P value | |
| Age, y | 13.09 (3.00) | 13.03 (2.87) | 13.27 (3.43) | 0.588 |
| IFX trough, μg/mL | 6.07 (7.27) | 2.70 (1.82) | 15.03 (8.72) | <0.001 * |
| BMI | 17.33 (2.97) | 17.14 (3.03) | 17.82 (2.85) | 0.171 |
| Weight, kg | 42.84 (14.28) | 41.47 (13.44) | 46.5 (16.22) | 0.248 |
| No. (%) | No. (%) | No. (%) | P value | |
| Sex | 1 | |||
| Female | 17 (31) | 12 (26) | 5 (38) | |
| Male | 38 (69) | 28 (74) | 10 (62) | |
| Race | 0.502 | |||
| Black | 20 (36) | 16 (40) | 4 (27) | |
| Caucasian | 27 (49) | 17 (43) | 10 (67) | |
| Other | 8(15) | 7(17) | 1 (7) | |
| Immunomodulator at first dose | 1 | |||
| No | 43 (78) | 31 (78) | 12 (80) | |
| Yes | 12 (22) | 9 (22) | 3 (20) | |
| Systemic steroids at first dose | 1 | |||
| No | 40 (73) | 29 (73) | 11 (73) | |
| Yes | 15 (27) | 11 (28) | 4 (27) | |
| Antibodies to infliximab present | 1 | |||
| No | 54 (98) | 39 (98) | 15 (100) | |
| Yes | 1 (2) | 1(1) | 0 (0) | |
| Crohn’s disease Phenotype | 0.076 | |||
| Inflammatory | 44 (80) | 29 (73) | 15 (100) | |
| Stricturing | 6(11) | 6(15) | 0(0) | |
| Penetrating | 5 (7) | 5(13) | 0 (0) | |
| Disease location | 0.056 | |||
| Ileal | 7(13) | 7(18) | 0 (0) | |
| Colonic | 7(13) | 3 (8) | 4 (27) | |
| Ileocolonic | 41 (75) | 30 (75) | 11 (73) | |
| UGI modifier | 1 | |||
| No | 39 (71) | 28 (70) | 11 (73) | |
| Yes | 16 (29) | 12 (30) | 4 (27) | |
Continuous variables expressed as mean (± SD). Differences among groups analyzed using unpaired t test for normally distributed variables (weight) and Mann-Whitney U test for nonnormally distributed variables (age, IFX trough, BMI). Categorical variables expressed as No., %. Differences among groups analyzed using Fisher Exact Test (2 categories) or chi-squared test (3+ categories). Disease location and phenotype presented according to Montreal classification. IFX = infliximab; SD = standard deviation; UGI = upper gastrointestinal.
Statistically significant where P<0.05.
TABLE 2.
Serum markers of disease activity at baseline and week 2 stratified by post-induction infliximab trough <7 μg/mL
| Total | IFX trough <7 μg/mL (‘‘inadequate’’) | IFX trough >7 μg/mL (‘‘adequate’’) | |||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | P value | |
| Baseline | |||||||
| Albumin, g/dL | 55 | 3.22 (0.58) | 40 | 3.10 (0.74) | 15 | 3.53 (0.69) | 0.015 * |
| CRP, mg/dL | 55 | 2.38 (2.83) | 40 | 2.66 (2.88) | 15 | 1.62 (2.65) | 0.044 * |
| ESR, mm/hour | 54 | 37.44 (25.47) | 39 | 40.13 (24.37) | 15 | 30.47 (27.79) | 0.134 |
| Week-2 | |||||||
| Albumin, g/dL | 53 | 3.54 (0.48) | 38 | 3.41 (0.44) | 15 | 3.85 (0.46) | 0.003 * |
| CRP, mg/dL | 52 | 0.51 (0.85) | 37 | 0.65 (0.97) | 15 | 0.16 (0.12) | 0.027 * |
| ESR, mm/hour | 52 | 17.46 (13.41) | 38 | 19.82 (14.36) | 14 | 11.07 (7.65) | 0.021 * |
Continuous variables expressed as mean (± SD). Differences among groups (inadequate vs adequate trough) analyzed using unpaired t-test for normally distributed variables (albumin) and Mann-Whitney U test for nonnormally distributed variables (CRP and ESR). CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; IFX = infliximab; SD = standard deviation.
Statistically significant where P<0.05. Bold represents P values < 0.05.
