Abstract
The diverse cerebral consequences of preterm birth create significant challenges for understanding pathogenesis or predicting later outcome. Instead of focusing on describing effects common to the group, comparing individual infants against robust normative data offers a powerful alternative to study brain maturation. Here we used Gaussian process regression to create normative curves characterizing brain volumetric development in 274 term-born infants, modeling for age at scan and sex. We then compared 89 preterm infants scanned at term-equivalent age with these normative charts, relating individual deviations from typical volumetric development to perinatal risk factors and later neurocognitive scores. To test generalizability, we used a second independent dataset comprising of 253 preterm infants scanned using different acquisition parameters and scanner. We describe rapid, nonuniform brain growth during the neonatal period. In both preterm cohorts, cerebral atypicalities were widespread, often multiple, and varied highly between individuals. Deviations from normative development were associated with respiratory support, nutrition, birth weight, and later neurocognition, demonstrating their clinical relevance. Group-level understanding of the preterm brain disguises a large degree of individual differences. We provide a method and normative dataset that offer a more precise characterization of the cerebral consequences of preterm birth by profiling the individual neonatal brain.
Keywords: early brain development, heterogeneity, normative modeling, preterm birth, volumetric MRI
Introduction
Preterm birth (or birth before 37 weeks gestational age, GA) affects approximately 10% of pregnancies worldwide (Chawanpaiboon et al. 2019) and is a significant risk predisposing to atypical brain development and lifelong cognitive difficulties including a higher incidence of neurodevelopmental and psychiatric disorders (Nosarti et al. 2012; Agrawal et al. 2018; Thompson et al. 2020). Although early brain correlates of preterm birth have been identified at a group level (Volpe 2019), this vulnerable population is highly heterogeneous, with individuals following diverse clinical and neurocognitive trajectories (Sled and Nossin-Manor 2013; Dimitrova et al. 2020). Indeed, the assumption that prematurity has a homogenous effect on brain development might help account for the relatively poor predictive power of neonatal magnetic resonance imaging (MRI) for later outcome (de Bruïne et al. 2011; Edwards et al. 2018). To better understand brain development, provide accurate prognosis of later functionality, and study the effect of clinical risks and interventions, it is important to provide an individualized assessment of cerebral maturation (O’Muircheartaigh et al. 2020). Comparing individuals against robust normative data avoids the requirement to define quasi-homogenous groups in a search for effects common to the group and offers a powerful alternative to investigate brain development with high sensitivity to pathology at an individual infant level (Towgood et al. 2009; Holland et al. 2014; O’Muircheartaigh et al. 2020).
In this study, we used Gaussian process regression (GPR) to create normative charts of typical volumetric development using a large sample of healthy term-born infants scanned cross-sectionally within the first month of life. Analogous to the widely employed pediatric height and weight growth charts, this technique allows the local imaging features of individuals to be referred to typical variation while simultaneously accounting for variables such as age and sex (Marquand et al. 2016, 2019). Having established normative values for brain growth of 14 brain regions, we aimed to 1) quantify deviations from typical development in individual preterm infants, 2) investigate the heterogeneity of these deviations, and 3) examine the association between individual deviations, perinatal clinical factors, and later neurocognitive abilities. To test generalizability, we used a second large independent preterm dataset acquired on a different magnetic resonance (MR) scanner using different imaging parameters.
Materials and Methods
Participants
This study utilized data from two cohorts. A total of 363 (89 preterm) infants recruited for the developing Human Connectome Project (dHCP; http://developingconnectome.org/) were scanned at term-equivalent age (TEA, 37–45 weeks postmenstrual age, PMA) during natural unsedated sleep at the Evelina London Children’s Hospital between 2015 and 2019. The second cohort comprised of further 253 preterm infants born before 33 weeks GA that underwent MRI between 37- and 45-week PMA at the neonatal intensive care unit (NICU) in Hammersmith Hospital between 2010 and 2013 for the Evaluation of Preterm Imaging (EPrime) study. Detailed description of these studies and the scanning procedure used has been previously reported (dHCP, Hughes et al. 2017; EPrime, Edwards et al. 2018). All MRI images were examined by a neonatal neuroradiologist. Exclusion criteria for term-born infants are described in Supplementary Methods. There were no exclusion criteria for the preterm infants, except for major congenital malformations and data included infants from nonsingleton pregnancies. Both studies were approved by the National Research Ethics Committee (dHCP, REC: 14/Lo/1169; EPrime, REC: 09/H0707/98). Informed written consent was given by parents prior to scanning.
MRI Acquisition and Preprocessing
MRI data for the dHCP were collected on a Philips Achieva 3T (Philips Medical Systems) using a dedicated 32-channel neonatal head coil (Hughes et al. 2017). T2-weighted scans were acquired with TR/TE of 12 s/156 ms, SENSE = 2.11/2.58 (axial/sagittal), 0.8 × 0.8 mm in-plane resolution, 1.6-mm slice thickness (0.8-mm overlap). Images were motion corrected and super-resolution reconstructed resulting in 0.5-mm isotropic resolution (Cordero-Grande et al. 2018). MRI data collected for EPrime were acquired on a Philips 3T system using an 8-channel phased array head coil. T2-weighted turbo spin echo was acquired with TR/TE of 8670/160 ms, in-plane resolution 0.86 × 0.86 mm, 2-mm slice thickness (1-mm overlap).
Both datasets were preprocessed using the dHCP structural pipeline (Makropoulos et al. 2018). In brief, motion-corrected, reconstructed T2-weighted images were corrected for bias-field inhomogeneities, brain extracted, and segmented. Tissue labels included cerebrospinal fluid (CSF), white matter (WM), cortical gray matter (cGM), deep gray matter (dGM), ventricles (including the cavum, a transient fluid-filled cavity located in the midline of the brain between the left and right anterior horns of the lateral ventricles, which if present, usually disappears during the neonatal period), cerebellum, brainstem, hippocampus, and amygdala. dGM was further parcellated into left/right caudate, lentiform, and thalamus. Total tissue volume (TTV) incorporated all brain GM and WM volumes; total brain volume (TBV) included TTV and ventricles; intracranial volume (ICV) included TBV and CSF (Table 1.). Given the high correlation between TTV and TBV (ρ = 0.98), we reported only TTV. Due to their size and lower tissue contrast in the neonatal brain, the amygdala and the hippocampus are prone to segmentation errors and higher partial voluming, especially in the EPrime dataset, where the image resolution was lower (1 mm) compared with dHCP (0.5 mm). Therefore, these structures were excluded from the present analyses. The quality of the preprocessing was visually evaluated using a scoring system detailed elsewhere (Makropoulos et al. 2018) to ensure no images severely affected by motion or with poor segmentation were included (Supplementary Methods and Supplementary Fig. 1).
Table 1.
