Abstract
Individuals with autism spectrum disorder (ASD) show motor impairment into adulthood and risk decline during aging, but little is known about brain changes in aging adults with ASD. Few studies of ASD have directly examined the corticospinal tract (CST)—the major descending pathway in the brain responsible for voluntary motor behavior—outside its primary motor (M1) connections. In 26 middle-aged adults with ASD and 26 age-matched typical comparison participants, we used diffusion imaging to examine the microstructure and volume of CST projections from M1, dorsal premotor (PMd), supplementary motor area (SMA), and primary somatosensory (S1) cortices with respect to age. We also examined relationships between each CST sub-tract (-cst), motor skills, and autism symptoms. We detected no significant group or age-related differences in tracts extending from M1 or other areas. However, sub-tracts of the CST extending from secondary (but not primary) motor areas were associated with core autism traits. Increased microstructural integrity of left PMd-cst and SMA-cst were associated with less-severe restricted and repetitive behaviors (RRB) in the ASD group. These findings suggest that secondary motor cortical areas, known to be involved in selecting motor programs, may be implicated in cognitive motor processes underlying RRB in ASD.
Keywords: aging, diffusion MRI, motor, RRB, white matter
Introduction
Autism spectrum disorders (ASD) are defined by socio-communicative deficits and repetitive and restricted patterns of behavior (American Psychiatric Association 2013). Individuals with ASDs also frequently display a variety of motor abnormalities, including impaired fine and gross motor skills, reduced balance, coordination and postural control, and abnormal gait (Jansiewicz et al. 2006; Dewey et al. 2007; Dziuk et al. 2007; Green et al. 2009; Fournier et al. 2010; Bhat et al. 2011; Nobile et al. 2011; Downey and Rapport 2012; Cassidy et al. 2016; Kaur et al. 2018). These motor difficulties (or delays) may be detected even prior to an ASD diagnosis (Iverson et al. 2019) and persist across the life span (Minshew et al. 2004; Ming et al. 2007; Dawson et al. 2010; Abu-Dahab et al. 2013; Lloyd et al. 2013; Travers et al. 2017; Linke et al. 2019).
During toddlerhood, longitudinal studies show that motor abilities further diverge from typical development from the age of 1–3 years with fine and gross motor skills falling even further behind in ASD (Dawson et al. 2010; Lloyd et al. 2013). In childhood and adolescence, increasing impairment in grip strength and motor speed was reported with age but decreasing incoordination in a sample aged 5–21 years (Abu-Dahab et al. 2013). Another study found that the incidence of hypotonia, apraxia, and gross motor deficits in ASD was lower in older children/adolescents (aged 7–18 years) than younger children (aged 2–6 years) (Ming et al. 2007), suggesting improvement in some motor skills in adolescence.
Motor function declines in healthy aging (Potvin et al. 1980) and, in ASD, this may exacerbate preexisting motor deficits, described above. A longitudinal study in a sample aged 5–40 years found that participants with ASD did not keep up with gains seen in typical comparison (TC) peers such that the ASD group fell further behind from adolescence to middle age (Travers et al. 2017). A study in a similar age range (5–52 years) found delayed development of postural stability in ASD, which remained well below typical adult levels (Minshew et al. 2004). Among the few studies in middle-aged to older adults with ASD, our group found impaired basic motor skills (manual dexterity, strength and flexibility, and coordination) in the same middle-aged adults with ASD as in the present study when compared with typical controls, suggesting persistent motor difficulties (Linke et al. 2019). Additionally, there may be an increased risk of neurodegenerative disease in this population as a few studies have found that adults with ASD may be at heightened risk of parkinsonism and other adult-onset neurological disorders (Croen et al. 2015; Starkstein et al. 2015). Reduced mobility, especially in older persons, can significantly impact the quality of life (Ferrucci et al. 2016). Therefore, an understanding of the age-related trajectories of motor systems in middle-aged and older individuals with ASD is needed (Piven and Rabins 2011; Torres et al. 2020; Wise 2020).
Despite the strong behavioral evidence of impaired motor function in ASD, relatively little is known regarding the underlying neural mechanisms, particularly in adults. Among the few adult studies, interhemispheric functional underconnectivity of primary motor (M1), primary somatosensory and premotor areas in middle-aged adults with ASD (Linke et al. 2019), increased activation in right pericentral and premotor cortex during visuomotor learning (Müller et al. 2004), and reduced functional and structural connectivity between the pre- and postcentral gyri in young to middle-aged adults with ASD have been found (Thompson et al. 2017; Linke et al. 2019). Compromised microstructure of right-hemisphere u-fibers underlying the hand knob area of the primary motor cortex has also been associated with poorer motor performance in young to middle-aged adults with ASD (Thompson et al. 2017).
The cortical motor system, modulated by subcortical and cerebellar circuits, is involved in planning, selecting, and controlling voluntary movements which are crucial for interacting with the external environment (Rizzolatti and Luppino 2001). The corticospinal tract (CST) is the major output pathway from the motor cortices to the peripheral nervous system. While best known for carrying axons from bilateral primary motor cortex to the spinal cord, the CST descends from functionally more diverse cortical motor and even sensory areas, including bilateral primary somatosensory, premotor, and supplementary motor areas (SMAs) (Dum and Strick 1991; He et al. 1993; Galea and Darian-Smith 1994; Ueno et al. 2018). How this diverse corticospinal activity is transformed into motor output within the ventral horn is still not fully understood and appears to integrate excitatory and inhibitory inputs including “mirror-like” processes. For example, corticospinal neurons are active during action observation and could play an important role in suppressing movements during mental rehearsal/action observation (Kraskov et al. 2009; Vigneswaran et al. 2013) or predicting/anticipating movements (Amoruso et al. 2018). Therefore, the CST appears to play a complex role in modulating neural sensorimotor activity between the brain and body, which could be implicated in ASD where abnormalities in sensorimotor processing are frequent.
Several ASD studies have examined the CST or its passage through the posterior limb of the internal capsule (PLIC), with mostly consistent findings. Elevated fractional anisotropy (FA) in the PLIC has been found in infants later diagnosed with ASD and in toddlers with ASD (Wolff et al. 2012; Andrews et al. 2019). A longitudinal study found that infants later diagnosed with ASD showed a slower increase in FA from 6–24 months when compared with an ASD-negative at-risk group (Wolff et al. 2012). In children and adolescents with ASD, studies have found reduced FA and increased mean diffusivity (MD) and radial diffusivity (RD) in bilateral CST (Carper et al. 2015), within the white matter skeleton corresponding to the CST (Jou et al. 2011; Shukla et al. 2011) and within the regions of interest corresponding to right CST and bilateral PLIC (Brito et al. 2009). However, at least one study of children with ASD did not detect a difference in FA or diffusivity measures of the CST (Koldewyn et al. 2014). Additionally, one study found a loss of typical leftward lateralization of CST volume in children and adolescents with ASD (Carper et al. 2015). A longitudinal study found that reduced FA of bilateral PLIC in children with ASD normalized in adolescence and adulthood, while (somewhat paradoxically) the gap in motor skills increased between ASD and typically developing individuals (McLaughlin et al. 2018). As with cortical motor findings, in adults, CST findings remain rather sparse. One study in a sample with a wide age range (30–74 years) found increased MD and RD in ASD in right CST but found no age-related group effects (Koolschijn et al. 2016).
