Abstract
Introduction
After first stroke, the transition from rehabilitation to home can be confronting and fraught with challenges. Although stroke clinical practice guidelines recommend predischarge occupational therapy home visits to ensure safe discharge and provision of appropriate equipment, there is currently limited evidence to support this recommendation.
Methods and analysis
The HOME Rehab trial is a national, multicentre, phase III randomised controlled trial with concealed allocation, blinded assessment and intention-to-treat analysis being conducted in Australia. The trial aim is to determine the effect and potential cost-effectiveness of an enhanced occupational therapy discharge planning intervention that involves pre and postdischarge home visits, goal setting and occupational therapy in the home (the HOME programme) in comparison to an in-hospital predischarge planning intervention. Stroke survivors aged ≥45 years, admitted to a rehabilitation ward, expected to return to a community (private) dwelling after discharge, with no significant prestroke disability will be randomly allocated 1:1 to receive a standardised discharge planning intervention and the HOME programme or the standardised discharge planning intervention alone. The primary outcome is participation measured using the Nottingham Extended Activities of Daily Living. Secondary outcome areas include hospital readmission, disability, performance of instrumental activities of daily living, health-related quality of life, quality of care transition and carer burden. Resources used/costs will be collected for the cost-effectiveness analysis and hospital readmission. Recruitment commenced in 2019. Allowing for potential attrition, 360 participants will be recruited to detect a clinically important treatment difference with 80% power at a two-tailed significance level of 0.05.
Ethics and dissemination
This study is approved by the Alfred Health Human Research Ethics Committee and site-specific ethics approval has been obtained at all participating sites. Results of the main trial and the secondary endpoint of cost-effectiveness will be submitted for publication in peer-reviewed journals
Trial registration number
Keywords: stroke, rehabilitation medicine, stroke medicine
Strengths and limitations of this study.
The HOME Rehab trial will be conducted as a powered randomised controlled trial to measure the effect of adding an enhanced occupational therapy discharge planning intervention; it will provide clinicians and hospital administrators with important information about supporting people with stroke to transition from hospital to home.
This is a phase III trial with concealed allocation, blinded assessment and intention-to-treat analysis and includes a process evaluation and economic evaluation.
The trial will be adequately powered to detect a clinically important treatment difference in functional independence at 4-week postdischarge from hospital after first stroke.
Owing to the type of interventions, blinding of the participants and treatment providers is not possible.
Introduction
Transitioning to home from hospital is a critical time for people poststroke.1–3 Hospital-community communication and coordination can be inadequate during the discharge phase,4–6 increasing the risk of poor return to community activity, low satisfaction, adverse events and unplanned readmission. The most effective method for supporting hospitalised people who have experienced stroke to transition from hospital to home is not yet known7 8 which has led to variability in practice within the rehabilitation context.9 10
As a rehabilitation programme draws close to discharge, it is usual for people with stroke to be involved in discharge planning where they receive an occupational therapy predischarge home assessment. While it is recommended in national clinical guidelines that occupational therapy predischarge home visits occur ‘to ensure safety and provision of appropriate aids, support and community services’,11 research to date suggests that there may not be any difference in outcomes for people who do and do not receive predischarge occupational therapy home visits.12–16 What remains unknown is whether a comprehensive discharge support programme that crosses the boundaries between hospital inpatient and community outpatient services may be more beneficial than usual care, which may lack coordination and effective communication. Therefore, we have designed the HOME Rehab trial to address this research gap.
The aim of this phase III randomised trial is to determine the clinical effect (disability, participation, instrumental activities of daily living), change in the number of unplanned readmissions and the potential cost-effectiveness of an enhanced occupational therapy discharge planning intervention that involves pre and postdischarge home visits, goal setting and occupational therapy in the home (the HOME programme) in comparison to an in-hospital predischarge planning intervention. The specific research questions are:
In survivors of stroke, does the addition of the HOME programme to an in-hospital predischarge planning intervention improve activity participation at 4-week postdischarge (primary aim)?
Does it reduce unplanned hospital readmissions (secondary aim)?
