Abstract
Introduction
Givosiran is an RNA interference therapeutic designed to block the synthesis of the aminolevulinic acid (ALA) synthase 1 (ALAS1) enzyme in patients with acute intermittent porphyria (AIP). Givosiran may have adverse effects on the kidney.
Methods
We performed a descriptive case series of renal function parameters of all the patients who received givosiran in France. Twenty patients receiving givosiran between March 2018 and July 2020 in France were analyzed: 7 patients in the ENVISION trial and 13 patients treated in collaboration with the Centre de Référence Maladies Rares Prophyries.
Results
A transient decrease in renal function was observed in all but 2 patients (90%) within the 3 months following givosiran initiation. None of the patients developed acute kidney injury or disease. Patients of the ENVISION cohort were followed for at least 30 months: 2 patients did not experience estimated glomerular filtration rate (eGFR) loss, 3 patients experienced a modest decline in renal function (–3.4 ml/min per 1.73 m2 per year in average), and 2 patients had a clearly abnormal eGFR loss (–5.8 ml/min per 1.73 m2 per year in average). None of the patients had biochemical signs of active tubular or glomerular injury. One patient’s kidney was biopsied without finding any signs of an active kidney disease and with normal ALAS1 tubular expression.
Conclusions
Givosiran is associated with a transient moderate increase in serum creatinine (sCr) without sign of kidney injury. A long-term deleterious impact of ALAS1 inhibition on renal function is not excluded. Because AIP promotes chronic kidney disease, it is difficult to separate the long-term effects of givosiran from the natural progression of the renal disease.
Keywords: acute intermittent porphyria, acute kidney injury, chronic kidney disease, givosiran, renal function
AIP is due to partial deficiency of the third enzyme of heme biosynthesis, hydroxymethylbilane synthase. Acute attacks are precipitated by events that induce hepatic ALAS1, the first enzyme of the pathway, resulting in the accumulation of toxic porphyrin precursors, ALA and porphobilinogen.1,2 The systemic accumulation of ALA and porphobilinogen causes injury to many organs including the nervous system, resulting in potentially life-threatening acute neurovisceral attacks and chronic manifestations such as chronic kidney disease, hypertension, neuropathy, and hepatocellular carcinoma.
Intravenous hemin represses ALAS1 induction and is the treatment of reference for acute attack. Nevertheless, its numerous shortcomings and side effects, including vein thrombosis and iron overload, are a limitation for its long-term use for patients experiencing recurrent attacks. Givosiran (Alnylam Pharmaceuticals, Cambridge, MA) is a small interfering RNA (siRNA) derivatized with N-acetylgalactosamine for hepatocyte targeting that silences ALAS1 messenger RNA (mRNA). On delivery to the liver, givosiran is incorporated into the RNA-induced silencing complex and uses the naturally occurring RNA interference mechanisms to specifically target ALAS1 mRNA, thereby preventing the synthesis of the corresponding ALAS1 protein.3,4 Givosiran is subcutaneously administered and prevents the accumulation of ALA and porphobilinogen.5 Its clinical efficacy is remarkable in the prevention of acute attacks. Treatment with givosiran reduced the annualized mean rate of porphyria attacks by 74% compared with placebo.6 Thus, the siRNA approach is a substantial improvement over intravenous heme in preventing recurrence of acute attacks.
Patients who received givosiran in the phase III, open-label, clinical trial ENVISION were 3 times more likely to have a decreased eGFR and worsening of chronic kidney disease, the cause of which is unknown.6 Long-term data analysis and an understanding of givosiran-related renal effects are critical to better appreciate the nephrotoxic potential of this therapeutic. The purpose of this study is to describe renal function parameters of all the patients in France followed for up to 30 months after initiation of givosiran for the treatment of severe AIP.