Baseline and week-2 serum markers of disease activity data are presented in Table 2. T tests comparing group means (stratified by adequate versus inadequate post-induction IFX trough) demonstrated statistically significant differences in baseline albumin (P=0.015), baseline CRP (P=0.044), week-2 albumin (P=0.003), week-2 CRP (P=0.027), and week-2 ESR (P=0.021). Baseline ESR was not significantly different among groups. In patients with both baseline and week-2 biomarker data available, there was a significant increase in mean serum albumin and a mean decrease in CRP and ESR in both cases and controls (Table, Supplemental Digital Content 1, http://links.lww.com/MPG/C15).
Employing univariable logistic regression of biomarkers as continuous variables, baseline albumin was significantly associated with inadequate post-induction IFX concentration (OR 0.26; 95% CI 0.08–0.83), whereas baseline CRP and ESR were not (Table, Supplemental Digital Content 2, http://links.lww.com/MPG/C16). Odds ratios for week 2 albumin and week 2 ESR as continuous variables were significantly associated with inadequate post-induction IFX trough, whereas week 2 CRP was not. When assessing the relationships between baseline covariables and post-induction IFX trough via univariable linear regression, all were nonsignificant. For this reason, in addition to relatively small sample size, these covariables were neither included as independent predictors of IFX concentration nor in multivariable regression analysis. Multicollinearity was confirmed by univariable linear regression, which demonstrated all biomarker variables were significantly correlated to one another at their respective time points (all P ≤0.001, data not shown). Due to this, multivariable logistic regression involving multiple biomarkers was not assessed.
Odds ratios of developing an inadequate post-induction IFX trough among patients meeting versus not meeting biomarker thresholds are shown in Table 3. Many of these thresholds had significant odds ratios over 4. For example, patients with a CRP over 1 mg/dL had an OR of an inadequate trough of 4.58 (95% CI 1.24–17.01) and patients with an albumin <3.5 g/dL had an odds ratio of 4.57 (95% CI 1.28–16.38). When we combined biomarkers using multiple thresholds, the odds ratios increased without sacrificing statistical significance. The odds ratio for inadequate trough in patients with a CRP >1 mg/dL and albumin <3.5 g/dL was 8.31 (95% CI 1.99–34.63). Many week 2 thresholds also reached statistical significance. Patients meeting week 2 thresholds of CRP >0.5 mg/dL or albumin <3.5 mg/dL or ESR >25 mm/hour had an OR of 11.08 (95% CI 2.14–57.22) of developing an inadequate post-induction IFX trough versus those not meeting any of these thresholds. For multiple week 2 thresholds, all patients meeting the threshold went on to have an inadequate post-induction IFX trough. For instance, 11/52 subjects had a week 2 CRP >0.5 mg/dl and 5/53 subjects had a week 2 albumin <3 g/dL, with 4 patients meeting both criteria. No patients meeting these week 2 thresholds went on to have an adequate trough. In these cases, an OR was unable to be calculated and instead chi-square P values are reported in Table 3.
TABLE 3.
Univariable logistic regression odds ratios of inadequate (<7 μg/mL) infliximab trough for baseline and week-2 thresholds of serum markers of disease activity, in isolation and combination
| Baseline | N | Odds ratio IFX <7 (95% confidence interval) | P value |
|---|---|---|---|
| CRP > 0.5 mg/dL | 55 | 3.01 (0.88–10.30) | 0.079 |
| CRP > 1 mg/dL | 55 | 4.58 (1.24–17.01) | 0.023 * |
| CRP > 2 mg/dL | 55 | 2.25 (0.61–8.28) | 0.223 |
| Albumin <3 g/dL | 55 | 2.67 (0.65–10.97) | 0.174 |
| Albumin <3.5 g/dL | 55 | 4.57 (1.28–16.38) | 0.020 * |
| CRP > 0.5 mg/dL and Albumin < 3 g/dL | 55 | 5.31 (0.62–45.32) | 0.127 |
| CRP > 1 mg/dL and Albumin <3 g/dL | 55 | 5.31 (0.62–45.32) | 0.127 |
| CRP > 2 mg/dL and Albumin <3 g/dL | 55 | 5.31 (0.62–45.32) | 0.127 |
| CRP > 0.5 mg/dL and Albumin <3.5 g/dL | 55 | 8.31 (1.99–34.63) | 0.004 * |
| CRP > 1 mg/dL and Albumin <3.5 g/dL | 55 | 5.41 (1.32–22.21) | 0.019 * |
| CRP > 2 mg/dL and Albumin <3.5 g/dL | 55 | 2.96 (0.72–12.13) | 0.132 |
| Week-2 | N | Odds ratio IFX <7 (95% confidence interval) | P value |
| CRP > 0.5 mg/dL | 41 | - | 0.017 * |
| Albumin <3 g/dL | 48 | - | 0.140 |
| Albumin <3.5 g/dL | 53 | 8.94 (1.77–45.25) | 0.008 * |
| ESR > 25 mm/hour | 52 | 4.03 (0.46–35.23) | 0.207 |
| CRP > 0.5 mg/dL and Albumin <3.5 g/dL | 43 | - | 0.036 * |
| CRP > 0.5 mg/dL or Albumin <3.5 g/dL | 52 | 10.68 (2.09–54.51) | 0.004 * |
| CRP > 0.5 mg/dL or Albumin <3 g/dL | 41 | - | 0.017 * |
| CRP > 0.5 mg/dL or Albumin <3.5 g/dL or ESR > 25 mm/hour | 51 | 11.08 (2.14–57.22) | 0.004 * |
| CRP > 0.5 mg/dL or Albumin <3 g/dL or ESR > 25 mm/hour | 51 | 7.91 (0.93–67.24) | 0.058 |