Brain regions of interest, the structures they include, and what global brain measures they are taken as a proportion from when calculating relative brain volumes
| Brain regions | Brain structures included | Relative volume (% from) |
|---|---|---|
| cGM | — | TTV |
| WM | — | TTV |
| Cerebellum | — | TTV |
| Brainstem | — | TTV |
| CSF | — | ICV |
| Ventricles | Lateral ventricles + cavum | TBV |
| Caudate (left/right) | — | TTV |
| Lentiform (left/right) | Pallidum + putamen | TTV |
| Thalamus (left/right) | — | TTV |
| TTV | All brain GM + WM tissue | — |
| TBV | All brain GM + WM tissue + ventricles | — |
| ICV | All brain GM + WM tissue + ventricles + CSF | — |
We estimated regional volumes in absolute (cm3) and relative (%) values. Relative volumes were calculated as the proportion of each tissue volume from TTV, the ventricles from TBV and CSF from ICV (Table 1). To capture the effect of preterm birth, we used relative volumes to 1) ensure results are not driven by extreme individual differences in nonbrain ICV, often seen in preterm infants, 2) partially alleviate differences in data acquisition.
Modeling Volumetric Development Using GPR
To characterize neonatal volumetric development, we used GPR, a Bayesian nonparametric regression, implemented in GPy (https://sheffieldml.github.io/GPy/). GPR simultaneously provides point estimates and measures of predictive confidence for every observation representing the distance of each individual observation from the normative mean at that point on the “curve” accounting for modeled covariates (Marquand et al. 2016).
We first trained a GPR model to describe typical development in the term-born dataset (274 infants) using PMA at scan and sex to predict 14 brain structures separately. Regions included ICV, TTV, cGM, WM, cerebellum, brainstem, CSF, ventricles, and left/right caudate, lentiform, and thalamus. Model accuracy was tested under 5-fold cross-validation, with each fold stratified to cover the whole PMA range (37–45 weeks). The relationship between the volume outputs and model predictors was estimated with a sum of radial basis function, linear, and white noise covariance kernels. Model hyperparameters were optimized using log marginal likelihood. Prediction performance was evaluated using the mean absolute error (MAE) between the predicted and the observed value derived from the 5-fold cross-validation.
To assess the effects of preterm birth, we retrained the model on the entire term-born dataset and applied the model to 89 dHCP preterm infants scanned at TEA. To assess generalizability, we applied the same model to 253 preterm infants from the EPrime study. A Z-score was derived for every infant by estimating the difference between the model prediction and the observed value normalized by the model uncertainty (the square root of the predicted variance). To quantify extreme deviations, prior to analyses, we chose a threshold of |Z| > 2.6 (corresponding to P < 0.005) following the convention adopted in previous GPR analyses modeling adult brain development (Wolfers et al. 2020). We examined the proportion of infants with volumes lying >2.6 standard deviations (SD) above/below the model mean (indicating the top/bottom 0.5% of the typical group values, hereafter described as extreme positive or negative deviations, respectively).
To quantify the effect of image spatial resolution differences between the dHCP and EPrime, we first downsampled the dHCP data to 1-mm isotropic resolution using FSL flirt (-applyisofxm, spline interpolation), reran the tissue segmentation on the new dHCP resolution data, and trained the GPR model. We examined the difference in 1) model means and 2) the number of EPrime infants who deviated significantly from the predicted model means.
Deviations From Normative Development, Perinatal Risks and Later Neurocognition
We tested the association between deviations from normative development (in relative volumes) and recognized perinatal clinical risks (Boardman and Counsell 2020), including GA at birth, birth weight Z-score, total days receiving mechanical ventilation, continuous positive airway pressure (CPAP), and total parenteral nutrition (TPN, available only for EPrime). Birth weight Z-scores were calculated using the population data from the uk90 growth charts implemented in sitar R package (Cole et al. 2010). Oxygen/respiratory support and nutrition were administered at the NICU. These data were obtained from electronic hospital records and days were counted if the infant spent any part of the day on ventilation, CPAP, or TPN, with higher number of days indicative of poorer health. Bayley III Scales of Infant Development (BSID-III) (Bayley 2006) assessment was carried out by trained developmental pediatricians/psychologists at 18 months for the dHCP and at 20 months for EPrime (corrected age). We used the composite scores for motor, cognitive, and language development (mean [SD] = 100[15]). Associations were examined using Spearman rho (ρ) or Mann–Whitney U test combined with Cliff’s delta (d, ranging from −1 to 1), under Bonferroni–Holm multiple comparison corrections.
Data Availability
Normative term-born data and the GPR code used in this study are freely available on GitHub (https://github.com/ralidimitrova). All imaging data collected for the dHCP will be publicly available in early 2021 at http://developingconnectome.org/.
Results
The perinatal, demographic and neurocognitive characteristics are presented in Table 2. EPrime infants were born earlier (p < 0.05, d = 0.28) and had lower birth weight (p < 0.05, d = 0.19) compared with dHCP preterm infants. On average, they had poorer motor (p < 0.05, d = 0.22) skills at follow-up (language p = 0.1; cognition p = 0.12). There were no differences in days on CPAP (p = 0.57) but dHCP preterm infants required mechanical ventilation for longer (p < 0.05, d = 0.16). The 2 preterm cohorts did not differ in punctate WM lesions (PWMLs, p = 0.09) or cerebellar hemorrhages (p = 0.82) incidence, nor in proportion of infants with intrauterine growth restriction (IUGR, p = 0.15); yet hemorrhagic parenchymal infarction (HPI) and periventricular leukomalacia (PVL) were observed only in the EPrime cohort (Table 2.).
Table 2.