Intriguingly, there is now growing evidence of a link between motor skills and autism symptom severity (Fulceri et al. 2019; Ohara et al. 2019), but few studies have investigated the neural correlates linking these domains using brain imaging. One study found a correlation between grip strength performance and social skills deficits and that the relationship between them was mediated by FA of CST white matter at the level of the brainstem in children and adults aged 5–33 years with ASD (Travers et al. 2015). Functional connectivity of motor cortex has been linked with severity of both social communication deficits (Nebel et al. 2016; Oldehinkel et al. 2019) and restricted and repetitive behaviors (Abbott et al. 2018) in children with ASD. Indeed, understanding how and which motor systems relate to different aspects of autism symptoms will be crucial for understanding the mechanisms underlying this relationship.
The CST comprises connections between primary motor, primary somatosensory, premotor, and supplementary areas with the spinal cord. Advances in diffusion magnetic resonance imaging (MRI) methods modeling more than a single fiber population per voxel permit the in vivo study of the CST in humans more comprehensively than before (Farquharson et al. 2013; Archer et al. 2017; Chenot et al. 2019). However, none of the previously mentioned studies that examined the CST (via tractography) in ASD discriminated between connections from primary motor cortex and those from other cortical areas (Koldewyn et al. 2014; Carper et al. 2015; Koolschijn et al. 2016). But retrograde tracer studies in the macaque estimate that less than 50% of CST projections originate from the primary motor cortex (Dum and Strick 1991; Galea and Darian-Smith 1994). Thus far, no diffusion study has examined the CST with respect to specific cortical areas that may each have different effects on the motor behavior in ASD and the different rates of change during development and aging. The present study examines for the first time the diverse projections of the CST and their relation to age in middle-aged adults with ASD compared with a control group matched for age, gender, handedness, and in-scanner head motion as well as their relationship with autism symptoms and motor performance. Given the previous reports of compromised CST microstructure in adolescents and adults with ASD and the increased risk of neurodegenerative disease (e.g., parkinsonism) in middle-aged and older adults with ASD, we hypothesize CST from the primary motor cortex (M1-cst) to show compromised microstructure (reduced FA and increased MD) and a steeper age-related decline in the ASD group. Although previous studies have not specifically looked at the nonprimary motor CST, that is, primary somatosensory and secondary motor portions, in ASD, we hypothesize them to show similar effects as for M1-cst.
Materials and Methods
Participants
Seventy-three participants (35 ASD, 37 TC), aged 40–70 years, were recruited from San Diego County communities through referrals from autism clinics, service providers (e.g., day programs, group homes, and meetups), and local advertisement as part of an ongoing longitudinal study. Individuals with comorbid autism-related medical conditions (e.g., Fragile-X syndrome, tuberous sclerosis, and epilepsy) or other neurological conditions (e.g., Tourette syndrome) were not eligible for participation. TC participants had no family history of autism or personal history of other neurological conditions or serious mental illness. There was no formal minimum intelligence quotient (IQ) requirement, although all participants needed sufficient functional communication to cooperate with magnetic resonance imaging (MRI) and complete neuropsychological assessments. Seventeen datasets were excluded after quality assessment of the imaging data (12 were affected by a hardware issue, 2 scans were incomplete, and 3 had low quality anatomical images) and three participants were excluded for incidental brain findings. The final sample consisted of 26 ASD and 26 TC participants.
A preexisting diagnosis was not required for recruitment. All ASD diagnoses were made or confirmed by a clinical psychologist according to the DSM-5 criteria (American Psychiatric Association 2013) and were supported by module 4 of the Autism Diagnostic Observation Schedule, second edition (Lord et al. 2002) which was administered as part of the research study (current Autism Diagnostic Observation Scale [ADOS] scores were not available for one participant due to administrator error, however, this individual met the ASD criteria on a research-related ADOS within the preceding 5 years). IQ was assessed with the Wechsler Abbreviated Scale of Intelligence, second edition (WASI-II) (Wechsler 2011). The Edinburgh Handedness Inventory was used to determine manual preference (Oldfield 1971). Motor function was assessed using the Bruininks Motor Ability Test (BMAT) Short Form (Bruininks and Bruininks 2012).
The study was approved by the San Diego State University and University of California, San Diego institutional review boards. All participants (or their conservators) gave written informed consent before participation.
Image Acquisition
MRI data were collected at the Center for Functional Magnetic Resonance Imaging (CFMRI, University of California, San Diego) on a 3T GE Discovery MR750 scanner. Diffusion-weighted images were acquired with 46 noncolinear directions at one of two b-values, b = 1500 (14 ASD and 9 TC participants) or 1000 s/mm2 (12 ASD and 17 TC participants) and 6 interspersed volumes at b = 0 s/mm2 (time repetition [TR] = 4000 ms, time echo [TE] = 89 ms, multiband factor = 3, 27 slices/band, field of view = 24 cm, 1.7 mm3 spatial resolution) with reversed (anterior–posterior) phase encoding to correct for susceptibility distortions and to improve the signal-to-noise ratio. A high-resolution anatomical T1-weighted MPRAGE image (TR = 8.776 ms, TE = 3.656 ms, flip angle = 8°, matrix 320 × 320, 0.8 mm3 spatial resolution) was also acquired.
Data Preprocessing
The diffusion-weighted images were preprocessed using the FMRIB Software Library (FSL) (Jenkinson et al. 2012). The susceptibility-induced off-resonance field was estimated and corrected from the reversed phase encoding image pairs using “topup” (Andersson et al. 2003). Inter- and intra-volume head motion, signal dropout, and eddy current distortions were corrected with “eddy,” including outlier slice replacement, using information from both individual slices and multiband slice groupings (Andersson et al. 2016; Andersson and Sotiropoulos 2016; Andersson et al. 2017). Rotation corresponding to the motion parameters was applied to the diffusion direction matrix (b-vectors). Due to the difference in b-values between the two protocols, the data were harmonized (see Statistical Analysis), and a protocol variable was included in all statistical analyses. The diagnostic groups were matched on protocol.
Root mean square displacement of the diffusion-weighted volumes relative to the first nondiffusion-weighted volume and the percentage of slices (out of the total number of slices in diffusion-weighted volumes) affected by the signal dropout from motion were used to quantify and match the groups on the head motion. Diffusion tensor imaging measures, including FA and MD, were estimated using a weighted least-squares fit.
