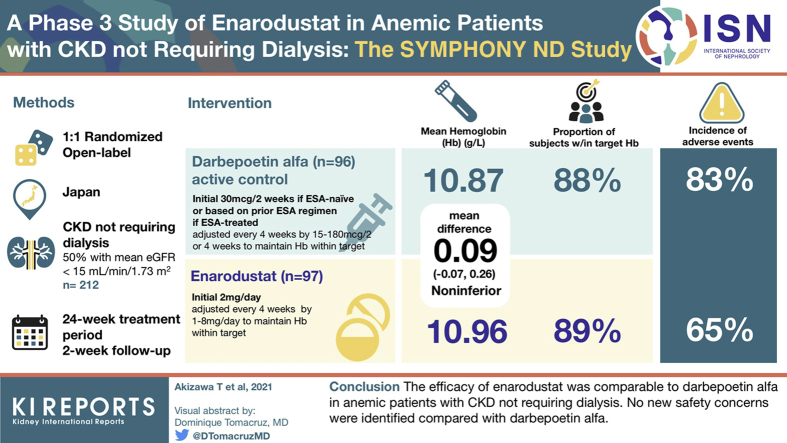
Abstract
Introduction
Enarodustat (JTZ-951) is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that might be a new therapeutic approach for managing anemia in patients with chronic kidney disease (CKD). We evaluated the efficacy (noninferiority to darbepoetin alfa [DA]) and safety of enarodustat in Japanese anemic patients with CKD not requiring dialysis.
Methods
Erythropoiesis-stimulating agent (ESA)–naïve patients and ESA-treated patients were randomized at a 1:1 ratio to receive enarodustat orally once daily or DA subcutaneously every 2 or 4 weeks for 24 weeks, respectively. Subjects in each arm had dose adjustments every 4 weeks to maintain their hemoglobin (Hb) level within the target range (10 to 12 g/dl). The primary endpoint was the difference in the mean Hb level between arms during the evaluation period defined as weeks 20 to 24 (noninferiority margin: –0.75 g/dl).
Results
The mean Hb level during the evaluation period in the enarodustat arm was 10.96 g/dl (95% confidence interval [CI]: 10.84 to 11.07 g/dl) with a difference of 0.09 g/dl (95% CI: −0.07 to 0.26 g/dl) between arms, establishing its noninferiority to DA. Nearly 90% of subjects in both arms maintained a mean Hb level within the target range. Compared with DA, enarodustat was associated with decreased hepcidin and ferritin, and increased total iron-binding capacity. There were no apparent differences in the incidence of adverse events between arms (65.4% [enarodustat], 82.6% [DA]).
Conclusions
The efficacy of enarodustat was comparable to DA in anemic patients with CKD not requiring dialysis. No new safety concerns were identified compared with DA.
Keywords: anemia in chronic kidney disease, comparative study, enarodustat, hepcidin, hypoxia-inducible factor prolyl hydroxylase inhibitor
Graphical abstract
See Commentary on Page 1751
CKD is a major health issue that affects approximately 9% of the population worldwide, with a significant increase in the number of people receiving renal replacement therapy from 1990 to 2017.1 Anemia is a common complication of CKD and its prevalence increases as the estimated glomerular filtration rate (eGFR) decreases.2 Anemia in CKD is associated with increased risk of CKD progression, cardiovascular disease, and mortality.3, 4, 5 Therefore, several clinical practice guidelines recommend the management of anemia in CKD patients.6, 7, 8
ESAs are the standard therapy for anemia in CKD together with oral or intravenous iron supplementation. It has been reported that treatment of anemia in CKD patients with ESAs improved their quality of life9 and might restrain the progression of renal dysfunction.10,11
However, the administration of ESAs with a high Hb target did not result in a marked difference in quality of life of CKD patients.12 Rather, this was associated with an increased risk of cardiovascular events, stroke, and mortality.13, 14, 15 The results of subsequent analyses in the Correction of Anemia With Epoetin Alfa in Chronic Kidney Disease (CHOIR) study showed that the administration of high doses of recombinant human erythropoietin, rather than high target Hb level, was associated with poor prognoses.16,17 In addition, a meta-analysis demonstrated that ESA treatment did not delay the progression of CKD.18
Hypoxia-inducible factor (HIF)–prolyl hydroxylase inhibitors are a new class of agents currently under investigation for the treatment of anemia in CKD patients.19 HIF is a transcription factor that has a general role in the physiological response to hypoxia by regulating the expression of several genes including those associated with endogenous erythropoietin production and iron mobilization.20, 21, 22
Enarodustat (JTZ-951) is an orally available HIF–prolyl hydroxylase inhibitor for the treatment of anemia in CKD patients.23, 24, 25 Enarodustat is likely to broaden treatment options for anemic patients not on dialysis because of its different mode of administration, thereby avoiding pain associated with parenteral ESA administration and allowing for a reduced number of hospital visits for an injection. In a phase 2b study in Japanese anemic patients with CKD not requiring dialysis, enarodustat dose-responsively increased Hb levels, and maintained Hb levels with physiological erythropoietin levels.24 The objective of this phase 3 study (phase 3 StudY to compare the efficacy and safety of enarodustat with darbepoetin alfa in aneMic Patients with cHrOnic kidNeY disease Not requiring Dialysis [SYMPHONY ND]) was to evaluate and compare the relative efficacy and safety of enarodustat in Japanese anemic patients with CKD not requiring dialysis with DA.
