Abstract
Introduction
Erythropoiesis-stimulating agents, standard of care for anemia of end-stage kidney disease, are associated with cardiovascular events. We evaluated the efficacy and safety of roxadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that stimulates erythropoiesis.
Methods
SIERRAS was a phase 3, randomized, open-label, active-controlled study enrolled adults on dialysis for end-stage kidney disease receiving erythropoiesis-stimulating agents for anemia. Patients were randomized (1:1) to thrice-weekly roxadustat or epoetin alfa. Doses were based on previous epoetin alfa dose and adjusted in the roxadustat arm to maintain hemoglobin at ∼11 g/dl during treatment. Epoetin alfa dosing was adjusted per US package insert. Primary efficacy endpoint was mean hemoglobin (g/dl) change from baseline averaged over weeks 28 to 52. Treatment-emergent adverse events were monitored.
Results
Enrolled patients (roxadustat, n = 370 and epoetin alfa, n = 371) had similar mean (SD) baseline hemoglobin levels (10.30 [0.66] g/dl). Mean (SD) hemoglobin changes for weeks 28 to 52 were 0.39 (0.93) and −0.09 (0.84) in roxadustat and epoetin alfa, respectively. Roxadustat was noninferior (least squares mean difference: 0.48 [95% confidence interval: 0.37, 0.59]; P < 0.001) to epoetin alfa. Tolerability was comparable between treatments.
Conclusion
In end-stage kidney disease, roxadustat was noninferior to epoetin alfa in up to 52 weeks of treatment in this erythropoietin-stimulating agent conversion study. Roxadustat had an acceptable tolerability profile.
Keywords: dialysis, epoetin alfa, hemoglobin, kidney failure, roxadustat
See Commentary on Page 1751
Chronic kidney disease (CKD) is progressive and debilitating, affecting ∼15% of adults in the United States (∼37 million people). Mortality rates increase with advanced stages of CKD, ranging from 10 to 198 deaths per 1000 person-years.1 CKD is complicated by anemia that increases in prevalence as estimated glomerular filtration rate declines.2 CKD often progresses to end-stage kidney disease (ESKD), when dialysis or kidney transplant is needed for survival.2
The majority of patients with ESKD are anemic and require treatment.3 Patients with anemia of CKD often have increased healthcare costs,3 cardiovascular disease, hospitalizations, and mortality.4 The pathogenesis of anemia is multifactorial, and impaired oxygen-dependent regulation of erythropoiesis contributes to inadequate erythropoietin production.5, 6, 7 In anemia of CKD, erythropoiesis also is suppressed by inflammation and iron deficiency. Hypoxia-inducible factor (HIF) is the body’s main oxygen tension sensor8 mediating a hemoglobin response.
Management of anemia of CKD was revolutionized in 1989 by recombinant human erythropoietin. Some studies have suggested that erythropoiesis-stimulating agents (ESAs) improve symptoms and reduce dependence on transfusions and their complications.9 Currently, ESAs combined with oral or i.v. iron supplementation is the standard of care for patients with ESKD and anemia.2 Safety studies have demonstrated that high-dose ESA therapy is associated with increased risk for cardiovascular (CV) events,10, 11, 12 resulting in safety warnings on ESA product labels13 and regulatory guidance advising physicians to prescribe the lowest ESA dose to achieve adequate hemoglobin levels and reduce the need for transfusions. Subsequently, achieved hemoglobin levels have decreased while transfusions have increased in US-based patients with ESKD.14
Roxadustat (FG-4592) is a potent, reversible HIF prolyl hydroxylase inhibitor15 that blocks HIF-α hydroxylation, preventing its degradation. HIF-α dimerizes with HIF-β, activates erythropoietin and iron availability genes, and mimics the body’s natural response to hypoxia to increase RBC production under normoxic conditions in patients with anemia.
Phase 3 studies of roxadustat in China and Japan led to its approval to treat anemia in patients with non–dialysis-dependent16, 17, 18 and dialysis-dependent (DD) CKD.19, 20, 21 We report the results of a US-based, pivotal, phase 3 study of roxadustat versus epoetin alfa in patients with ESKD and anemia on dialysis.
Methods
Study Design and Oversight
SIERRAS (FGCL-4592-064) was a randomized, open-label, epoetin alfa–controlled, phase 3 study evaluating the efficacy and safety of roxadustat for the treatment of anemia in patients with DD-CKD at 76 US-based sites (NCT02273726). The study was approved by ethics committees and conducted in accordance with regulatory requirements and the Declaration of Helsinki. Written informed consent was obtained from all patients.
The sponsor (FibroGen) designed the trial, provided financial support, and was responsible for data collection and analysis. All authors had full access to the data and analyses, approved the final draft of the manuscript, and signed off on its accuracy. A FibroGen employee wrote the first draft. All authors reviewed the manuscript and vouched for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Study Population
Eligible patients (≥18 years) had ESKD and were on dialysis for ≥3 months before screening. Protocol Amendment 2 allowed for patients incident to dialysis (ID; on dialysis for ≥2 weeks to ≤4 months at randomization). All patients were taking stable doses (≤30% change) of an ESA during the 4 weeks (8 weeks for Mircera) before randomization (Protocol Amendment 2: patients with ID-CKD must have been on ESA ≥4 weeks before screening), had a mean hemoglobin value from the last 3 screening assessments of 9.0 to 12.0 g/dl (Protocol Amendment 2: ≥8.5 and ≤12.0 g/dl for patients with ID-CKD), with a difference ≤1.3 g/dl between the highest and lowest value. Study inclusion and exclusion criteria are provided in Supplementary Table S1.
Study Treatment
Patients were randomized (1:1) to oral roxadustat or parenteral epoetin alfa thrice weekly (TIW). Under Protocol Amendments 1 and 2, the initial minimum 1-year treatment period could be shortened to complete the phase 3 program in a timely manner. Randomization was stratified by screening hemoglobin (≤10.5 vs. >10.5 g/dl); history of CV, cerebrovascular, or thromboembolic disease; and mean prescribed epoetin alfa dose (or equivalent; ≤150 vs. >150 IU/kg/week) in the prior 4 weeks. Automated randomization and treatment assignments were performed using an Interactive Web Response System. Details are provided in the Supplementary Methods.
Roxadustat starting doses were 70, 100, 150, or 200 mg TIW based on the patient’s prescribed prestudy ESA dose (Supplementary Table S2). For patients randomized to epoetin alfa, the initial dose was continued or converted from another ESA to epoetin alfa based on mean ESA dose (Supplementary Table S3). Roxadustat was supplied by FibroGen; epoetin alfa was supplied by each study site from commercial sources. Scheduled visits were weekly for the first 2 weeks, every 2 weeks until week 24, and every 4 weeks thereafter.