Fiber Tractography
Probability distribution functions of the local fiber orientations at each voxel were built using a ball-and-stick model, allowing up to three fiber orientations per voxel (Behrens et al. 2007). Probabilistic tractography was performed in diffusion space with FSL’s “Probtrackx2” by sampling from the orientation distributions starting from seed regions, as described below, using the following parameters: 5000 seeds per voxel, modified Euler integration, step length = 0.5 mm, curvature = 0.2 (Behrens et al. 2003, 2007), and was run on GPUs for faster computing times (Hernandez-Fernandez et al. 2019).
To reconstruct the CST sub-tracts, we seeded from functionally defined sensorimotor areas: left and right primary motor (M1), dorsal premotor (PMd), ventral premotor (PMv), SMA, and primary somatosensory (S1) regions from the Human Motor Area Template (HMAT, Fig. 1A) (Mayka et al. 2006). (The pre-SMA was not included, given the lack of anatomical data supporting corticospinal projections from this region (Luppino et al. 1994).) The template regions were nonlinearly warped from the Montreal Neurological Institute (MNI) space to subject diffusion space via the anatomical image using ANTs registration (Avants et al. 2008) followed by boundary-based registration within subjects (Greve and Fischl 2009). Regions of interest covering the left and right PLIC and left and right cerebral peduncles (CP) were drawn in the MNI space on three consecutive axial slices: z = 7–9 mm and − 29 to −31 mm in the MNI coordinates, respectively (Fig. 1A), and were warped to the diffusion space using the same MNI–diffusion space transformation. Tracking was constrained to pass through ipsilateral PLIC and to pass through and stop in ipsilateral CP.
Figure 1 .
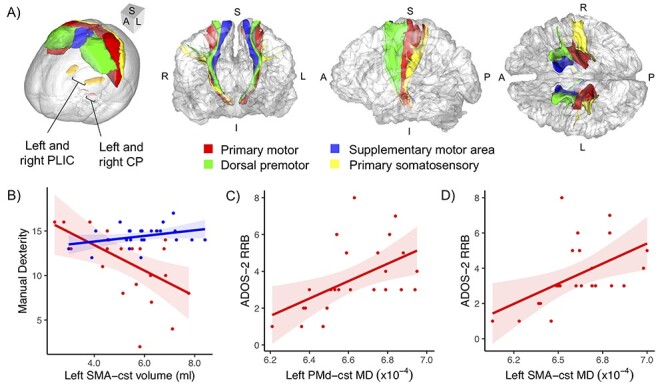
(A) The seed regions from the HMAT (Mayka et al. 2006), labeled by color, and waypoint/stop regions (PLIC and CP, labeled) used for tractography, shown in MNI space (far left), and the cst connections, in corresponding colors, in anterior, lateral, and superior views (left to right) shown for a right-handed ASD male, 49 years old. (B–D) Associations between cst measures and behavior (BMAT and ADOS-2 subscores). (B) Left SMA-cst volume was differentially associated with manual dexterity across diagnostic groups, being negative in ASD and positive in TC. Greater RRB severity was associated with increased MD of the (C) left PMd-cst and (D) left SMA-cst in the ASD group. Shaded areas represent the 95% confidence interval. S, superior; I, inferior; A, anterior; P, posterior; L, left; R, right.
The tract probability maps were threshold at 1% of their maximum values and were binarized to create a tract mask. Tract volume was calculated as the total volume of the binarized tract mask. Tract-specific diffusivity measures were calculated by averaging the values across voxels within the binary tract mask.
Statistical Analysis
Statistical analyses were conducted in R (R Core Team 2016), and plots were created using the package “ggplot2” (Wickham 2016). t-tests and chi-square tests (for categorical variables) were used to assess the differences between the ASD and TC groups on age, sex, handedness, IQ, and head motion measures. The diffusion scalar maps (FA and MD) were harmonized across the acquisition protocol (b-value) using an empirical Bayes’-based method (Johnson et al. 2007) that was implemented for imaging data and demonstrated to be robust for small sample sizes and biased samples (ComBat; Fortin et al. 2017). Harmonization was performed at a voxel-wise level on the scalar maps warped to MNI space, which were warped back to the diffusion space to extract the tract measures. The individual values for tract volume were also harmonized across protocol using ComBat. Subjects’ tracts that exceeded +/− 3 standard deviations (SDs) from the mean on any of the measures were removed as outliers. Tracts affected included: both right PMd-cst and right SMA-cst (two TC participants), both left PMd-cst and left SMA-cst (one ASD participant), right PMd-cst only (one ASD participant), right S1-cst only (one TC participant), and right M1-cst only (one ASD participant).
Permutation testing with 5000 iterations was used for all tests to control for type I error. Analyses of covariance (ANCOVAs) were performed for each tract and measure (FA, MD, and volume) to assess the effects of group, age, and group-by-age interactions. Post hoc partial correlations were run to explore age and group-by-age effects. Motion-related slice dropout and a dummy variable coded for the different b-value protocols were included as covariates in all analyses involving tract measures to control for imaging protocol and head motion, respectively. To examine brain–behavior relationships, partial Pearson correlations were performed on each tract measure with the coordination, manual dexterity, and strength and flexibility subscores of the BMAT, in the ASD and TC groups separately. To account for the ordinal, noncontinuous nature of Autism Diagnostic Observation Scale, second edition (ADOS-2) measures, partial Spearman’s correlations were performed on each tract measure with social affect (SA) and restricted and repetitive behaviors (RRB) subscores in the ASD group. Partial correlations adjusted for age, motion, and protocol. The fine motor and balance and mobility subscores of the BMAT were not tested due to their very limited ranges (ceiling effect). False discovery rate (FDR) correction (Benjamini and Hochberg 1995) was applied to the ANCOVA and partial correlation analyses for each variable of interest (e.g., diagnosis by age, age, and ADOS subscore) separately, controlling for the number of left and right cst tracts and measures (24 tests). All analyses were additionally performed excluding the left-handed individuals to investigate effects that may depend on handedness, as brain activation and morphology have been linked to handedness (Dassonville et al. 1997; Amunts et al. 2000).
Results
The groups were matched on age, sex, handedness, nonverbal IQ, protocol, and head motion (Table 1). All tracts were reconstructed successfully (Fig. 1A), with the exception of the ventral premotor cst. That tract was highly variable across subjects regardless of group, appearing very extensive in some subjects and very thin and narrow in others. This was reflected in large coefficients of variation (CV) in both volume (CV = 0.42, average CV for other tracts = 0.27) and streamline count (CV = 2.45, average CV for other tracts = 0.87) averaged across hemispheres. The ventral premotor cst was therefore excluded from further analyses. Table 2 shows the means and SDs of cst sub-tract volumes by diagnostic group.
