See Clinical Trial on Page 1939
Focal segmental glomerulosclerosis (FSGS) is a histologic pattern characterized by scarring of segments of glomeruli and is a leading cause of end-stage renal disease. Podocytes are a key target cell for injury in the evolution of segmental sclerosing glomerular lesions and have been studied extensively as fusion of their tertiary foot process and depletion correlates closely with the development of proteinuria. Recently, glomerular endothelial injury and crosstalk with podocytes have been suggested to play a role in the pathogenesis of FSGS and progressive disease. It is well established that podocytes control glomerular endothelial cell growth and survival via crosstalk of essential paracrine factors, such as vascular endothelial growth factor. However, how the neighboring glomerular endothelial cells provide feedback to modulate podocyte (patho)biology remains largely unknown. However, there are reports demonstrating structural and biochemical alterations in endothelial cells that precede podocyte injury in experimental FSGS,1,2 which suggests that the glomerular endothelium could be actively involved in early pathogenesis.
Endothelin-1 is a potent vasoconstrictor when binding to the endothelin receptor type A (ETAR) and has opposing actions through binding to the endothelin receptor type B (ETBR), and both receptors are expressed on many cell types.3 Endothelin receptor antagonists have been evaluated in patients with proteinuric glomerular diseases including diabetic kidney disease and FSGS, and have demonstrated encouraging anti-proteinuric effects and to stabilize kidney function. In this issue of KI Reports, van de Lest et al.4 performed a retrospective study to examine the expression of ETAR in biopsy samples of patients with FSGS by immunohistochemistry and immunofluorescence analysis. The authors compared 39 kidney biopsies from patients with idiopathic FSGS to 8 controls obtained from deceased donors, and found that there was significantly higher numbers of glomeruli staining positive for ETAR among the FSGS cases, and ETAR was shown to be colocalized with endothelial cell marker CD31. When staining FSGS biopsies for nephrin, the authors report a significant correlation between the percentage of glomeruli with ETAR and the percentage of glomeruli with nephrin loss on adjacent podocytes. This association persisted after exclusion of nephrin loss due to glomerulosclerotic lesions. The authors conclude that upregulation of ETAR is a common finding in biopsies from FSGS patients and that it correlates with podocyte damage.
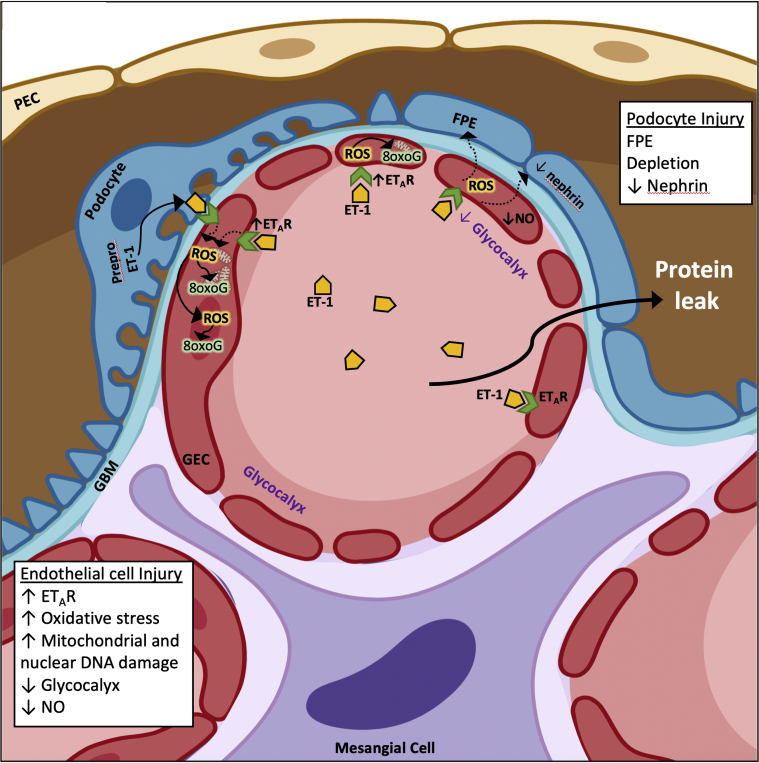
The findings by van de Lest et al.4 support previous reports showing increased glomerular endothelial-specific ETAR expression in experimental podocyte injury models (e.g., Pod-TgfbrI, Pod-Dicer, and adriamycin) that developed FSGS.5 ETAR-dependent signaling in glomerular endothelial cells caused endothelial dysfunction characterized initially by loss of glomerular endothelial cell fenestrations and loss of the glycocalyx, decreased nitric oxide synthase activity, followed by subsequent podocyte injury and loss of filtration barrier integrity in experimental FSGS2,5 (Figure 1). As the investigators point out, the increase in ETAR expression in biopsies from FSGS cases implies endothelin-1 signaling; however, staining for endothelin-1 in their patient cohort yielded inconclusive results, which is not surprising given that it is a secreted molecule. An increase in pre-proendothelin-1 expression in podocytes has been demonstrated to be transient in preclinical models, where its expression was detected after a few days of podocyte activation in early disease.5 Detection of RNA in situ in biopsy samples could help depict the cell population responsible for local endothelin-1 production and release, though increased ETAR expression in glomerular endothelial cells could respond to higher circulating endothelin-1 in the milieu.