To maintain a hemoglobin level of ∼11 g/dl, roxadustat dose adjustments were permitted after week 4 and every 4 weeks thereafter. Roxadustat dose was adjusted using a predefined algorithm (Supplementary Table S4). Epoetin alfa dose was adjusted by investigators based on package insert guidelines.
Supplemental Iron Use
During the treatment period, all patients were encouraged to take oral iron without restriction and to start oral iron therapy before becoming iron depleted. In both treatment groups, i.v. iron (≤250 mg per dosing cycle; no limit under protocol Amendment 2) was permitted if the patient did not respond adequately to oral iron, could not tolerate oral iron, and was considered iron deficient (i.e., ferritin <100 ng/mL or transferrin saturation [TSAT] <20%). Treatment with study drug continued during i.v. iron administration. Iron was discontinued once the patient was iron replete (i.e., ferritin ≥100 ng/mL and TSAT ≥20%).
Rescue Therapy
Rescue therapy included RBC transfusion, ESA therapy, or a combination. Transfusion was considered if rapid correction of anemia was required to stabilize the patient or as medically necessary. Study treatment continued during or after transfusion. For roxadustat patients, ESA use was not permitted unless their hemoglobin levels had not responded to ≥2 dose increases (or maximum dose was reached), hemoglobin was <8.5 g/dl on 2 consecutive assessments (≥5 days apart), other causes for lack of a response had been ruled out, and risk reduction for alloimmunization in transplant-eligible patients was a goal. If >1 cycle of ESA rescue was required, roxadustat was permanently discontinued.
Efficacy Endpoints
The primary efficacy endpoint in the United States was mean hemoglobin change from baseline averaged over weeks 28 to 52 regardless of rescue therapy while receiving study drug.
Secondary efficacy endpoints were analyzed in a fixed sequence. Key secondary endpoints included patients (%) with mean hemoglobin ≥10.0 g/dl during weeks 28 to 52 (United States) and patients (%) achieving a hemoglobin response with hemoglobin 10.0 to 12.0 g/dl from weeks 28 to 36 without rescue therapy within 6 weeks of and during weeks 28 to 36 (European Union). Noninferiority of roxadustat to epoetin alfa was established if the lower limit of 95% confidence interval (CI) for the treatment difference was greater than −15%.
Other secondary endpoints in the fixed-sequence testing: mean change from baseline in low-density lipoprotein cholesterol to the average during weeks 12 to 28; mean change from baseline in hemoglobin averaged over weeks 18 to 24 in patients with baseline high-sensitivity C-reactive protein (hs-CRP) greater than the upper limit of normal (ULN); mean monthly i.v. iron use averaged over weeks 28 to 52; time to first transfusion during treatment; mean change from baseline in mean arterial pressure (MAP) averaged over weeks 20 to 28; and time to first exacerbation of hypertension during weeks 28 to 52.
Additional efficacy endpoints included measurements of hepcidin and iron-related parameters at baseline and week 52.
Safety
Safety measures included treatment-emergent adverse events (TEAEs), treatment-emergent serious adverse events (TESAEs), vital signs, electrocardiograms, clinical laboratory tests, and physical examinations, assessed during treatment and for 28 days after the last dose of study drug. Measures were assessed in the safety population (all randomized patients who received ≥1 dose of study drug). If the treatment received was different from the random assignment, the actual treatment received was used for analysis.
Statistical Analysis
Sample size determinations are provided in the Supplementary Methods. The US and EU primary efficacy endpoint analyses for noninferiority were conducted on the intent-to-treat population (all randomized patients) and the per-protocol set (patients in the full analysis set who received ≥8 weeks of treatment, had ≥1 valid postdose hemoglobin assessment, and were without major protocol violations). To establish the noninferiority of roxadustat, the lower boundary of the 2-sided 95% CI for the difference between the values in the roxadustat and epoetin alfa groups was greater than or equal to –0.75 g/dl.
A multiple-imputation analysis of covariance model was used for the US primary efficacy endpoint. Hemoglobin values affected by rescue therapy were not censored. The multiple-imputation analysis of covariance model contained terms for treatment, baseline hemoglobin, and stratification factors (Supplementary Methods). The difference between groups was calculated with least squares mean, that is, group means difference was adjusted by the covariates to make the interferential comparison.
A mixed model of repeated measures was used for the EU primary efficacy endpoint, with baseline hemoglobin value as a covariate and treatment, visit (up to week 52), treatment-by-visit interaction, and stratification factors except mean qualifying screening hemoglobin (≤10.5 vs. >10.5 g/dl) as fixed effects. Hemoglobin values were censored for rescue therapy.
The study provided ≥99% power to demonstrate noninferiority of roxadustat versus epoetin alfa for the US and EU primary efficacy endpoints, assuming a treatment difference (roxadustat – epoetin alfa) of −0.30 g/dl, a noninferiority margin for this difference of −0.75 g/dl, and an SD of 1.25 g/dl. Therefore, roxadustat was noninferior if the lower bound of the 95% CI for the treatment difference in the change hemoglobin was greater than or equal to −0.75 g/dl. Although the sample size considerations for efficacy assessment were the same as those used in the phase 3 trial in China,21 the sample size for the global program was designed to examine cardiovascular outcomes in a separate, pooled analysis that required a greater number of patients.22
Sensitivity analysis for the primary endpoints used a pattern-mixture model using a last-mean-carried-forward multiple imputation method.23 Missing data were imputed based on the last nonmissing mean from the applicable treatment group. This process was repeated 200 times to generate 200 imputed data sets.
A 2-sided 95% CI for the treatment difference for patients (%) achieving a hemoglobin response was based on a published approach24 adjusted for treatment group and stratification factors. Noninferiority was declared if the lower limit of the 95% CI was greater than −15%.
Secondary endpoints were tested using a fixed-sequence procedure on the full analysis set to maintain the overall 2-sided type I error of 0.05. If the claim of superiority or non-inferiority was successful, the test progressed to the next comparison in sequence (Supplementary Table S5). Additional efficacy endpoints were not adjusted for multiple comparisons; P values are provided for reference. All statistical analyses were performed using SAS Version 9.1.3 or higher.