Perinatal, demographic, and neurocognitive characteristics of the study sample
| Term (dHCP) n = 274 |
Preterm (dHCP) n = 89 |
Preterm (EPrime) n = 253 |
|
|---|---|---|---|
| GA at birth (weeks), median (IQR) | 40.3 (39.1–41) | 31.6 (28.7–34) | 30.3 (28–31.6) |
| PMA at scan (weeks), median (IQR) | 40.7 (39.4–41.4) | 41.3 (40.1–42.7) | 42.4 (41–43.4) |
| Female, No. (%) | 129 (47%) | 43 (46%) | 115 (45%) |
| HC at scan (cm), median (IQR) | 35 (33.5–36) | 35 (33.7–36.1) | 36.3 (35–37.2) |
| Weight at scan (kg), median (IQR) | 3.4 (3–3.8) | 3.1 (2.7–3.7) | 3.4 (2.8–3.8) |
| Weight at birth (kg), median (IQR) | 3.4 (3–3.7) | 1.6 (1–2) | 1.3 (1–1.59) |
| Nonsingleton, No. infants, (%) | — | 25 (28%) | 62 (25%) |
| Days of ventilation, No. infants, (%) median (IQR)* |
— | 45 (51%) 6 (2.5–20) |
116 (46%) 2 (1–5) |
| Days of TPN, No. infants, (%) median (IQR)* |
— | — | 160 (63%) 10 (6–15) |
| Days of CPAP, No. infants, (%) median (IQR)* |
— | 63 (71%) 7 (1–30) |
204 (81%) 11 (4–30) |
| IUGR, No. infants, (%) | — | 22 (25%) | 43 (17%) |
| PWMLs, No. infants, (%) | 34 (12%) | 27 (30%) | 52 (21%) |
| Cerebellar hemorrhages, No infants, (%) | — | 5 (6%) | 16 (6%) |
| HPI, No. infants, (%) | — | — | 11 (4%) |
| PVL, No. infants, (%) | — | — | 6 (2%) |
| Behavioral follow-up, No. infants (%) | 222 (81%) | 68 (76%) | 237 (94%) |
| Age (months), median (IQR) | 18.4 (18–18.7) | 18.5 (18–19.1) | 20.1 (20–20.6) |
| BSID-III Motor, median (IQR) | 103 (97–107) | 100 (94–107) | 95 (85–100) |
| BSID-III Language, median (IQR) | 97 (88–106) | 97 (86–106) | 91 (79–103) |
| BSID-III Cognition, median (IQR) | 100 (95–105) | 100 (90–105) | 95 (88–103) |
Notes: GA – Gestational age; IQR – interquartile range; PMA – Postmenstrual age; HC – head circumference; TPN – total parenteral nutrition; CPAP – continuous positive airway pressure; IURG – intrauterine growth restriction; PWMLs – punctate white matter lesions; HPI-Haemorrhagic Parenchymal Infarction; PVL – Periventricular leukomalacia; BSID-III – Bayley Scales of Infant Development III; Missing data: Birth HC – 11 preterm infants; birth weight-1 preterm. TPN data were not available for dHCP preterm infants. *median (IQR) calculated only for infants on ventilation/CPAP/TPN.
Typical Volumetric Development in Term-Born Infants During the Neonatal Period
We found an increase in all absolute volumes except the ventricles, where no change was detected (Fig. 1A, Supplementary Fig. 2). The increase was greatest in cGM (10.4% per week [pw]) and cerebellum (9.9% pw) compared with ICV (6.1% pw), TTV (6% pw), and CSF (7% pw). Subcortical structures increased between 4% and 6% pw (caudate L:4.1%, R:4.3%; lentiform L:6.6%, R:5.3%; thalamus L:4.1%, R:4.7% pw) with smaller increases in brainstem (3.9% pw) and WM (2% pw).
Figure 2 .
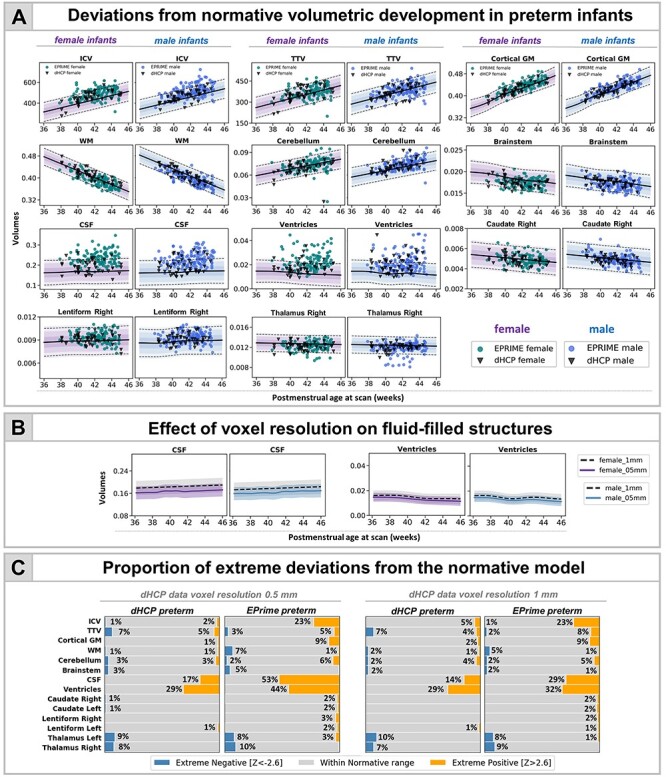
Characterizing the effects of preterm birth on the developing brain. (A) Deviations from normative volumetric development in preterm infants. Observations for individual preterm infants from both dHCP and EPrime cohorts are shown with model means for both female and male term-born infants together with ±1, ±2, and ±3 SDs. ICV, TBV, and TTV are in cm3; cGM, WM, cerebellum, brainstem, and subcortical structures shown as a proportion of TTV; CSF as a proportion of ICV and ventricles as a proportion of TTV. Horizontal lines show Z > |2.6|, the threshold used to define extreme deviations. The normative curves for the ventricles show data within 10 SD from the mean, full range is shown in Fig. 4 and discussed below. (B) Mean differences in fluid filled structures between GPR models build using 0.5- and 1-mm dHCP imaging resolution. (C) Proportion (%) of extreme deviations from the normative model in preterm infants. Extreme negative deviations (Z < -2.6) are depicted in blue, whereas extreme positive deviations (Z > 2.6) are shown in orange.
Figure 1 .
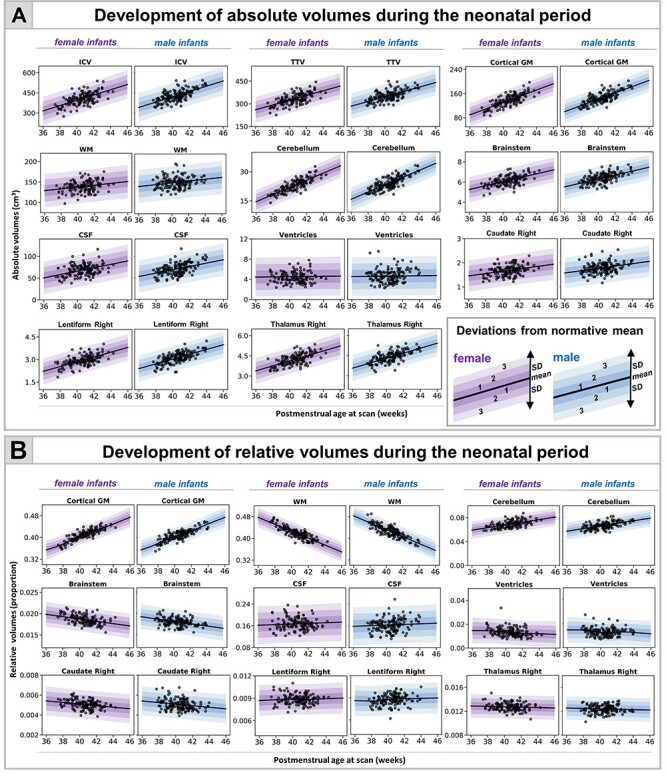
Normative modeling of volumetric development during the neonatal period. The model means for both female and male term infants are shown in purple and blue respectively, together with ±1, ±2, and ±3 SDs from the model means for absolute (A) and relative (B) volumes (tissue volumes represented as a proportion from TTV, ventricles from TBV, and CSF from ICV). Normative charts are shown only for right dGM structures (left structures are shown in Supplementary Fig. 2).
