See Clinical Research on pages 1850 and 1858
In recent years, our understanding of epidemiology and the impact of acute kidney injury (AKI) has grown exponentially in pediatrics. Seminal longitudinal studies in neonates as well as pediatric and congenital heart disease populations have clearly shown that AKI occurs commonly and is associated with adverse patient outcomes and increased mortality.1, 2, 3, 4 At the same time, the limitations of both serum creatinine and urine output as biomarkers have become clear, as we have struggled to translate this work to improved patient outcomes. As a result, there has been a shift in the AKI paradigm to understanding risk stratification, disease-specific phenotypes, and the incorporation of novel biomarkers to optimize clinical care and outcomes.
Given the limitations of serum creatinine and urine output as biomarkers, bedside risk stratification tools, with and without incorporation of urinary biomarkers, have been developed to identify patients at risk for developing severe AKI without 48 to72 hours of evaluation.5,6 The renal angina index was designed to be a highly sensitive tool for ruling out severe AKI that occurs 72 hours after intensive care admission, and it has been systematically validated in an international cohort of critically ill children. In parallel to this work, researchers have sought to understand the role of novel biomarkers. As recently delineated in a biomarker position paper by the Acute Disease Quality Initiative (ADQI), use of biomarkers has resulted in a better understanding of AKI pathophysiology, and, importantly, there have been improved outcomes with biomarker-guided management.7 The ADQI group highlighted the need for improved risk stratification of high-risk patients and identifying AKI phenotypes for the acute management of patients with AKI. The ADQI group also highlighted the need to prospectively validate biomarkers and to understand the role of biomarkers in identifying those at risk for subsequent chronic kidney disease (CKD) staging and long-term outcomes after AKI. In this issue of Kidney International Reports, we comment on 2 studies that use the concepts of phenotypic refinement and biomarker use to identify patients at risk for adverse outcomes related to AKI.8,9
In the article “Recalibration of the Renal Angina Index for Pediatric Septic
Shock,” Stanski et al. report on the performance and recalibration of the renal angina index (RAI) to predict AKI in a large multicenter cohort of children with severe sepsis. In the original validated form, the RAI score, when assessed at 12 hours after intensive care unit admission with a cutoff of ≥8, has been shown to be highly sensitive to predict severe AKI at intensive care unit day 3. The RAI clearly outperformed serum creatinine alone and severity of illness scoring systems. Since this seminal work, there have been several derivations of the RAI refined to specific pediatric populations.S1 Each of these refinements has improved the precision and accuracy of the original RAI. Sepsis represents a unique disease state with a pathophysiology that contributes to unique phenotypes of AKI referred to as sepsis associated AKI (SA-AKI). Recent work from the Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology (AWARE) study suggest that unique phenotypes of SA-AKI exist and contribute differently to outcomes.S2 The work by Stanski et al. recalibrates RAI to predict SA-AKI by incorporating platelet count—a marker known to be associated with sepsis-associated AKI.S3 In this study, the optimal cutoff was found to be ≥20, but the sensitivity was reduced. Separately, similar to other studies, platelet count <150 × 103/μl was an independent predictor of SA-AKI. Incorporating the platelet threshold allowed for restoration of the high sensitivity of the original threshold and improved specificity.
Although this work and the concepts of AKI risk stratification precision are exciting, 1 of the concerns with developing disease-specific risk stratification tools is that they become cumbersome for clinical use. As the authors point out, it might be prudent to limit the modification of these indices to certain high-risk states (sepsis, congenital heart surgery, etc.) and high-risk exposures such as nephrotoxic medication use in patients with sepsis. The electronic health record provides clinicians with a unique opportunity to automate disease-specific risk stratification, to optimize care, and to implement care bundles. Unlike other risk scores such as the Pediatric Risk of Mortality (PRISM) score that are primarily used for research and not automatically calculated, using the electronic health record to calculate a disease-specific RAI, would be simple to incorporate and would be incredibly valuable for clinical decision making.
In the article, “Long-Term Renal Outcomes in Children with Acute Kidney
Injury Post−Cardiac Surgery,” Sethi et al. evaluated several urinary biomarkers as an assessment of kidney health in children with and without AKI who had previously undergone congenital heart surgery.9 At the opposite end of the risk stratification spectrum is identifying patients at the highest risk for CKD after critical illness. It is well known that AKI is a risk factor for subsequent CKD, but there remains an incomplete understanding of identifying the highest-risk patients and disease states. Children undergoing cardiac surgery are known to have a high AKI incidence and to be at increased risk for CKD. Unfortunately, there remains an extraordinary variation in the rates of AKI that varies by a multitude of factors (institution, fluid management strategies, surgical complexity, etc.).4 This variation has made the prediction of those at highest risk for subsequent CKD difficult. It is possible that refining the cardiac surgery−associated AKI (CS-AKI) phenotype will enhance our ability to understand who is most at risk for CKD.