Is it cost-effective (secondary aim)?
Primary end point is assessed at 4-weeks postdischarge; secondary aims, clinical outcomes and health economics are assessed at 6-month and 12-months postdischarge.
Method and analysis
Design
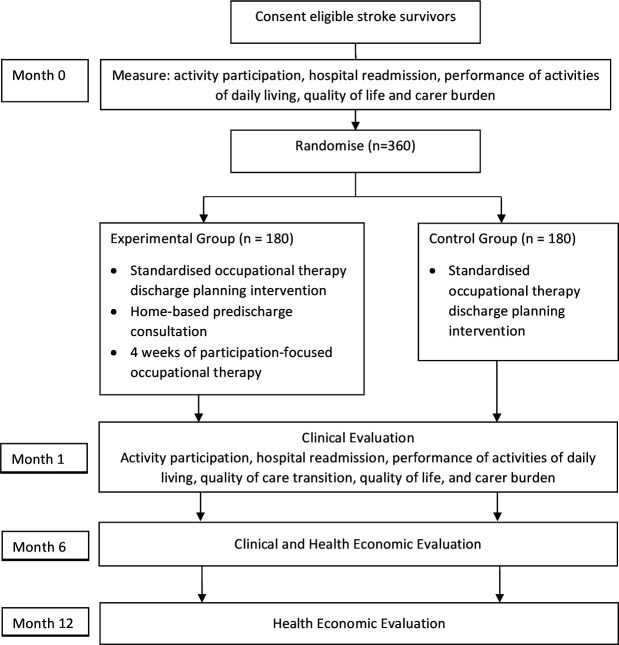
The HOME Rehab trial is a multicentre, phase III randomised controlled trial being conducted in Australia with concealed allocation, blinded measurement and intention-to-treat analysis. Adults who have experienced stroke will be recruited from inpatient rehabilitation wards across multiple states in Australia; the list of sites is available on the trial registry. Participants will be randomly allocated to receive in-hospital predischarge planning and the HOME programme or in-hospital discharge planning alone. Clinical outcomes will be measured at baseline, 1-month (4-weeks) postdischarge (end of intervention) and 6 months postdischarge (beyond the intervention); health economic outcomes will be measured at 6 and 12 months postdischarge (figure 1). Measurements will be collected by assessors blind to group allocation. It is not possible to blind participants or therapists to group allocation. The protocol has been approved by the relevant Human Research Ethics Committees and is registered at www.ANZCTR.org.au (ACTRN12618001360202).
Figure 1.
Design of the trial.
Participants, therapists, sites
People with stroke will be included if they are aged ≥45 years; admitted to a rehabilitation ward, which includes referral for occupational therapy; expected to return to live in a community (private) dwelling after discharge from hospital and have no significant prestroke disability (prestroke-modified Rankin Scale (mRS) score 0–2). Participants will be excluded if they need major home modifications or receive daily assistance with all care so as to enable discharge, have severe comorbid disease (as assessed by a score <8 on the Charlson Comorbidity Index), have an illness likely to be associated with a life expectancy of <12 months, have a significant cognitive impairment (>5 adjusted errors on the Short Portable Mental Status Questionnaire), have a body mass index of 45 or higher, have moderate or severe aphasia or have a planned discharge to an address 2 hours or greater from a recruiting site.
Therapists will be eligible to deliver the intervention if they are occupational therapists with ≥3 years of experience and have completed training in the standardised delivery of the HOME programme.
Rehabilitation wards will be included if they have a stroke throughput of ≥20/year.