Materials and Methods
Study Design
This was a randomized, open-label, active controlled, parallel-arm trial conducted in Japan from February 2018 to June 2019. Active control, DA, was supplied by Kyowa Kirin Co., Ltd. The study consisted of a 4-week screening period, 24-week treatment period, and a 2-week follow-up period. The treatment period consisted of a 4-week initial treatment period with fixed initial dose and 20-week maintenance treatment period with dose adjustments based on the Hb level.
The study was registered with the Japan Pharmaceutical Information Center (JapicCTI-183870) and was conducted in accordance with the ethical principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice of the Japanese Ministerial Ordinance. The study was approved by the Institutional Review Board of each participating study site. All patients provided written informed consent before participation.
Subjects
Patients (≥20 years old) with CKD not requiring dialysis (eGFR <60 ml/min/1.73 m2) who were unlikely to receive renal replacement therapy during the study were recruited. The patients consisted of two subpopulations: ESA-naïve patients (not received ESA within 12 weeks before screening visit 1) and ESA-treated patients (received stable ESA within 8 weeks before screening visit 1). Main inclusion criteria were predefined Hb level for each subpopulation (ESA-naïve and ESA-treated patients), and transferrin saturation (TSAT) >20% or ferritin >50 ng/ml at screening visit 1. Patients who had received erythrocyte transfusion within 12 weeks before screening visit 1, were suspected to have anemia caused by noninfectious chronic inflammatory disease, or had intact-parathyroid hormone ≥500 pg/ml at screening visit 1 were excluded (see Supplementary Summary of Eligibility Criteria).
Intervention
ESA-naïve and ESA-treated subjects were randomly assigned at a ratio of 1:1 to the enarodustat arm (once daily oral administration) or DA arm (subcutaneous administration every 2 or 4 weeks). Subjects assigned to the enarodustat arm received 2 mg/d as the initial dose. In the DA arm, ESA-naïve patients received 30 μg/2 wks as the initial dose whereas dose and frequency administered to ESA-treated patients were determined based on their prior ESA regimen. From week 4 onward, the dose was adjusted every 4 weeks within the range of 1 to 8 mg/d in the enarodustat arm or in the range of 15 to 180 μg/2 or 4 wks in the DA arm to maintain Hb levels within the target range (≥10.0 g/dl and ≤12.0 g/dl).
While subjects in the enarodustat arm were in the screening period or the initial treatment period (or subjects in the DA arm were in the screening period), intravenous iron preparations were prohibited. However, stable oral iron preparations were permitted if they have been used before the screening period. During the maintenance treatment period, iron replacement therapy was implemented in principle, in consideration of Hb level, if ferritin was ≤100 ng/ml or TSAT was ≤20%.
Assessments
The primary endpoint was difference in the mean Hb level between arms during the evaluation period. Hb level during the evaluation period was defined as the mean Hb level at weeks 20, 22, and 24 or at discontinuation corresponding to week 24. Additionally, the difference in the mean Hb level at the end-of-treatment (EOT) period defined as the mean of the last two or three measurements (e.g., EOT, the previous timepoint and the second to last timepoint before EOT if efficacy was assessed three times or more from week 4 onward) was evaluated as the sensitivity analysis.
The secondary endpoints included the time course of Hb levels, proportion of subjects who maintained Hb levels within the target range, mean prescribed dose, and number of dose adjustments. Furthermore, the Hb level increase rate per week and the proportion of Hb level within ± 1.0 g/dl from week 0 were evaluated during the initial treatment period in ESA-naïve subjects and ESA-treated subjects, respectively. In addition, iron-related parameters were examined as other efficacy endpoints.
Safety assessments included adverse events (AEs) occurring after the start of treatment, laboratory tests, vital signs, standard 12-lead electrocardiogram, chest X-ray, and fundoscopy, although no primary safety endpoint was set. The investigator confirmed the findings of fundoscopy performed before the start of treatment and the end of treatment, and determined whether the new or exacerbated findings were clinically significant. Other assessments included vascular endothelial growth factor (VEGF) and renal function–related parameters.