Results
Participants
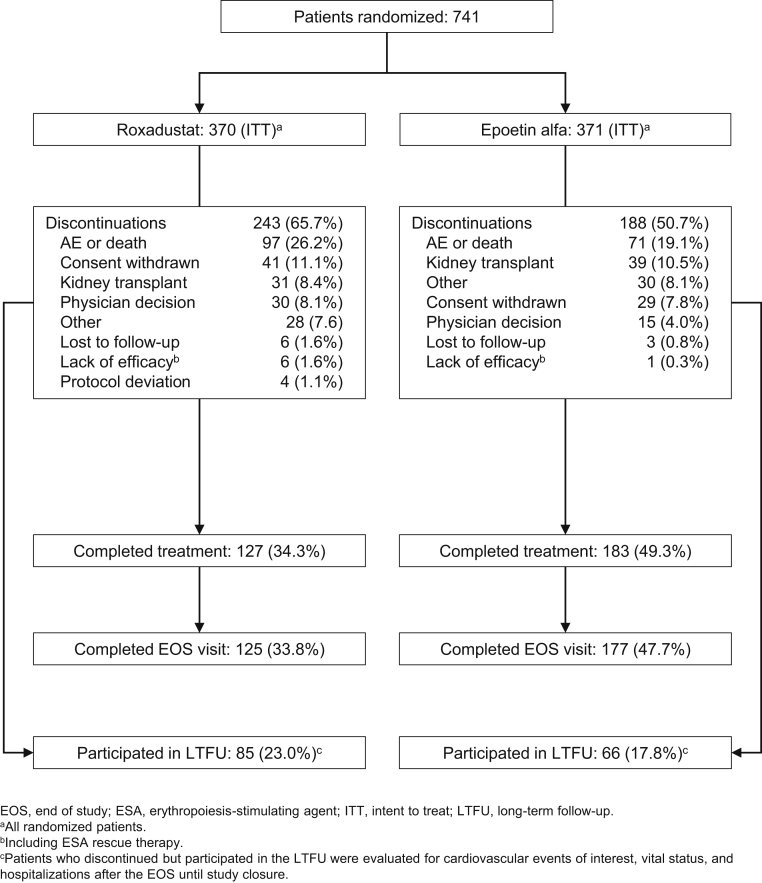
This US-based study was conducted between January 2015 and September 2018. A total of 741 patients were randomized (roxadustat = 370, epoetin alfa = 371), and 431 patients (243 and 188) discontinued (Figure 1). Analysis populations by protocol version are provided in Supplementary Table S6. Baseline demographic and clinical characteristics were similar between groups (Table 1).
Figure 1.
CONSORT flow diagram.
Table 1.
Baseline demographic and clinical characteristics (intent-to-treat population)
| Characteristic | Roxadustat (n = 370) | Epoetin alfa (n = 371) |
|---|---|---|
| Age, mean (SD), yearsa | 57.6 (13.6) | 58.4 (13.3) |
| Male sex, n (%) | 187 (50.5) | 215 (58.0) |
| Race, n (%) | ||
| White | 165 (44.6) | 184 (49.6) |
| Black or African American | 158 (42.7) | 156 (42.0) |
| Asian | 21 (5.7) | 15 (4.0) |
| Other | 26 (7.0) | 16 (4.3) |
| Weight, kg | 84.3 (22.3) | 86.6 (23.0) |
| BMI, mean (SD), kg/m2 | 30.2 (7.4) | 30.5 (7.5) |
| Hemoglobin, mean (SD), g/dl | 10.30 (0.66) | 10.31 (0.66) |
| Hemoglobin cohort, n (%) | ||
| ≤10.5 g/dl | 230 (62.2) | 235 (63.3) |
| >10.5 g/dl | 140 (37.8) | 136 (36.7) |
| Previous ESA therapy, n (%) | ||
| Epoetin alfa | 290 (78.4) | 293 (79.0) |
| Darbepoetin alfa | 65 (17.6) | 65 (17.5) |
| Mircera | 14 (3.8) | 8 (2.2) |
| Other | 1 (0.3) | 4 (1.1) |
| Weekly epoetin alfa dose category, n (%) | ||
| ≤150 IU/kg | 298 (80.5) | 301 (81.1) |
| >150 IU/kg | 72 (19.5) | 70 (18.9) |
| Dialysis modality, n (%) | ||
| Hemodialysis | 354 (95.7) | 354 (95.4) |
| Peritoneal dialysis | 16 (4.3) | 17 (4.6) |
| Dialysis duration, mean (SD), years | 4.0 (3.5) | 3.9 (3.8) |
| Iron repletion status, n (%) | ||
| Ferritin ≥100 ng/mL and TSAT ≥20% | 360 (97.3) | 363 (97.8) |
| Ferritin <100 ng/mL or TSAT <20% | 10 (2.7) | 8 (2.2) |
| CRP, n (%) | ||
| Less than or equal to ULN | 178 (48.1) | 192 (51.8) |
| Greater than ULN | 189 (51.1) | 177 (47.7) |
| Missing | 3 (0.8) | 2 (0.5) |
| Total cholesterol, mean (SD), mg/dl | 162.62 (43.61) | 162.13 (41.64) |
| LDL cholesterol, mean (SD), mg/dl | 84.46 (33.99) | 84.52 (34.11) |
| LDL/HDL ratio, mean (SD) | 2.01 (0.90) | 2.04 (0.92) |
| Diabetes, n (%) | 250 (67.6) | 254 (68.5) |
| Cardiovascular history, n (%) | ||
| Hypertension | 366 (98.9) | 367 (98.9) |
| CHF (NYHA 1 or 2) | 133 (35.9) | 124 (33.4) |
| MI (STEMI or NSTEMI) | 52 (14.1) | 46 (12.4) |
| Stroke | 41 (11.1) | 38 (10.2) |
BMI, body mass index; CHF, congestive heart failure; CRP, C-reactive protein; HDL, high-density lipoprotein; IU, International Units; LDL, low-density lipoprotein; MI, myocardial infarction; NSTEMI, non-ST segment elevation myocardial infarction; NYHA, New York Heart Association; STEMI, ST segment elevation myocardial infarction; TSAT, transferrin saturation; ULN, upper limit of normal.
Age was calculated in years from birth date to date of informed consent.
