Abstract
Objective
To compare the incidence of atopic dermatitis in children aged from 6 months to 3 years across birth seasons and climate conditions.
Design
Cohort study.
Setting
Fifteen regional centres across Japan.
Participants
A total of 100 304 children born from 2011 to 2014.
Exposure
Birth month, and mean sunshine duration (short/long) and humidity (high/low) in the first 6 months of life.
Primary outcome measure
Incidence of atopic dermatitis.
Results
The highest incidence of atopic dermatitis was in children born in the months of October to December. The lowest incidence of atopic dermatitis was in the months of April to June and in periods with a long duration of sunshine and high humidity. Low humidity was significantly associated with a higher incidence of atopic dermatitis. However, this significant difference disappeared when the birth season and parental history of allergic disease were considered in multivariate analysis.
Conclusions
In Japan, being born in the late autumn to early winter months is associated with a risk of developing atopic dermatitis until the age of 3 years. Sunshine duration and humidity from birth to 6 months of age are not associated with the incidence of atopic dermatitis.
Keywords: paediatric dermatology, epidemiology, community child health, preventive medicine, paediatric clinical genetics & dysmorphology, immunology
Strengths and limitations of this study.
Six-monthly meteorological data were used, corresponding to individual children.
The parental history of allergic disease was considered.
A history of bacterial infection was not considered theoretically as a confounder.
Metrological data of children’s residence were not individual but averaged.
The severity of atopic dermatitis was not analysed.
Introduction
Approximately 10%–20% of children have atopic dermatitis (AD).1 2 Many genetic and environmental factors may be associated with the incidence and aggravation of AD.3 An epidemiological investigation identifying factors associated with AD would be helpful for reducing the number of child patients who suffer from this disease from infancy.
Possible environmental factors of AD include seasonal climate conditions, chemical irritants, bacterial colonisation, psychological stress4 and birth month.4 Among environmental factors, being born from autumn to winter in the northern hemisphere increases the risk of developing AD, while birth from spring to summer may decrease the incidence of AD.5 The mechanism of this association could be confounded by prevailing seasonal viruses, airborne natural antigens, or sunshine duration and humidity, which could be related to psychological stress, dry skin, and itchiness. Exposure to ultraviolet may improve skin barrier performance,6 and may reduce the risk of developing AD.7 Low humidity may be related to a high incidence of AD.8 Relating birth cohort data9–12 and metrological data13 may help answer the question of how birth month is associated with the incidence of AD. Therefore, this study aimed to investigate how birth month is associated with the incidence of AD.
Methods
Measures
Details on the Japan Environment and Children’s Study (JECS) project are published elsewhere.9 Approximately 100 000 expecting mothers who lived in designated study areas were recruited over 3 years from January 2011. Participating children were followed until they reached 13 years old. Exposure to environmental factors was assessed by chemical analyses of biospecimens (blood, cord blood, urine, breast milk and hair), household environment measurements and computational simulations using monitoring data and questionnaires. One of the JECS’ priority outcomes was immune system disorders (allergic diseases).9 The JECS comprises a cohort of 104 062 children born from 2011 to 2014 in 15 Regional Centres covering 18 prefectures across Japan.14 We used the JECS data ‘jecs-ta-20190930-qsn’ for generating questionnaires, which were sent to caregivers by post when their children were aged 6 months, 1 year, 2 years and 3 years. We asked if physicians had diagnosed the children with AD.
In Japan, sunshine duration almost peaks on 21 June (the summer solstice) and is at its shortest on 21 December (the winter solstice). Humidity, which is lowest in winter, rises proportionally with a rise in sunshine duration to summer. Sunshine duration varies depending on regions from approximately 125–180 hours/month. Humidity also varies depending on region and month from approximately 50%–80% in Japan. Therefore, sunshine duration and humidity of the 18 studied prefectures from the northern to southern regions of Japan varied. Overall in Japan, among seasons, summer has the longest sunshine duration and highest humidity, and winter has the shortest sunshine duration and lowest humidity.