Figure 1.
Increased endothelial ETAR expression and oxidative stress is associated with podocyte injury in patients with FSGS. In a biopsy cohort of human FSGS, there was an increase in expression of endothelin receptor A (ETAR) on glomerular endothelial cells (GECs), and an increase in oxidative stress shown by detection of DNA 8-oxoG in the nucleus and possibly in the mitochondria. Podocyte derived endothelin-1 (ET-1) and increased paracrine activation of ETAR has been previously shown to decrease nitric oxide (NO) synthase activity and NO production, decreased number of GEC fenestrae and degradation of the glycocalyx.2,5 In the human FSGS, the increased GEC ETAR was associated with adjacent podocyte injury and decreased nephrin expression and proteinuria. FPE, foot process effacement; GBM, glomerular basement membrane; PEC, parietal epithelial cell. (Created using BioRender.)
In addition, the study by van de Lest et al.4 demonstrated that individual glomeruli positive for ETAR were associated with an increase of the DNA-modification 8-oxoguanine (8-oxoG), an indicator of oxidative stress, and this was less observed in the ETAR-negative glomeruli or in control biopsies. When stratifying the FSGS patients as 8-oxoG positive or negative based on a threshold mean positive area, and comparing clinical parameters for both groups, the authors found higher levels of proteinuria among 8-oxoG–positive patients, whereas eGFR was comparable between the groups. This human data support the notion that an oxidant/antioxidant imbalance occurs, and the resulting excess reactive oxygen species in the glomerulus could drive the pathophysiology of glomerular diseases.6 Increased markers of oxidative DNA and RNA damage have been shown to be correlated to albuminuria even among apparently healthy individuals,7 and in the urine of patients with progressive diabetic kidney disease compared with diabetes patients without kidney disease.S1 Accumulating experimental and clinical studies add further credence to the notion that excess reactive oxygen species and oxidative damage may be a common pathophysiological pathway shared by different aggressors at the cellular level. However, the cell-specific context of where the oxidative stress occurs within the glomerulus is important and is under much debate. The study by van de Lest et al. confirms that the location of the oxidative DNA damage (i.e., 8-oxoG) is within the glomerular endothelium in patients with FSGS. Oxidative stress is possibly downstream from ETAR activation (Figure 1) and may not be exclusive to mitochondrial DNA as previously shown.5 Increased oxidative stress and damage accumulation in endothelial cells have many consequences and have been shown to release factors that can mediate podocyte injury and cell death,2,5 and hence the possibility of a pathologic crosstalk resulting in loss of barrier function.