Primary and Key Secondary Efficacy Endpoints
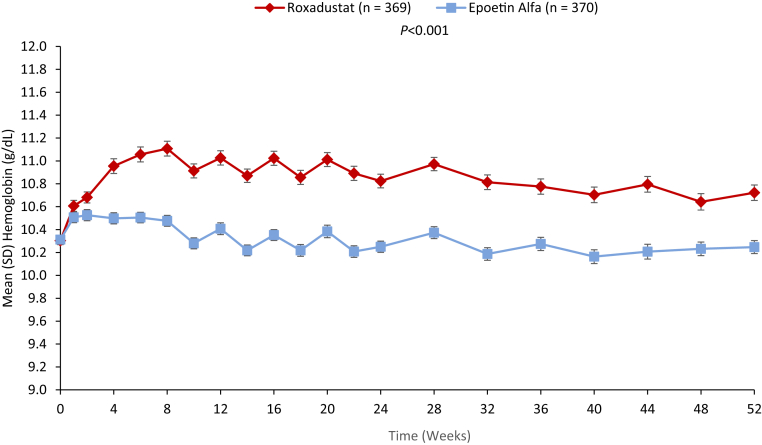
Mean baseline hemoglobin was 10.3 g/dl in both treatment groups. Mean (SD) changes in hemoglobin averaged over weeks 28 to 52 were 0.39 (0.93) and −0.09 (0.84) g/dl in roxadustat and epoetin alfa groups (least squares mean [LSM] difference: 0.48 [95% CI: 0.37, 0.59]; P < 0.001) (Figure 2). Roxadustat was noninferior to epoetin alfa for hemoglobin maintenance (Table 2).
Figure 2.
Mean hemoglobin levels (g/dl) ± SD in roxadustat-treated patients (red) compared with epoetin alfa-treated patients (blue) were significantly increased from baseline to week 52 (full analysis set). The number of patients (n) with nonmissing values is indicated for each treatment.
Table 2.
Primary efficacy endpoint: mean hemoglobin change from baseline averaged over weeks 28 to 52 regardless of rescue therapy (intention to treat)
| Roxadustat (n = 370) |
Epoetin alfa (n = 371) |
Treatment difference | P value | |||
|---|---|---|---|---|---|---|
| Observed values | Change from baseline | Observed values | Change from baseline | |||
| Baseline hemoglobin,a mean (SD), g/dl | 10.30 (0.66) | 10.31 (0.66) | ||||
| Weeks 28–52 hemoglobin, mean (SD), g/dl | 10.69 (0.76) | 0.39 (0.93) | 10.22 (0.68) | −0.09 (0.84) | ||
| Treatment comparisonb | ||||||
| LSM (SEM) | 0.28 (0.07) | −0.19 (0.06) | 0.48 (0.06) | <0.01 | ||
| (95% CI) | (0.15, 0.41) | (−0.32, −0.07) | (0.37, 0.59) | |||
CI, confidence interval; ESA, erythropoiesis-stimulating agent; LSM, least squares mean; SEM, standard error of the mean.
Defined as the mean of up to 4 last central laboratory values before the first dose of study drug.
Multiple-imputation analysis of covariance model with baseline hemoglobin as a covariate, and treatment, ESA-dependent incident dialysis within ≤4 months versus >4 months of starting dialysis when randomized, and other randomization stratification factors except mean qualifying screening hemoglobin (≤10.5 vs. >10.5 g/dl) as fixed effects.
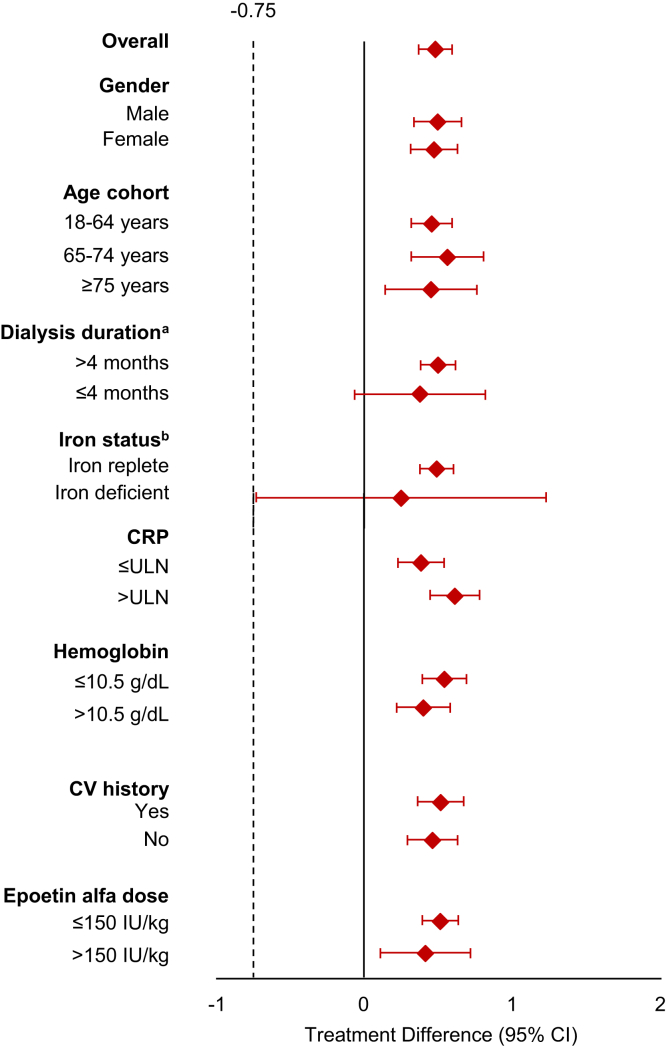
Subgroup analyses (i.e., gender, age cohort, duration of dialysis, iron status, hs-CRP, hemoglobin, CV history, epoetin alfa dose) were consistent with the primary analyses (Figure 3).
Figure 3.
Primary efficacy endpoint treatment differences by subgroup.
Throughout the study, epoetin alfa–treated patients maintained mean hemoglobin values closer to baseline; roxadustat-treated patients maintained slightly higher mean hemoglobin values (Figure 2).
Secondary Efficacy Endpoints
The percentage of patients with mean hemoglobin ≥10.0 g/dl averaged over weeks 28 to 52 was 66.1% (95% CI: 61.0, 70.9) and 58.6% (95% CI: 53.4, 63.7) in the roxadustat and epoetin alfa groups (responder rate difference: 7.6% [95% CI: 0.9, 14.3]). Patients with a hemoglobin response between 10.0–12.0 g/dl averaged over weeks 28 to 36 was 64.1% (95% CI: 58.7, 69.2) and 60.8% (95% CI: 55.5, 65.9) in the roxadustat and epoetin alfa groups (responder rate difference: 2.7% [95% CI: −4.3, 9.7]). For both endpoints, roxadustat was noninferior to epoetin alfa, as the lower limits of the 95% CIs were above the prespecified margin of −15% (Supplementary Table S8).
