Abstract
Background:
Skin reactions due to medical devices for diabetes management have become a common problem in diabetes technology. There is a varying degree in how detailed skin reactions are described in scientific literature and diabetes practice, and no uniform structured documentation is given. Whereas most articles only describe findings, some others already document final diagnoses, such as contact dermatitis. Furthermore, inconsistent wordings for comparable issues were used.
Methods:
A more detailed and standardized documentation, possibly facilitated by a generally accepted guideline for structured descriptions, of skin reactions could be helpful to enable better differentiations between the described skin reactions. Therefore, a report form to assess skin reactions due to medical devices in diabetes therapy was developed and will be presented in this article.
Results:
The one-page report form is divided into four categories and a separate instruction paper. Beside general information the form includes the location, size, severity and duration of skin appearances, the grading of itching, and suspected diagnoses.
Conclusion:
A consistent use of the form in daily practice and clinical trials could facilitate a fast and standardized documentation and help to evaluate the occurrence and severity of different skin reactions due to medical devices in diabetes management.
Keywords: skin reaction, skin-related issues, report form
Background
Most patients with diabetes type 1 use various technological devices like continuous glucose monitoring (CGM) systems or insulin pumps. Usually these medical products consist of several components: besides an external receiver, a CGM system comprises a casing including a transmitter and a sensor, which is inserted through the skin as well as an adhesive fixing the casing on the skin. In traditional insulin pumps, the pump including the reservoir and pump mechanism is worn external. A subcutaneous connection is given by a Teflon or steel cannula of the infusion set, which is attached on the skin. Tubeless insulin pumps (patch pumps) do not have an infusion set, thus the pump, including pump mechanism, electronics, reservoir, and cannula, is directly attached to the body by means of an adhesive.1 To maintain expected wear time, overtapes for better fixing the device on the skin are used frequently.2 The increasing usage and wear time of those medical devices may contribute to an increased occurrence of skin reactions.3-5 Of patients using an insulin pump or CGM system, more than one third report one or more skin lesions due to device use.5
Mechanical or physical effects, like long skin occlusion under the sensor, sweating or damaged epidermis due to tearing off the adhesive, or an injury due to insertion can lead to skin reactions. Furthermore, allergens in the medical device, like the adhesives or the plastic material itself, can trigger allergic skin reactions. Additionally, individual factors like age or dry skin influence the risk of skin reactions.3 Actually, there are several internet portals for patients to talk about their skin reactions and exchange handling suggestions6 emphasizing the increased occurrence of skin reactions.
However, a previous publication showed that in studies with medical devices reports about skin reactions are rare and, if reported, descriptions varied substantially.7 There were no uniform structured reporting procedures and for comparable issues inconsistent terms or only general wordings were used. Furthermore, description details varied regarding occurrence and severity of the skin reaction or the number of afflicted persons.
In clinical studies, adverse events (AE) have to be documented. However, the number of skin reactions observed in a study may be limited by a small subject population, a short study duration, or optimal study conditions. Furthermore, subjects with known skin problems often are excluded.
In diabetes practices a skin reaction may be observed and treated in daily practice, but systematic assessments and incident reports to national competent authorities are unlikely. A report includes the detailed description of side effects, but also information about the medical history of the patients and their actual medication, representing a very time and cost consuming action.6
The time consuming documentation of each skin reaction might contribute to the information being restricted.4 Additionally, clear evaluation criteria for skin reactions are missing and due to limited dermatological expertise of diabetes nurses, physicians, or principal study investigators, inconsistent terms for comparable issues result.
A structured and validated plan to guide a comprehensive and standardized assessment and quick report may help to explore and compare the prevalence of skin reactions. Thus, a report form for uniform descriptions of skin reactions due to medical devices for diabetes management was designed.
Methods
For the development of a standardized report form, already existing methods to assess and scale skin reactions as well as terminologies were screened and evaluated.