The greatest changes in relative volumes were observed in cGM and WM (Fig. 1B, Supplementary Fig. 2). cGM represented 36% of TTV at 37-week PMA and increased to 44% at 44-week PMA, whereas the relative WM volume decreased from 48% to 38% of TTV. The relative cerebellar volume increased from 6% to 7%. There was an increase in relative lentiform volume, a subtle decrease in caudate, and no change in thalamus. We observed a slight increase in CSF proportion of ICV and a steady decrease in the proportion that ventricles contributed to TBV. MAE for all models is shown in Supplementary Table 1.
Image Resolution and Volumetric Development
Overall, the majority of observations in both dHCP and EPrime preterm samples fit within 2.6 SD of the term-born model, indicating good agreement between the two studies (Fig. 2A). Differences were most profound in fluid-filled structures, likely attributable to partial voluming of high T2-signal CSF. In agreement, when compared with the models built using the original dHCP resolution of 0.5 mm, the matched 1-mm resolution models showed a mean shift (increase) for the CSF and ventricular volumes (Fig. 2B; Supplementary Figs 3 and 4). As a result, when using the lower resolution normative charts, the proportion of extreme positive deviations in EPrime decrease from 53% to 29% for CSF and from 44% to 32% for ventricles (Fig. 2C). All infants who showed extreme deviations in the matched 1-mm resolution showed extreme deviations in the original 0.5-mm resolution. Changes in the proportion of extreme deviations associated with image resolution for the rest of the structures were more subtle. Unless stated otherwise, data are presented for the 0.5-mm resolution models. The overall proportion of extreme deviations in the term-born sample was very low in all brain structures, with no more than 2% of the sample with Z-scores > |2.6| in the original 0.5-mm resolution.
Figure 5 .
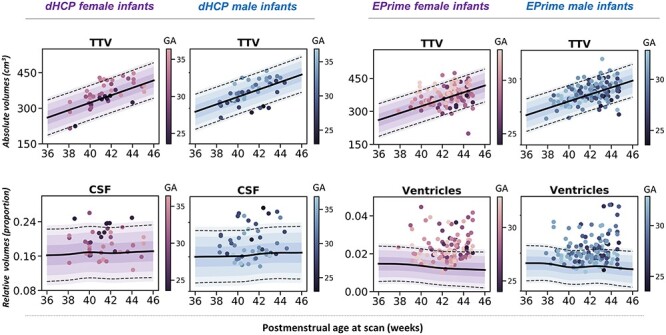
Association between degree of prematurity and deviations from normative brain development. In the dHCP preterm sample, increased degree of prematurity (lower GA at birth) was related to reduced TTV and increased CSF. In the EPrime sample, increased degree of prematurity was associated with reduced TTV and increased ventricular volume. Individual preterm observations are plotted against the normative model mean for female (purple) and male (blue) term infants. The plots also show ±1, ±2, and ±3 SDs from the normative means together with lines indicating Z > |2.6|, the threshold used to define extreme deviations. Ventricular data are shown only for infants with volume ± 10 SDs from the model mean.
Infants With Reduced TTV Suffered More Extreme Prematurity
Six dHCP preterm infants (7%) and 8 EPrime (3%) infants showed extreme negative deviations in TTV (Fig. 2C); all of which (except 1 EPrime infant) were born at GA < 30 weeks and weighed less than 1 kg at birth. Significantly reduced TTV was accompanied by enlarged CSF and ventricles (dHCP: 3/6; EPrime: 7/8). Four infants (dHCP: 1/6; EPrime 3/8) also had associated reduction in cerebellar volumes. A further 3 preterm infants (dHCP: 1/6; EPrime 2/8) had associated reduction in the thalami, bilaterally. All infants required oxygen support after birth. Infants who had TTV 2.6 SD above the model mean (dHCP: 4 [5%]; EPRIME: 18 [7%]) were born GA > 30 weeks and had no incidental findings, other than PWMLs, and short need for oxygen support and TPN after birth.
Infants With Reduced Thalamic Volume Also Had PWMLS
In the dHCP preterm sample, all 8 infants with extreme negative thalamic deviations had PWMLs, 7 of 8 had multiple lesions. Four out of these 7 infants had lesions involving the corticospinal tract (Fig. 3). Seven out of the 8 infants were on CPAP, but none of them for a long period of time (5 infants <4 days; 1 infant 11 days; 1 infant 18 days), and all 7 did not require ventilation. None of these infants had a birthweight of less than 1 kg. In EPrime, 17 infants had bilateral reduced thalamic volume and 10 unilateral extreme deviations (with structure in the other hemisphere close to but not reaching Z < -2.6). In total, 78% of these infants had PWMLs compared with 16% incidence in the rest of the sample. Overall, across the whole cohort, infants with PWMLs had significantly reduced left (d = 0.56) and right (d = 0.53) thalamic volumes, compared with infants without (both p < 0.05). In EPrime, infants with reduced thalamic volumes often had CSF or ventricular volumes significantly bigger than the normative values for their age/sex. In 5 infants, this was associated with PVL and in a further 2, with HPI.
Figure 3 .
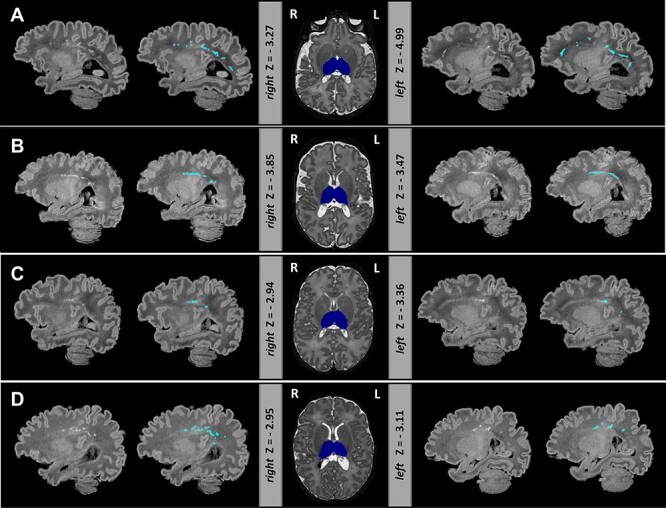
Extreme negative deviations in thalamic volume were often accompanied by PWML in the preterm brain. Depicted are four infants (A–D) with bilateral thalamic volumes significantly below the model mean. Thalamic segmentation (dark blue) is overlaid onto the T2-weighted images. T1-weighted images are shown with and without the manual outlined PWMLs (light blue). Note T1-weighted images were not used in the preprocessing but are shown here due to better contrast for detecting PWMLs.