Patients and Methods
Study Population
We searched for all patients treated at the Centre de Reference Maladies Rares Porphyries (CRMR Porphyries, http://www.porphyrie.net/) in Paris in whom givosiran therapy was introduced between March 2018 and July 2020. Two cohorts were analyzed: 7 French patients included in the phase 3 ENVISION trial,6 and 15 patients who received initially givosiran under temporary authorization of use (TAU) regimen, which allows the exceptional use of pharmaceutical specialties that do not benefit from a marketing authorization. Since June 2020, givosiran is available in France in routine care. This cohort will be refereed to as the “CRMR” cohort. Patients of the CRMR cohort were followed in health care centers throughout France, but the dispensation demand can only be made by the CRMR in Paris.
All patients received a diagnosis of AIP following the European Porphyria Network guidelines, and all suffered from recurrent acute attacks (≥4 attacks per year).7 AIP diagnosis was performed at the CRMR. Patients in the ENVISION cohort received monthly subcutaneous givosiran injections (at a dose of 2.5 mg/kg of body weight). In the ENVISION cohort, 4 patient received a placebo for 6 months and thereafter givosiran injections in the open-label phase. Patients in the CRMR cohort received at least 2 injections with an interval of 1 month (median 5 injections, min: 2, max: 9). Electronic health records were retrospectively analyzed, and clinical and biological data were collected in a pseudonymized database. Baseline and follow-up data were analyzed. Measurements of sCr and of other clinical and biological parameters were performed at the time of monthly visits. Two patients in the CRMR cohort were not analyzed because of the lack of repeated assessment of sCr. GFR was estimated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. GFR was measured in some patients, by using urinary clearance of Technetium-99mTc-diethylenetriaminepentaacetic acid (99mTc-DTPA) and urinary clearance of creatinine. Patients were informed that their health records data would be used for research studies. Patients were not directly involved in the current research, and no clinical or biological data were collected in addition to those needed for routine care.
Immunohistochemistry for ALAS1
Immunohistochemistry was performed on paraffin-embedded tissues. Target retrieval was carried out by heating the tissue in a citrate buffer (DakoCytomation, Glostrup, Denmark) at pH 6. Endogenous peroxidase was inactivated by incubating the specimen for 10 minutes at room temperature in 0.03% H2O2. The sections were incubated overnight at 4°C with phosphate-buffered saline containing 1:50 rabbit anti-ALAS1 (HPA035860, Sigma-Aldrich, St Louis, MO). The immunoreactive proteins were visualized using the Envision + HRP system (AEC; DakoCytomation). Finally, the tissue sections were counterstained with hematoxylin and mounted using aqueous mounting medium (Dako, Glostrup, Denmark). The primary antibodies were replaced by equal concentrations of rabbit or mouse IgG (Dako) as negative controls. A blocking peptide (ABIN973092; Antibodies-online GmbH, Aachen, Germany) has been used to monitor the specificity of the staining.
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted from kidney biopsy specimen and cell cultures using the RNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. Four AIP patients were analyzed: AIP_1, a 60-year-old woman, eGFR 29 ml/min per 1.73 m2; AIP_2, a 66-year-old woman, eGFR 36 ml/min per 1.73 m2; AIP_3: 45-year-old woman, eGFR 25 ml/min per 1.73 m2. The index patient was patient 7. The yield and purity of the RNA were measured using a NanoDrop ND-1000 spectrophotometer (Nanodrop Technologies). Transcripts expression levels were quantified by SYBR green real-time quantitative polymerase chain reaction using an ABI PRISM 7900 sequence detector system (Applied Biosystems, Foster City, CA). Vehicle-treated samples were used as the controls, and fold changes for each tested gene were normalized to the ribosomal protein L13A (RPL13A) housekeeping gene. The relative expression levels were calculated using the 2(–ΔC(T)) (threshold cycle number) method.