Statistical Analysis
All analyses were performed using SAS version 9.2 or higher (SAS Institute, Cary, North Carolina, USA). The noninferiority margin for the difference in the mean Hb level between arms was set to 0.75 g/dl based on the clinical study results of existing ESAs.26 Specifically, the difference in Hb level between with and without ESA treatment was approximately 1.5 g/dl. Thus, the noninferiority margin was set as 0.75 g/dl, which corresponds to one-half of the difference. A sample size of 86 subjects per arm was required to demonstrate that enarodustat was as effective as DA, assuming the noninferiority limit of −0.75 g/dl, a 5% significance level with 90% power, and estimated a treatment difference of −0.4 ± 0.7 g/dl. The target number of subjects was set as 100 per arm in consideration of a 15% dropout rate. Simulation data generated from 10,000 runs with 86 subjects per arm indicated 100% power to demonstrate the 95% CI of the mean Hb level during the evaluation period was within the target range (see Supplementary Simulation).
The primary endpoint was evaluated using the per protocol set (PPS) consisting of subjects with a treatment compliance rate of ≥75% who were assessed for Hb level at all points during the evaluation period and who met the protocol requirements. The secondary endpoints were evaluated using the full analysis set consisting of subjects who received the investigational drug and were assessed for efficacy at least twice from week 4 onward.
Regarding the primary endpoint, in accordance with the closed testing procedure, once it was confirmed that the 95% CI for the mean Hb level in the enarodustat arm was within the target range of Hb level, then analysis to verify noninferiority was performed. The point estimate of treatment difference (enarodustat arm − DA arm) and its 95% CI for Hb level during the evaluation period were determined using analysis of covariance with the treatment arm as the factor and baseline value of Hb level (the mean Hb level at screening visit 1, screening visit 2 [2 weeks after screening visit 1], and week 0) as the covariate (significance level: 5%, two-sided). When the lower limit of the 95% CI was above −0.75 g/dl, noninferiority was judged to have been demonstrated. Sensitivity analysis was performed using the full analysis set.
A post hoc analysis of the change in iron-related parameters and renal function–related parameters at week 24 was performed using the Wilcoxon rank-sum test for a comparison between the enarodustat arm and DA arm (significance level: 5%, two-sided). Because this was an exploratory analysis, adjustment for multiplicity was not taken into consideration.
AEs reported after the start of study treatment were coded using MedDRA/J version 20.0 and tabulated. For additional tabulations, AEs of interest (“embolic and thrombotic events,” “hypertension,” “malignant or unspecified tumors,” and “retinal disorders”) were categorized with reference to the Standardised MedDRA Queries.
Results
Study Subject Characteristics
In total, 216 subjects (102 ESA-naïve and 114 ESA-treated subjects) were randomly assigned to the enarodustat arm (n = 107; 50 ESA-naïve and 57 ESA-treated subjects) or DA arm (n = 109; 52 ESA-naïve and 57 ESA-treated subjects) and 195 subjects completed the study. The PPS included 193 subjects after the exclusion of 23 subjects (10 in the enarodustat arm and 13 in the DA arm) with <3 Hb measurements during the evaluation period. Overall, 212 subjects were included in the full analysis set after the exclusion of four subjects (2 in the enarodustat arm and 2 in the DA arm) with <2 efficacy measurements from week 4 onward. Subject disposition is shown in Supplementary Figure S1.
Baseline characteristics of subjects in the PPS are shown in Table 1. Overall, baseline characteristics were balanced between the arms. The mean eGFR was 18.6 ml/min/1.73 m2 in the enarodustat arm and 17.3 ml/min/1.73 m2 in the DA arm, with approximately half of the subjects having CKD stage 5. Baseline characteristics of subjects in the full analysis set were similar to those in the PPS (Supplementary Table S1).
Table 1.