Although conclusions about intercellular crosstalk between glomerular endothelial cells and podocytes in the pathogenesis of FSGS cannot be drawn based solely on the results of this study, the study adds to the mechanistic rationale for the use of selective pharmacologic inhibition of ETAR to preserve the glomerular filtration barrier—especially as selective ETAR antagonism has been demonstrated to reduce proteinuria and risk of renal events in patients with diabetic kidney disease and FSGS,S2,S3 and has also been shown to prevent loss of the glycocalyx in mice with type 2 diabetic kidney disease and FSGS.2,8 However adverse events such as fluid retention and anemia have been reported, leading to the early termination of a clinical trial assessing the effect of ETAR antagonist avosentan in patients with type 2 diabetes and kidney disease (the ASCEND trial). In the subsequent SONAR trial with ETAR antagonist astrasentan, included an open label enrichment period, excluding participants with fluid retention before randomization. Recently, in a post hoc analysis of the SONAR trial, Heerspink et al.9 showed that patients starting on sodium-glucose cotransporter–2 (SGLT2) inhibitors during the enrichment period had a greater reduction of albuminuria than patients only taking astrasentan. Although the effect of combining ETAR antagonists and SGLT2 inhibitors in diabetic kidney disease needs to be investigated further in a larger cohort, their separate mechanisms of action could have an additive effect on proteinuria reduction. In patients with primary FSGS, the promising outcomes from the phase 2 DUET trial, where combined ETAR/angiotensin receptor blocker resulted in ~50% reduction of proteinuria compared with angiotensin receptor blockade alone, has lead to the launch of a phase 3 DUPLEX trial (NCT03541174). Importantly, fluid retention–related adverse events have not been reported in nondiabetic kidney disease trials, possibly because of those patients being less likely to have significant cardiovascular comorbidities than patients with diabetic kidney disease.
There are no specific Food and Drug Administration–approved therapy for patients with FSGS. With the mounting preclinical data and the recent clinical outcomes trials, antagonizing ETAR is a promising therapeutic avenue for decreasing proteinuria and tissue damage in FSGS. With their study, van de Lest et al.4 provide clues to potential intercellular chatter between stressed glomerular endothelial cells and podocyte injury that lead to protein leak in FSGS.
Disclosure
All the authors declared no competing interests.
Acknowledgments
ISD is supported by the National Institutes of Health grant R01DK097253 and Department of Defense CDMRP grants W81XWH-20-1-0836. Illustrations were created using BioRender.
Footnotes
Supplementary References
Supplementary Material
Supplementary References
References
- 1.Sun Y.B., Qu X., Zhang X., Caruana G., Bertram J.F., Li J. Glomerular endothelial cell injury and damage precedes that of podocytes in adriamycin-induced nephropathy. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebefors K., Wiener R.J., Yu L. Endothelin receptor-A mediates degradation of the glomerular endothelial surface layer via pathologic crosstalk between activated podocytes and glomerular endothelial cells. Kidney Int. 2019;96:957–970. doi: 10.1016/j.kint.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai T., Yanagisawa M., Masaki T. Molecular characterization of endothelin receptors. Trends Pharmacol Sci. 1992;13:103–108. doi: 10.1016/0165-6147(92)90038-8. [DOI] [PubMed] [Google Scholar]
- 4.van de Lest N.A., Bakker A.E., Dijkstra K.L. Endothelial endothelin receptor a expression is associated with podocyte injury and oxidative stress in patients with focal segmental glomerulosclerosis. Kidney Int Rep. 2021;6:1939–1948. doi: 10.1016/j.ekir.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daehn I., Casalena G., Zhang T. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014;124:1608–1621. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markan S., Kohli H., Sud K. Oxidative stress in primary glomerular diseases: A comparative study. Mol Cell Biochem. 2008;311:105–110. doi: 10.1007/s11010-008-9701-0. [DOI] [PubMed] [Google Scholar]
- 7.Schei J., Fuskevåg O.M., Stefansson V.T.N. Urinary markers of oxidative stress are associated with albuminuria but not GFR decline. Kidney Int Rep. 2018;3:573–582. doi: 10.1016/j.ekir.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garsen M., Lenoir O., Rops A.L. Endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx. J Am Soc Nephrol. 2016;27:3545–3551. doi: 10.1681/ASN.2015091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heerspink H.J.L., Kohan D.E., de Zeeuw D. New insights from SONAR indicate adding sodium glucose co-transporter 2 inhibitors to an endothelin receptor antagonist mitigates fluid retention and enhances albuminuria reduction. Kidney Int. 2021;99:346–349. doi: 10.1016/j.kint.2020.09.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.