Results
The clinical and biological parameters of the patients are detailed in Table 1. The 2 cohorts were broadly similar, except for the duration of follow-up, which was 30 months after the introduction of givosiran in the ENVISION cohort and up to 9 months (median 6, range 5–9) in the CRMR cohort. At introduction, patients ranged in age from 19 to 68 years, and about half of them had a medical history of hypertension. All but 2 were female. At givosiran initiation, all patients in the ENVISION had an eGFR >60 ml/min per 1.73 m2, except 1 (14%) with CKD stage 3A. Six patients in the CRMR cohort (46 %) had an eGFR <60 ml/min per 1.73 m2: 5 with CKD stage 3A and 1 with CKD stage 3B. This high rate of patients with CKD is likely the consequence of acute and chronic kidney injuries mediated by urinary porphyrin precursors, which we previously referred to as porphyria-associated kidney disease.8 On the day of givosiran initiation, the mean CKD-EPI eGFR was 66 ± 16 ml/min per 1.73 m2 in the ENVISION cohort and 66.5 ± 28 ml/min per 1.73 m2 in the CRMR cohort.
Table 1.
Baseline characteristics of patients receiving givosiran
| ENVISION cohort (n=7) | CRMR cohort (n=13) | |
|---|---|---|
| Sex ratio (female) | 7 (100) | 11 (85) |
| Age (yr) | 46 ± 11 | 39.5 ± 15 |
| AIP duration (yr) | 22 ± 14 | 15.5 ± 14.6 |
| Family history | 5 (71) | 10 (90) |
| HMBS allelic variants | c.1013T>C c.346C>T c.673C>T c.724_743dup20 c.849G>A c503_504insT c.76C>T |
c.749A>C c.673C>T c.296A>G c.291delG c.673C>T c.1004_1051del c.517C>T c.748G>A c.766C>T c.849G>A c.88C>T c.913-2A>G |
| Number of crises per month | 1.8 ± 1.4 | 1.1 ± 0.6 |
| AIP symptoms | ||
| Digestive | 7 (100) | 13 (100) |
| Neurologic | 4 (57) | 7 (54) |
| Both | 4 (57) | 7 (54) |
| Hypertension | 4 (57) | 6 (46) |
| Serum creatinine at givosiran introduction (μmol/l) | 93 ± 21 | 96 ± 25 |
| CKD-EPI eGFR at givosiran introduction (ml/min per 1.73 m2) | 66 ± 16 | 70 ± 26 |
| SF-12 scorea | 47 ± 23 | 62 ± 21 |
| Preventive heme-arginate infusions | 6 (85) | 8 (61) |
| Time from diagnosis to givosiran (yr) | 20 ± 14 | 16.4 ± 14 |
| Follow-up (mo) | 30 ± 0 | 7.8 ± 2.9 |
AIP, acute intermittent porphyria; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; SF-12, 12-Item Short Form Health Survey.
Continuous variables are expressed as mean ± standard deviation, and nominal variables are expressed as n (%).
Scores on the Physical Component Summary of the SF-12 range from 0 (worst functioning) to 100 (best functioning)
After givosiran introduction, renal function typically decreased (Supplementary Figure S1). The average max sCr increase over the follow-up period was 1.25 and 1.22 times baseline values (i.e., at givosiran initiation) in the ENVISION and CRMR cohorts, respectively. At the last follow-up visit, the average sCr increase was 1.11 and 1.16 times baseline in the ENVISION and CRMR cohorts, respectively.
The kinetics of renal function evolution are better appreciated at an individual level, although large fluctuations in sCr are observed (Figure 1 and Supplementary Figure S2). Consistent with previous findings,6 a transient sCr increase (compared with sCr level at the moment of givosiran introduction) was observed in all but 2 patients (90%) within 3 months However, sCr started to increase during the months preceding initiation of the therapeutic in 11 of these patients (61%). The amplitude of the early sCr peak was modest, and none of the patients developed acute kidney injury or disease according to KDIGO criteria (sCr increase >1.5-fold baseline or >26.5 μmol/l).
Figure 1.
Evolution of renal function under givosiran in the ENVISION cohort. (a–g) Repeated sCr measurements in patients of the ENVISION cohort before and after givosiran introduction. (h) eGFR differences between month 30 and the day of givosiran initiation. (h) Repeated eGFR measurement before and after givosiran initiation in patients 6 and 7. eGFR, estimated glomerular filtration rate; sCr, serum creatinine.