Subject characteristics (per protocol set)
| Characteristic | Enarodustat arm (n = 97) | DA arm (n = 96) | ||
|---|---|---|---|---|
| Age, mean (SD), yrs | 70.4 | (9.1) | 68.9 | (9.1) |
| Male | 61 | (62.9) | 47 | (49.0) |
| Body weight, mean (SD), kg | 60.2 | (10.0) | 60.9 | (12.7) |
| eGFR, mean (SD), ml/min/1.73 m2 | 18.6 | (10.1) | 17.3 | (8.3) |
| <15 | 47 | (48.5) | 48 | (50.0) |
| ≤15 to <30 | 37 | (38.1) | 41 | (42.7) |
| ≤30 | 13 | (13.4) | 7 | (7.3) |
| Primary disease of CKD | ||||
| Chronic glomerulonephritis | 28 | (28.9) | 23 | (24.0) |
| Diabetic nephropathy | 30 | (30.9) | 32 | (33.3) |
| Nephrosclerosis | 29 | (29.9) | 28 | (29.2) |
| Other | 10 | (10.3) | 13 | (13.5) |
| Use of prior ESA | ||||
| ESA-naïve | 45 | (46.4) | 45 | (46.9) |
| ESA-treated | 52 | (53.6) | 51 | (53.1) |
| Prior ESA | ||||
| rHuEPO | 0 | 0 | ||
| DA | 25 | (25.8) | 33 | (34.4) |
| Epoetin beta pegol | 27 | (27.8) | 18 | (18.8) |
| Prior ESA dose, mean (SD) | ||||
| rHuEPO, IU/2 wks | NA | NA | ||
| DA μg/4 wks | 75.6 | (51.3) | 72.7 | (45.0) |
| Epoetin beta pegol, μg/4 wks | 64.8 | (47.2) | 79.2 | (47.9) |
| Oral iron | 15 | (15.5) | 16 | (16.7) |
CKD, chronic kidney disease; DA, darbepoetin alfa; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; rHuEPO, recombinant human erythropoietin; NA, not applicable.
Body weight and eGFR at screening visit 1 are shown. The number of patients taking oral iron at screening visit 1 is shown. Values shown are n (%) unless otherwise stated.
Primary Endpoint
In the PPS, the mean Hb level during the evaluation period was 10.96 g/dl (95% CI: 10.84 to 11.07 g/dl) in the enarodustat arm and 10.87 g/dl (95% CI: 10.75 to 10.99 g/dl) in the DA arm. The 95% CI of the mean Hb level in the enarodustat arm was within the target range of Hb level (≥10.0 g/dl and ≤12.0 g/dl). The least squares mean of the difference between arms was 0.09 g/dl (95% CI: −0.07 to 0.26 g/dl), establishing its noninferiority to DA. Results of sensitivity analysis were in line with the primary analysis results (difference: 0.13 g/dl, 95% CI: –0.04 to 0.30 g/dl).
Hb Levels
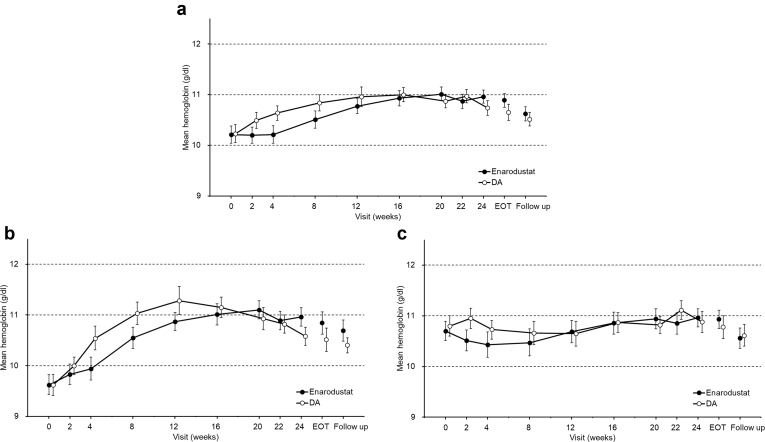
In each arm, 24-week treatment with dose adjustments maintained Hb levels within the target range (Figure 1a). The proportion that achieved a mean Hb level within the target range during the EOT period was 88.6% (95% CI: 80.9% to 94.0%) in the enarodustat arm and 87.9% (95% CI: 80.1% to 93.4%) in the DA arm.
Figure 1.
Time course of Hb levels over time (full analysis set). (a) All subjects. (b) ESA-naïve subjects. (c) ESA-treated subjects. Each point indicates the mean Hb level in each treatment arm and bars indicate the 95% confidence interval. Hb, hemoglobin; ESA, erythropoiesis-stimulating agent; DA, darbepoetin alfa; EOT, end of treatment.
During the overall treatment period, the mean prescribed dose of enarodustat was 2.68 mg/d, which was comparable to the initial dose (2 mg/d). More than 80% of subjects required two or fewer dose adjustments in the enarodustat arm (no dose adjustment: 18.1%, one: 34.3%, two: 29.5%). The mean weekly prescribed doses of DA were 15.701 μg from week 0 to week 4 and 16.230 μg during the treatment period. Similarly, in the DA arm, more than 80% of subjects required two or fewer dose adjustments.
The time course of Hb levels in ESA-naïve subjects and ESA-treated subjects is shown Figure 1b and c, respectively. The mean Hb levels during the evaluation period in ESA-naïve subjects and ESA-treated subjects of the enarodustat arm were comparable: 10.99 g/dl (95% CI: 10.84 to 11.13 g/dl) and 10.93 g/dl (95% CI: 10.75 to 11.11 g/dl), respectively. Changes in Hb level from week 0 to week 4 in each subpopulation are shown in Supplementary Table S2.