For decades, patch testing with visual sign assessment represented the cornerstone of dermatological evaluation and methods to evaluate if a substance has an allergic or toxic effect when contacting the skin were already reported in 1847.8
However, most often only the patch testing techniques and no details on the visual assessment were described.8 A scoring of dermatological changes like erythema, papules, vesicles, bullae, and edema by grading scales was introduced later.
A main role for the toxicological classification of test substances was presented by the Draize eye test developed in 1944.9 Originally, the test was developed for eye irritation tests on rabbits to evaluate the safety of materials meant for use in and around the eyes.9,10
Modifications of the test, including the assessment of erythema and edema, were used as skin irritation test. A scoring enabled the product classification from nonirritant to very irritant.8,9,11 Techniques of the Draize test were generally accepted and also used by the Food and Drug Administration (FDA) to evaluate substance safety for long time. However, species differences between human and rabbit cause large variations in results, leading to criticism of the test.9,11
Currently, a score or form especially developed to assess skin reactions due to medical devices like insulin pumps or CGM systems is not available. Thus, in some clinical trials using CGM systems, the Draize scale is used to assess and categorize skin reactions in context of the AE documentation.
Berg et al developed a patient questionnaire to directly focus on skin problems associated with insulin pumps and CGM. Patients were asked about their previous and current dermatological complications. Each complication was categorized into predefined items like “red eczema,” “dry wound,” or “changes in pigmentation” by the patients themselves. To help categorizing the type of skin problem, example pictures of the categories were given. A five-point scale (“no problem” to “very big problem”) was used to rate the discomfort caused by each visible skin lesion.5 The categorization and scaling of complications by the patients themselves may be critical. Furthermore, the predefined items already include consequences of preceding reactions like “old scars,” which are not relevant for a report form that assesses current skin reactions.
An index to enable the comparability of diagnosed atopic dermatitis was developed by the European Task Force on Atopic Dermatitis.12 The SCORAD (SCORing Atopic Dermatitis) index collects objective information as well as subjective symptoms. The objective part consists of information about the extent and intensity of appearances like erythema, edema/papulation, or oozing/crust. The subjective part is about pruritus and sleep loss.12 For documenting the extent of the symptoms, a figure with numbered areas is given. The intensity ranges from 0 = absence to 3 = severe. Subjective symptoms are graded with a visual analogue scale. The final index score can be calculated by using a formula which includes the determined severities for objective and subjective symptoms.13
Results
Based on long-standing experience of the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany (IfDT) in clinical trials using the previously described medical devices and the accompanying assessment and documentation of skin reactions, a report form for the assessment of skin reactions due to medical devices for diabetes management was designed.
Developmental versions of the form were tested by experienced physicians of the IfDT by using previous skin reactions captured on photos. The filled forms from different physicians were compared to check standardized outcomes, and general feedback regarding the form was integrated into subsequent versions.
To bring along dermatological experience and ensure dermatologically correct terms, input was also requested from a dermatologist experienced in diabetes-related skin reactions. Furthermore, feedback from members of the working group diabetes and technology (AGDT) of the German diabetes association, including experienced diabetologists and diabetes advisors, was integrated.
The final form consists of four parts: general information (A), findings (B), further action (C), and suspected diagnosis (D) (see Figure 1).
Figure 1.
Report form.
The form is meant to be filled by health care professionals (HCP) and not necessarily professional dermatologists. Therefore, a separate instruction form is attached to guide HCPs filling out the form (supplement 1). The instruction form includes comprehensible definitions for the predefined skin appearances, severity categories, and suspected diagnoses as well as general information on how to fill out the form.
As soon as a skin reaction becomes known to the HCP, the report form should be filled. On the report form itself, primarily general information about the affected person like gender, age, and current system use will be asked (Part A).