Odds ratios and P values calculated using univariable logistic regression, exact confidence intervals reported. – Denotes cells in which there were 0 subjects in which the given threshold was reached and later went on to develop an adequate trough; therefore, unable to calculate OR or 95% confidence interval. Chi-squared test used to generate P values of the association between post-induction infliximab concentration <7 μg/mL and meeting the baseline lab threshold. CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; IFX = infliximab.
Statistically significant where P<0.05. Bold represents P values < 0.05.
Sensitivity analyses examining markers of disease activity in relation to IFX trough of 5 μg/mL are shown in Tables, Supplemental Digital Content 3–6, http://links.lww.com/MPG/C17, http://links.lww.com/MPG/C18, http://links.lww.com/MPG/C19, http://links.lww.com/MPG/C20. Overall, these results were similar to analyses using an IFX trough of 7 μg/mL.
DISCUSSION
Low-serum drug concentrations are responsible for the majority of therapeutic failures of anti-TNFα medications in adults and children with IBD (14). Higher post-induction IFX troughs are associated with improved clinical and biochemical remission rates in children with CD (5). Minar et al (15) demonstrated the utility of reactive therapeutic drug monitoring through assessment of 72 children with CD who presented with signs and symptoms concerning for loss of response to IFX therapy. In this cohort, the median post-induction trough was only 2.1 μg/mL. When routine week 14 troughs are monitored proactively, suboptimal IFX concentrations are common (16). Our study supports these findings, as 72.7% of patients in our cohort treated with standard induction dosing did not achieve an adequate post-induction IFX trough. Furthermore, patients who had severe disease prompting rapid dose-escalation, regimen-intensification, or surgery before the fourth dose were excluded. Thus, our analysis excluded patients with particularly severe disease, and represents a more typical patient in whom such a week 14 monitoring approach would be considered. As children with IBD who achieve remission in the first 90 days from diagnosis achieve better 52-week outcomes, and week 14 IFX trough levels strongly predict clinical and/or biochemical remission at 1 year, these findings are supportive of proactive monitoring or a selective preemptive dose escalation strategy (17–19).
Elevated serum CRP, hypoalbuminemia, and prolonged ESR, have been shown to predict lower IFX concentrations during maintenance therapy using population data and pharmacokinetic modeling (11–13,20–22). Our logistic regression data showed similar associations of baseline and week-2 albumin, CRP, and ESR in relation to post-induction IFX trough. Decreased CRP level and increased infliximab concentration post-induction have been associated with improved durable outcomes, with elevated CRP having been shown to be predictive of nonresponse in CD (8,23). There is, however, a relative paucity of data regarding pretreatment biochemical predictors of inadequate post-induction IFX trough.
Our study demonstrates that in routine pediatric CD patients, baseline and week-2 serum markers of disease activity at relatively common and clinically relevant thresholds are highly associated with development of an inadequate post-induction IFX trough (7). Use of a single biomarker is prone to false negatives given that 25% of patients with active CD do not elevate their CRP because of genetic polymorphisms and ESR can be affected by co-existing anemia (24). Attempting to overcome this, we combined multiple thresholds for several commonly utilized markers of disease activity. In doing so, we increased the sensitivity of our screening process to avoid missing patients who met thresholds of only 1 biomarker but not others (for patient-specific factors). Use of two time points provides an additional opportunity to identify patients who would potentially benefit from an escalated dosing strategy early in the treatment course. For example, baseline CRP >0.5 mg/dL (but less than 1.0 mg/dL) was not significantly associated with later development of inadequate post-induction IFX trough, but a CRP >1.0 mg/dL was, exhibiting the usefulness of multiple thresholds clinically. Further, the persistence of CRP >0.5 mg/dL, albumin <3.5 or ESR >25 at week 2 was significantly associated with inadequate post-induction IFX trough and could prompt a physician to intensify dosing, a strategy demonstrated to be successful in improving clinical outcomes in patients with IFX treatment failure (25). Using this approach, we may fail to identify patients with normal inflammatory markers despite active disease. Further studies may focus on other novel biomarkers with a better accuracy of predicting inadequate drug exposure.