In ESA-naïve subjects, the mean change in Hb from week 0 after 4 weeks of enarodustat treatment was 0.32 g/dl (95% CI: 0.13 to 0.51 g/dl) with no evidence suggesting a rapid Hb increase (i.e., >2.0 g/dl in 4 weeks). The increase rate in Hb level per week estimated using the mixed effect model was 0.079 g/dl per week (95% CI: 0.033 to 0.125 g/dl per week).
In ESA-treated subjects, the mean change in Hb after 4 weeks of enarodustat treatment was –0.26 g/dl (95% CI: –0.45 to –0.08 g/dl). The proportion of subjects who achieved an Hb level within ± 1.0 g/dl from week 0 was 87.7% (95% CI: 76.3 to 94.9%), with a stable Hb level after switching from existing ESA therapy.
Iron-Related Parameters
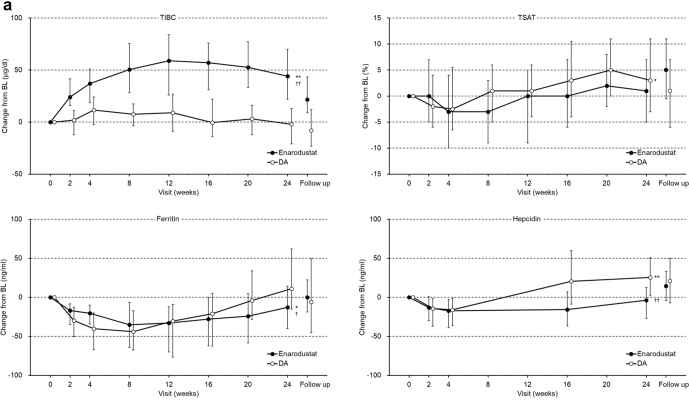
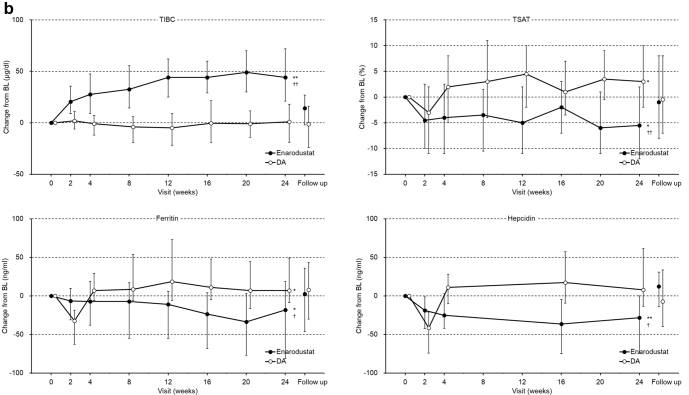
The time-courses of change in iron-related parameters are shown in Figure 2. Iron-related parameters at week 0 and week 24 are shown in Supplementary Table S3. In the enarodustat arm, ferritin and hepcidin were decreased and total iron-binding capacity (TIBC) was increased during the treatment period in ESA-naïve and ESA-treated subjects. Post hoc analysis revealed that changes in ferritin, hepcidin, and TIBC at week 24 were significantly different in each subpopulation between arms. TSAT was continuously decreased compared with week 0 in ESA-treated subjects and was related to increased TIBC and unchanged serum iron, but did not fall below 20% during the treatment period. One subject in the enarodustat arm received intravenous iron preparations. Overall, 42 of 105 (40.0%) subjects in the enarodustat arm and 44 of 107 (41.1%) subjects in the DA arm received oral iron preparations during the study period.
Figure 2.
Changes in iron-related parameters (full analysis set). (a) ESA-naïve subjects; (b) ESA-treated subjects. Each point indicates the median value in each treatment arm and bars indicate Q1 and Q3. Post hoc analysis was performed for changes in week 24, which were compared with week 0 using the Wilcoxon signed-rank test and between both arms using the Wilcoxon rank sum test (significance level: 5%, two-sided). Adjustment for multiplicity was not performed. ∗P < 0.05 and ∗∗P < 0.0001 for comparisons with week 0, †P < 0.05 and ††P < 0.0001 for comparisons between both arms. BL, baseline; DA, darbepoetin alfa; ESA, erythropoiesis-stimulating agent; TIBC, total iron-binding capacity; TSAT, transferrin saturation.