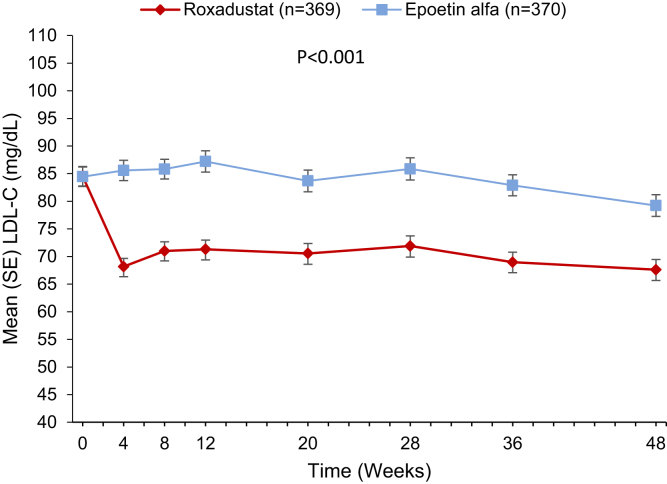
Patients in the roxadustat group experienced a decrease in low-density lipoprotein cholesterol (mg/dl) from baseline to week 48 (Figure 4). Mean (SD) changes from baseline averaged over weeks 12 to 28 was −13.7 (23.1) versus 1.2 (22.4) in the roxadustat versus epoetin alfa group (LSM difference: −14.7 [95% CI: −17.6, −11.7]). Roxadustat was superior to epoetin alfa in lowering low-density lipoprotein cholesterol (P < 0.001). In patients with baseline low-density lipoprotein ≥100 mg/dl, a larger percentage of those in the roxadustat versus epoetin alfa group achieved a target of <100 mg/dl averaged over weeks 12 to 28 (52.8% vs. 26.3%; odds ratio: 3.27 [95% CI: 1.75, 6.13]). Mean (SD) change from baseline in total cholesterol (mg/dl) averaged over weeks 12 to 28 was −23.9 (30.0) versus −1.7 (29.5) in the roxadustat versus epoetin alfa group (LSM difference: −22.4 [95% CI: −26.5, −18.3]; P < 0.001 [nominal]).
Figure 4.
Mean low-density lipoprotein cholesterol levels in roxadustat-treated patients (red) compared with epoetin alfa–treated patients (blue) were significantly decreased from baseline to week 48 (full analysis set).
At baseline, the proportion of patients with hs-CRP level greater than ULN was slightly higher in the roxadustat versus epoetin alfa group (Table 1). During 52 weeks of treatment with roxadustat, mean increases in hemoglobin levels were comparable between patients with baseline hs-CRP greater than ULN and hsCRP less than or equal to ULN with stable mean weekly dosing (Supplementary Figure S1A and B). By contrast, mean epoetin alfa dosing increased by ∼30% during weeks 21 to 24 and ∼60% during weeks 41 to 52. Patients with baseline hsCRP greater than ULN required larger increases in mean weekly epoetin alfa doses versus those with baseline hs-CRP ≤less than or equal to ULN (Supplementary Figure S1C and D).
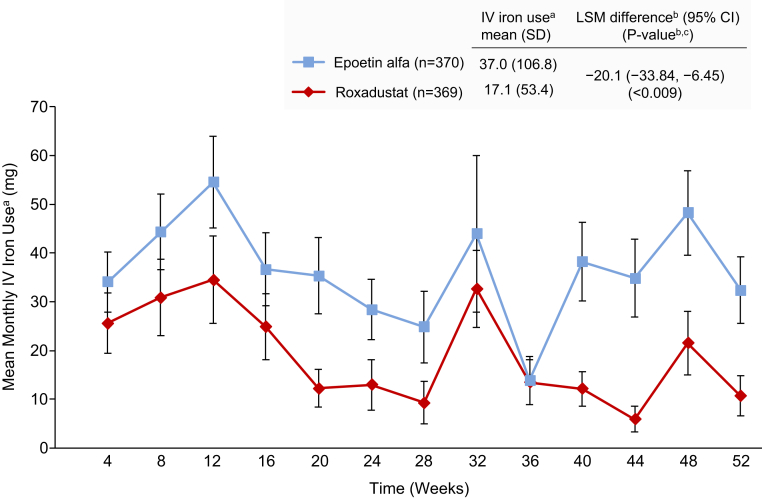
Mean (SD) monthly i.v. iron use per patient-exposure month during weeks 28 to 52 was 17.1 (53.4) mg versus 37.0 (106.8) mg in the roxadustat versus epoetin alfa group (LSM difference: −20.1 [95% CI: −33.8, −6.45]; P <0.009) (Figure 5).
Figure 5.
Mean monthly i.v. iron use in roxadustat-treated patients (red) compared with epoetin alfa-treated patients (blue) was significantly reduced from baseline to week 52 (full analysis set).
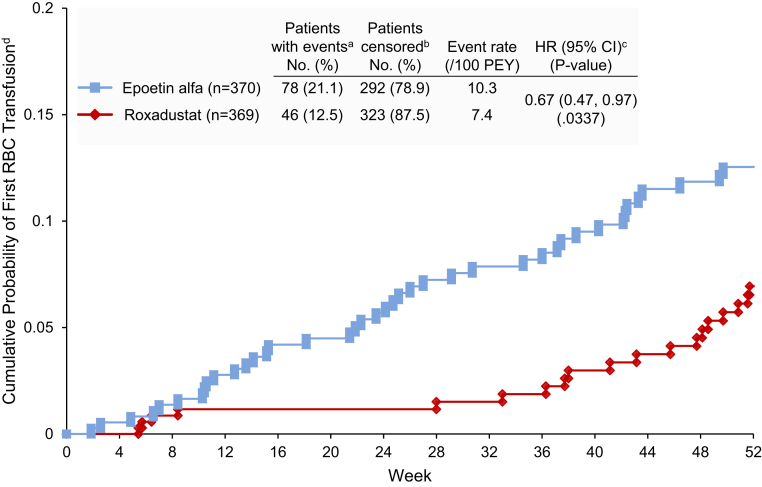
Fewer patients in the roxadustat versus epoetin alfa group received transfusions during treatment (12.5% vs. 21.1%; hazard ratio [HR] 0.67 [95% CI: 0.47, 0.97]). Roxadustat was noninferior to epoetin alfa as the upper limit of the 95% CI was <1.8 (Figure 6). The statistical test for superiority yielded P < 0.05.
Figure 6.
Cumulative percentage of in roxadustat-treated patients (red) compared with epoetin alfa–treated patients (blue) having a first blood/red blood cell transfusion was significantly reduced from baseline to week 52 (full analysis set).
The mean (SD) changes in MAP from baseline averaged over weeks 20 to 28 were 0.46 (10.9) mm Hg and 0.04 (10.5) mm Hg in the roxadustat and epoetin alfa groups (LSM difference: 0.69 [95% CI: −0.76, 2.14]; P = 0.35) (Supplementary Table S9). Fixed-sequence testing was stopped.