Randomisation and blinding procedure
Assessors will be blinded to treatment allocation. Participants will be randomly allocated to one of the two groups using a fixed allocation ratio of 1:1 following consent and baseline assessment. We anticipate that the response to both discharge programmes may be associated with pretreatment motor ability and whether their inpatient occupational therapist conducts the postdischarge visits, and so participants will be stratified by baseline Functional Independence Measure (FIM)17 motor score (≤38 vs >38) and mode of delivery of occupational therapy (inpatient therapist vs community therapist) to minimise group imbalances on these variables. To maintain sequential recruitment balance between groups throughout the trial, a permuted block randomisation process will be used within each strata using random block sizes. The randomisation creation process (including block sizes) and resulting schedule will be set, held and managed centrally external to the investigators (LCh, The University of Melbourne) and will be managed using Research Electronic Data Capture (REDCap).18 19
Intervention
Participants in both groups (control and experimental) will undergo a standardised predischarge planning intervention led by an occupational therapist, and the experimental group will additionally receive the HOME programme.
This standardised in-hospital predischarge planning intervention will include one 30 minute in-hospital discharge planning assessment using the Discharge Planning Assessment Tool (DPAT)20 and a family-led home environment assessment. The DPAT is a client-centred assessment to prepare for discharge to a home environment; DPAT is to be completed by a client, significant other and occupational therapist and/or other team members early in hospitalisation and before discharge. The DPAT includes two rating scales of confidence (client and family member), and captures subscales related to returning home and managing care (including mobility in the home, mobility in the community, bathroom, bedroom, kitchen, household management, medication management, nutrition and diet, skin management and leisure). Results of the predischarge confidence and discharge plan evaluation (for participants in each group) will be shared with the inpatient rehabilitation team prior to discharge. The family-led home environment assessment is a standardised checklist that is completed by the family, who are also loaned a tablet computer or digital camera to take photos of areas of the home that the participant would need or wish to access on return home. Using digital photographs taken by family members, patient information and an equipment list has previously been shown to be an accurate method of collecting necessary information for occupational therapy home modifications/equipment prescriptions.21 22
In addition, participants will receive written instructions outlining their recommended home modifications and education for use of prescribed equipment prior to discharge.
Participants in the experimental group will then receive the HOME programme immediately following the standardised discharge planning intervention. Previously tested in an older, acute population,15 23 and piloted in general rehabilitation,16 the HOME programme is centred in the occupational therapy understanding that the interactions between a person and their environment drive meaningful participation in activity after hospitalisation.24–26 The HOME programme commences during hospitalisation and dovetails with the standardised discharge planning intervention, but unlike the control intervention, the HOME programme continues posthospitalisation (box 1). During hospitalisation, there is a focus on safety and transition from hospital, while posthospitalisation, the focus is on increasing a person’s capacity to deal with demands from the environment and their newly acquired disability to maximise independence.
Box 1. Clinical aims of the HOME occupational therapy programme.
Prepare the person to return home and resume their desired lifestyle
Assess the individual person’s occupational needs respecting their personal beliefs, needs and goals and understand the older person’s patterns of daily living.27 42
Recommend functional adaptations that will maximise the person’s abilities as they reintegrate back to usual living.43
Optimise the person-environment fit.44
Recommend and implement environmental modifications.
Prescribe adaptive equipment and observe its use in situ.42
Facilitate effective communication between the individual person and their General Practitioner (GP) / health partners to support the transfer of medical information from hospital to community.28
Enhance self-efficacy beliefs and promote independence and sense of control through mastery of meaningful tasks
Transfer altered skills to the home situation and assist in the adjustment to these changes.45
Habitual retraining in situ using strategies such as situational cues and targeting behaviours for change.
Encourage one-on-one education about the safe performance of activities in and around their home and immediate community.
Facilitate joint problem-solving and solution generation.42 45
Lessen a person’s fear during the transition from hospital to home.46
Use goal setting and motivational interviewing as therapeutic tools
Develop client-centred goals that address individual occupational needs.27 42
Develop goals that aim to maximise the person’s potential to participate in meaningful activities.43
Include goals that enable the person to participate in activities both in the home and in the community42 and incorporate primary health and physical activity goals.
