Introduction
Membranous glomerulopathy (MGN) is among the most common glomerular diseases identified in adult patients with nephrotic syndrome. Traditionally, MGN is classified into primary and secondary forms depending on the clinical context (i.e., renal-limited vs. associated with a systemic disorder), with a presumed secondary etiology identified in approximately 30% of cases. Since the discovery of phospholipase A2 receptor (PLA2R) as a major target antigen in primary MGN,1 multiple additional antigenic targets have been discovered. Most if not all of these antigens are expressed on podocytes, including thrombospondin type-1 domain-containing 7A2 and neural epidermal growth factor–like 1 (NELL1), both of which have been described in both primary MGN and in MGN in the setting of malignancy.3, 4, 5 The discovery of these and other new antigens has led some to advocate for classification based on the deposited antigen.6
In contrast to PLA2R-associated MGN where deposits are globally distributed and immunoglobulin G4 (IgG4)–dominant, NELL1-associated MGN is characterized by IgG1-dominant subepithelial deposits that are often segmental or segmental to global in distribution and can occur in association with malignancy.3, 4, 5 To date, there have been no reports of NELL1-associated MGN in the setting of hematopoietic stem cell transplantation (HSCT) or with findings of tubular basement membrane (TBM) deposits.3, 4, 5 Herein, we report the first case of NELL1-associated segmental MGN with TBM deposits following allogeneic HSCT complicated by graft-versus-host disease (GVHD).
Case
A 74-year-old White male presented with nephrotic syndrome. Past medical history included chronic myelomonocytic leukemia status post myeloablative haploidentical peripheral blood HSCT from his son 2 years prior, longstanding hypertension, and chronic kidney disease (predating HSCT; associated with 1+ proteinuria on urinalysis), uric acid nephrolithiasis, hyperlipidemia, benign prostatic hypertrophy, and gout. The patient’s post-HSCT course was complicated at 3 months by neutropenic fever, inguinal rash, and upper gastroesophageal symptoms, suspicious for GVHD and treated with sirolimus; 3 months later he developed oligoclonal large granular lymphocytosis that resolved with steroids. Throughout the post-transplantation course, the patient required occasional transfusions and was treated with filgrastim and romiplostim for granulocytopenia and thrombocytopenia. There was no evidence of recurrent chronic myelomonocytic leukemia.
Nine months post-transplantation, the patient developed a painful, pruritic maculopapular rash involving the chest and back, with skin biopsy findings consistent with GVHD. The rash responded to steroids, which were subsequently discontinued. The patient was noted to have mild edema without hypoalbuminemia. Intravenous immunoglobulin was administered for low serum IgG levels. As the edema improved following furosemide therapy, no further workup was performed. One year later, in the setting of increasing edema, the patient was found to have nephrotic syndrome. At the time, the patient’s medications included intravenous Ig, tamsulosin, rosuvastatin, and verapamil. Physical exam showed blood pressure of 120/74 mm Hg and body mass index of 25.1 kg/m2.
Laboratory evaluation revealed serum creatinine of 1.46 mg/dl (baseline, 1.2 to 1.4 mg/dl), 24-hour urine protein of 6.8 g/day, and serum albumin of 2.5 g/dl. The patient had a hemoglobin of 13.8 g/dl, white blood cell count 4.1 thousand/μl with normal differential, platelet count 136 thousand/μl, normal C3 and C4 complement levels, bland urine sediment, and negative serologies including anti-nuclear antibody (Ab), anti–double-stranded DNA Ab, antineutrophil cytoplasmic Ab, anti-PLA2R Ab, anti–glomerular basement membrane Ab, hepatitis B surface antigen, and hepatitis C Ab. Serum and urine protein electrophoresis demonstrated an IgA-lambda M-protein.