Atypical Ventricular Development in Preterm Infants: Frequent But Highly Heterogeneous
Widening of the fluid-filled structures was the most frequently observed deviation from normative development in both cohorts. In the dHCP 29% and 17% of the preterm infants showed extreme deviations in ventricular and CSF volumes, respectively. This number was higher in EPrime where increased ventricles and CSF were seen in 44% and 53% of infants with the original 0.5-mm dHCP resolution and in 29% and 32% with the downsampled 1-mm resolution. Figure 4 shows the most extreme cases where infants’ ventricles were 10 SD above the model mean. These extreme deviations in ventricular volume were associated with overt focal brain injuries including HPI (infants 1, 2, 4, 6) and PVL (infants 5, 7). In all of these infants we also observed significant negative deviations in TTV or thalamus and increased CSF. These infants performed poorly at follow-up (Fig. 4).
Figure 4 .
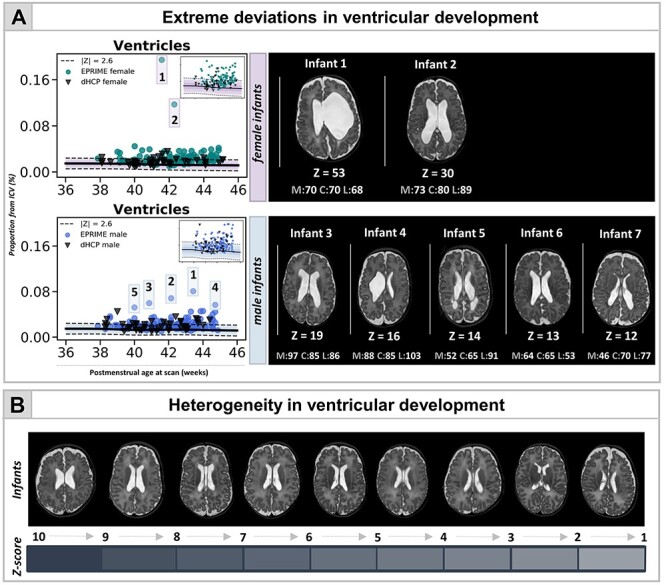
Capturing heterogeneity and extreme deviations in ventricular development in the preterm brain at TEA. (A) Normative curves are shown for both female and male infants (in upper right corner curves excluding the outliers, also shown in Fig. 2A). The figure also depicts the T2-weighted images for infants with ventricular volume lying 10 SD above the mean, separate for females (top) and males (bottom), together with their neurocognitive scores (M—motor, C—cognitive, L—language). Ventricular development in EPrime preterm infants is highly heterogeneous both in shape and size as illustrated in (B) showing ventricular volumes of various Z-scores.
Association Between Perinatal Risks and Deviations From Normative Development
In the dHCP cohort, decreased GA at birth related to reduced TTV (ρ = 0.45) and increased proportion of CSF (ρ = −0.44), whereas in EPrime to reduced TTV (ρ = 0.27) and increased relative ventricular volumes (ρ = −0.26) (all pcorr < 0.05) (Fig. 5; Supplementary Table 2). In both samples, greater birth weight Z-score was related to bigger ICV (dHCP: ρ = 0.41, EPrime: ρ = 0.36) and TTV (ρ = 0.40, ρ = 0.37) at TEA and in EPrime alone, to reduced relative brainstem (ρ = −0.26) and bilateral thalamic volumes (right: ρ = −0.28, left: ρ = −0.25) (all pcorr < 0.05; Supplementary Table 3).
In the dHCP preterm sample, longer requirement for CPAP related to smaller TTV (ρ = −0.47) and ICV (ρ = −0.37) as well as to increased proportion CSF (ρ = 0.39) and ventricles (ρ = 0.35). Longer need for mechanical ventilation was associated with reduced TTV (ρ = −0.44) and relative left caudate volume (ρ = −0.34) as well as with increased relative CSF (ρ = 0.39) and ventricular volumes (ρ = 0.42) (all pcorr < 0.05). Consistent with this, in EPrime, longer requirement for both CPAP and mechanical ventilation were related to reduced TTV (ρ = −0.30, ρ = −0.38, respectively) and increased relative ventricular volume (both at ρ = 0.22) (all pcorr < 0.05). Increased number of days requiring TPN were related to reduced TTV and ICV (ρ = −0.43, ρ = −0.35, pcorr < 0.05) and increased relative ventricular volume (ρ = 0.25, pcorr < 0.05; Supplementary Table 4).
Association Between Deviations From Normative Development and Neurocognitive Outcome
In the dHCP preterm sample, increased ventricular volume was related to poorer motor (ρ = −0.40) and language (ρ = −0.37) scores at 18 months (all pcorr < 0.05). In EPrime, increased CSF (ρ = −0.22) and cGM (ρ = −0.21) and reduced WM (ρ = 0.20) were associated with poorer language abilities, whereas reduced TTV (ρ = 0.20), increased CSF (ρ = −0.25), and cGM (ρ = −0.20) were linked to poorer cognitive performance (all pcorr < 0.05). While most of these associations were of similar effect size in the dHCP, they did not survive multiple comparison correction (Supplementary Table 5).
Discussion
The diverse cerebral consequences of preterm birth create significant challenges for understanding pathogenesis or predicting later neurocognitive outcomes. Focusing on individuals and their unique cerebral development can offer new insights. In this study, we first characterized normative volumetric development during the neonatal period, to then describe the effect of preterm birth at an individual infant level. We showed deviations from the normative curves consistent with previous studies but with marked variability among individuals. These individual deviations were associated with perinatal risks and later neurocognition.
We previously demonstrated that GPR could be used to detect subtle WM injury with high sensitivity (O’Muircheartaigh et al. 2020) and to characterize the heterogeneous consequences of preterm birth on the developing brain microstructure (Dimitrova et al. 2020). The present application of GPR to volumetric data offers more straightforward clinical translation. GPR provides normative charts describing typical volumetric development and can detect and quantify atypical maturation in individual infants (Ou et al. 2017). The GPR approach generalized to a cohort of infants with MRI data collected on a different MR scanner with different acquisition parameters. In the future, this method could be integrated into automatic tools that complement radiological decisions regarding infant development (Duerden and Thompson 2020). Our method and normative dataset are freely available for researchers to use for understanding pathogenesis, trialing interventions, and defining neurocognitive prognosis for vulnerable preterm infants.