Patients of the ENVISION cohort (Figure 1a to h) were followed for at least 30 months, which provides critical information on the long-term evolution of renal function. Three evolution profiles could be distinguished. Two patients did not experience overall sCr increase between initiation and month 30 (patients 1 and 2 in Figure 1a and b). Three patients (patients 3–5 in Figure 1c, d and e) experienced a modest decline of renal function (–3.4 ml/min per 1.73 m2 per year in average) during this period, which is slightly higher than the physiological decline, and can be explained by the natural progression of porphyria-associated kidney disease. Finally, 2 patients (patients 6 and 7 in Figure 1f and g) had an average eGFR loss of 12.8 ml/min per 1.73 m2 (–5.8 ml/min per 1.73 m2 per year in average), which is clearly abnormal. Notably, the rate of renal function decline under givosiran was higher in patients with lower baseline eGFR, and patients who experienced the largest decrease in renal function over time were also those with an eGFR <60 ml/min per 1.73 m2 (i.e., CKD stage 3A) at initiation of givosiran (Figure 1h).
Pre-givosiran eGFR trajectories are difficult to draw because renal function has not been systematically measured for each individual patient, and the measurements were carried out in a very heterogeneous manner, with very varied frequencies and delays. This heterogeneity prevented a systematic interindividual comparison. In addition, measurements before givosiran were often performed at the time of attacks, when there is functional impairment of renal function. We focused on patients 6 and 7 in the ENVISION cohort, who have the more severe decline after givosiran (Figure 1i). Patient 6 seemed to have an improvement in kidney function in the previous year, which was interrupted by treatment, with a return to baseline afterward; and patient 7 has a progressive deterioration of its renal function, not affected by givosiran, followed by a stabilization.
No patient had significant hematuria or leukocyturia. Average urine protein concentration was always <0.3 g/L (urinary protein/creatinine ratio were unavailable, which is a limitation) (Supplementary Figure S3 and Supplementary Table S1) indicating that givosiran does not induce obvious renal structural damage. In line with this, urinary retinol-binding protein, a sensitive marker of proximal tubular injury, which was measured in 3 patients under givosiran, was <0.6 mg/l in all evaluated cases, suggesting that givosiran does not induce tubular injury. The therapeutic did not impact blood pressure (Supplementary Figure S3 and Supplementary Table S1).
Measurement of GFR using urinary clearance of 99mTc-DTPA besides urinary clearance of creatinine has been performed in 3 patients having received at least 15 injections (Table 2). Under givosiran, urinary creatinine clearance overestimated measured GFR by 45% in average, making an interaction with tubular creatinine transporters unlikely. Indeed, had givosiran inhibited tubular creatinine secretion (thereby increasing sCr and reducing urinary creatinine concentration), creatinine clearance would have underestimated, rather than overestimate, measured GFR.
Table 2.
Urinary clearances and measured glomerular filtration rate in 3 patients in the ENVISION cohort
| Number of injections to evaluation | sCr (μmol/l) | eGFR CKD-EPI (ml/min per 1.73 m2) | Urinary 99mTc-DTPA clearance (ml/min per 1.73 m2)a | Urinary creatinine clearance (ml/min per 1.73 m2)a | Ratiob | |
|---|---|---|---|---|---|---|
| Patient 2 | 22 | 81 | 78 | 83 | 104 | 126 |
| Patient 4 | 16 | 89 | 60 | 44 | 69 | 156 |
| Patient 7 | 29 | 149 | 39 | 32 | 48 | 148 |
| Mean | 53 | 73 | 143 |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DTPA, diethylenetriaminepentaacetic acid; eGFR, estimated glomerular filtration rate; sCr, serum creatinine.
Christensen and Groth formula.
Urinary creatinine clearance and urinary 99mTc-DTPA clearance.