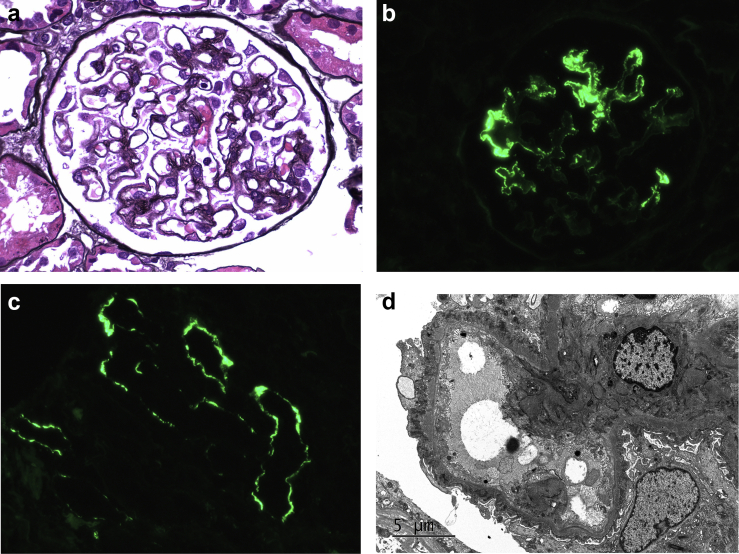
Kidney biopsy demonstrated 39 glomeruli, 16 of which were globally sclerotic. Glomeruli appeared normal in cellularity and exhibited segmental to global glomerular basement membrane thickening with “spikes” highlighted by periodic acid-Schiff and Jones methenamine silver stains (Figure 1a). Proximal tubules demonstrated protein resorption droplets. Mild to moderate tubular atrophy and interstitial fibrosis involved approximately 30% of the cortex sampled. There was mild interstitial lymphocytic inflammation, confined to areas of tubulointerstitial scarring, and mild arteriosclerosis and arteriolosclerosis. Congo red stain for amyloid was negative.
Figure 1.
Neural epidermal growth factor–like 1 (NELL1)–associated membranous glomerulopathy in a patient with graft-versus-host disease. Light microscopy shows segmental glomerular basement membrane thickening with subepithelial spike formation (a, Jones methenamine silver, original magnification ×600). Immunofluorescence staining for immunoglobulin G (IgG) shows granular segmental glomerular capillary wall staining for IgG (b, original magnification ×400) and along tubular basement membranes (c, original magnification ×400). Ultrastructural examination confirms the presence of subepithelial electron dense deposits (d, original magnification ×6000).
Immunofluorescence demonstrated 3+ granular segmental to global glomerular capillary wall staining for IgG (Figure 1b) and κ and λ light chains, accompanied by 2+ IgM, 1+ C3, and trace C1 in a similar distribution. In addition, there were focal granular TBM deposits which stained 1+ for IgG, κ, and λ (Figure 1c). No mesangial, subendothelial, or arterial vascular wall staining was observed. Ultrastructural examination confirmed the presence of segmental to global subepithelial electron dense deposits separated by intervening glomerular basement membrane spikes (Figure 1d), with some of the deposits undergoing partial resorption (Ehrenreich and Churg stage 2-3). Podocytes demonstrated 70% foot process effacement. No subendothelial or mesangial electron dense deposits, organized substructure, or endothelial tubuloreticular inclusions were identified.
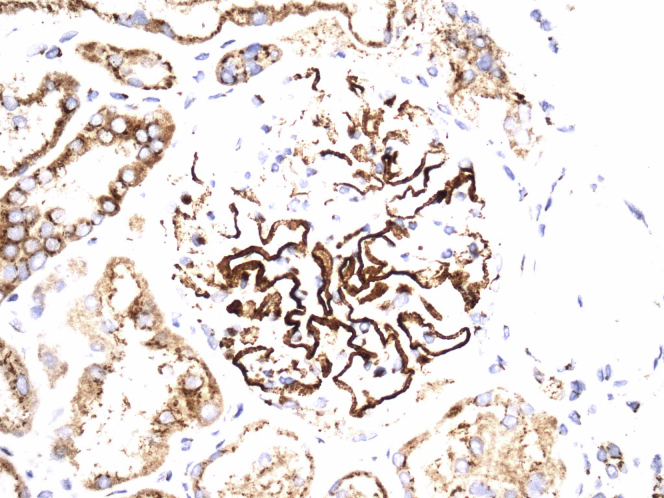
Immunofluorescence (on frozen sections) was negative for PLA2R (Abcam, Cambridge, MA, ab188028). Immunohistochemical staining for NELL1 (Sigma-Aldrich, St. Louis, MO, HPA051535), performed on formalin-fixed paraffin-embedded tissue, highlighted segmental to global subepithelial deposits (Figure 2a), but was not present in TBMs. Immunofluorescence staining for anti–LRP-2 (Sigma-Aldrich, MABS489) was also negative in TBMs.