Safety
All 216 subjects (107 in the enarodustat arm and 109 in the DA arm) were included the safety population. No death occurred in the enarodustat arm and one death from drowning, considered unrelated to the investigational drug, occurred in the DA arm. Except for death, 15 serious adverse events (SAEs) occurred in 13 subjects in the enarodustat arm, and 14 SAEs occurred in 11 subjects in the DA arm. Four SAEs (fluid retention, hyperkalemia, edema, and pneumonia) were judged to be related to enarodustat. Overall, 65.4% of subjects receiving enarodustat and 82.6% of subjects receiving DA experienced at least one AE. No apparent differences are noted between ESA-naïve and ESA-treated subjects in the enarodustat arm in terms of the incidence of AE. The most frequent AE in the enarodustat arm was viral upper respiratory tract infection (17.8% vs. 22.9% for DA). Four subjects in the enarodustat arm discontinued the study treatment because of AEs versus five subjects in the DA arm. AEs reported in at least 5% of subjects in any treatment arm and AEs of interest (“embolic and thrombotic events,” “hypertension,” “malignant or unspecified tumors,” and “retinal disorders”) are summarized in Table 2. There were no clinically significant changes in laboratory tests, VEGF, vital signs, standard 12-lead electrocardiogram, chest X-ray, and fundoscopy.
Table 2.
Adverse events reported in 5% or more subjects and adverse events of interest
| Enarodustat arm |
DA arm |
|||||
|---|---|---|---|---|---|---|
| ESA-naïve (n = 50) | ESA-treated (n = 57) | Total (n = 107) | ESA-naïve (n = 52) | ESA-treated (n = 57) | Total (n = 109) | |
| AEs (≥5% subjects), | ||||||
| Any AEs | 36 (72.0) | 34 (59.6) | 70 (65.4) | 45 (86.5) | 45 (78.9) | 90 (82.6) |
| Viral upper respiratory tract infection | 9 (18.0) | 10 (17.5) | 19 (17.8) | 8 (15.4) | 17 (29.8) | 25 (22.9) |
| Diarrhea | 1 (2.0) | 2 (3.5) | 3 (2.8) | 3 (5.8) | 6 (10.5) | 9 (8.3) |
| Upper respiratory tract inflammation | 1 (2.0) | 1 (1.8) | 2 (1.9) | 5 (9.6) | 2 (3.5) | 7 (6.4) |
| Contusion | 1 (2.0) | 0 (0) | 1 (0.9) | 2 (3.8) | 4 (7.0) | 6 (5.5) |
| Embolic and thrombotic events | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypertension | 2 (4.0) | 3 (5.3) | 5 (4.7) | 2 (3.8) | 3 (5.3) | 5 (4.6) |
| Blood pressure increased | 2 (4.0) | 2 (3.5) | 4 (3.7) | 1 (1.9) | 1 (1.8) | 2 (1.8) |
| Hypertension | 0 (0) | 1 (1.8) | 1 (0.9) | 1 (1.9) | 2 (3.5) | 3 (2.8) |
| Malignant or unspecified tumors | 0 (0) | 0 (0) | 0 (0) | 2 (3.8) | 1 (1.8) | 3 (2.8) |
| Malignant neoplasm of renal pelvis | 0 (0) | 0 (0) | 0 (0) | 1 (1.9) | 0 (0) | 1 (0.9) |
| Gastric cancer | 0 (0) | 0 (0) | 0 (0) | 1 (1.9) | 0 (0) | 1 (0.9) |
| Soft tissue neoplasm | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.8) | 1 (0.9) |
| Retinal disorders | 3 (6.0) | 1 (1.8) | 4 (3.7) | 1 (1.9) | 0 (0) | 1 (0.9) |
| Retinal hemorrhage | 2 (4.0) | 0 (0) | 2 (1.9) | 0 (0) | 0 (0) | 0 (0) |
| Retinal tear | 0 (0) | 1 (1.8) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Retinal detachment | 1 (2.0)a | 0 (0) | 1 (0.9)a | 0 (0) | 0 (0) | 0 (0) |
| Macular edema | 1 (2.0)a | 0 (0) | 1 (0.9)a | 0 (0) | 0 (0) | 0 (0) |
| Diabetic retinal edema | 0 (0) | 0 (0) | 0 (0) | 1 (1.9) | 0 (0) | 1 (0.9) |
AE, adverse event; DA, darbepoetin alfa; ESA, erythropoiesis-stimulating agent.
Values shown are n (%).
AEs occurred in the same subject.
Renal Function–Related Parameters
Renal function–related parameters (eGFR and urine protein), measured at week 0 and week 24, are shown in Supplementary Table S4. eGFR at week 24 was not decreased compared with week 0, although urine protein at week 24 was increased in the enarodustat arm. Post hoc analysis revealed that changes in urine protein at week 24 did not reach statistically significant differences between arms.