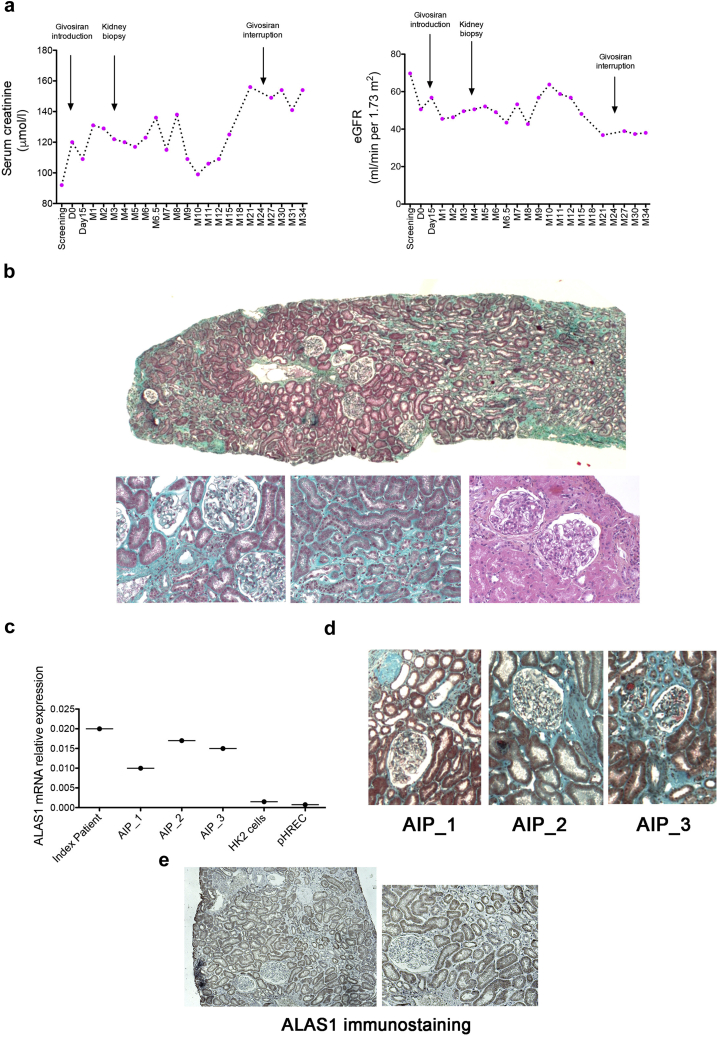
Kidney biopsy was performed 3 months after givosiran initiation (20 days after the last givosiran dose) in a patient with a progressive renal function decline (patient 7 in Figure 1, with details in Figure 2a). Microscopic examination identified mild tubular atrophy and interstitial fibrosis without sign of active tubular, glomerular, or arteriolar injury (Figure 2b). Immunofluorescence analysis did not show immune deposits (data not shown). ALAS1 transcripts were expressed in the kidney specimen at similar levels compared with AIP patients not receiving givosiran (Figure 2c). In line with this, ALAS1 was expressed by tubules (Figure 2d and Supplementary Figure S4). Givosiran was interrupted in this patient 29 months after introduction because of a pregnancy plan. Renal function deterioration under givosiran was followed by a stabilization of aftertreatment interruption.
Figure 2.
Pathologic examination of a kidney biopsy specimen. (a) Repeated sCr and eGFR measurements in a patient before and after givosiran introduction. (b) Masson’ trichrome and periodic acid–Schiff stains show tubular atrophy mild interstitial fibrosis. An active lesion is defined by the presence of active tubular necrosis, tubulitis, interstitial inflammation, glomerular proliferation, or thrombotic microangiopathy (original magnification × 20). (c) ALAS1 mRNA expression in kidney biopsy samples of the index patient, of 3 AIP patients not treated with givosiran (AIP_1, AIP_2, and AIP_3), and in renal tubular cells in culture (pHERC: primary cultured renal epithelial cells, and the HK2 cell line). (d) Representative photomicrographs of Masson trichrome staining of kidney biopsy specimen from patients AIP_1, AIP_2, and AIP_3. (e) ALAS1 immunostaining in the kidney biopsy specimen of the index patient (original magnification × 40). AIP, acute intermittent porphyria; eGFR, estimated glomerular filtration rate; sCr, serum creatinine.