Figure 2.
Immunoperoxidase stain for neural epidermal growth factor–like 1 shows incomplete glomerular capillary wall positivity in the distribution of the subepithelial deposits (original magnification ×400) with no convincing positivity along tubular basement membranes.
Discussion
GVHD is a common complication of allogeneic HSCT due to immune response by engrafted cells from the donor against the recipient via innate and adaptive immunity. GVHD most commonly affects the skin, gastrointestinal tract, and liver, and is traditionally classified into acute and chronic forms.7,8 Pathologic diagnosis of acute GVHD is based largely on the presence of lymphocytic inflammation associated with apoptosis of host parenchymal cells, consistent with mechanisms of self-recognition by graft-derived cytotoxic T cells.8 In contrast, the clinical and pathologic manifestations of chronic GVHD are more variable and its pathogenesis is more complex and less well-defined.7,9
While involvement of the kidney by GVHD is believed to be uncommon, a wide spectrum of kidney diseases have been described in patients with HSCT, likely reflecting the complex interplay of alloimmunity, immunosuppressive therapy, radiation exposure, opportunistic infection, underlying neoplasia, as well as comorbid medical conditions in these complex patients.S1-S5 In a kidney biopsy series of 20 patients with allogenic and autologous HSCT by Chang et al., thrombotic microangiopathy was the most frequent pathology identified, whereas MGN was the most prevalent finding in a biopsy series of 15 cases reported by Troxell et al.S3,S5 Development of nephrotic range proteinuria post-HSCT is uncommon with biopsy-based evidence showing that the most common underlying glomerular pathology is MGN (especially in those with allogenic HSCT, accounting for approximately two-thirds of patients with nephrotic range proteinuria).S1
Since the first description by Hiesse et al. in 1988, more than 60 cases of MGN post-HSCT have been reported.S6,S7 Pathologic features of HSCT-associated MGN, which more aptly may be referred to as GVHD-associated MGN, include most prominently subepithelial immune complex deposition, although subendothelial, mesangial, and TBM deposits are rarely observed.S1-S4 In most cases, the MGN is of an early stage (stage 1-2).S2-S4, S8 Because most studies predate the implementation of staining for PLA2R, description of the targeted antigens in GVHD-associated MGN are sparse. Byrne-Dugan et al. described positive staining for PLA2R in one of five cases of MGN occurring after GVHD.S2
Review of the archives of the Renal Pathology Laboratory at Columbia University Irving Medical Center from 2000 to 2021 revealed 71 native kidney biopsy specimens from patients who previously underwent HSCT. Among this cohort, the most frequent diagnoses were thrombotic microangiopathy (15 of 71 [21%]) followed by MGN (9 of 71 [13%]) and acute tubular injury (9 of 71 [13%]) (Table 1). Among the six with MGN and available frozen tissue, staining for PLA2R was positive in one. Remarkably, two of nine biopsy specimens (22%), including the case reported herein, exhibited positive staining for NELL1, and both cases had segmental subepithelial deposits (“segmental MGN”) and TBM deposits (which stained negative for NELL1). In the remaining seven biopsy specimens, including the one with PLA2R positivity, the subepithelial deposits were globally distributed, and one of the seven contained TBM deposits. None of the patients had active malignancy. Based on these findings, we believe that there may be an etiologic association between GVHD and development of NELL1-associated MGN. Future investigation should help to better define the frequency of this occurrence. The target antigen of the TBM deposits, which does not appear to be NELL1, is another area of potential study.
Table 1.