We collected climate condition data per prefecture and month from the Japan Meteorological Agency website.13 The sunshine duration was defined as hours with ≥120 Watt/m2 of direct sunshine, as measured by a solarimeter. The mean sunshine duration and humidity of the 18 prefectures from April 2011 to March 2015 were 166.1 (SD: 45.4) hours/month and 68.4% (SD: 7.6%), respectively. These values were used as cut-off values for categorising children as being exposed to a long/short sunshine duration and high/low humidity while taking into account their resident area and birth month. For this categorisation, the mean sunshine duration and humidity in the child’s resident area over 6 months beginning with the child’s birth month were compared with the cut-off values. An example of this categorisation is that a child who was born in April 2011 would be categorised on the basis of meteorological data from April to September 2011.
For considering a child’s genetic predisposition to AD, we used a parental history of asthma, allergic rhinitis, pollen allergy, AD, allergic conjunctivitis and/or food allergy. The father and mother were asked individually about a history of allergic diseases to determine their experience of each diagnosed disease by a physician.
Analyses
The 12 months of the year were categorised into four seasons in three different ways (ie, starting with January to March, February to April or March to May). We compared Kaplan-Meier curves of the incidence of AD among seasons by performing log-rank tests. The incidence of AD by birth month and season until 3 years of age was calculated. We also compared the incidence of AD using Kaplan-Meier curves for children who were born in regions with long versus short sunshine, with high versus low humidity and with a long/short sunshine duration and high/low humidity. Because we found a difference in the incidence of AD among birth seasons, we compared this incidence among seasons by strata of children whose parents had a history of allergic disease and those who did not have a history of allergic disease. We performed Cox regression by birth season, humidity, and parental history of allergy, which were considered to be risk factors of AD from Kaplan-Meier curves. Data from participants who were lost to follow-up or developed AD after 3 years of age were considered as being censored. We conducted all statistical analyses using SAS statistical software V.9.4 (SAS Institute). Kaplan-Meier curves were drawn using RStudio V.1.2.1335 (R Project for Statistical Computing, Vienna, Austria). Two-sided p values of <0.05 were considered to indicate a significant difference.
Patient and public involvement
Written informed consent was obtained from all participants. Patient consent for publication was not required. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Results
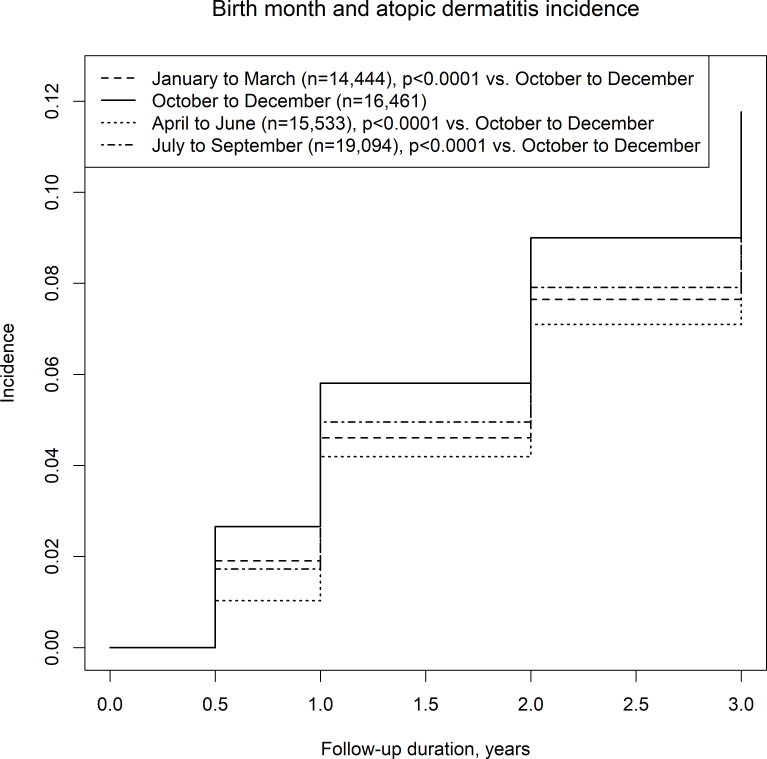
The number of participants who answered the questionnaire decreased with the child’s age. By the ages of 6 months, 1 year, 2 years and 3 years, 1715 of 100 304 children (response rate: 96.4%), 4505 of 90 549 children (response rate: 87.0%), 7299 of 84 859 children (response rate: 81.5%), and 9704 of 80 176 children (response rate for 77.0%), respectively, had developed AD. Online supplemental table 1 shows the characteristics of the participants. The highest and lowest incidence of AD was observed in the October to December group and the April to June group, respectively (figure 1). The overall incidence per 100 person-years varied from 5.27 (October to December) to 4.31 (April to June) (online supplemental table 1). By month, the highest incidence was in children born in October and December (online supplemental figure 1). Online supplemental figures 2 and 3 show the results after varying how the months were grouped. The order of accumulated incidence of AD among the seasons did not change much from 6 months to 3 years old.