Table 1.
Participant characteristics and group matching
| ASD (n = 26)a | TC (n = 26) | P b | |||
|---|---|---|---|---|---|
| Mean ± SD [range] | Mean ± SD [range] | ||||
| Gender (M/F) | 21/5 | 23/3 | 0.44 | ||
| Handedness (R/L) | 24/2 | 23/3 | 0.64 | ||
| Age (years) | 51.1 ± 6.0 | [41–64] | 51.6 ± 6.1 | [40–61] | 0.77 |
| WASI-II verbal IQ | 103 ± 24 | [52–160] | 115 ± 14 | [85–144] | 0.04 |
| Nonverbal IQ | 115 ± 14 | [85–144] | 114 ± 12 | [93–138] | 0.15 |
| Full-scale IQ | 106 ± 22 | [51–143] | 116 ± 12 | [92–138] | 0.04 |
| BMAT summary score | 435 ± 57 | [310–510] | 489 ± 20 | [424–516] | <0.01 |
| ADOS-2 social affect | 10.4 ± 4.0 | [5–19] | — | — | |
| Repetitive behavior | 3.6 ± 1.8 | [1–8] | — | — | |
| Symptom severity | 7.4 ± 2.0 | [3–10] | — | — | |
| Motion dropout (% slices) | 0.04 ± 0.13 | [0–0.64] | 0.01 ± 0.02 | [0–0.08] | 0.14 |
| RMSD (head motion) | 1.31 ± 1.16 | [0.34–5.34] | 1.10 ± 0.56 | [0.43–2.78] | 0.40 |
Notes: M, male; F, female; R, right; L, left.
aADOS-2 subscores were unavailable for one ASD participant due to administrator error (see text), and three ASD participants did not complete the BMAT.
b t-tests; chi-square test for sex and handedness.
Table 2.
Means and SDs of CST sub-tract volumes (in mm3) by diagnostic group
| Left hemisphere | Right hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
| M1-cst | PMd-cst | SMA-cst | S1-cst | M1-cst | PMd-cst | SMA-cst | S1-cst | |
| ASD mean | 7650 | 7090 | 5347 | 5461 | 7719 | 6674 | 5690 | 5974 |
| ASD SD | 1412 | 1570 | 1385 | 1786 | 1474 | 1561 | 1346 | 2371 |
| TC mean | 8044 | 6853 | 5805 | 5823 | 7852 | 7011 | 6227 | 6441 |
| TC SD | 1082 | 1500 | 1247 | 1045 | 1218 | 1522 | 1337 | 1721 |
Note: SD, standard deviation of the mean.
No significant group, age, or group-by-age effects were found in the ANCOVA results. However, since the literature on middle age and aging in ASD is extremely limited, we report effect sizes (Supplementary Table S1) as an important contribution to the knowledge base. Effect sizes were small bilaterally for M1-cst, the canonical motor tract, and largest portion of the CST by volume, with no apparent group, age, or group-by-age effects. By contrast, right PMd-cst, the next largest tract, showed several results with medium effect sizes (partial η2 ≥ 0.06), including: for the right PMd-cst FA, the group-by-age interaction (reflecting a positive relationship with age in the ASD but not in the TC group; Supplementary Fig. S1A) as well as the group effect (TC < ASD). A medium age effect was also found for right PMd-cst MD (positively related to age). In the smaller tracts, medium effects were found for left S1 FA (group: ASD > TC; group × age: negatively related to age in TC but not in the ASD group; Supplementary Fig. S1B). When excluding left-handed participants, all of the above medium effects remained. We also observed additional medium effects in left M1-cst volume (group: ASD < TC) and right M1-cst MD (group: ASD > TC; group × age: positively related to age in the ASD but not in the TC group; see Supplementary Table S2) and group-by-age interaction in left S1-cst FA (positively related to age in ASD but not in the TC group; see Supplementary Table S2).
For the brain–behavior associations, no significant correlations between the M1-cst and BMAT measures were found after FDR correction. However, at a subthreshold level (P < 0.05, uncorrected), nominally significant correlations between left SMA-cst volume and the manual dexterity BMAT subscore were observed in the ASD (partial r = −0.499, P = 0.030) and TC (partial r = 0.444, P = 0.033) groups. Interestingly, the direction of the relationship between tract volume and behavior differed between the two groups with the lower volume of left SMA-cst linked to better performance in the ASD group but to slightly poorer performance in the TC group (Fig. 1B). Supplementary Tables S3 and S4 show the partial correlation results of the BMAT measures for the ASD and TC groups, respectively. These correlations remained in the same direction when excluding left-handed individuals and when they were run separately for each diffusion protocol.
For associations between sub-tracts and ADOS-2 measures, after FDR correction, several significant correlations were found (Table 3). The RRB subscore of the ADOS-2 was positively associated with the diffusivity measures of left PMd-cst (MD: partial r = 0.684, pFDR = 0.009; Fig. 1C) and left SMA-cst (MD: partial r = 0.628, pFDR = 0.038; Fig. 1D). These correlation effects remained significant or marginally significant when excluding left-handed participants from the analyses. When the correlation analyses were run separately for each protocol, the same trends emerged within protocol, with all of the above correlations showing partial r > 0.35.
Table 3.