We quantified rapid postnatal brain growth consistent with previous imaging and postmortem studies describing change in the size, organization, and complexity of the brain during the perinatal period (Huttenlocher and Dabholkar 1997; Hüppi et al. 1998; Knickmeyer et al. 2008; Kersbergen et al. 2016; Makropoulos et al. 2016). This dramatic growth is a sum product of a number of heterochronous developmental processes that take place in the developing brain including synaptogenesis, dendritic arborization, and early stages of myelination (Huttenlocher 1990; Huttenlocher and Dabholkar 1997; Travis et al. 2005; Petanjek et al. 2008; Lebenberg et al. 2019). Abrupt preterm extrauterine exposure represents a significant stressor to these events and may lead to widespread deviations from the normative trajectories in any or many of these processes as seen in pathology and preclinical models (Elovitz and Mrinalini 2004; Burd et al. 2012; Volpe 2019) associated with atypical trajectory of brain growth and a wide range of neurodevelopmental consequences (Inder et al. 2005; Bora et al. 2014; Ball et al. 2017; Gui et al. 2019). However, these alterations are not a result of loss of intrauterine environment alone but are a product of the cumulative effects of clinical and genetic factors creating individualized circumstances for every infant. GPR applied to a large normative dataset offers a powerful approach to study how preterm birth shapes the brain at an individual infant level and offers the means to capture important differences in single infants that may be missed by analysis of the means/medians of quasi-homogenous groups, which “averages-out” personal effects.
By quantifying this interindividual variability, our analysis clarified the relationship between reduced global brain growth and preterm birth. Many but not all studies show group-level differences in TTV between preterm and term-born infants (Boardman et al. 2007). We report a subset of infants in both preterm cohorts that deviated significantly from normative brain volumes. These infants were born very early, very small and had prolonged need for supplemental oxygen. Consistent with this, lower GA at birth, birthweight Z-score, longer requirement for respiratory support, and TPN were related to reduced TTV and enlarged CSF/ventricles in both preterm cohorts. Longitudinal studies suggest that these effects are not only evident at TEA but might also persist to childhood and later life (Nosarti 2002; Allin et al. 2004; Ment et al. 2009; de Kieviet et al. 2012; El Marroun et al. 2020). Not all extremely preterm infants had TTV deviations significantly below the model mean, which could explain the discrepancies found between previous group analyses studying the association between preterm birth and reduced brain volume. An individualized approach is now possible to address the important question of which protective factors or lack of adverse perinatal risks lead to typical global brain growth in these at-risk infants.
The period encompassing mid gestation and the last trimester of pregnancy is a critical phase for the development and establishment of the thalamocortical network (Kostović et al. 2014). During this short period, there are dynamic changes in thalamocortical efferent fiber organization and cortical lamination, including rapid axonal growth and the dissolution of the subplate (Kostovic and Rakic 1990; Vasung et al. 2011; Kostović et al. 2014). This makes the thalamus and connecting WM projections particularly vulnerable to injury as a result of preterm birth (Boardman et al. 2006; Ball et al. 2015) with studies suggesting abnormal development may persist beyond TEA (Lin et al. 2001). We reported a subset of preterm infants with thalamic volumes significantly below the model mean (Z < -2.6). These infants had a high load of PWMLs, and 5 of the EPrime infants had PVL, supporting previous findings of a close link between thalamic development and WM abnormalities, including a previous group analysis of the EPrime dataset (Boardman et al. 2006; Pierson et al. 2007; Ligam et al. 2009; Volpe 2009; Ball et al. 2015; Wisnowski et al. 2015; Tusor et al. 2017). The exact mechanisms that underlie reduced thalamic growth, possibly including neuronal loss and/or atypical developmental trajectory triggered by preterm extrauterine exposure, however, remain elusive (Volpe 2009).
Compared with the dHCP preterm cohort, the EPrime study comprised extremely preterm infants, which were sicker during clinical care, had overall poorer motor outcomes, and were scanned using different acquisition parameters. These factors in combination likely underlie some of the differences in associations between extreme deviations and later neurocognitive scores observed between the 2 datasets. The lower spatial resolution in EPrime in particular, contributed to the mean shift (increase) in CSF and ventricular volumes observed in the EPrime. With all this in mind, it was reassuring that deviations in brain development and their association with perinatal risks found in the dHCP broadly replicated in EPrime, indicating good generalization of the model to independent data collected on a different MRI scanner. We chose to use volumetric measures that are easy to calculate in research studies or routine clinical examinations. This could offer a direct clinical application, although given the regional heterochrony of early brain development (Lebenberg et al. 2019), future work should focus on more finely parcellated regions or more sophisticated MRI-derived features, including cortical thickness and surface area. We reported an association between deviations from normative brain development at TEA and behavior at 18–20 months. An important step for future research is to investigate whether these early brain deviations persist in later life and are predictive of childhood and later neurodevelopment (Boardman et al. 2020; George et al. 2020).
The argument that every brain is different is not novel, and the expectation that the effects of preterm birth are homogeneous and exactly alike in every infant is equally untenable. Individualized methodologies have been successfully applied in other fields (e.g., neuropsychology, Towgood et al. 2009; aging, Ziegler et al. 2014) and hold significant promise for the preterm infant. Although a group-mean difference is detectable using the conventional case–control approach, the significant heterogeneity would not be captured and effects of clinical significance to individual infants would be averaged out (Sled and Nossin-Manor 2013). Additionally, visually subtle effects may have prognostic significance when combined with other deviations from normative brain growth, for example reduced thalamic volume, and further analytic power may be gained by including covariates in the GPR model.
In summary, our approach offers a readily interpretable, generalizable, and more precise understanding of the cerebral consequences of preterm birth by focusing on the individual rather than the group average atypicality and in future might improve the predictive power of neuroimaging.
Funding
The dHCP project was funded by the European Research Council (ERC) under the European Union Seventh Framework Programme (FR/2007-2013)/ERC grant agreement no. 319456. The EPrime study was funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (grant reference no. RP-PG-0707-10154). The results leading to this publication have received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777394 for the project AIMS-2-TRIALS. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA and AUTISM SPEAKS, Autistica, SFARI. The authors acknowledge infrastructure support from the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' Hospitals NHS Foundation Trust. The study was supported in part by the Wellcome Engineering and Physical Sciences Research Council Centre for Medical Engineering at King's College London (grant WT 203148/Z/16/Z) and the Medical Research Council (UK) (grant MR/K006355/1). J.O. is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant 206675/Z/17/Z). G.M. received support from the Sackler Institute for Translational Neurodevelopment at King's College London and from National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre (BRC). J.O., A.D.E. and G.M. received support from the Medical Research Council Centre for Neurodevelopmental Disorders, King's College London (grant MR/N026063/1). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health and IMI 2JU.
Notes
The authors would like to thank all the families who dedicated their time to take part in these studies. Conflict of Interest: The authors declare no conflict of interest.
Supplementary Material
Contributor Information
Ralica Dimitrova, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK; Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK.
Sophie Arulkumaran, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Olivia Carney, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Andrew Chew, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Shona Falconer, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Judit Ciarrusta, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK; Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK.