Figure 1.
Birth season and incidence of atopic dermatitis in a Japanese birth cohort with the seasons starting from January.
bmjopen-2020-047226supp001.pdf (131.6KB, pdf)
bmjopen-2020-047226supp002.pdf (854.4KB, pdf)
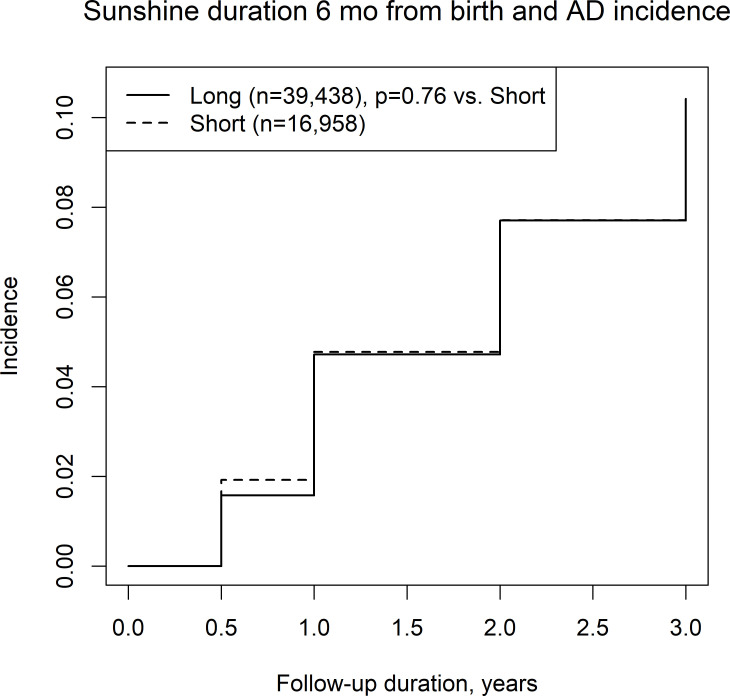
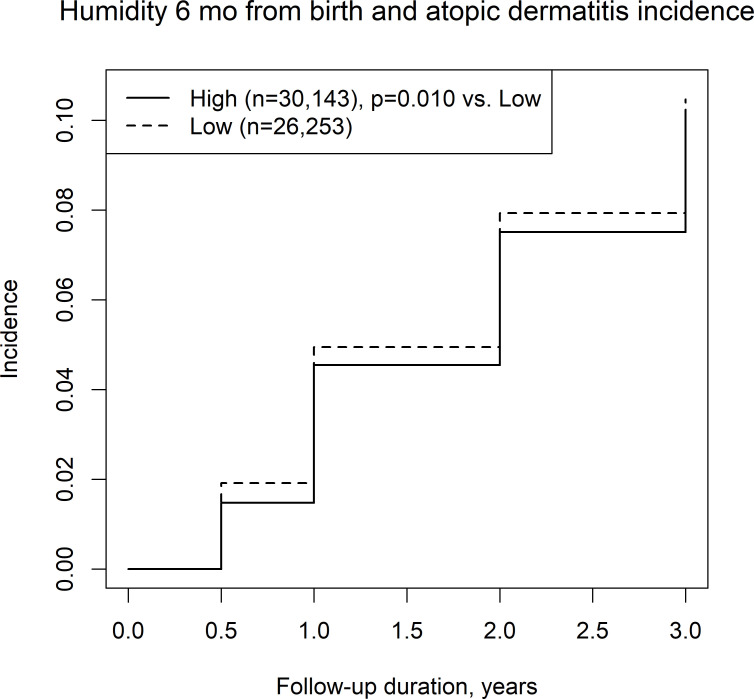
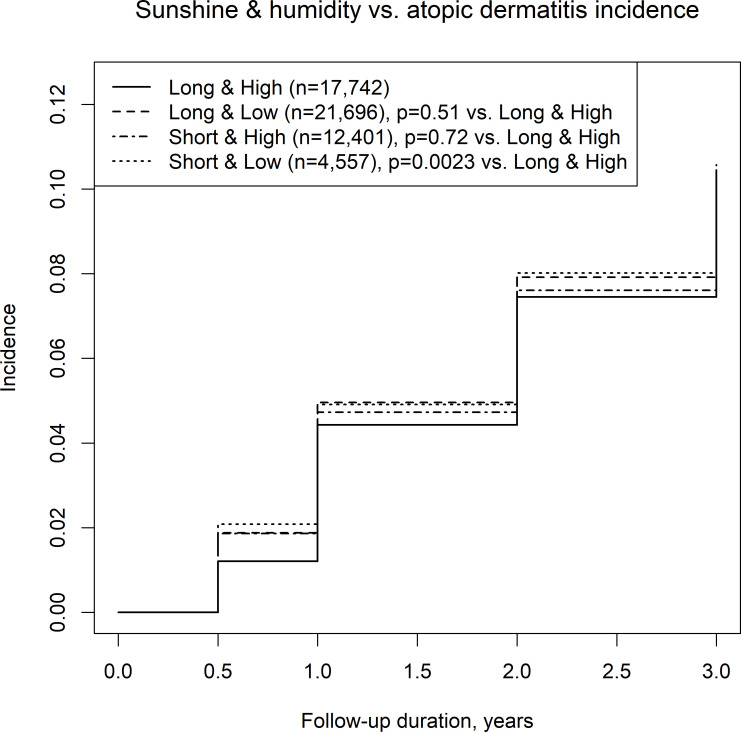
We also assessed the incidence of AD among four climate categories, including combinations of a long/short sunshine duration and high/low humidity, in the first 6 months of life. High or low sunshine duration was not associated with the incidence of AD (figure 2), while low humidity was associated with the incidence of AD compared with high humidity (figure 3). Among the long/short sunshine duration and high/low humidity groups, the short sunshine duration and low humidity group had a significantly higher incidence of AD than the long sunshine duration and high humidity group (figure 4).
Figure 2.
Incidence of atopic dermatitis in regions with a long/short mean sunshine duration.
Figure 3.
Incidence of atopic dermatitis in regions with a high/low mean humidity.
Figure 4.
Incidence of atopic dermatitis in regions with a long/short mean sunshine duration and high/low mean humidity.
We also analysed the incidence of AD based on parental history of any allergic disease. Online supplemental figures 4 and 5 show the incidence of AD by birth season, as stratified by parental history of allergic disease. The observed highest and lowest incidence of AD shown in online supplemental figures 4 and 5 was consistent with that shown in figure 1.
Table 1 shows the results of Cox proportional regression for risk factors of the incidence of AD. The adjusted HR showed that birth between October and December and the father’s and mother’s history of allergy were risk factors of AD until the age of 3 years in the child.
Table 1.