Discussion
In this SYMPHONY ND study, 216 subjects with anemia associated with CKD not requiring dialysis were randomly assigned to receive enarodustat or DA. Subjects in the study successfully maintained Hb levels and its 95% CI within the 10.0 to 12.0 g/dl target range when adjusting for enarodustat dose in the range of 1 to 8 mg. The primary efficacy analysis demonstrated the noninferiority of enarodustat relative to DA. During the EOT period, the proportion that achieved a mean Hb level within the target range was high in the enarodustat arm and comparable between treatment arms.
Consistent with a previous report,24 a positive Hb level increase rate per week and a change in Hb levels were observed in ESA-naïve subjects administered 2 mg/d enarodustat without any evidence of rapid Hb increase. In addition, a simple dosing conversion scheme was effective at providing stable Hb maintenance, regardless of previous ESA dose, in subjects converting from existing ESAs to enarodustat. These findings suggest that Hb levels in subjects with enarodustat were properly controlled regardless of prior ESA use, and despite change in therapy, especially the route of drug administration.
SYMPHONY ND is the first study in which changes in iron-related parameters during enarodustat treatment were evaluated in anemic patients with CKD not requiring dialysis and then compared with DA, which has a relatively long half-life. In the enarodustat arm, changes in TIBC, ferritin, and hepcidin occurred in the 4-week initial treatment period, and sustained effects on these iron-related parameters were confirmed in the 20-week maintenance treatment period. In the DA arm, similar effects on ferritin and hepcidin were noted 2 weeks after treatment initiation, and a trend toward returning to baseline levels appeared as early as week 4. Although it was reported that erythropoietin production induced by HIF leads to the production of erythroferrone by erythroblasts, which limits the gene expression of hepcidin from the liver,27 differences in changes of iron parameters between the two arms may be attributed to different mechanisms of action and dosing intervals. Therefore, it is expected that enarodustat might be a new therapy to treat anemia with CKD and provide better iron availability. However, no apparent differences between the two arms was noted in terms of the number of subjects who were administered iron replacement therapy during the study because the study was not designed to identify this and iron supplementation was allowed when ferritin was <100 ng/ml or TSAT was <20% under the discretion of the investigators. Thus, future well-designed investigations are required to clarify the iron use effects of enarodustat.
Regarding safety, no AEs were more frequently reported in the enarodustat arm compared with the DA arm. For AEs of interest, “embolic and thrombotic events” did not occur in either arm. The occurrence of “hypertension” was not different between the treatment arms, and no clinically significant changes in mean blood pressure were observed during the treatment period (data not shown). Theoretically, HIF is associated with tumorigenesis and proangiogenic effects mediated by VEGF.28, 29, 30 However, no “malignant or unspecified tumors” were reported in the enarodustat arm. Although “retinal disorders” were numerically higher in the enarodustat arm, no moderate or severe AEs were reported, and no AEs resulted in treatment discontinuation. As previously reported in nonclinical studies,23 retinal VEGF mRNA levels and retinal vascular permeability were unchanged after enarodustat administration although increased plasma VEGF levels were confirmed. In the current study, no subjects with event(s) categorized as “retinal disorders” demonstrated increased VEGF (Supplementary Table S5). It has been reported that there are relationships between CKD and ocular disease (e.g., age-related macular degeneration or diabetic retinopathy), and that the prevalence of fundus pathology increased as CKD worsened.31, 32, 33 Thus, VEGF-related retinochoroidal risk is considered low, but should be carefully assessed in clinical studies with longer durations.
HIF activation has the potential to protect against kidney disease by the optimization of cellular adaptive response to hypoxic conditions, which is supported by the fact that enarodustat has shown renal protective effects in both acute kidney disease and CKD models (e.g., ischemia-reperfusion, CKD, and diabetic kidney disease).34, 35, 36, 37 Furthermore, enarodustat suppressed the transformation of renal interstitial fibroblasts, indicating its potential as a treatment option for renal fibrosis, which is the terminal pathway involved in the progression of CKD characterized by tubulointerstitial fibrosis.38 In the present study, the decrease of eGFR in the DA arm appeared to be within the natural course of CKD progression39 and was numerically slower that in the enarodustat arm. No subjects in the enarodustat arm discontinued the study because of the introduction of dialysis.
We acknowledge certain limitations of the SYMPHONY ND study. First, the study period was relatively short. The long-term safety of enarodustat should be evaluated, especially events related to “retinal disorders” and “malignant or unspecified tumors.” Second, the sample size was insufficient to characterize effects on CKD progression. A large-scale event study will be required to determine potential renoprotective effects mediated by enarodustat.
In conclusion, the results of SYMPHONY ND demonstrate that orally administered enarodustat was as effective as subcutaneously administered DA for increasing and maintaining Hb levels. Enarodustat was well-tolerated, and no new safety related concerns were identified compared with DA.