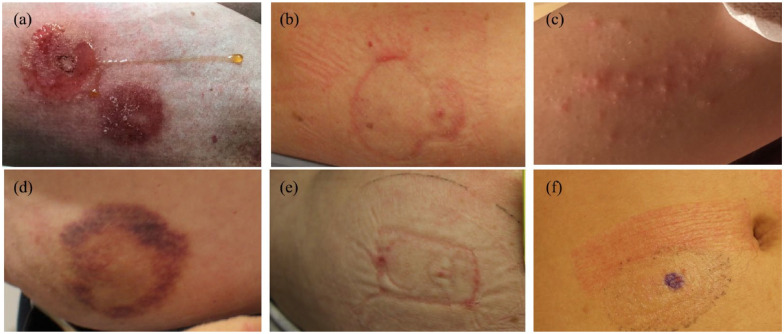
Essential aspects when documenting skin-related issues are mainly objective and subjective skin appearances, including their localization, duration, severity, and size (Part B). For objective skin appearances common efflorescences like erythema, papules, desquamation, crust formation, pustules/vesicles, weeping, pressure mark, hematoma, and induration were allocated (B6). To enable a standardized wording, the skin appearances were predefined. Terms were chosen, together with a dermatologist enabling dermatologically correct, but also comprehensive descriptions. Example pictures for the listed appearances are given in Figure 2 (a–f), and the separate instruction form includes comprehensible definitions for the skin appearances (supplement 1).
Figure 2.
(a) Erythema and weeping across the device casing (an allergic contact dermatitis was diagnosed later), (b) erythema and skin lesion at device casing edges, (c) pustules/vesicles, (d) hematoma around adhesive area, (e) pressure mark, and (f) erythema at the overtape.
To draw conclusions about the triggering component of the medical device, it should be documented whether the appearances are located at the insertion site, in the area of the casing (if available), at the area of the (additional, eg, overtapes) adhesive, or outside the borders of the adhesive (B6). Therefore, a number (one to five) representing the different location possibilities has to be entered for each occurred appearance behind the predefined term. In case a skin appearance is located in more than one of the predefined five locations, several numbers can be entered on the report form.
Sometimes HCPs might like to document additional information. To prevent additional and thus several documents for one patient, a free text space is given for additional information.
The size of the skin reaction is asked separately and has to be documented in millimeter on the report form (B7).
Skin changes can impair user’s daily life significantly.14 Especially the subjective symptom “itching” is a common reported symptom when using CGM systems.7,15 Thus, by using a scale with a maximum score of 10, affected persons will grade their feeling of itching between 0 (=no itching) and 10 (=intense itching).
To assess the temporal progression (B8) of the skin appearances, the first occurrence of symptoms and the solvation of the last are asked. Whereas itching can be noticed earlier some skin appearances may only be detectable after system removal. Adding the date of system removal will therefore enable the differentiation whether the skin appearance was detected before or only after sensor removal, allowing more detailed information about the duration of skin appearance. Additionally, to identify a potential immediate type I allergic reaction, the start date of the last system application has to be documented as well.
Due to the varying degrees of reactions,8 a grading into mild, moderate, or severe of the skin reactions in sum can be done. The definitions of the grading categories are based on the adverse event severity grading scale by the FDA16 and are given in the separate instruction of the report form. Mild is defined as asymptomatic or mild symptoms. Only clinical or diagnostic observations have to be performed and no intervention is indicated. For moderate appearances, minimal, local, or noninvasive interventions are indicated. The appearances limit age-appropriate activities of an independent daily life. Severe skin reactions are defined as severe or medically significant but not immediately life threatening. A hospitalization or prolongation of hospitalization is indicated. The appearances are disabling and limit self-care activities of the daily life.
Serious AE in clinical trials as well as serious incidents assessed in daily practice have to be reported to national competent authorities.17 An allergic contact dermatitis (ACD) for example is expected to reoccur under similar conditions and, thus, constitutes a permanent impairment of daily life. The diagnosis of an ACD has to be graded as severe and serious and must be reported to national competent authorities.
For documenting further actions, like change to an alternative system or use of a protective adhesive, as well as information about whether actions were effective, the form provides some free text space (Part C).