HRs (95% CIs) of possible factors involved in the incidence of atopic dermatitis until 3 years old
| Factors | Crude HR (95% CI) | Adjusted HR (95% CI) |
| Short versus long sunshine duration | 1.03 (0.99 to 1.08) | ― |
| Low versus high humidity | 1.06 (1.01 to 1.11) | 0.99 (0.95 to 1.04) |
| Birth month between January and March | 1.02 (0.95 to 1.09) | 1.02 (0.95 to 1.09) |
| Birth month between April and June | Reference | Reference |
| Birth month between July and September | 1.06 (0.99 to 1.13) | 1.05 (0.98 to 1.13) |
| Birth month between October and December | 1.20 (1.12 to 1.28) | 1.20 (1.12 to 1.29) |
| Father’s history of allergy | 1.21 (1.16 to 1.28) | 1.18 (1.12 to 1.24) |
| Mother’s history of allergy | 1.70 (1.62 to 1.78) | 1.69 (1.61 to 1.77) |
Discussion
The current study showed that the incidence of AD until the age of 3 years was highest when children were born between October and December and lowest when they were born between April and June. Consideration of parental history of allergic disease did not alter this association. Multivariate analysis showed that sunshine duration or humidity was not associated with the incidence of AD.
Potential aetiologies of this association are bacterial infection, dry air, high solar radiation/sunshine hours and atmospheric pressure. Dry skin and itchiness, which develop into AD, are common in low humidity. In the USA, a higher incidence of AD is associated with indoor heating use and low humidity, ultraviolet exposure and outdoor temperature.15 In Japan, a negative correlation between humidity and dermatological visits concerning AD was observed.16 In our study, we did not observe a clear correlation between high or low humidity in the first 6 months of life and the risk of AD, which suggested that humidity is not critical for inducing AD.
In Japan, AD shows remittance in 50% of 4-month-old patients before the age of 18 months. However, a previous study reported a high cumulative incidence rate of AD of 30% in children who were younger than 3 years.17 In mice, low humidity caused a higher cutaneous immune reaction by an increase in Langerhans cells and penetration of allergen.18 The mean humidity in Japan was 70% in 2016, which is not high compared with other countries.19 High humidity is clearly associated with a higher incidence of hand, foot and mouth disease with fever, ulcers and vesicles.20 Dry and itchy skin are caused by low humidity and low temperature.8 However, in this study, there was no effect of high or low humidity on the risk of AD when birth month and parental history of allergic disease were considered (table 1). Therefore, in the present children, humidity did not appear to be important for inducing AD. Our study also did not suggest that a long sunshine duration induces AD.
Ultraviolet lighting is sometimes used clinically for reducing itchiness from severe AD.21 However, a long sunshine duration can also cause dry skin, which is a risk factor of AD. Furthermore, heat from a long sunshine duration can cause sweating, leading to itchiness and psychological stress from discomfort, both of which can exacerbate AD. In Japan, humidity is fairly constant. Therefore, we initially suspected that in Japan, itchiness and psychological stress from a long duration of sunshine may be a cause of AD in children. However, in contrast to our speculation, a combination of short sunshine duration and low humidity was associated with the highest incidence of AD (figure 4).
Notably, the association between the incidence rate of AD across birth seasons, sunshine duration, and humidity levels hardly changed from 6 months to 3 years old (figures 1 and 4). This finding indicates that environmental factors with an adverse or preventive effect persist through early childhood. Allergic predisposition may not be determined until 6 months of age. Before that age, most infants only drink milk and do not eat allergenic solid products. A meta-analysis did not show clear evidence for a protective effect of breast feeding on the incidence of AD.22 Our analysis did not examine feeding behaviour of the children.
Confounding factors of our analysis need to be considered. When we considered genetic predisposition, we found a high incidence of AD in children who were born in October to December, regardless of whether their parents had allergic diseases (figure 1, online supplemental figures 4, 5 and table 1). Because parents do not plan the birth month of a newborn with a genetic predisposition to AD, this factor could not have theoretically confounded the observed associations.