Plan for increasing independence/capacity postdischarge, setting goals for increasing activity.45 47
The experimental group will receive one predischarge (approximately 90 min) and two postdischarge visits by an occupational therapist, followed by two booster telephone support sessions. Although performance gaps addressed are participant-specific (tailored), the process to identify and address the issues limiting independence and return to activity will be systematic and reproducible across all participants in the experimental group. Thus, all participants will receive identical intervention components. While still in hospital, a predischarge home assessment is conducted to assess the person-environment fit as well as observe the use of prescribed equipment in situ. Using the I-HOPE, activities that are valued but difficult to perform in the home environment will be identified and then prioritised, and the magnitude of the influence of the environment on performance of these activities will be assessed by the occupational therapist.27 Participation goals will then be set and results were shared with the inpatient rehabilitation team. These same I-HOPE goals will then shape the two postdischarge occupational therapy sessions with the aim of enhancing self-efficacy beliefs and promoting independence and the sense of control through mastery of meaningful activities. The two booster telephone support sessions will reinforce goal performance, enhance intrinsic motivation to return to activity28 and facilitate effective communication between the participant, family/carers and GP.29
Participants in the control group will receive only the standardised discharge planning intervention. Contamination from the experimental intervention will be determined by examining the resource diary at the end of 4-week intervention period, specifically to identify occupational goals and interventions.
Primary outcome
Activity participation will be measured using the Nottingham Extended Activities of Daily Living (NEADL).30 The NEADL is a self-reported measure of 22 activities representing four domains of daily living (mobility, kitchen, domestic and leisure) considered to be important to people with stroke who have been discharged home.
Secondary outcomes
Quality of the care transition that is associated with hospitalisation will be assessed using the 3-item Care Transitions Measure,31 which is a validated measure reflecting the quality of a person’s care transition that is associated with hospital utilisation.
Disability will be assessed by administering the mRS.32 33
Functional ability will be assessed using the Functional Autonomy Measurement System,34 which measures in five areas: activities of daily living, mobility, communication, mental functions and instrumental activities of daily living.
Health-related quality of life will be assessed using the EQ-5D-5L35 and will be used to also estimate quality-adjusted life years for the economic evaluation.
Carer burden will be assessed using the Carer Experience Scale (CES).36 The CES focuses on six domains: activities outside caring, support from family and friends, assistance from the government and other organisations, fulfilment from caring, control over caring and getting on with the care recipient; this measure is administered to the carer.
Descriptive information will include demographic and socioeconomic information, details of the index stroke and prior health-related resource use (including occupational therapy). Date and cause of death will be obtained from linkages with the National Death Index held by the Australian Institute of Health and Welfare, including those who may be lost to follow-up at 6 months and 12 months.
Economic evaluation
Direct costs for delivering each intervention over and above standard care (staff time and transportation, consumables and equipment), participant-related direct and indirect costs (participant time and transportation, change in employment status and impact of the intervention on the activities of carers) and health system costs (ie, costs of health services used, readmissions) will be collected at each assessment time point and at 12 months. Hospital admissions (inclusive of emergency presentations and hospital admissions) will be collected from two sources at all timepoints, self-report by the participant and data obtained from hospital administrative data sets. Cost of each treatment pathway, resources used and their costs will be collected. Self-reported data related to health service utilisation and medications will be confirmed through person-level linkages of participant data with data held by state and commonwealth health departments. This will include the Pharmaceutical Benefits Scheme and Medicare Benefits Schedule for the 12 month period following discharge and a 12 month period prior to the index stroke event (to permit adjustment for prestroke utilisation trends that may be unrelated to the interventions being studied or unbalanced between the groups).
Data monitoring
Data safety and monitoring will be overseen by two health professionals and one statistician independent of the trial. The committee will review data related to safety and trial conduct within 3 months of enrolment of the first participant and then annually; an interim analysis will be undertaken at n=240. The committee will be responsible for stopping recruitment in the case of multiple serious, trial-related adverse events. For the purposes of this study, a serious adverse event will be defined as an event that (1) is fatal or life threatening, (2) results in persistent or significant disability or (3) results in hospitalisation. A nonserious adverse event would include such undesirable experiences such as noninjurious fall.