After having assessed and described the skin reactions, a suspected diagnosis may optionally be added (Part D). Common diagnoses like irritant contact dermatitis (ICD) or ACD and infections are already given. The definitions are described on the separate instruction form. Often no distinction is made between ICD and ACD.3 Due to mostly stronger intensity compared with skin irritations, an allergic reaction is frequently reported,7 but usually occurs seldom.3 ACD is a type 4 allergy due to one or more allergens (most acrylates); after sensitization a lifelong immunological memory exists. Thus, symptoms will reoccur after repeated contact and usually get even stronger.3 Therefore, previous skin reactions and their kind of severity are requested as general information at the beginning of skin assessment. To ensure a final diagnosis of an ACD, a patch test should be performed.18 Further steps may be the lifelong contact avoidance of identified allergens.
Conclusion
To summarize, beside general information the form includes the location, size, severity and duration of skin appearances, the grading of itching, and suspected diagnoses.
To keep the form as short as possible but also comprehensive, the objective and subjective categories of the report form were chosen based on their clinical importance and the high incidence and variation in previous studies reporting skin reactions. To allow a fast and uniform completion of the form, check or one word options are in focus of this form. Free text spaces are given for additional information that should not be neglected and may be relevant for more accurate assessment of the skin-related issues but will not be usable for an evaluation of occurred skin reactions.
Although the form was designed to be as simple as possible and a separate instruction form is given, an initial training may be useful for a quick filling out during daily practice stress.
Currently, the form is not validated and inter- and intraindividual variations by different HCPs cannot be excluded. To assess variations, the form should be used widespread in clinical trials as well as in diabetes practices to enable a comprehensive evaluation.
A consistent use of the form in daily practice and clinical trials could facilitate a fast and standardized documentation for physicians and diabetes nurses. A standardized documentation primarily helps to evaluate the occurrence and severity of different skin reactions due to medical devices in diabetes management. Furthermore, it may help to identify and reduce triggering factors, such as potent allergens and thus help patients to avoid skin reactions in the future. The further development toward a score, for example, resulting in one final number could be the next step to simplify the evaluation of the assessed skin reactions and it may be easier to get a final diagnosis.
Supplemental Material
Supplemental material, Freckmann_Supplement_1 for Skin Reaction Report Form: Development and Design of a Standardized Report Form for Skin Reactions Due to Medical Devices for Diabetes Management by Guido Freckmann, Sina Buck, Delia Waldenmaier, Eva Zschornack, Manuela Link, Nina Jendrike, Ines Obstfelder, Sara Vetrugno, Stefanie Kamann and Cornelia Haug in Journal of Diabetes Science and Technology
Acknowledgments
The authors thank the IfDT staff and the Arbeitsgemeinschaft Diabetes & Technologie der Deutschen Diabetes Gesellschaft e.V. (AGDT) members who contributed to data collection or assisted in preparation of this article.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GF is general manager of the IDT (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies on the evaluation of BG meters and medical devices for diabetes therapy on its own initiative and on behalf of various companies. GF/IDT have received speakers’ honoraria or consulting fees from Abbott, Ascensia, Dexcom, LifeScan, Menarini Diagnostics, Metronom Health, Novo Nordisk, PharmaSense, Roche, Sanofi, Sensile, and Ypsomed. EZ, DW, SB, SV, ML, NJ, IO, and CH are employees of IDT. SK has received speakers’ honoraria from Abbott, Lilly, Novo Nordisk, and Roche.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Guido Freckmann  https://orcid.org/0000-0002-0406-9529
https://orcid.org/0000-0002-0406-9529
Sina Buck  https://orcid.org/0000-0001-8428-1038
https://orcid.org/0000-0001-8428-1038
Delia Waldenmaier  https://orcid.org/0000-0003-3280-2369