Our study has several weaknesses, including a relatively small sample size, which precludes subset analyses, such as those with different CD phenotypes. The retrospective nature of our study created a heterogeneous population that did not specifically match for factors including disease phenotype, concomitant medications, and demographics; however, these factors were similar among cases and controls. Our case-control study design could only analyze patients who had an IFX trough drawn. It is possible that our cohort of patients in which IFX concentrations were drawn post-induction may be different than CD patients as a whole, although using odds ratios should account for this. We lacked validated clinical and endoscopic outcomes to correlate with post-induction troughs. Multiple IFX drug assays were used (Mayo and Prometheus), which could theoretically introduce an unmeasured covariable; however, prior studies have shown that while differences exist, most IFX drug concentration assays perform similarly (26).
To the best of our knowledge, our study represents the first report of routinely collected baseline and week 2 serum markers of disease activity at clinically useful and prevalent thresholds demonstrated to be significantly associated with inadequate post-induction IFX trough in pediatric CD patients receiving standard on-label, weight-based dosing. Still, over one-fourth of the patients in our cohort did achieve an adequate trough with standard induction dosing, exhibiting that empiric escalated dosing is not indicated in all children, and that an objective, thoughtful, and predictive approach is needed. Bayesian dashboard dosing strategies to individualize pharmacokinetics and dosing of IFX are being developed and evaluated (27,28). Until these are readily available, use of an intensified regimen in select patients who are highly unlikely to achieve adequate IFX concentrations at the end of induction may improve outcomes in pediatric CD. Accordingly, prospective studies are needed to better understand approaches to dose selection and therapeutic dose monitoring in the pediatric IBD population, including the assessment of effects on later outcomes.
Supplementary Material
What Is Known
Post-induction infliximab concentrations predict clinical and biochemical remission at week 52 in pediatric Crohn’s disease, yet inadequate post-induction concentrations are prevalent.
Low-serum drug concentrations are responsible for the majority of therapeutic failures of anti-TNFα medications in pediatric inflammatory bowel disease.
Low-serum albumin and increased serum C-reactive protein levels are associated with low infliximab trough concentrations during maintenance therapy in inflammatory bowel disease.
What Is New
Baseline and week 2 levels of albumin, C-reactive protein, and erythrocyte sedimentation rate are associated with inadequate post-induction infliximab concentration in pediatric Crohn’s disease patients receiving standard dosing.
Acknowledgments:
We would like to acknowledge the participating patients and our division colleagues for their support. We are grateful to Dr. Frank Scott for his helpful suggestions on the manuscript.
This work was supported by the National Institute of Child Health and Human Development (grant number 5U54HD090254 to L.S.C.).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
L.S.C. is an employee of ReveraGen BioPharma. For the remaining authors, no conflicts of interest are declared.
REFERENCES
- 1.Hyams J, Crandall W, Kugathasan S, et al. , REACH Study Group. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007;132:863–73. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Feagan BG, Lichtenstein GR, et al. , ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 3.Feuerstein JD, Nguyen GC, Kupfer SS, et al. , American Gastroenterological Association Institute Clinical Guidelines Committee. (2017) American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34. [DOI] [PubMed] [Google Scholar]
- 4.Improve Care Now. Model IBD Care—a Guideline for Consistent Reliable Care Website. https://d3n8a8pro7vhmx.cloudfront.net/improvecarenow/pages/283/attachments/original/1464375801/Model_IBD_-Care_Guideline_2016.pdf?1464375801. Accessed December 30, 2019.