Patient and public involvement
Principles of the National Health and Medical Research Council Consumer and Community Involvement in Health and Medical Research statement37 have informed our approach to consumer and public engagement, with collaborative engagement with people with stroke, clinicians and policymakers from trial inception and design, to conduct and dissemination. This trial is supported by an end-user advisory panel, inclusive of advisors living with stroke, carers, occupational therapists, health managers and policymakers, who meet on a regular basis throughout the study. We will consult this panel to voice end-user concerns, review process evaluation data and to identify end-user-oriented solutions to any concerns; multiple opportunities for involvement and feedback will be made available during the analyses around emergent concepts and trial implications to ensure engagement through to dissemination. Trial results will be interpreted by the end-user advisory panel, before a summary will be shared with participants who have indicated this to be their preference. All advisory panel members are paid an honorarium and will be thanked in the contributorship statement of any publications.
Process evaluation
The process evaluation plan was informed by the Medical Research Council Guidance on Process Evaluations of complex interventions38 and will focus on the evaluation of fidelity and implementation context. Intervention fidelity will be monitored throughout the study through annual site review with participating therapists to ensure that key components of the intervention are delivered, adherence to the protocol, and completeness of outcome assessments is maintained throughout the trial. Implementation will be explored using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) framework39 components of Reach (measures of participant participation and representativeness) with collection of both quantitative and qualitative data to provide insights into the acceptance and burden from the perspectives of both participants and their carers; effectiveness (success rate at an individual level); adoption (programme acceptance/uptake across the trial); implementation (fidelity of the programme to the protocolised intervention and factors which may potentially affect the trial outcome) and maintenance (long-term effects at the individual and organisational level) to both support explanation of trial findings and inform scale-up should the programme be effective.
Sample size estimates
Our pilot data demonstrated a mean change at 90 days postdischarge of 2.7 (SD 5.5) in activity participation measured by the NEADL in the home visit group and 0.8 (0.5) in the control group. For the purposes of power analysis, we have hypothesised a potentially smaller but still clinically important effect, where the control group would exhibit a change score of 1 and a common SD of 5.5. Recruitment of a total of n=330 (equally distributed between groups) would yield 80% power to detect such an effect using independent t-test with two-tailed alpha of 0.05. Allowing for potential attrition, the final total sample size of n=360 is adopted for this study. This sample size estimation is conservative, as in addition to the smaller hypothesised effect size, we would also expect an additional increase in power due to the inclusion of the baseline NEADL scores as a covariate in a corresponding analysis of covariance (ANCOVA) model prespecified for the primary analysis.
Once the outcomes for n=240 participants are obtained, an adaptive sample size estimation procedure will be undertaken as per the ‘promising zone’ methodology Mehta and Pocock40 with a potential increase in the total sample size to the prespecified maximum of 360 participants.
Statistical analyses
All randomised participants will be included in the analyses following intention-to-treat principles. Treatment of missing data will be based on the satisfiability of missingness at random assumptions and will be based on the intention-to-treat strategy as per White et al.41 Outcomes will be analysed using appropriate analysis of covariance or logistic regression models, controlling for baseline values, and presented as mean between-group differences (95% CI). For the primary outcome analysis, differences in mean change in NEADL (baseline minus follow-up) will be compared between groups using ANCOVA model with change as an outcome, treatment group as a factor and baseline value of NEADL as a covariate. The outcome will be presented as mean between-group difference with respective 95% CI. Effect of participant characteristics on outcomes will be explored by including relevant interaction terms in regression models. The heterogeneity of effect across sites will be tested using respective mixed-effect models with individual centres as a random effect.
Similar adjusted analyses with appropriate regression models will be conducted for continuous secondary outcomes. Dichotomous secondary outcomes (ie, readmission) will be analysed using a logistic regression model with the readmission as the dependent variable and treatment group as independent variable, adjusted for relevant prespecified covariates. The outcomes will be presented as ORs with respective 95% CIs. Adjustment covariates will be prespecified in a separate Statistical Analysis Plan document that will provide the details of the analysis strategy prior to the lock of the trial data.