Partial Spearman’s correlation results between CST sub-tract and ADOS-2 measures in the ASD group. Significant partial correlations (pFDR < 0.05) are shown in bold
| Left hemisphere | Right hemisphere | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Social affect | RRB | Social affect | RRB | ||||||||
| df | Partial r | p FDR | Partial r | p FDR | df | Partial r | p FDR | Partial r | p FDR | ||
| M1-cst | FA | 19 | 0.15 | 0.99 | −0.28 | 0.53 | 18 | 0.26 | 0.99 | 0.12 | 0.77 |
| MD | 19 | −0.01 | 0.99 | 0.45 | 0.29 | 18 | −0.08 | 0.99 | 0.20 | 0.66 | |
| Volume | 19 | 0.17 | 0.99 | −0.05 | 0.83 | 18 | −0.07 | 0.99 | 0.14 | 0.75 | |
| PMd-cst | FA | 18 | 0.02 | 0.99 | −0.33 | 0.51 | 18 | −0.13 | 0.99 | −0.06 | 0.83 |
| MD | 18 | 0.13 | 0.99 | 0.68 | 0.01 | 18 | 0.23 | 0.99 | 0.33 | 0.51 | |
| Volume | 18 | −0.10 | 0.99 | −0.26 | 0.57 | 18 | 0.02 | 0.99 | 0.05 | 0.83 | |
| SMA-cst | FA | 18 | −0.14 | 0.99 | −0.42 | 0.31 | 19 | 0.12 | 0.99 | −0.08 | 0.83 |
| MD | 18 | 0.23 | 0.99 | 0.63 | 0.04 | 19 | 0.06 | 0.99 | 0.30 | 0.53 | |
| Volume | 18 | −0.07 | 0.99 | 0.06 | 0.83 | 19 | 0.10 | 0.99 | −0.13 | 0.77 | |
| S1-cst | FA | 19 | 0.03 | 0.99 | −0.20 | 0.66 | 19 | 0.00 | 0.99 | 0.17 | 0.66 |
| MD | 19 | 0.16 | 0.99 | 0.43 | 0.31 | 19 | 0.05 | 0.99 | 0.18 | 0.66 | |
| Volume | 19 | 0.18 | 0.99 | −0.18 | 0.66 | 19 | −0.06 | 0.99 | −0.28 | 0.53 | |
Discussion
The present study examined sub-tracts of the CST including the canonical M1-cst and broader, nonprimary motor portions of the tract (PMd-cst, SMA-cst, and S1-cst) as well as links between these tracts and autism symptomology and motor abilities in middle-aged adults with ASD. We segmented the CST based on functionally specific primary motor, secondary motor, and S1 areas to gain anatomical specificity. Although we expected significant group differences, no significant group-by-age, group, or age effects were detected for the canonical M1-cst. Neither were there significant effects found for nonprimary corticospinal connections, although medium group-by-age, group, and age effects were observed for PMd-cst (but not M1-cst). However, we identified, for the first time, significant links between the secondary motor (PMd and SMA) corticospinal connections and autism traits (specifically RRB) in ASD. Nominally significant correlations between left SMA-cst volume and manual dexterity skills were also found, where the direction of the relationship differed between diagnostic groups (negative and positive relationships in the ASD and TC groups, respectively), but did not survive the FDR correction. This highlights the need for greater anatomical specificity in the study of such a large pathway as the CST, which has contributions from functionally distinct areas serving both primary and modulatory motor functions (Dum and Strick 1991; Galea and Darian-Smith 1994).
Aging Trajectories of the CST
Longitudinal and cross-sectional diffusion MRI studies in the typical population have shown that the CST as a whole undergoes protracted development, with FA estimated to peak in early to midadulthood, and follows a slow decline in aging (Lebel and Beaulieu 2011; Lebel et al. 2012; Yeatman et al. 2014; Cox et al. 2016). In the published literature, reported age effects in diffusivity measures of the CST from middle to older age are relatively subtle (Cox et al. 2016). In our sample of middle-aged adults, we did not detect group, age, or group-by-age interaction effects in the canonical M1-cst. This is not consistent with the overall literature on typical aging and is most likely due to our study being underpowered to detect age effects in M1-cst. It is also largely consistent with another cross-sectional study on the CST in adults with ASD that included an overlapping, but wider age range (30–74 years). That study also found neither age nor group-by-age effects but did identify increased diffusivity in the ASD group when compared with controls (Koolschijn et al. 2016). While we did not detect comparable group differences in microstructural indices, this could be due to the smaller sample size (n = 52 vs. n = 100) as well as the more focused age range (24 vs. 44 year range) in our study. Based on the calculated effect size from Koolschijn and colleagues, power analysis conducted using G*Power 3.1 (Faul et al. 2009) revealed a limited statistical power of 0.46 in our sample. However, when excluding left-handed participants, we observed medium (but nonsignificant) effects of group and group-by-age in right M1-cst MD that showed elevated MD in the ASD relative to the TC group, consistent with Koolschijn and colleagues, and a positive relationship with age in the ASD but not the TC group. This is consistent with a trajectory of accelerated decline of right M1-cst with age in the ASD group. We also found a medium effect size of group in left M1-cst volume that was reduced in ASD, but did not reach the level of significance, which is also compatible with reduced leftward lateralization in CST volume previously reported in ASD adolescents (Carper et al. 2015). CST volume was not available in the Koolschijn et al. study for comparison.
Examination of nonprimary motor CST sub-tracts showed age and group-by-age effects of medium size that did not reach the level of significance. A medium age effect was observed in right PMd-cst microstructure (positively associated for MD). There was a medium group-by-age interaction effect with a negative association in microstructural integrity (decreased FA) with age of the right PMd-cst in the TC group, which was not the case in the ASD group where the opposite pattern was found (increased FA associated with age). A similar medium size interaction effect was observed for the left S1-cst FA, showing an associated increase with age in the ASD but not the TC group. At a neurobiological level, it is unlikely that microstructural integrity of the CST increases in middle age. A more plausible explanation is that these effects derive from relative sparing of the CST combined with selective degeneration of other tracts (e.g., thalamo-cortical) traveling within the internal capsule that intersect the CST. Indeed, paradoxical findings of increased FA driven by loss of crossing fibers have been reported in neurodegenerative disorders (Douaud et al. 2009, 2011) and in a longitudinal aging study (De Groot et al. 2016). As suggested in Pierpaoli et al. (2001), MD can be informative in differentiating the relative contribution of the tract of interest from crossing tracts on FA. Specifically, a simultaneous increase in MD and FA points to degeneration of crossing tracts, whereas a decrease in MD concomitant with increasing FA indicates improving microstructural integrity of the tract of interest. The concomitant increase in right PMd-cst MD we observed with age (across groups) supports the likelihood that the finding of age-associated increase in FA is driven by crossing tracts combined with sparing of PMd-cst. Decreased FA as found with age in the TC group, likely indicates demyelination, axonal degeneration, and/or axonal loss (Beaulieu 2002).
Studies on the PLIC, which encompasses all corticospinal connections (primary and nonprimary), are most readily compared with the collective corticospinal connections examined in the present study rather than the individual sub-tracts. The medium-to-large size interaction effects of atypical age-related correlations of CST connections in ASD we observed, intriguingly, are consistent with a longitudinal study in a younger age range than ours (3–41 years) that projected FA of the PLIC in ASD to surpass their typical peers in adulthood (McLaughlin et al. 2018). However, the fact that we do see continued deficits in motor skills relative to TC individuals in our middle-aged adult sample combined with the additional caveat regarding the interpretation of diffusion indices with respect to crossing tracts suggests that a positive age-related association in FA reflects rather the degeneration of tracts crossing a relatively preserved CST or a cohort effect within the ASD group.
Links Between CST and Motor Performance in ASD
We found nominally significant correlations that did not survive the FDR correction between left SMA-cst volume and manual dexterity skills in both ASD and TC groups which differed in the direction of the relationship (negative in the ASD and positive in the TC group). It is interesting to note that a somewhat similar pattern of findings in primary versus secondary motor systems was found in a study of cortical white matter volume and motor performance in ASD. In a study of children, increased volume of the radiate white matter (white matter just beneath the cortex) of bilateral primary motor cortex was associated with improved motor skills in typically developing participants, whereas increased radiate white matter volumes of left primary and premotor cortices were associated with poorer motor skills in the ASD group (Mostofsky et al. 2007). While our findings were in SMA-cst rather than PMd-cst and M1-cst, the reversed direction of associations between volume and motor performance in the ASD group in both studies is striking and could reflect more widespread differences in organization within the motor network and particularly nonprimary motor inputs.