Thomas Wolfers, Donders Centre for Cognitive Neuroimaging, Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen 6525EN, the Netherlands; Department of Cognitive Neuroscience, Radboud University Medical Centre, Nijmegen 6525EN, the Netherlands.
Dafnis Batalle, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK; Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK.
Lucilio Cordero-Grande, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK; Biomedical Image Technologies, ETSI Telecomunicacion, Universidad Politecnica de Madrid and CIBER-BBN, Madrid 28040, Spain.
Anthony N Price, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Rui P A G Teixeira, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Emer Hughes, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Alexia Egloff, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Jana Hutter, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Antonios Makropoulos, Biomedical Image Analysis Group, Department of Computing, Imperial College London, London SW7 2AZ, UK.
Emma C Robinson, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Andreas Schuh, Biomedical Image Analysis Group, Department of Computing, Imperial College London, London SW7 2AZ, UK.
Katy Vecchiato, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK; Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK.
Johannes K Steinweg, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Russell Macleod, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Andre F Marquand, Donders Centre for Cognitive Neuroimaging, Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen 6525EN, the Netherlands; Department of Cognitive Neuroscience, Radboud University Medical Centre, Nijmegen 6525EN, the Netherlands.
Grainne McAlonan, Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK; MRC Centre for Neurodevelopmental Disorders, King’s College London, London SE1 1UL, UK; South London and Maudsley NHS Foundation Trust, London SE5 8AZ, UK.
Mary A Rutherford, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Serena J Counsell, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Stephen M Smith, Centre for Functional MRI of the Brain (FMRIB), Wellcome Centre for Integrative Neuroimaging, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford OX3 9DU, UK.
Daniel Rueckert, Biomedical Image Analysis Group, Department of Computing, Imperial College London, London SW7 2AZ, UK.
Joseph V Hajnal, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK.
Jonathan O’Muircheartaigh, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK; Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK; MRC Centre for Neurodevelopmental Disorders, King’s College London, London SE1 1UL, UK.
A David Edwards, Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London SE1 7EH, UK; MRC Centre for Neurodevelopmental Disorders, King’s College London, London SE1 1UL, UK.
References
- Agrawal S, Rao SC, Bulsara MK, Patole SK. 2018. Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics. 142:e20180134. [DOI] [PubMed] [Google Scholar]
- Allin M, Henderson M, Suckling J, Nosarti C, Rushe T, Fearon P, Stewart AL, Bullmore ET, Rifkin L, Murray R. 2004. Effects of very low birthweight on brain structure in adulthood. Dev Med Child Neurol. 46:46–53. [DOI] [PubMed] [Google Scholar]
- Ball G, Aljabar P, Nongena P, Kennea N, Gonzalez-Cinca N, Falconer S, Chew ATM, Harper N, Wurie J, Rutherford MA et al. 2017. Multimodal image analysis of clinical influences on preterm brain development. Ann Neurol. 82:233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Pazderova L, Chew A, Tusor N, Merchant N, Arichi T, Allsop JM, Cowan FM, Edwards AD, Counsell SJ. 2015. Thalamocortical connectivity predicts cognition in children born preterm. Cereb Cortex. 25:4310–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. 2006. Bayley scales of infant and toddler development. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Boardman JP, Counsell SJ. 2020. Invited review: factors associated with atypical brain development in preterm infants: insights from magnetic resonance imaging. Neuropathol Appl Neurobiol. 46:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JP, Counsell SJ, Rueckert D, Hajnal JV, Bhatia KK, Srinivasan L, Kapellou O, Aljabar P, Dyet LE, Rutherford MA et al. 2007. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol. 62:185–192. [DOI] [PubMed] [Google Scholar]
- Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P, Hajnal J, Allsop JM, Rutherford MA, Edwardsa AD. 2006. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage. 32:70–78. [DOI] [PubMed] [Google Scholar]
- Boardman JP, Hall J, Thrippleton MJ, Reynolds RM, Bogaert D, Davidson DJ, Schwarze J, Drake AJ, Chandran S, Bastin ME et al. 2020. Impact of preterm birth on brain development and long-term outcome: protocol for a cohort study in Scotland. BMJ Open. 10:e035854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora S, Pritchard VE, Chen Z, Inder TE, Woodward LJ. 2014. Neonatal cerebral morphometry and later risk of persistent inattention/hyperactivity in children born very preterm. J Child Psychol Psychiatry Allied Discip. 55:828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Balakrishnan B, Kannan S. 2012. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 67:287–294. [DOI] [PubMed] [Google Scholar]
- Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M et al. 2019. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Heal. 7:e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Donaldson MDC, Ben-shlomo Y. 2010. SITAR—a useful instrument for growth curve analysis. Int J Epidemiol. 39:1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Grande L, Hughes EJ, Hutter J, Price AN, Hajnal JV. 2018. Three-dimensional motion corrected sensitivity encoding reconstruction for multi-shot multi-slice MRI: application to neonatal brain imaging. Magn Reson Med. 79:1365–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruïne FT, van den Berg-Huysmans AA, Leijser LM, Rijken M, Steggerda SJ, van der Grond J, van Wezel-Meijler G. 2011. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology. 261:899–906. [DOI] [PubMed] [Google Scholar]
- de Kieviet JF, Zoetebier L, Van Elburg RM, Vermeulen RJ, Oosterlaan J. 2012. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 54:313–323. [DOI] [PubMed] [Google Scholar]
- Dimitrova R, Pietsch M, Christiaens D, Ciarrusta J, Wolfers T, Batalle D, Hughes E, Hutter J, Cordero-Grande L, Price AN et al. 2020. Heterogeneity in brain microstructural development following preterm birth. Cereb Cortex. 30:4800–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Thompson DK. 2020. Can you see what I see? Assessing brain maturation and injury in preterm and term neonates. Brain. 143:383–386. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Redshaw ME, Kennea N, Rivero-Arias O, Gonzales-Cinca N, Nongena P, Ederies M, Falconer S, Chew A, Omar O et al. 2018. Effect of MRI on preterm infants and their families: a randomised trial with nested diagnostic and economic evaluation. Arch Dis Child Fetal Neonatal Ed. 103:F15–F21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Zou R, Leeuwenburg MF, Steegers EAP, Reiss IKM, Muetzel RL, Kushner SA, Tiemeier H. 2020. Association of gestational age at birth with brain morphometry. JAMA Pediatr. 174:1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz MA, Mrinalini C. 2004. Animal models of preterm birth. Trends Endocrinol Metab. 15:479–487. [DOI] [PubMed] [Google Scholar]