For the economic evaluation, there will be a cost description analysis of each treatment pathway and the incremental difference for costs and quality-adjusted life years determined. A full economic evaluation protocol will be published prior to study recruitment being completed.
Study sponsorship and funding
The study is funded by the National Health and Medical Research Council, Australia, grant ID 1141561). Trial organisation, data management and monitoring are supported by Monash University, Melbourne, Australia.
Discussion
The HOME trial will provide information that will assist survivors of stroke returning home after rehabilitation and their families, rehabilitation clinicians and policymakers make more informed decisions about the benefits of home assessments and postdischarge support for adults early after stroke. Findings will lead to evidence-based clinical practise guideline recommendations, rather than expert opinion, allowing for clearer health policy, which in turn will improve outcomes for consumers and produce greater cost-efficiency in the rehabilitation sector.
The HOME Trial is the first prospective, randomised clinical trial to investigate the effect of adding an enhanced occupational therapy discharge planning intervention that involves pre and postdischarge home visits, goal setting and occupational therapy in the home to an in-hospital predischarge planning intervention within a rehabilitation setting. The trial has concealed allocation, blinded assessment and intention-to-treat analysis and includes a process evaluation and economic evaluation.
Supplementary Material
Footnotes
Twitter: @A-4195-2013, @AvrilDrummond1, @stanley_mandy, @AndrewNadine
Correction notice: Figure 1 has been updated.
Contributors: NAL and LC conceived the study; NAL, LC, AD, MS, LCh, KL, SOK, IC, MC, TU, NA, LJ and DAC contributed to the design of the study. NAL, LC, AD, MS, LCh, KL, IC, MC, TU, NA and DAC procured the funding. NAL, DAC, SOK and LCh drafted the manuscript and all authors have read and approved the final manuscript.
Funding: The study is supported by the National Health and Medical Research Council, grant numberGNT1141561; NAL is supported by National Heart Foundation of Australia (GNT102055), DAC is supported by National Health and Medical Research Council (GNT 1063761), KL is supported by an Australian Research Council Fellowship (DE200101494).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Lichtman JH, Leifheit-Limson EC, Jones SB, et al. Preventable readmissions within 30 days of ischemic stroke among Medicare beneficiaries. Stroke 2013;44:3429–35. //. 10.1161/STROKEAHA.113.003165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ottenbacher KJ, Karmarkar A, Graham JE, et al. Thirty-day Hospital readmission following discharge from postacute rehabilitation in fee-for-service Medicare patients. JAMA 2014;311:604–14. 10.1001/jama.2014.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitzman P, Hudson K, Sylvia V, et al. Care coordination for community transitions for individuals post-stroke returning to low-resource rural communities. J Community Health 2017;42:565–72. 10.1007/s10900-016-0289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stroke Foundation . National stroke audit – rehabilitation services report 2016. Melbourne, Australia: Stroke Foundation, 2016. [Google Scholar]
- 5.Andrew NE, Kilkenny M, Naylor R, et al. Understanding long-term unmet needs in Australian survivors of stroke. Int J Stroke 2014;9 Suppl A100:106–12. 10.1111/ijs.12325 [DOI] [PubMed] [Google Scholar]
- 6.Tyson S, Turner G. Discharge and follow-up for people with stroke: what happens and why. Clin Rehabil 2000;14:381–92. 10.1191/0269215500cr331oa [DOI] [PubMed] [Google Scholar]
- 7.Gonçalves-Bradley DC, Lannin NA, Clemson LM, et al. Discharge planning from hospital. Cochrane Database Syst Rev 2016;23. Cd000313. 10.1002/14651858.CD000313.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepperd S, McClaran J, Phillips CO. Discharge planning from hospital to home. Cochrane Database of Systematic Reviews 2010;. 2010;22. //CD000313. [DOI] [PubMed] [Google Scholar]
- 9.Drummond A, Whitehead P, Fellows K, et al. Occupational therapy predischarge home visits for patients with a stroke: what is national practice? British Journal of Occupational Therapy 2012;75:396–402. 10.4276/030802212X13470263980711 [DOI] [Google Scholar]
- 10.Lannin NA, Clemson L, McCluskey A. Survey of current pre-discharge home visiting practices of occupational therapists. Aust Occup Ther J 2011;58:172–7. 10.1111/j.1440-1630.2010.00911.x [DOI] [PubMed] [Google Scholar]
- 11.Stroke Foundation . Clinical guidelines for stroke management 2017, 2017. Available: https://informme.org.au/Guidelines/Clinical-Guidelines-for-Stroke-Management-2017 [Accessed 13 February 2019].