Link Between Secondary Motor CST and RRB Severity
Lower MD of left PMd and SMA corticospinal connections were significantly linked to a lower severity of restricted and repetitive patterns of behavior and interests in ASD. RRB can vary from repetitive sensory and motor behaviors (e.g., hand-flapping, rocking) to “higher-order” behaviors, such as an insistence on sameness or restricted interests (Leekam et al. 2011). The PMd cortex and SMA have distinct roles that are relevant to RRB. The PMd cortex is involved in the selection of motor programs based on learned associations with external stimuli (Halsband and Passingham 1985), while the SMA is involved in internally remembered motor sequences (Roland et al. 1980) and is prompted by proprioceptive cues (Passingham 1987). There are direct monosynaptic projections from the SMA and primary motor cortex that innervate and exert influence on common motoneurons (Maier 2002), and the SMA has been shown to play a role in the reactive inhibition of unwanted movements (Chen et al. 2010). Thus, these secondary motor inputs may play an important role in modifying the characteristics or the frequency of repetitive behaviors. The correlation found between RRB and the left PMd and SMA tracts in adults with ASD may reflect a greater ability to appropriately select actions based on associated stimuli and to inhibit inappropriate (e.g., stereotyped) behaviors at the periphery in individuals with more effective secondary motor relays.
Limitations
There were several limitations in the present study. One is the small sample size, limiting the statistical power to detect subtle effects such as age in the CST. However, this is an important population that is understudied but difficult to recruit, given changing nosology (American Psychiatric Association 1968, 1987, 1994, 2013) and underdiagnosis in this age range (Brugha et al. 2011). Another limitation is the cross-sectional design, which does not allow for us to track changes in the CST longitudinally. The age-related differences in CST measures of medium effect size we observed are therefore susceptible to potential cohort effects between younger and older participants and are not necessarily attributed to the differences in rates of change. The ASD sample in this study also consisted largely of participants without a co-occurring intellectual disability, therefore the results may not be generalizable to ASD populations with an intellectual disability.
While multifiber probabilistic tractography was used, the diffusion data were limited to a single q-shell (b-value). This could be one reason why the ventral premotor corticospinal connections could not be reliably reconstructed in many of the participants, given that the ventral premotor descending connections contribute only a small percent of corticospinal output from the frontal lobe ~4% (Dum and Strick 1991). The reconstruction of the CST and its sub-tracts would benefit from running tractography on the multishell diffusion data.
Conclusion
Although we did not find significant alterations in any of the CST sub-tracts examined in a sample of middle-aged adults with ASD, effects of medium size were detected. We also found an association between secondary motor connections and autism symptoms in ASD. Reduced microstructural integrity of left PMd and SMA-cst was linked to higher severity of RRB in ASD and could be related to a diminished ability to select actions via these tracts. The differential relationship found between left SMA-cst volume and manual dexterity in ASD and TC groups could suggest altered inputs to secondary motor areas in ASD. Widespread motor deficits and alterations in brain motor networks in ASD are consistently reported in the literature, but their relevance to autism symptoms is not well understood. These findings suggest that the projection pathways of the motor network, and in particular connections with secondary motor cortices, are implicated in ASD and show unexpected associations with the core ASD symptoms. Understanding how these secondary motor areas are involved in or affected by ASD may help guide research on interventions related to RRB and motor skills and may have particular relevance for middle-aged (and older) adults with ASD as they begin to experience typical aging-related effects on motor function.
Notes
The authors would like to thank the individuals and families who participated in this study. Conflict of Interest: None declared.
Funding
National Institutes of Health (R01 MH103494).
Supplementary Material
Contributor Information
Janice Hau, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
Jiwandeep S Kohli, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
Ian Shryock, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
Mikaela K Kinnear, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
Adam Schadler, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
Ralph-Axel Müller, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
Ruth A Carper, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
References
- Abbott AE, Linke AC, Nair A, Jahedi A, Alba LA, Keown CL, Fishman I, Müller RA. 2018. Repetitive behaviors in autism are linked to imbalance of corticostriatal connectivity: a functional connectivity MRI study. Soc Cogn Affect Neurosci. 13(1):32–42. doi: 10.1093/scan/nsx129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Dahab SMN, Skidmore ER, Holm MB, Rogers JC, Minshew NJ. 2013. Motor and tactile-perceptual skill differences between individuals with high-functioning autism and typically developing individuals ages 5-21. J Autism Dev Disord. 43(10):2241–2248. doi: 10.1007/s10803-011-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (Ed.) . 1968. Diagnostic and statistical manual of mental disorders (DSM-II). 2nd ed. Washington, D.C.: American Psychiatric Association Publishing. [Google Scholar]
- American Psychiatric Association (Ed.) . 1987. Diagnostic and statistical manual of mental disorders (DSM-III-R). 3rd ed. Washington, D.C.: American Psychiatric Association Publishing. [Google Scholar]
- American Psychiatric Association (Ed.) . 1994. Diagnostic and statistical manual of mental disorders (DSM-IV). 4th ed. Arlington, VA: American Psychiatric Association Publishing. [Google Scholar]
- American Psychiatric Association (Ed.) . 2013. Diagnostic and statistical manual of mental disorders (DSM-5). 5th ed. Washington, D.C.: American Psychiatric Association Publishing. [Google Scholar]
- Amoruso L, Finisguerra A, Urgesi C. 2018. Autistic traits predict poor integration between top-down contextual expectations and movement kinematics during action observation. Sci Rep. 8(1):1–10. doi: 10.1038/s41598-018-33827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Jäncke L, Mohlberg H, Steinmetz H, Zilles K. 2000. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 38(3):304–312. doi: 10.1016/S0028-3932(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Drobnjak I, Zhang H, Filippini N, Bastiani M. 2017. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: within volume movement. NeuroImage. 152(February:450–466. doi: 10.1016/j.neuroimage.2017.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. 2016. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage. 141:556–572. doi: 10.1016/j.neuroimage.2016.06.058. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Skare S, Ashburner J. 2003. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN. 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DS, Lee JK, Solomon M, Rogers SJ, Amaral DG, Nordahl CW. 2019. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 11(1):1–12. doi: 10.1186/s11689-019-9291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DB, Vaillancourt DE, Coombes SA. 2017. A template and probabilistic atlas of the human sensorimotor tracts using diffusion MRI. Cereb Cortex. 28(5):1685–1699. doi: 10.1093/cercor/bhx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. 2002. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Jbabdi S, Rushworth MFS, Woolrich MW. 2007. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. 2003. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 57(1):289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- Bhat AN, Landa RJ, Galloway JC. 2011. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys Ther. 91(7):1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino Da Cruz LC, Rodrigues LDS, Gasparetto EL, Calçada CABP. 2009. Diffusion tensor imaging findings in school-aged autistic children. J Neuroimaging. 19(4):337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, Bebbington P, Jenkins R, Meltzer H. 2011. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 68(5):459–466. doi: 10.1001/archgenpsychiatry.2011.38. [DOI] [PubMed] [Google Scholar]