- 5.Van Hoeve K, Dreesen E, Hoffman I, et al. Adequate infliximab exposure during induction predicts remission in paediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2019;68: 847–53. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy NA, Heap GA, Green HD, et al. , UK Inflammatory Bowel Disease Pharmacogenetics study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019;4:341–53. [DOI] [PubMed] [Google Scholar]
- 7.Clarkston K, Tsai YT, Jackson K, et al. Development of infliximab target concentrations during induction in pediatric Crohn disease patients. J Pediatr Gastroenterol Nutr 2019;69:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magro F, Rodrigues-Pinto E, Santos-Antunes J, et al. High C-reactive protein in Crohn’s disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis 2014;8:129–36. [DOI] [PubMed] [Google Scholar]
- 9.Hibi T, Sakuraba A, Matanabe M, et al. C-reactive protein is an indicator of serum infliximab level in predicting loss of response in patients with Crohn’s disease. J Gastroenterol 2014;49:254–62. [DOI] [PubMed] [Google Scholar]
- 10.Fasanmade AA, Adedokun OJ, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinietics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther 2010;48:297–308. [DOI] [PubMed] [Google Scholar]
- 11.Boyle MP, Moss AC, O’Toole AM, et al. C-reactive protein as a predictor of low trough infliximab concentrations in patients who lost response to infliximab. J Dig Dis 2017;18:678–83. [DOI] [PubMed] [Google Scholar]
- 12.Frymoyer A, Hoekman DR, Piester TL, et al. Application of population pharmacokinetic modeling for individualized infliximab dosing strategies in Crohn disease. J Pediatr Gastroenterol Nutr 2017;65:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauman LE, Xiong Y, Mizuno T, et al. Improved population pharmacokinetic model for predicting optimized infliximab exposure in pediatric inflammatory bowel disease. Inflamm Bowel Disease 2020;26:429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang B, Choi SY, Choi YO, et al. Infliximab trough levels are associated with mucosal healing during maintenance treatment with infliximab in paediatric Crohn’s Disease. J Crohns Colitis 2019;13:189–97. [DOI] [PubMed] [Google Scholar]
- 15.Minar P, Saeed SA, Afreen M, et al. Practical use of infliximab concentration monitoring in pediatric Crohn disease. J Pediatr Gastroenterol Nutr 2016;62:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lega S, Phan BL, Rosenthal CJ, et al. Proactively optimized infliximab monotherapy is as effective as combination therapy in IBD. Inflamm Bowel Dis 2019;25:134–41. [DOI] [PubMed] [Google Scholar]
- 17.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 2017;389:1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naviglio S, Lacorte D, Lucafò M, et al. Causes of treatment failure in children with inflammatory bowel disease treated with infliximab: a pharmacokinetic study. J Pediatr Gastroenterol Nutr 2019;68:37–44. [DOI] [PubMed] [Google Scholar]
- 19.Bodini G, Giannini EG, Savarino V, et al. Infliximab trough levels and persistent vs transient antibodies measured early after induction predict long-term clinical remission in patients with inflammatory bowel disease. Dig Liver Dis 2018;50:452–6. [DOI] [PubMed] [Google Scholar]
- 20.Brandse JF, Mould D, Smeekes O, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis 2017;23:650–60. [DOI] [PubMed] [Google Scholar]
- 21.Buurman DJ, Maurer JM, Keizer RJ, et al. Population pharmacokinetics of infliximab in patients with inflammatory bowel disease: potential implications for dosing in clinical practice. Aliment Pharmacol Ther 2015;42:529–39. [DOI] [PubMed] [Google Scholar]
- 22.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis 2014;20:2247–59. [DOI] [PubMed] [Google Scholar]
- 23.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology 2015;149:1275.e2–85.e2. [DOI] [PubMed] [Google Scholar]
- 25.Steenholdt C, Bendtzen K, Brynskov J, et al. Changes in serum trough levels of infliximab during treatment intensification but not in anti infliximab antibody detection are associated with clinical outcomes after therapeutic failure in Crohn’s disease. J Crohns Colitis 2015;9:238–45. [DOI] [PubMed] [Google Scholar]
- 26.Marini JC, Sendecki J, Cornillie F, et al. Comparisons of serum infliximab and antibodies-to-infliximab tests used in inflammatory bowel disease clinical trials of remicade. AAPS J 2017;19:161–71. [DOI] [PubMed] [Google Scholar]
- 27.Mould DR, Dubinsky MC. Dashboard systems: pharmacokinetic/pharmacodynamic mediated dose optimization for monoclonal antibodies. J Clin Pharmacol 2015;55(Suppl 3):S51–9. [DOI] [PubMed] [Google Scholar]
- 28.Dubinksy MC, Phan BL, Singh N, et al. Pharmacokinetic dashboard-recommended dosing is different than standard of care dosing in infliximab-treated pediatric IBD patients. AAPS J 2017;19:215–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.