Time to first exacerbation of hypertension was not formally assessed because the fixed-sequence testing was stopped. This endpoint was reported in 32.0% and 29.7% of patients in the roxadustat and epoetin alfa groups (HR 1.26 [95% CI: 0.97, 1.64]; P = 0.08) (Supplementary Table S10).
Additional Efficacy Endpoints
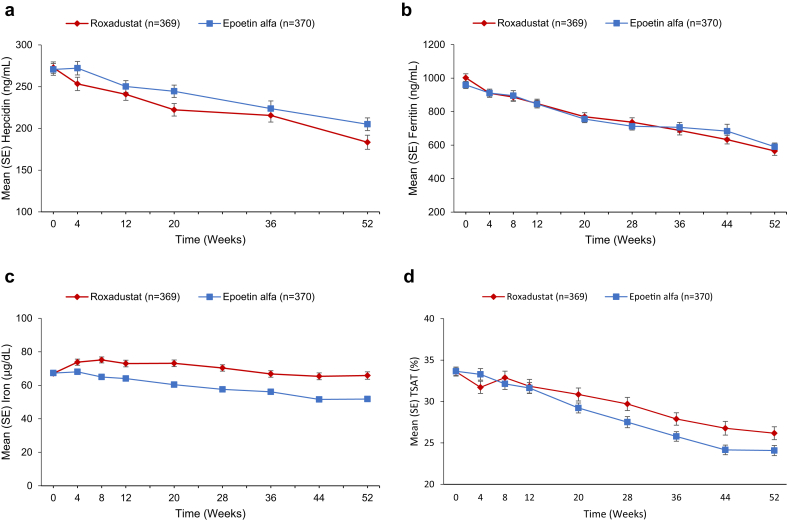
At baseline, mean (SD) hepcidin levels were 272.85 (129.70) and 270.67 (134.52) μg/L in the roxadustat and epoetin alfa groups (Figure 7A). By week 4, the mean (SD) change from baseline was −19.70 (130.19) and −0.45 (128.7) in the roxadustat and epoetin alfa group. This larger hepcidin reduction in the roxadustat group persisted through week 52, when mean (SD) changes from baseline were −95.53 (148.27) and −66.66 (141.61) (LSM difference: −19.12 [95% CI: −39.52, 1.28]; P = 0.07 [nominal]) (Supplementary Table S11).
Figure 7.
Levels of hepcidin (A), ferritin (B), iron (C), and transferrin saturation (TSAT) (D) in the roxadustat group were lower (hepcidin, iron, and TSAT) or similar (ferritin) to the epoetin alfa group from baseline to week 52 (full analysis set). Total iron binding capacity for roxadustat significantly increased from baseline to week 52 compared with epoetin alfa (least squares mean [SEM] 36.28 [2.32], 95% confidence interval 31.73, 40.82; P < 0.001).
At baseline, mean (SD) ferritin levels (ng/mL) were 1002.02 (459.68) and 959.24 (414.30) in the roxadustat and epoetin alfa groups, gradually declining in both groups over 52 weeks (Figure 7B). Mean (SD) changes from baseline were −429.91 (340.16) and −389.52 (341.11) in the roxadustat and epoetin alfa group (LSM difference: −41.71 [95% CI: −96.51, 13.09]; P = 0.14 [nominal]). Mean (SD) week 52 reductions were larger among roxadustat and epoetin alfa patients with mean baseline ferritin ≥400 ng/mL (−461.68 [334.14] and −408.39 [341.37]) versus baseline levels <400 ng/mL (−101.63 [203.07] and −105.40 [171.40]) (Supplementary Figure S2A).
At baseline, mean (SD) serum iron levels were 67.09 (22.36) μg/dl and 67.31 (21.59) μg/dl in the roxadustat and epoetin alfa groups (Figure 7C). By week 4, mean (SD) changes from baseline were 6.95 (32.47) and 0.35 (28.81) (LSM difference: 6.33 [95% CI: 2.20, 10.45]; P = 0.003 [nominal]). In both groups, iron levels remained stable, with levels that were significantly higher in the roxadustat versus epoetin alfa group.
At baseline, mean TSAT levels were the same (∼33%) in both treatment groups. TSAT reductions were observed in both groups with mean (SD) changes from baseline to week 52 of −7.96% (13.70%) and −9.78% (13.07%) in the roxadustat and epoetin groups (LSM difference: 2.18% [95% CI: 0.16, 4.20]; P = 0.03 [nominal]). Mean (SD) TSAT reductions were more pronounced in roxadustat and epoetin alfa patients with baseline TSAT ≥40% (−18.0% [12.5%] and −23.2% [10.4%]) versus those with baseline TSAT 20% to <40% (−4.8% [12.0%] and −5.6% [10.9%]) (Supplementary Figure S2B).
Safety
At least 1 TEAE was experienced by 91.6% (event rate/100 PEY: 728.1) and 91.4% (event rate/100 PEY: 728.5) of patients in the roxadustat and epoetin alfa groups. TEAEs occurring in ≥5% of patients in either group are listed in Supplementary Table S12. The most frequently reported TEAE in the roxadustat group was nausea, occurring in 17.0% of patients in the roxadustat group and 16.2% in the epoetin alfa group. This was followed by hypertension, occurring in 16.8% of the roxadustat group and 12.7% of the epoetin alfa group. At the end of the study, the mean (SD) change from baseline in systolic/diastolic blood pressure was similar between study groups: –2.8 (24.0)/–1.7 (12.5) and –2.8 (21.8)/–1.6 (11.9) in roxadustat and epoetin alfa, respectively. There were no between-group differences in hyperkalemia (16.2% and 15.1%) or neoplasms (4.3% and 5.9%). Sixty-five percent of roxadustat- and 67% of epoetin alfa-treated patients experienced ≥1 TESAE during treatment. TESAEs occurring in ≥1% of patients in either treatment group are summarized in Supplementary Table S13. TSEAEs of hypertension were similar between treatment groups (1.6% vs. 1.1%, roxadustat and epoetin alfa, respectively). Fatal TEAEs occurred in 16.8% and 15.7% of patients in the roxadustat and epoetin alfa groups.
Discussion
This US-based phase 3 trial demonstrated the efficacy of roxadustat versus epoetin alfa for achieving and maintaining higher hemoglobin levels in patients with DD-CKD. Roxadustat met the primary endpoints for increasing hemoglobin levels regardless of rescue therapy and censored for rescue therapy within 6 weeks of and during weeks 28 to 36. Compared with epoetin alfa, roxadustat safely increased hemoglobin, reduced the need for i.v. iron and transfusions, while maintaining comparable ferritin and TSAT levels. These results suggest that roxadustat, as a first-in-class HIF-PH inhibitor, provides for more efficient iron use compared with epoetin alfa.