- Bruininks RH, Bruininks BD. (2012). Bruininks motor abilities test. Bloomington, MN: Pearson. [Google Scholar]
- Carper RA, Solders S, Treiber JM, Fishman I, Müller RA. 2015. Corticospinal tract anatomy and functional connectivity of primary motor cortex in autism. J Am Acad Child Adolesc Psychiatry. 54(10):859–867. doi: 10.1016/j.jaac.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy S, Hannant P, Tavassoli T, Allison C, Smith P, Baron-Cohen S. 2016. Dyspraxia and autistic traits in adults with and without autism spectrum conditions. Mol Autism. 7(1):1–6. doi: 10.1186/s13229-016-0112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Scangos KW, Stuphorn V. 2010. Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci. 30(44):14657–14675. doi: 10.1523/JNEUROSCI.2669-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenot Q, Tzourio-Mazoyer N, Rheault F, Descoteaux M, Crivello F, Zago L, Mellet E, Jobard G, Joliot M, Mazoyer B et al. 2019. A population-based atlas of the human pyramidal tract in 410 healthy participants. Brain Struct Funct. 224(2):599–612. doi: 10.1007/s00429-018-1798-7. [DOI] [PubMed] [Google Scholar]
- Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, Deary IJ. 2016. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat Commun. 7:1–13. doi: 10.1038/ncomms13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, Kripke C. 2015. The health status of adults on the autism spectrum. Autism. 19(7):814–823. doi: 10.1177/1362361315577517. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu X-H, Ugurbil K, Kim S-G, Ashe J. 1997. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci. 94:14015–14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. 2010. Randomized, controlled trial of an intervention for toddlers with autism: the early start Denver model. Pediatrics. 125(1):e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M, Cremers LGM, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Niessen WJ, Vernooij MW. 2016. White matter degeneration with aging: longitudinal diffusion MR imaging analysis. Radiology. 279(2):532–541. doi: 10.1148/radiol.2015150103. [DOI] [PubMed] [Google Scholar]
- Dewey D, Cantell M, Crawford SG. 2007. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 13(2):246–256. [DOI] [PubMed] [Google Scholar]
- Douaud G, Behrens TE, Poupon C, Cointepas Y, Jbabdi S, Gaura V, Golestani N, Krystkowiak P, Verny C, Damier P et al. 2009. In vivo evidence for the selective subcortical degeneration in Huntington’s disease. NeuroImage. 46(4):958–966. doi: 10.1016/j.neuroimage.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Douaud G, Jbabdi S, Behrens TEJ, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Kindlmann G, Matthews PM et al. 2011. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. NeuroImage. 55(3):880–890. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey R, Rapport MJK. 2012. Motor activity in children with autism: a review of current literature. Pediatr Phys Ther. 24(1):2–20. doi: 10.1097/PEP.0b013e31823db95f. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. 1991. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 11(3):667–689 doi: S0022510X0200268X [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Larson JCG, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. 2007. Dyspraxia in autism: association with motor, social, and communicative deficits. Dev Med Child Neurol. 49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Farquharson S, Tournier J-D, Calamante F, Fabinyi G, Schneider-Kolsky M, Jackson GD, Connelly A. 2013. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg. 118(6):1367–1377. doi: 10.3171/2013.2.JNS121294. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. 2009. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. 2016. Age-related change in mobility: perspectives from life course epidemiology and geroscience. Journals of Gerontology: Medical Sciences. 71(9):1184–94. doi: 10.1093/gerona/glw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, Roalf DR, Satterthwaite TD, Gur RC, Gur RE et al. 2017. Harmonization of multi-site diffusion tensor imaging data. NeuroImage. 161(August:149–170. doi: 10.1016/j.neuroimage.2017.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. 2010. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord. 40(10):1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Fulceri F, Grossi E, Contaldo A, Narzisi A, Apicella F, Parrini I, Tancredi R, Calderoni S, Muratori F. 2019. Motor skills as moderators of core symptoms in autism spectrum disorders: preliminary data from an exploratory analysis with artificial neural networks. Front Psychol. 9(JAN:1–12. doi: 10.3389/fpsyg.2018.02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. 1994. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb Cortex. 4(2):166–194. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, Baird G. 2009. Impairment in movement skills of children with autistic spectrum disorders. Dev Med Child Neurol. 51:311–316. doi: 10.1111/j.1469-8749.2008.03242.x. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. 2009. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsband U, Passingham RE. 1985. Premotor cortex and the conditions for movement in monkeys (Macaca fascicularis). Behav Brain Res. 18(3):269–277. doi: 10.1016/0166-4328(85)90035-X. [DOI] [PubMed] [Google Scholar]
- He S-Q, Dum RP, Strick PL. 1993. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 15(5):3284–3306. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Fernandez M, Reguly I, Jbabdi S, Giles M, Smith S, Sotiropoulos SN. 2019. Using GPUs to accelerate computational diffusion MRI: from microstructure estimation to tractography and connectomes. NeuroImage. 188(December 2018:598–615. doi: 10.1016/j.neuroimage.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, Shic F, Wall CA, Chawarska K, Curtin S, Estes A, Gardner JM, Hutman T, Landa RJ, Levin AR et al. 2019. Early motor abilities in infants at heightened versus low risk for ASD: a baby siblings research consortium (BSRC) study. J Abnorm Psychol. 128(1):69–80. doi: 10.1037/abn0000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. 2006. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. J Autism Dev Disord. 36(5):613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. 2012. Fsl. NeuroImage. 62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015 (no access date, paper citation). [DOI] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A. 2007. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, Pelphrey KA. 2011. Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. Am J Neuroradiol. 32(9):1607–1613. doi: 10.3174/ajnr.A2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Srinivasan SM, Bhat AN. 2018. Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without autism spectrum disorder (ASD). Res Dev Disabil. 72(October 2017:79–95. doi: 10.1016/j.ridd.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Yendiki A, Weigelt S, Gweon H, Julian J, Richardson H, Malloy C, Saxe R, Fischl B, Kanwisher N. 2014. Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proc Natl Acad Sci U S A. 111(5):1981–1986. doi: 10.1073/pnas.1324037111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, Caan MWA, Teeuw J, Olabarriaga SD, Geurts HM. 2016. Age-related differences in autism: the case of white matter microstructure. Hum Brain Mapp. 00(April:1–15. doi: 10.1002/hbm.23345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraskov A, Dancause N, Quallo MM, Shepherd S, Lemon RN. 2009. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression? Neuron. 64(6):922–930. doi: 10.1016/j.neuron.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. 2011. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Leekam SR, Prior MR, Uljarevic M. 2011. Restricted and repetitive behaviors in autism spectrum disorders: a review of research in the last decade. Psychol Bull. 137(4):562–593. doi: 10.1037/a0023341. [DOI] [PubMed] [Google Scholar]