Givosiran was interrupted in another patient because of acute liver injury (Supplementary Figure S5). Withdrawal was followed by return to baseline levels of liver enzymes. However, sCr increase preceded liver enzymes elevation by about 2 months and begun to decrease before givosiran interruption, arguing against a common mechanism for kidney and liver injury.
Discussion
These results indicate that a transient increase in sCr usually occurs in the 3 months following the introduction of givosiran and is limited in amplitude. No biochemical or structural evidence of acute glomerular, vascular, or tubular injury related to givosiran was identified. A long-term deterioration of renal function was observed in some patients, but a lack of control group does not allow us to formally conclude as to the imputability of givosiran. In addition, as AIP promotes CKD, especially in patients with a severe disease (those who are candidates for givosiran), it is difficult to separate the effect of the study drug from the natural evolution of porphyria-associated kidney disease, which is associated with a progressive decline of renal function.8
The mechanisms by which givosiran could alter renal function remain to be characterized. Givosiran does not seem to inhibit tubular creatinine secretion and is not known to be a substrate of the transporters MATE-1 and MATE-2, which inhibition is expected to cause a rise in the sCr level. An alternative hypothesis could be that givosiran penetrates the proximal tubule through endocytosis and inhibits ALAS1 expression in proximal tubular cells. These cells are metabolically highly active and strongly depend on the activity of hemoproteins such as mitochondrial cytochromes and also antioxidant catalases and peroxidases. Therefore, ALAS1 inhibition, by affecting the production of these hemoproteins, could have deleterious consequences on tubular cell homeostasis. However, we provide evidence that givosiran does not promote tubular injury and does not affect ALAS1 expression in the kidney. This is consistent with findings in mice exposed to high doses of this siRNA.9
It might be conceivable that early renal dysfunction under givosiran treatment reflects intrarenal hemodynamic modifications, an effect reminiscent of other drugs such as renin-angiotensin-aldosterone system blockers, nonsteroidal anti-inflammatory drugs, or calcineurin inhibitors. ALA is a potent vasoconstrictor, and as such, this molecule, when present in a high concentration in plasma, could affect intrarenal hemodynamics and renal blood flow and modulate renal function. The abrupt and profound drop in systemic ALA concentrations observed soon after givosiran injection could reduce renal arteriolar tonus (e.g., efferent arteriolar vasodilatation) and reduce glomerular perfusion, leading to an increase in sCr. Moreover, renal microcirculation is also dependent on the activity of cyclo-oxygenases and NO synthases, which also are hemoproteins, and which expression could be affected by inhibition of ALA production. However, the specific effects of ALA on intrarenal physiology are unknown and have not been addressed as of this writing. Finally, an impact of health improvement under givosiran and modifications of lifestyle (for example feeding) on renal function parameter cannot be excluded.
Our data indicate that over the long term, renal function of some patients treated with givosiran deteriorates, and that givosiran seems to affect the renal function of patients with pre-existing CKD (patients 6 and 7), which complicates the interpretation of renal effects. Indeed, these patients could be more at risk to develop renal side effects of givosiran because of preexistent alterations in intrarenal homeostasis. Individuals without prior altered renal function could be less prone to develop those adverse effects. Potential confounders include the natural evolution of CKD or unrelated factors that might affect an already compromised renal function. The comparison with a control group could help answer this question. The interpretation of the progression of CKD in the patient in whom givosiran has been interrupted (patient 7) is complicated by the long-lasting effect of givosiran: in this patient, more that 7 months after its interruption, urinary ALA concentration is still undetectable and the patient is still asymptomatic, indicating that hepatic ALAS1 mRNA translation remains prevented.3 Assuming that givosiran promotes CKD, or drives CKD progression in some patients through ALAS1 inhibition, this effect is likely to persist for some time after the therapeutic interruption, and we should be exploring all options in order to mitigate these harmful effects.