In their study, Sethi et al. evaluated 3 AKI biomarkers (neutrophil gelatinase-associated lipocalin [NGAL], interleukin-18, and kidney injury molecule−1) in a cohort of 93 subjects, 44 with a history of AKI and 49 controls over a median follow-up time of 41 months.9 At follow-up, AKI patients had higher urinary concentrations of the aforementioned biomarkers compared to controls, but none of the AKI patients had proteinuria or hypertension, and there was no significant difference in glomerular filtration rate between AKI and control patients.9 Among the studies that have evaluated biomarkers in follow-up, the Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury consortium study found no differences in biomarker concentrations or associations with CKD or hypertension at 5-year follow-up.S4 In contrast, the Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury study found persistent elevations of interleukin-18, kidney injury molecule−1, and liver fatty acid binding protein (L-FABP) at 7-year follow-up, coupled with hypertension in 20% of the cohort and CKD in nearly 15%.S5 Other studies have similarly demonstrated substantial heterogeneity in the incidence of CKD and hypertension with varying follow-up times.S6−S8 The findings of Sethi et al., along with the existing literature, may similarly point to the need for refining the CS-AKI phenotype. Not all CS-AKI is created equal, there may be differences in the long-term impact as it pertains to incident AKI severity and duration, defined by either transient (≤48 hours) or persistent (lasting >48 hours) as described by the 16th Acute Disease Quality Initiative consensus statement.S9 Early biomarker assessments may similarly facilitate improved phenotypic characterization for AKI and delineate who will go on to develop CKD, hypertension, and proteinuria. The time to follow-up may also be important. It may take years before any effects of CKD are seen, although in 1 study, signs of CKD were seen in some patients as early as 6 months after AKI.S10
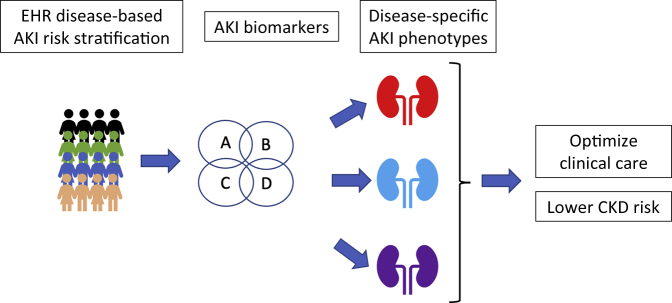
In summary, AKI epidemiology is now well described in discrete cohorts of critically ill children. The focus of research now has to shift more to risk stratification for early and long-term outcomes, potentially by delineating phenotypes (Figure 1). These 2 studies serve as examples of how we can, and must, continue to improve and refine our ability to diagnose and predict AKI in order to improve short and long-term outcomes.
Figure 1.
Schematic representation incorporating patient risk factors and biomarkers for identifying disease-specific acute kidney injury (AKI) phenotypes for optimizing clinical care and reducing risk for chronic kidney disease (CKD). EHR, electronic health record.
Disclosure
KMG is a consultant for Bioporto Diagnostics. Bioporto has no influence in the writing or review of this commentary. DTS declares no competing interests.
Footnotes
Supplementary References
Supplementary Material
Supplementary References
References
- 1.Jetton J.G., Boohaker L.J., Sethi S.K. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–194. doi: 10.1016/S2352-4642(17)30069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddourah A., Basu R.K., Bagshaw S.M. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S., Krawczeski C.D., Zappitelli M. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alten J, Cooper D, Blinder J, et al. Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the multicenter Neonatal and Pediatric Heart and Renal Outcomes Network (NEPHRON). Crit Care Med. in press. [DOI] [PubMed]
- 5.Basu R.K., Zappitelli M., Brunner L. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85:659–667. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon S., Goldstein S.L., Mottes T. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant. 2016;31:586–594. doi: 10.1093/ndt/gfv457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostermann M., Zarbock A., Goldstein S. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19209. [DOI] [PubMed] [Google Scholar]
- 8.Stanski N.L., Wong H.R., Basu R.K. Recalibration of the renal angina index for pediatric septic shock. Kidney Int. 2021;6:1858–1867. doi: 10.1016/j.ekir.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S., Sharma R., Gupta A. Long-term renal outcomes in children with acute kidney injury post−cardiac surgery. Kidney Int Rep. 2021;6:1850–1857. doi: 10.1016/j.ekir.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.