These results are consistent with those demonstrated in phase 3 randomized controlled trials in China18,21 and Japan.17,19,20 Roxadustat maintained hemoglobin levels with stable mean doses that were comparable in patients with normal and elevated hs-CRP levels until the end of the study, supporting the durability of roxadustat’s effects regardless of hs-CRP, a proxy measure for inflammation.
By contrast, epoetin doses were increased in patients with baseline hs-CRP levels greater than ULN. Despite the use of higher doses of epoetin alfa, hemoglobin levels were still lower than those in patients with baseline hs-CRP levels less than ULN. These findings are consistent with phase 3 studies in China and with others showing inflammation suppresses ESA response.18,21,25,26
Differences in hemoglobin, iron stores, i.v. iron, and transfusion should be considered together. ESAs reduced transfusions when initially adopted; however, i.v. iron is required to maintain iron stores with ESAs, high doses of ESAs are required to maintain hemoglobin in inflammation, and there is an increased risk of CV morbidity and mortality associated with higher doses.10, 11, 12,27 Although the roxadustat-associated higher hemoglobin levels are arguably a function of the algorithm tested, these levels were associated with a reduction in transfusion and i.v. iron use. The underlying mechanisms may be related to roxadustat’s efficacy in inflammation, mobilization of internal iron stores, and increased absorption of oral iron.
Inflammation increases hepcidin levels, resulting in functional iron deficiency.28 Consistent with previous studies,18,21 roxadustat lowered hepcidin. We postulate that hepcidin reduction mobilizes internal iron stores, enhancing RBC production. Between–treatment-group TSAT levels were comparable; iron and transferrin were increased with roxadustat. Under the study conditions, mean monthly i.v. iron use was ∼40% less in roxadustat versus epoetin alfa patients. The percentage of roxadustat versus epoetin alfa patients who received transfusion was ∼33% lower; roxadustat patients maintained functional iron stores. The ability to mobilize internal iron stores with less i.v. iron should be interpreted in the setting of clinically stable TSAT. While TSAT is stable, it is important to note that the components of TSAT (serum iron and TIBC) both increased. These data, particularly the higher serum iron in the setting of less i.v. iron administration, support the thesis that roxadustat promotes robust erythropoiesis by combining the drug’s favorable impact on iron absorption and mobilization with its ability to induce erythropoietin.28
The comparison of roxadustat and epoetin alfa in this study is more than just a comparison of medications but rather a comparison of medications plus their algorithms. The ability of each medication to increase hemoglobin has been well documented in multiple trials and in clinical practice. The treatment algorithms for both have been adjusted independently and by different factors based on experience in phase 2 data26,29,30 in the recent decade for roxadustat and based on phase 4 studies completed more than a decade ago for epoetin alfa.11,12 Therefore, the comparison of dosing strategies needs to be interpreted in the context of certain key points. The algorithms for each drug are designed to achieve different hemoglobin levels. The roxadustat algorithm before the study was anticipated to achieve a population mean of approximately 11 g/dl, whereas the package insert for epoetin alfa and clinical practice suggest that the likely achieved hemoglobin levels would be lower in patients treated with epoetin alfa. Patients receiving darbepoetin and randomized to epoetin alfa were converted to their study treatment based on the conversion table in the Aranesp package insert31 the ability of this table to predict the dose of epoetin alfa they needed would affect their hemoglobin measures. The findings of this study to include all the secondary endpoints should be interpreted in this context.
The current study also showed that roxadustat was well tolerated, extending the safety profile beyond the 26-week period evaluated in previous studies.18,21 TEAE, TESAE, and fatal TEAE rates were well balanced between the treatment groups. Hyperkalemia rates were comparable in the treatment groups, in contrast to results from other phase 3 roxadustat studies.18 Larger numbers of patients discontinued roxadustat versus epoetin alfa, which may reflect a potential bias of the open-label design with an investigational drug versus the standard of care. When enrolled in a trial of an investigative agent compared with the standard of care, patients are informed that if they discontinue the drug being received, they would return to receiving standard of care. The knowledge that they are currently receiving the therapy that they would receive if they dropped out of the trial may affect this consideration.
Although this study was designed to evaluate the efficacy of roxadustat in patients previously treated for anemia, this imposes restrictions with respect to generalizability. The conversion study design is frequently used for recombinant erythropoietin trials owing to its convenience. This design does not allow the study of the universe of patients who initiate dialysis due to the selection bias introduced by the variable vintage of patients enrolled. Further affecting generalizability, it is important to note that this study enrolled patients only in the United States. Additionally, the rate of study drug discontinuation should be noted in the evaluation of these data. Finally, because the endpoints are calculated for regulatory purposes of assessing efficacy, their methods of censoring hemoglobin values after rescue should be noted.
In conclusion, this phase 3 study of roxadustat versus epoetin alfa in patients with DD-CKD demonstrated the noninferiority of roxadustat overall, with a greater degree of hemoglobin change and a reduction in transfusion and i.v. iron use. These beneficial effects were sustained for up to 52 weeks of treatment.
Disclosure
CC serves on Advisory Boards for AstraZeneca and FibroGen and received research support for Akebia, AstraZeneca, FibroGen, and GlaxoSmithKline. RMK owns FibroGen stock. MB received grants from FibroGen during the conduct of the study. GS, CB, ME, RL, KGS, CL, LS, and KHPY are employees of FibroGen and hold stock and/or stock options in FibroGen. EM, DS, SLD, and MM have no conflicts of interest to disclose. Roxadustat is in clinical development for the treatment of anemia of CKD in collaboration with Astellas Pharma and AstraZeneca.
Acknowledgments
This study was funded by FibroGen, Inc. Medical writing assistance was provided by Linda Goldstein, PhD, CMPP, from The Write Source MSC, LLC, and Tracey Fine, MS, ELS, of FibroGen and was funded by FibroGen.
FibroGen Inc. was the sponsor of this study and was responsible for data collection and analysis.
Author Contributions
Research idea and study design: GS, RL, KS, CL, LS, and KHPY. Data acquisition: CC, RMK, EM, DS. MB, SLD, MM, GS, CB, and ME. Data analysis/interpretation: CC, RMK, EM, DS. MB, SLD, MM, GS, CB, ME, RL, KS, CL, LS, and KHPY, Statistical analysis: GS, RL, KS, and CL. Supervision or mentorship: KHPY. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Supplemental Methods
CONSORT Checklist
Supplementary References
Table S1. Inclusion and Exclusion Criteria.
Table S2. Initial Roxadustat Dosing: Conversion from ESAs to Roxadustat.