Membranous glomerulopathy in patients with hematopoietic stem cell transplantation
| Patient no. | Demographics |
HSCT |
Clinical parameters at time of renal biopsy |
Renal biopsy findings |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, yrs | Sex | Race | Indication for HSCT | GVHD | Interval between HSCT and renal biopsy, mo | sCr, mg/dl |
UPCR, g/g | Serum albumin, g/dl | Edema | Segmental MGN | TBM deposits | PLA2R | NELL1 | Mesangial deposits | |
| 1a | 74 | M | W | CMML | Y | 36 | 1.46 | 6.8 | 2.5 | Y | Y | Y | neg | pos | N |
| 2 | 58 | M | Leukemia NOS | Y | 60 | 0.8 | 2.2 | 3.9 | Y | Y | Y | NA | pos | Y | |
| 3 | 68 | M | MDS | Y | N | N | NA | neg | N | ||||||
| 4 | 70 | F | W | AML | Y | 54 | 0.8 | 3.3 | 2.6 | Y | N | N | neg | neg | Y |
| 5 | 53 | M | AML | Y | 48 | 1.3 | Y | N | N | neg | neg | N | |||
| 6 | 59 | F | W | AML | Y | 48 | 1.4 | 5 | 2.9 | Y | N | N | pos | neg | N |
| 7 | 55 | M | CML | NA | 60 | 2.3 | 15 | N | N | NA | neg | NA | |||
| 8 | 73 | F | W | APL | Y | 12 | 0.7 | 5.7 | 2.3 | Y | N | N | neg | neg | N |
| 9 | 68 | M | W | MCL | Y | 1.2 | 4.5 | 3.7 | N | N | Y | neg | neg | N | |
AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; F, female; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant; M, male; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MGN, membranous glomerulopathy; N, no; neg, negative; NA, not available/not performed; NELL1, neural epidermal growth factor–like 1; NOS, not otherwise specified; pos, positive; PLA2R, phospholipase A2 receptor; sCr, serum creatinine; TBM, tubular basement membrane; UPCR, urine protein to creatinine ratio; W, white; Y, yes.
Index case that is the subject of this report.
The pathogenesis of GVHD-associated MGN, while not well understood, presumably involves graft-derived Abs targeting host podocyte antigens. Based on our limited data and the available literature, the antigenic target in GVHD-associated MGN has been found to be PLA2R in 2 of 11 cases (18%) examined, whereas NELL1 was identified in two of nine cases (22%).S2 Further clues to the presence of NELL1 antibodies in this setting include findings of segmental MGN (Table 2). Whether the presence of TBM deposits is associated with higher likelihood of NELL1 positivity requires further study. Future studies should also elucidate whether other MGN-associated podocyte antigens, such as thrombospondin type-1 domain-containing 7A and exostosin1/exostosin2, will also be found in GVHD-associated MGN.
Table 2.
Teaching points
| NELL1-associated membranous glomerulopathy frequently exhibits a segmental or segmental to global distribution of subepithelial deposits. |
| Membranous glomerulopathy may occur in the setting of hematopoietic stem cell transplantation where it represents a form of graft-versus-host disease and is usually PLA2R-negative. |
| A subset of membranous glomerulopathy in patients with graft-versus-host disease shows a segmental distribution of deposits, tubular basement membrane deposits, and NELL1 positivity. |
NELL1, neural epidermal growth factor–like 1; PLA2R, phospholipase A2 receptor.
In summary, we present a unique case of GVHD-associated MGN with segmental to global subepithelial deposits, TBM deposits, and positive staining for NELL1, and we have identified a second, similar case from our archives. This is the first demonstration of NELL1-associated MGN occurring in the setting of HSCT complicated by GVHD.
Patient Consent
The authors declare that they have obtained consent from the patients discussed in the report.
Footnotes
References
Supplementary Material
References
References
- 1.Beck L.H., Jr., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomas N.M., Beck L.H., Jr., Meyer-Schwesinger C. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caza T., Hassen S., Dvanajscak Z. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99:967–976. doi: 10.1016/j.kint.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudose S., Santoriello D., Debiec H. The clinicopathologic spectrum of segmental membranous glomerulopathy. Kidney Int. 2021;99:247–255. doi: 10.1016/j.kint.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Sethi S., Debiec H., Madden B. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163–174. doi: 10.1016/j.kint.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S. New 'Antigens' in membranous nephropathy. J Am Soc Nephrol. 2021;32:268–278. doi: 10.1681/ASN.2020071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagasia M.H., Greinix H.T., Arora M. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Przepiorka D., Weisdorf D., Martin P. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 9.Zeiser R., Blazar B.R. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377:2565–2579. doi: 10.1056/NEJMra1703472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.