A lack of systematic pathologic examination of kidney biopsy specimen late after givosiran initiation is a limitation, but in a context of care, kidney biopsy was not indicated in almost all of these patients because of the absence of frank proteinuria or severely deteriorated renal function. On the other hand, a systematic screen for histologic lesion is unethical because the risk associated with kidney biopsies would be superior to the expected benefits in terms of treatment.
In conclusion, we provide evidence that givosiran is associated with an early and reversible decline of renal function likely mediated by alterations of intrarenal hemodynamics. A long-term deleterious renal impact of ALAS1 inhibition on renal function in some patients cannot by excluded, but separating the natural evolution of the severe forms of porphyria-associated kidney disease from the long-term renal effects of givosiran is challenging. Because the long-term effects of ALAS1 inhibition are unknown, a monitoring of renal function of patients under givosiran is recommended.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary File (Word and EPS)
Table S1. Proteinuria, systolic and diastolic blood pressure in the pre and post givosiran period in the ENVISION cohort.
Figure S1. Evolution of renal function under givosiran.
Figure S2. Evolution of renal function under givosiran in the CRMR cohort.
Figure S3. Evolution of proteinuria and blood pressure under givosiran.
Figure S4. ALAS1 immunostaining in the kidney.
Figure S5. Biological parameters of patients in the ENVISION trial who developed liver enzymes elevation, followed by givosiran interruption.
Supplementary Material
Table S1. Proteinuria, systolic and diastolic blood pressure in the pre and post givosiran period in the ENVISION cohort.
Figure S1. Evolution of renal function under givosiran.
Figure S2. Evolution of renal function under givosiran in the CRMR cohort.
Figure S3. Evolution of proteinuria and blood pressure under givosiran.
Figure S4. ALAS1 immunostaining in the kidney.
Figure S5. Biological parameters of patients in the ENVISION trial who developed liver enzymes elevation, followed by givosiran interruption.
References
- 1.Bissell D.M., Anderson K.E., Bonkovsky H.L. Porphyria. N Engl J Med. 2017;377:862–872. doi: 10.1056/NEJMra1608634. [DOI] [PubMed] [Google Scholar]
- 2.Puy H., Gouya L., Deybach J.C. Porphyrias. Lancet. 2010;375:924–937. doi: 10.1016/S0140-6736(09)61925-5. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal S., Simon A.R., Goel V. Pharmacokinetics and pharmacodynamics of the small interfering ribonucleic acid, givosiran, in patients with acute hepatic porphyria. Clin Pharmacol Ther. 2020;108:63–72. doi: 10.1002/cpt.1802. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda M., Gan L., Chen B. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. Proc Natl Acad Sci U S A. 2014;111:7777–7782. doi: 10.1073/pnas.1406228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sardh E., Harper P., Balwani M. Phase 1 Trial of an RNA interference therapy for acute intermittent porphyria. N Engl J Med. 2019;380:549–558. doi: 10.1056/NEJMoa1807838. [DOI] [PubMed] [Google Scholar]
- 6.Balwani M., Sardh E., Ventura P. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N Engl J Med. 2020;382:2289–2301. doi: 10.1056/NEJMoa1913147. [DOI] [PubMed] [Google Scholar]
- 7.Tollånes M.C., Aarsand A.K., Villanger J.H., on behalf of the European Porphyria Network (EPNET) Establishing a network of specialist Porphyria centres—effects on diagnostic activities and services. Orphanet J Rare Dis. 2012;7:93. doi: 10.1186/1750-1172-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallet N., Mami I., Schmitt C. High prevalence of and potential mechanisms for chronic kidney disease in patients with acute intermittent porphyria. Kidney Int. 2015;88:386–395. doi: 10.1038/ki.2015.97. [DOI] [PubMed] [Google Scholar]
- 9.Gomá-Garcés E., Pérez-Gómez M.V., Ortíz A. Givosiran for acute intermittent porphyria. N Engl J Med. 2020;383:1989. doi: 10.1056/NEJMc2026458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.