Table S3. Initial Epoetin Alfa Dosing: Conversion from Non-epoetin ESAs to Epoetin Alfa.
Table S4. Study Drug Dosing Adjustments.
Table S5. Fixed Sequence for Analysis of Study Endpoints.
Table S6. Analysis Population by Protocol Version.
Table S7. Mean Changes in Hemoglobin over Weeks 28–36 Censored for Rescue Therapy.
Table S8. Key Secondary Efficacy Endpoints.
Table S9. Mean Change in MAP from Baseline to the Average over Weeks 20–28 (FAS).
Table S10. Time to First Exacerbation of Hypertension between Weeks 28–52 (FAS).
Table S11. Changes from Baseline for Hepcidin and Iron-related Parameters at Week 52 (FAS).
Table S12. TEAEs Occurring in ≥5% of Patients in Either Treatment Group (SAF).
Table S13. TESAEs Occurring in ≥1% of Patients in Either Treatment Group (SAF).
Figure S1. Relationship between changes in hemoglobin and changes in study drug doses by baseline hsCRP level.
Figure S2. Subgroup analysis of ferritin (A) and TSAT (B) (FAS).
Supplementary Material
Supplemental Methods
CONSORT Checklist
Supplementary References
Table S1. Inclusion and Exclusion Criteria.
Table S2. Initial Roxadustat Dosing: Conversion from ESAs to Roxadustat.
Table S3. Initial Epoetin Alfa Dosing: Conversion from Non-epoetin ESAs to Epoetin Alfa.
Table S4. Study Drug Dosing Adjustments.
Table S5. Fixed Sequence for Analysis of Study Endpoints.
Table S6. Analysis Population by Protocol Version.
Table S7. Mean Changes in Hemoglobin over Weeks 28–36 Censored for Rescue Therapy.
Table S8. Key Secondary Efficacy Endpoints.
Table S9. Mean Change in MAP from Baseline to the Average over Weeks 20–28 (FAS).
Table S10. Time to First Exacerbation of Hypertension between Weeks 28–52 (FAS).
Table S11. Changes from Baseline for Hepcidin and Iron-related Parameters at Week 52 (FAS).
Table S12. TEAEs Occurring in ≥5% of Patients in Either Treatment Group (SAF).
Table S13. TESAEs Occurring in ≥1% of Patients in Either Treatment Group (SAF).
Figure S1. Relationship between changes in hemoglobin and changes in study drug doses by baseline hsCRP level.
Figure S2. Subgroup analysis of ferritin (A) and TSAT (B) (FAS).
References
- 1.Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance System—United States. Version 7.1; 2020. https://nccd.cdc.gov/CKD/ Available at.
- 2.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 3.Stauffer M.E., Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidney Disease Outcomes Quality Initiative Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(suppl 3):S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Girelli D., Marchi G., Busti F. Iron replacement therapy: entering the new era without misconceptions, but more research is needed. Blood Transfus. 2017;15:379–381. doi: 10.2450/2017.0152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koury M.J., Haase V.H. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol. 2015;11:394–410. doi: 10.1038/nrneph.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratcliffe L.E., Thomas W., Glen J. Diagnosis and management of iron deficiency in CKD: a summary of the NICE guideline recommendations and their rationale. Am J Kidney Dis. 2016;67:548–558. doi: 10.1053/j.ajkd.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Kaelin W.G., Jr., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Babitt J.L., Lin H.Y. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besarab A., Bolton W.K., Browne J.K. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer M.A., Burdmann E.A., Chen C.Y. Baseline characteristics in the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT) Am J Kidney Dis. 2009;54:59–69. doi: 10.1053/j.ajkd.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Singh A.K., Szczech L., Tang K.L. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 13.Amgen, Inc . 2012. Epogen® (epoetin alfa): Full Prescribing Information. Thousand Oaks, CA. [Google Scholar]
- 14.Gilbertson D.T., Monda K.L., Bradbury B.D. RBC transfusions among hemodialysis patients (1999–2010): influence of hemoglobin concentrations below 10 g/dl. Am J Kidney Dis. 2013;62:919–928. doi: 10.1053/j.ajkd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Semenza G.L., Agani F., Booth G. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int. 1997;51:553–555. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- 16.Akizawa T., Iwasaki M., Otsuka T. Roxadustat treatment of chronic kidney disease-associated anemia in Japanese patients not on dialysis: a phase 2, randomized, double-blind, placebo-controlled trial. Adv Ther. 2019;36:1438–1454. doi: 10.1007/s12325-019-00943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akizawa T., Yamaguchi Y., Otsuka T. A phase 3, multicenter, randomized, two-arm, open-label study of intermittent oral dosing of roxadustat for the treatment of anemia in Japanese erythropoiesis-stimulating agent-naive chronic kidney disease patients not on dialysis. Nephron. 2020;144:372–382. doi: 10.1159/000508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N., Hao C., Peng X. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 19.Akizawa T., Iwasaki M., Yamaguchi Y. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol. 2020;31:1628–1639. doi: 10.1681/ASN.2019060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akizawa T., Ueno M., Shiga T. Oral roxadustat three times weekly in ESA-naive and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: results from two phase 3 studies. Ther Apher Dial. 2019;24:628–641. doi: 10.1111/1744-9987.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N., Hao C., Liu B.C. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381:1011–1022. doi: 10.1056/NEJMoa1901713. [DOI] [PubMed] [Google Scholar]
- 22.Fishbane S., Provenzano R., Szczech L. Impact of roxadustat on cardiovascular events in dialysis patients: pooled results from three randomized, open-label, epoetin alfa-controlled studies. J Am Soc Nephrol. 2021 in press. [Google Scholar]
- 23.Carpenter J.R., Roger J.H., Kenward M.G. Analysis of longitudinal trials with protocol deviation: a framework for relevant, accessible assumptions, and inference via multiple imputation. J Biopharm Stat. 2013;23:1352–1371. doi: 10.1080/10543406.2013.834911. [DOI] [PubMed] [Google Scholar]
- 24.Miettinen O., Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 25.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 26.Provenzano R., Besarab A., Wright S. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67:912–924. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Drüeke T.B., Locatelli F., Clyne N. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 28.Wang C.-Y., Babitt J.L. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol. 2016;23:189–197. doi: 10.1097/MOH.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besarab A., Chernyavskaya E., Motylev I. Roxadustat (FG-4592): Correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besarab A., Provenzano R., Hertel J. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015;30:1665–1673. doi: 10.1093/ndt/gfv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amgen, Inc . 2019. Aranesp® (darbepoetin alfa): full prescribing information. Thousand Oaks, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.