- Linke A, Kinnear M, Kohli J, Fong C, Lincoln A, Carper R, Müller R-A. 2019. Impaired motor skills and atypical functional connectivity of the sensorimotor system in 40-65 year old adults with autism Spectrum disorders. Neurobiol Aging. 85:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M, MacDonald M, Lord C. 2013. Motor skills of toddlers with autism spectrum disorders. Autism. 17(2):133–146. doi: 10.1177/1362361311402230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. 2002. The autism diagnostic observation scale (ADOS). Los Angeles (CA): Western Psychological. [Google Scholar]
- Luppino G, Matelli M, Camarada R, Rizzolatti G. 1994. Corticospinal projections from mesial frontal and cingulate areas in the monkey. NeuroReport. 5(18):2545–2548. [DOI] [PubMed] [Google Scholar]
- Maier MA. 2002. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 12(3):281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. 2006. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. NeuroImage. 31(4):1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K, Travers BG, Dadalko OI, Dean DC, Tromp D, Adluru N, Destiche D, Freeman A, Prigge MD, Froehlich A et al. 2018. Longitudinal development of thalamic and internal capsule microstructure in autism spectrum disorder. Autism Res. 11(3):450–462. doi: 10.1002/aur.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. 2007. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 29(9):565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Sung KB, Jones BL, Furman JM. 2004. Underdevelopment of the postural control system in autism. Neurology. 63(11):2056–2061. doi: 10.1212/01.WNL.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. 2007. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 130(8):2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Müller RA, Cauich C, Rubio MA, Mizuno A, Courchesne E. 2004. Abnormal activity patterns in premotor cortex during sequence learning in autistic patients. Biol Psychiatry. 56(5):323–332. doi: 10.1016/j.biopsych.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, Choe AS, Barber AD, Pekar JJ, Mostofsky SH. 2016. Intrinsic visual-motor synchrony correlates with social deficits in autism. Biol Psychiatry. 79(8):633–641. doi: 10.1016/j.biopsych.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile M, Perego P, Piccinini L, Mani E, Rossi A, Bellina M, Molteni M. 2011. Further evidence of complex motor dysfunction in drug naïve children with autism using automatic motion analysis of gait. Autism. 15(3):263–283. doi: 10.1177/1362361309356929. [DOI] [PubMed] [Google Scholar]
- Ohara R, Kanejima Y, Kitamura M, Izawa KP. 2019. Association between social skills and motor skills in individuals with autism spectrum disorder: a systematic review. Eur J Investigat Health Psychol Educ. 10(1):276–296. doi: 10.3390/ejihpe10010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel M, Mennes M, Marquand A, Charman T, Tillmann J, Ecker C, Dell’Acqua F, Brandeis D, Banaschewski T, Baumeister S et al. 2019. Altered connectivity between cerebellum, visual, and sensory-motor networks in autism spectrum disorder: results from the EU-AIMS longitudinal European autism project. Biol Psychiatry Cogn Neurosci Neuroimaging. 4(3):260–270. doi: 10.1016/j.bpsc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Passingham RE. 1987. Two cortical systems for directing movement. Ciba Found Symp. 132:151–164. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. 2001. Water diffusion changes in wallerian degeneration and their dependence on white matter architecture. NeuroImage. 13(6):1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Piven J, Rabins P. 2011. Autism spectrum disorders in older adults: toward defining a research agenda. J Am Geriatr Soc. 59(11):2151–2155. doi: 10.1111/j.1532-5415.2011.03632.x. [DOI] [PubMed] [Google Scholar]
- Potvin AR, Syndulko K, Tourtellotte WW, Lemmon JA, Potvin JH. 1980. Human neurologic function and the aging process. Am Geriatr Soc. 28(1):1–9. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Retrieved May 22, 2019, from http://www.r-project.org/.
- Rizzolatti G, Luppino G. 2001. The cortical motor system. Neuron. 31(6):889–901. doi: 10.1016/S0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhoj E. 1980. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 43(1):118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Müller RA. 2011. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry Allied Discip. 52(3):286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein S, Gellar S, Parlier M, Payne L, Piven J. 2015. High rates of parkinsonism in adults with autism. J Neurodev Disord. 7(1):1–11. doi: 10.1186/s11689-015-9125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Murphy D, Dell’Acqua F, Ecker C, McAlonan G, Howells H, Baron-Cohen S, Lai MC, Lombardo MV. 2017. Impaired communication between the motor and somatosensory homunculus is associated with poor manual dexterity in autism spectrum disorder. Biol Psychiatry. 81:211–219. doi: 10.1016/j.biopsych.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres EB, Caballero C, Mistry S. 2020. Aging with autism departs greatly from typical aging. Sensors (Switzerland). 20(2):572. doi: 10.3390/s20020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Duffield TC, Prigge MDB, Froehlich AL, Lange N, Alexander AL, Lainhart JE. 2017. Longitudinal development of manual motor ability in autism spectrum disorder from childhood to mid-adulthood relates to adaptive daily living skills. Dev Sci. 20(4):1–15. doi: 10.1111/desc.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Tromp DPM, Adluru N, Destiche D, Samsin D, Froehlich A, Prigge MDB, Duffield TC, Lange N et al. 2015. Brainstem white matter predicts individual differences in manual motor difficulties and symptom severity in autism. J Autism Dev Disord. 45(9):3030–3040. doi: 10.1007/s10803-015-2467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Nakamura Y, Li J, Gu Z, Niehaus J, Maezawa M, Crone SA, Goulding M, Baccei ML, Yoshida Y. 2018. Corticospinal circuits from the sensory and motor cortices differentially regulate skilled movements through distinct spinal interneurons. Cell Rep. 23(5):1286–1300.e7. doi: 10.1016/j.celrep.2018.03.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran G, Philipp R, Lemon RN, Kraskov A. 2013. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr Biol. 23(3):236–243. doi: 10.1016/j.cub.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 2011. WASI-II: Wechsler abbreviated scale of intelligence. San Antonio, TX: Pearson. [Google Scholar]
- Wickham H. 2016. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag. [Google Scholar]
- Wise EA. 2020. Aging in autism spectrum disorder. Am J Geriatr Psychiatry. 28(3):339–349. doi: 10.1016/j.jagp.2019.12.001. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM et al. 2012. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatr. 169(6):589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Wandell BA, Mezer AA. 2014. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 5:1–12. doi: 10.1038/ncomms5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