- 12.Fukumoto M, Watanabe T, Yasufuku Y, et al. Home visits by occupational therapists in acute hospital care: a systematic review. Int J Rehabil Res 2019;42:205–10. 10.1097/MRR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 13.Drummond AER, Whitehead P, Fellows K, et al. Occupational therapy predischarge home visits for patients with a stroke (HOVIS): results of a feasibility randomized controlled trial. Clin Rehabil 2013;27:387–97. 10.1177/0269215512462145 [DOI] [PubMed] [Google Scholar]
- 14.Sampson C, James M, Whitehead P, et al. An introduction to economic evaluation in occupational therapy: cost-effectiveness of pre-discharge home visits after stroke (HOVIS). British Journal of Occupational Therapy 2014;77:330–5. 10.4276/030802214X14044755581664 [DOI] [Google Scholar]
- 15.Clemson L, Lannin NA, Wales K, et al. Occupational therapy predischarge home visits in acute hospital care: a randomized trial. J Am Geriatr Soc 2016;64:2019–26. 10.1111/jgs.14287 [DOI] [PubMed] [Google Scholar]
- 16.Lannin NA, Clemson L, McCluskey A, et al. Feasibility and results of a randomised pilot-study of pre-discharge occupational therapy home visits. BMC Health Serv Res 2007;7:42. 10.1186/1472-6963-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granger CV, Hamilton BB, Keith RA, Keith BB;, Zielezny RA;, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil 1986;1:59–74. 10.1097/00013614-198604000-00007 [DOI] [Google Scholar]
- 18.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neville M. Psychometric properties of the discharge planning assessment tool (DPAT). Arch Phys Med Rehabil 2018;99:e50. 10.1016/j.apmr.2018.07.174 [DOI] [Google Scholar]
- 21.Sim S, Barr CJ, George S. Comparison of equipment prescriptions in the toilet/bathroom by occupational therapists using home visits and digital photos, for patients in rehabilitation. Aust Occup Ther J 2015;62:132–40. 10.1111/1440-1630.12121 [DOI] [PubMed] [Google Scholar]
- 22.Daniel H, Oesch P, Stuck AE, Stuck Peter;, Born Andreas;, et al. Evaluation of a novel photography-based home assessment protocol for identification of environmental risk factors for falls in elderly persons. Swiss Med Wkly 2013;143:w13884. 10.4414/smw.2013.13884 [DOI] [PubMed] [Google Scholar]
- 23.Wales K, Clemson L, Lannin NA, et al. Occupational therapy discharge planning for older adults: a protocol for a randomised trial and economic evaluation. BMC Geriatr 2012;12:34. 10.1186/1471-2318-12-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawton MP, Nahemow L. Ecology and the aging process. In: The psychology of adult development and aging. Washington, DC, US: American Psychological Association, 1973. pp.: 619–74. [Google Scholar]
- 25.Lawton M. Competence, environmental press, and the adaptation of older people. In: Aging and the environment: theoretical approaches. New York: Springer Publishing Co, 1982: 33–59. [Google Scholar]
- 26.Wahl H, Lang F. Aging in context across the adult life course: integrating physical and social environmental research. In: Wahl HW, Scheidt RJ, Windley PG, et al., eds. Annual review of gerontology and geriatrics. New York: Springer, 2004. pp.: 1–22. [Google Scholar]
- 27.Stark SL, Somerville EK, Morris JC. In-Home occupational performance evaluation (I-HOPE). American Journal of Occupational Therapy 2010;64:580–9. 10.5014/ajot.2010.08065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Halloran PD, Blackstock F, Shields N, et al. Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin Rehabil 2014;28:1159–71. 10.1177/0269215514536210 [DOI] [PubMed] [Google Scholar]