Serum vitamin D levels may be involved in the association between birth month and the incidence of AD. Maternal vitamin D supplementation does not affect the risk of AD at 3 years old.23 However, in an Australian study, a far distance from the equator was associated with a higher prevalence of eczema.24 Ultraviolet B irradiation decreases inflammation and promotes skin barrier function.6 Our data showed that birth between April and June was the most protective period against the incidence of AD. A high or low prevalence of AD among birth seasons was preserved from 6 months to 3 years old.
Limitations
Our study has the following limitations. First, our results are limited by infection not being examined as a potential confounder. Second, we used 6 monthly means of sunshine duration and humidity. Individual experience was not considered. Third, dichotomisations of a long/short sunshine duration and high/low humidity were determined by single cut-off values. Fourth, the incidence of AD was reported by caregivers based on the physician’s diagnosis. There could have been recall bias of caregivers. Fifth, physicians who diagnosed children included specialists of AD and non-specialists. Physicians might have underdiagnosed AD in infancy because this diagnosis can cause stigma to children and caregivers.25 Sixth, we were unable to consider details of AD. Because AD can be caused by several mechanisms, analysis of disease severity may lead to a further understanding of its aetiology.
Conclusions
Births in October to December have the highest incidence of AD until the age of 3 years. This result is consistent when a parental history of allergic disease is considered. A high or low incidence of AD by birth season persists from 6 months through to 3 years in childhood. Although the lowest incidence of AD is found in residents in regions with a long sunshine duration and high humidity in crude data, multivariate analysis shows no association of sunshine duration and humidity with the incidence of AD.
Supplementary Material
Acknowledgments
We thank Dr Ellen Knapp for editing a draft of this manuscript. †Members of the JECS Group as of 2020: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Youichi Kurozawa (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Footnotes
Contributors: All the authors agreed with the manuscript’s results and conclusion and approved the final version of the manuscript. HY and MM conceived the study. HY and MM contributed to the design of the study and interpretation of the data analyses. HY analysed the data. HY, MM, AT, RK, SH, TO, YA and KM wrote the first draft of the manuscript. SH, SO, RS, HI and ZY contributed to data collection. All authors contributed to revision of the manuscript. ZY was responsible for data integrity. ZY obtained funding.
Funding: This work was supported by the Ministry of the Environment, Japan.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this email address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol was approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies (no. 100910001) and by the Ethics Committees of all participating institutions, in accordance with the ethical guidelines and regulations of the Declaration of Helsinki.
References
- 1. Deckers IAG, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One 2012;7:e39803. 10.1371/journal.pone.0039803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weidinger S, Novak N. Atopic dermatitis. Lancet 2016;387:1109–22. 10.1016/S0140-6736(15)00149-X [DOI] [PubMed] [Google Scholar]
- 3. Katayama I, Aihara M, Ohya Y, et al. Japanese guidelines for atopic dermatitis 2017. Allergol Int 2017;66:230–47. 10.1016/j.alit.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 4. Kusunoki T, Asai K, Harazaki M, et al. Month of birth and prevalence of atopic dermatitis in schoolchildren: dry skin in early infancy as a possible etiologic factor. J Allergy Clin Immunol 1999;103:1148–52. 10.1016/S0091-6749(99)70191-0 [DOI] [PubMed] [Google Scholar]
- 5. Calov M, Alinaghi F, Hamann CR, et al. The association between season of birth and atopic dermatitis in the Northern Hemisphere: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2020;8:674–80. 10.1016/j.jaip.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 6. Thyssen JP, Zirwas MJ, Elias PM. Potential role of reduced environmental UV exposure as a driver of the current epidemic of atopic dermatitis. J Allergy Clin Immunol 2015;136:1163–9. 10.1016/j.jaci.2015.06.042 [DOI] [PubMed] [Google Scholar]