https://orcid.org/0000-0003-3280-2369
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Paul N, Kohno T, Klonoff DC. A review of the security of insulin pump infusion systems. J Diabetes Sci Technol. 2011;5(6):1557-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Englert K, Ruedy K, Coffey J, et al. Skin and adhesive issues with continuous glucose monitors: a sticky situation. J Diabetes Sci Technol. 2014;8(4):745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamann S, Oppel E, Liu F, Reichl F-X, Heinemann L, Högg C. Evaluation of isobornyl acrylate content in medical devices for diabetes treatment. Diabetes Technol Ther. 2019;21(10):533-537. [DOI] [PubMed] [Google Scholar]
- 4. Kamann S, Aerts O, Heinemann L. Further evidence of severe allergic contact dermatitis from isobornyl acrylate while using a continuous glucose monitoring system. J Diabetes Sci Technol. 2018;12(3):630-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berg AK, Norgaard K, Thyssen JP, et al. Skin problems associated with insulin pumps and sensors in adults with type 1 diabetes: a cross-sectional study. Diabetes Technol Ther. 2018;20(7):475-482. [DOI] [PubMed] [Google Scholar]
- 6. Heinemann L, Kamann S. Adhesives used for diabetes medical devices: a neglected risk with serious consequences? J Diabetes Sci Technol. 2016;10(6):1211-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pleus S, Ulbrich S, Zschornack E, et al. Documentation of skin-related issues associated with continuous glucose monitoring use in the scientific literature. Diabetes Technol Ther. 2019;21(10):538-545. [DOI] [PubMed] [Google Scholar]
- 8. Farage MA, Maibach HI, Andersen KE, et al. Historical perspective on the use of visual grading scales in evaluating skin irritation and sensitization. Contact Dermatitis. 2011;65(2):65-75. [DOI] [PubMed] [Google Scholar]
- 9. Lee M, Hwang JH, Lim KM. Alternatives to in vivo Draize rabbit eye and skin irritation tests with a focus on 3D reconstructed human cornea-like epithelium and epidermis models. Toxicol Res. 2017;33(3):191-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilhelmus KR. The Draize eye test. Surv Ophthalmol. 2001;45(6):493-515. [DOI] [PubMed] [Google Scholar]
- 11. Vinardell MP, Mitjans M. Alternative methods for eye and skin irritation tests: an overview. J Pharm Sci. 2008;97(1):46-59. [DOI] [PubMed] [Google Scholar]
- 12. Wolkerstorfer A, de Waard van der Spek FB, Glazenburg EJ, Mulder PG, Oranje AP. Scoring the severity of atopic dermatitis: three item severity score as a rough system for daily practice and as a pre-screening tool for studies. Acta Derm Venereol. 1999;79(5):356-359. [DOI] [PubMed] [Google Scholar]
- 13. Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23-31. [DOI] [PubMed] [Google Scholar]
- 14. Christensen MO, Berg AK, Rytter K, et al. Skin problems due to treatment with technology are associated with increased disease burden among adults with type 1 diabetes. Diabetes Technol Ther. 2019;21(4):215-221. [DOI] [PubMed] [Google Scholar]
- 15. Weng AT, Zachariae C, Christensen KB, et al. Five-month follow-up shows no improvement in dermatological complications in children with type 1 diabetes using continuous glucose monitoring systems and insulin pumps [published online ahead of print 16 October 2019]. J Diabetes Sci Technol. doi: 10.1177/1932296819882425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Norquist JM, Khawaja SS, Kurian C, et al. Adaptation of a previously validated vaccination report card for use in adult vaccine clinical trials to align with the 2007 FDA Toxicity Grading Scale Guidance. Hum Vaccin Immunother. 2012;8(9):1208-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verordnung über die Erfassung, Bewertung und Abwehr von Risiken bei Medizinprodukten (Medizinprodukte-Sicherheitsplanverordnung -MPSV). 2002. [Google Scholar]
- 18. Iwasaki A. Local advantage: skin DCs prime; skin memory T cells protect. Nat Immunol. 2009;10(5):451-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Freckmann_Supplement_1 for Skin Reaction Report Form: Development and Design of a Standardized Report Form for Skin Reactions Due to Medical Devices for Diabetes Management by Guido Freckmann, Sina Buck, Delia Waldenmaier, Eva Zschornack, Manuela Link, Nina Jendrike, Ines Obstfelder, Sara Vetrugno, Stefanie Kamann and Cornelia Haug in Journal of Diabetes Science and Technology