- 29.Newnham H, Barker A, Ritchie E, et al. Discharge communication practices and healthcare provider and patient preferences, satisfaction and comprehension: a systematic review. Int J Qual Health Care 2017;29:752–68. 10.1093/intqhc/mzx121 [DOI] [PubMed] [Google Scholar]
- 30.Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301–5. 10.1177/026921558700100409 [DOI] [Google Scholar]
- 31.Coleman EA, Mahoney E, Parry C. Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care 2005;43:246–55. 10.1097/00005650-200503000-00007 [DOI] [PubMed] [Google Scholar]
- 32.Quinn TJ, Dawson J, Walters M. Dr John Rankin; his life, legacy and the 50th anniversary of the Rankin stroke scale. Scott Med J 2008;53:44–7. 10.1258/RSMSMJ.53.1.44 [DOI] [PubMed] [Google Scholar]
- 33.Quinn TJ, Dawson J, Walters MR, et al. Functional outcome measures in contemporary stroke trials. Int J Stroke 2009;4:200–5. 10.1111/j.1747-4949.2009.00271.x [DOI] [PubMed] [Google Scholar]
- 34.Hebert R, Carrier R, Bilodeau A. The functional autonomy measurement system (SMAF): description and validation of an instrument for the measurement of handicaps. Age Ageing 1988;17:293–302. 10.1093/ageing/17.5.293 [DOI] [PubMed] [Google Scholar]
- 35.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Janabi H, Flynn TN, Coast J. Estimation of a preference-based carer experience scale. Med Decis Making 2011;31:458–68. 10.1177/0272989X10381280 [DOI] [PubMed] [Google Scholar]
- 37.Statement on Consumer and Community Involvement in Health and Medical Research, National Health and Medical Research Council 2016. Consumers Health Forum of Australia. [Google Scholar]
- 38.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: medical Research Council guidance. BMJ 2015;350:h1258. 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322–7. 10.2105/AJPH.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta CR, Pocock SJ. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Stat Med 2011;30:3267–84. 10.1002/sim.4102 [DOI] [PubMed] [Google Scholar]
- 41.White IR, Horton NJ, Carpenter J, et al. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342:d40. 10.1136/bmj.d40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gitlin L. Environmental adaptations for older adults and their families in the home and community. In: Söderback I, ed. International Handbook of occupational therapy interventions. Dordrecht: Springer, 2009. pp.: 53–62. [Google Scholar]
- 43.Barras S, Grimmer-Somers K, May E. Consensus on 'core/essential' and 'ideal world' criteria of a pre-discharge occupational therapy home assessment. J Eval Clin Pract 2010;16:1295–300. 10.1111/j.1365-2753.2009.01331.x [DOI] [PubMed] [Google Scholar]
- 44.Davenport R, Helal S, Mann W, et al. Assistive environments for elder Care—Integrating smart phones with smart homes. In: International Conference on Aging, Disability and Independence Washington, DC, USA, December 2003, 2003. p.:128–9. [Google Scholar]
- 45.Gitlin LN, Winter L, Dennis MP, et al. A randomized trial of a multicomponent home intervention to reduce functional difficulties in older adults. J Am Geriatr Soc 2006;54:809–16. 10.1111/j.1532-5415.2006.00703.x [DOI] [PubMed] [Google Scholar]
- 46.Atwal A, McIntyre A, Craik C, et al. Occupational Therapists’ Perceptions of Predischarge Home Assessments with Older Adults in Acute Care. British Journal of Occupational Therapy 2008;71:52–8. 10.1177/030802260807100203 [DOI] [Google Scholar]
- 47.Crennan M, MacRae A. Occupational therapy discharge assessment of elderly patients from acute care hospitals. Phys Occup Ther Geriatr 2010;28:33–43. 10.3109/02703180903381060 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.