- 7. Rueter K, Jones AP, Siafarikas A, et al. Direct infant UV light exposure is associated with eczema and immune development. J Allergy Clin Immunol 2019;143:1012–20. 10.1016/j.jaci.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 8. Engebretsen KA, Johansen JD, Kezic S, et al. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol 2016;30:223–49. 10.1111/jdv.13301 [DOI] [PubMed] [Google Scholar]
- 9. Kawamoto T, Nitta H, Murata K, et al. Rationale and study design of the Japan environment and children's study (JECS). BMC Public Health 2014;14:25. 10.1186/1471-2458-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michikawa T, Nitta H, Nakayama SF, et al. Baseline profile of participants in the Japan environment and children's study (JECS). J Epidemiol 2018;28:99–104. 10.2188/jea.JE20170018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yokomichi H, Kojima R, Horiuchi S, et al. Effectiveness of influenza vaccination in infants and toddlers with and without prior infection history: the Japan environment and children's study. Vaccine 2021;39:1800–4. 10.1016/j.vaccine.2021.02.044 [DOI] [PubMed] [Google Scholar]
- 12. Kojima R, Yokomichi H, Akiyama Y, et al. Association between preterm birth and maternal allergy considering IgE level. Pediatr Int 2021. 10.1111/ped.14635. [Epub ahead of print: 04 Feb 2021]. [DOI] [PubMed] [Google Scholar]
- 13. Ministry of Internal Affairs and Communications . Statistics of Japan 2018, 2018. Available: https://www.e-stat.go.jp/en
- 14. Iwai-Shimada M, Nakayama SF, Isobe T, et al. Questionnaire results on exposure characteristics of pregnant women participating in the Japan environment and children study (JECS). Environ Health Prev Med 2018;23:45. 10.1186/s12199-018-0733-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol 2013;133:1752–9. 10.1038/jid.2013.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furue M, Yamazaki S, Jimbow K, et al. Prevalence of dermatological disorders in Japan: a nationwide, cross-sectional, seasonal, multicenter, hospital-based study. J Dermatol 2011;38:310–20. 10.1111/j.1346-8138.2011.01209.x [DOI] [PubMed] [Google Scholar]
- 17. Kohno Y. Identification of causative and exacerbation factors of atopic dermatitis and studies for improvment of living environment to prevent the development and exacerbation of symptoms. Reports of research on allegic disease and immunology by Ministry of Health, Labour and Wefare of Japan 2006-2007 2008:173–7. [Google Scholar]
- 18. Hosoi J, Hariya T, Denda M, et al. Regulation of the cutaneous allergic reaction by humidity. Contact Dermatitis 2000;42:81–4. 10.1034/j.1600-0536.2000.042002081.x [DOI] [PubMed] [Google Scholar]
- 19. El Dorado Weather, Inc . Current world humidity, 2020. Available: https://www.eldoradoweather.com/forecast/world-forecasts/world-humidity.html
- 20. Onozuka D, Hashizume M. The influence of temperature and humidity on the incidence of hand, foot, and mouth disease in Japan. Sci Total Environ 2011;410-411:119–25. 10.1016/j.scitotenv.2011.09.055 [DOI] [PubMed] [Google Scholar]
- 21. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000;4:1–191. 10.3310/hta4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang YW, Tsai CL, Lu CY. Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. Br J Dermatol 2009;161:373–83. 10.1111/j.1365-2133.2009.09049.x [DOI] [PubMed] [Google Scholar]
- 23. Chawes BL, Bønnelykke K, Stokholm J, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA 2016;315:353–61. 10.1001/jama.2015.18318 [DOI] [PubMed] [Google Scholar]
- 24. Osborne NJ, Ukoumunne OC, Wake M, et al. Prevalence of eczema and food allergy is associated with latitude in Australia. J Allergy Clin Immunol 2012;129:865–7. 10.1016/j.jaci.2012.01.037 [DOI] [PubMed] [Google Scholar]
- 25. Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol 2016;9:159–66. 10.2147/CCID.S91263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-047226supp001.pdf (131.6KB, pdf)
bmjopen-2020-047226supp002.pdf (854.4KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this email address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.