- George JM, Pagnozzi AM, Bora S, Boyd RN, Colditz PB, Rose SE, Ware RS, Pannek K, Bursle JE, Fripp J et al. 2020. Prediction of childhood brain outcomes in infants born preterm using neonatal MRI and concurrent clinical biomarkers (PREBO-6): study protocol for a prospective cohort study. BMJ Open. 10:e036480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui L, Loukas S, Lazeyras F, Hüppi PS, Meskaldji DE, Borradori Tolsa C. 2019. Longitudinal study of neonatal brain tissue volumes in preterm infants and their ability to predict neurodevelopmental outcome. Neuroimage. 185:728–741. [DOI] [PubMed] [Google Scholar]
- Holland D, Chang L, Ernst TM, Curran M, Buchthal SD, Alicata D, Skranes J, Johansen H, Hernandez A, Yamakawa R et al. 2014. Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurol. 71:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EJ, Winchman T, Padormo F, Teixeira R, Wurie J, Sharma M, Fox M, Hutter J, Cordero-grande L, Price AN et al. 2017. A dedicated neonatal brain imaging system. Magn Reson Med. 78:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. 1998. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 43:224–235. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. 1990. Morphometric study of human cerebral cortex development. Neuropsychologia. 28:517–527. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387:167–178. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. 2005. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 115:286–294. [DOI] [PubMed] [Google Scholar]
- Kersbergen KJ, Makropoulos A, Aljabar P, Groenendaal F, de Vries LS, Counsell SJ, Benders MJNL. 2016. Longitudinal regional brain development and clinical risk factors in extremely preterm infants. J Pediatr. 178:93–100e6. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. 2008. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 28:12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N, Radoš M, Sedmak G, Benjak V, Kostović-Srzentić M, Vasung L, Čuljat M, Radoš M, Hüppi P et al. 2014. Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct. 219:231–253. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. 1990. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 297:441–470. [DOI] [PubMed] [Google Scholar]
- Lebenberg J, Mangin JF, Thirion B, Poupon C, Hertz-Pannier L, Leroy F, Adibpour P, Dehaene-Lambertz G, Dubois J. 2019. Mapping the asynchrony of cortical maturation in the infant brain: a MRI multi-parametric clustering approach. Neuroimage. 185:641–653. [DOI] [PubMed] [Google Scholar]
- Ligam P, Haynes RL, Folkerth RD, Liu L, Yang M, Volpe JJ, Kinney HC. 2009. Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatr Res. 65:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Okumura A, Hayakawa F, Kato T, Kuno K, Watanabe K. 2001. Quantitative evaluation of thalami and basal ganglia in infants with periventricular leukomalacia. Dev Med Child Neurol. 43:481–485. [DOI] [PubMed] [Google Scholar]
- Makropoulos A, Aljabar P, Wright R, Hüning B, Merchant N, Arichi T, Tusor N, Hajnal JV, Edwards AD, Counsell SJ et al. 2016. Regional growth and atlasing of the developing human brain. Neuroimage. 125:456–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makropoulos A, Robinson EC, Schuh A, Wright R, Fitzgibbon S, Bozek J, Counsell SJ, Steinweg J, Vecchiato K, Passerat-Palmbach J et al. 2018. The developing Human Connectome Project: a minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage. 173:88–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquand AF, Kia SM, Zabihi M, Wolfers T, Buitelaar JK, Beckmann CF. 2019. Conceptualizing mental disorders as deviations from normative functioning. Mol Psychiatry. 24:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquand AF, Rezek I, Buitelaar J, Beckmann CF. 2016. Understanding heterogeneity in clinical cohorts using normative models: beyond case-control studies. Biol Psychiatry. 80:552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Kesler S, Vohr B, Katz KH, Baumgartner H, Schneider KC, Delancy S, Silbereis J, Duncan CC, Constable RT et al. 2009. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 123:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C. 2002. Adolescents who were born very preterm have decreased brain volumes. Brain. 125:1616–1623. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, Mac Cabe J, Rifkin L, Hultman CM. 2012. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 69:610–617. [DOI] [PubMed] [Google Scholar]
- O’Muircheartaigh J, Robinson E, Pietsch M, Wolfers T, Aljabar P, Grande LC, Teixeira RPAG, Bozek J, Schuh A et al. 2020. Modelling brain development to detect white matter injury in term and preterm born neonates. Brain. 143:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Zöllei L, Retzepi K, Castro V, Bates SV, Pieper S, Andriole KP, Murphy SN, Gollub RL, Grant PE. 2017. Using clinically acquired MRI to construct age-specific ADC atlases: quantifying spatiotemporal ADC changes from birth to 6-year old. Hum Brain Mapp. 38:3052–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Kostović I, Uylings HBM. 2008. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 18:915–929. [DOI] [PubMed] [Google Scholar]
- Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC. 2007. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 114:619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Nossin-Manor R. 2013. Quantitative MRI for studying neonatal brain development. Neuroradiology. 55:97–104. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Matthews LG, Alexander B, Lee KJ, Kelly CE, Adamson CL, Hunt RW, Cheong JLY, Spencer-Smith M, Neil JJ et al. 2020. Tracking regional brain growth up to age 13 in children born term and very preterm. Nat Commun. 11:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towgood KJ, Meuwese JDI, Gilbert SJ, Turner MS, Burgess PW. 2009. Advantages of the multiple case series approach to the study of cognitive deficits in autism spectrum disorder. Neuropsychologia. 47:2981–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K, Ford K, Jacobs B. 2005. Regional dendritic variation in neonatal human cortex: a quantitative Golgi study. Dev Neurosci. 27:277–287. [DOI] [PubMed] [Google Scholar]
- Tusor N, Benders MJ, Counsell SJ, Nongena P, Ederies MA, Falconer S, Chew A, Gonzalez-Cinca N, Hajnal JV, Gangadharan S et al. 2017. Punctate white matter lesions associated with altered brain development and adverse motor outcome in preterm infants. Sci Rep. 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Jovanov-Milošević N, Pletikos M, Mori S, Judaš M, Kostović I. 2011. Prominent periventricular fiber system related to ganglionic eminence and striatum in the human fetal cerebrum. Brain Struct Funct. 215:237–253. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. 2009. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. 2019. Dysmaturation of premature brain: importance, cellular mechanisms, and potential interventions. Pediatr Neurol. 95:42–66. [DOI] [PubMed] [Google Scholar]
- Wisnowski JL, Ceschin RC, Choi SY, Schmithorst VJ, Painter MJ, Nelson MD, Blüml S, Panigrahy A. 2015. Reduced thalamic volume in preterm infants is associated with abnormal white matter metabolism independent of injury. Neuroradiology. 57:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers T, Beckmann CF, Hoogman M, Buitelaar JK, Franke B, Marquand AF. 2020. Individual differences v. The average patient: mapping the heterogeneity in ADHD using normative models. Psychol Med. 50:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G, Ridgway GR, Dahnke R, Gaser C. 2014. Individualized Gaussian process-based prediction and detection of local and global gray matter abnormalities in elderly subjects. Neuroimage. 97:333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Normative term-born data and the GPR code used in this study are freely available on GitHub (https://github.com/ralidimitrova). All imaging data collected for the dHCP will be publicly available in early 2021 at http://developingconnectome.org/.