Disclosure
T.A. reports personal fees from Japan Tobacco Inc. during the conduct of the study and personal fees from Astellas, Bayer Yakuhin, Kyowa Kirin, Kissei Pharmaceutical, Ono Pharmaceutical, Fuso Pharmaceutical Industries, Torii Pharmaceutical, GlaxoSmithKline, Nipro Corporation, Otsuka Pharmaceutical, Sanwa Chemical, and Chugai Pharmaceutical outside of the submitted work. M.N. reports grants and personal fees from Japan Tobacco Inc. during the conduct of the study and grants and/or personal fees from Kyowa Kirin, Astellas, Astra Zeneca, GlaxoSmithKline, Mitsubishi Tanabe Pharma Corporation, Akebia Therapeutics, Bayer Yakuhin, and Torii Pharmaceutical outside of the submitted work. T.Y. reports personal fees from Japan Tobacco Inc. during the conduct of the study and grants and/or personal fees from Ono Pharmaceutical, Kowa, Chugai Pharmaceutical, TSUMURA&Co., CAC Croit Corporation, Kyowa Kirin, Daiichi Sankyo, ASAHI INTECC, Asahi Kasei, Kaken Pharmaceutical, 3H Clinical Trial, AC Medical, A2 Healthcare, Facet Biotech, Japan Media Corporation, Luminary Medical, Medidata Solutions, Senju Pharmaceutical, Otsuka Pharmaceutical, Eisai, FMD K&L Japan, Intellim, Welby, 3H Medi Solution, Nipro Corporation, Hemp Kitchen, NOBORI, Puravida Technologies and Medrio outside of the submitted work. H.H. reports personal fees from Japan Tobacco Inc. during the conduct of the study and personal fees from Kyowa Kirin, Chugai Pharmaceutical, and Torii Pharmaceutical outside of the submitted work. R.K., K.M., and Y.M. are employees of Japan Tobacco Inc.
Acknowledgments
The authors thank all the physicians, nurses, and patients at the participating centers for their support; and editorial support of ASCA Corporation (http://www.asca-co.com/english_site/) for proofreading a draft of this manuscript. Funding for this research was provided by Japan Tobacco Inc.
Author Contributions
All authors contributed to the final analysis and interpretation of data, and had full access to the study data and analyses. All authors contributed to revising the article, providing intellectual content of clinical importance to the work described, and gave final approval for the version to be published.
Footnotes
Summary of Eligibility Criteria
Simulation
Figure S1. Subject disposition.
Table S1. Subject characteristics (Full analysis set).
Table S2. Change in hemoglobin levels from week 0 at week 4 (full analysis set).
Table S3. Iron-related parameters at week 0 and week 24 (full analysis set).
Table S4. Renal function-related parameters at week 0 and week 24 (safety population).
Table S5. VEGF at week 0 and week 24 in the enarodustat arm (safety population).
Supplementary Material
Summary of Eligibility Criteria
Simulation
Figure S1. Subject disposition.
Table S1. Subject characteristics (Full analysis set).
Table S2. Change in hemoglobin levels from week 0 at week 4 (full analysis set).
Table S3. Iron-related parameters at week 0 and week 24 (full analysis set).
Table S4. Renal function-related parameters at week 0 and week 24 (safety population).
Table S5. VEGF at week 0 and week 24 in the enarodustat arm (safety population).
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astor B.C., Muntner P., Levin A. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2002;162:1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 3.Scrutinio D., Passantino A., Santoro D. The cardiorenal anaemia syndrome in systolic heart failure: prevalence, clinical correlates, and long-term survival. Eur J Heart Fail. 2011;13:61–67. doi: 10.1093/eurjhf/hfq167. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg D.S., Wexler D., Blum M. The use of subcutaneous erythropoietin and intravenous iron for the treatment of anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg D., Wexler D., Blum M. The cardio-renal anemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(suppl 8):viii7–viii12. doi: 10.1093/ndt/gfg1084. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 7.Locatelli F., Bárány P., Covic A. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant. 2013;28:1346–1359. doi: 10.1093/ndt/gft033. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H., Nishi S., Tomo T. 2015 Japanese society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017;3:36. [Google Scholar]
- 9.Gandra S.R., Finkelstein F.O., Bennett A.V. Impact of erythropoiesis-stimulating agents on energy and physical function in nondialysis CKD patients with anemia: a systematic review. Am J Kidney Dis. 2010;55:519–534. doi: 10.1053/j.ajkd.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Tsubakihara Y., Gejyo F., Nishi S. High target hemoglobin with erythropoiesis-stimulating agents has advantages in the renal function of non-dialysis chronic kidney disease patients. Ther Apher Dial. 2012;16:529–540. doi: 10.1111/j.1744-9987.2012.01082.x. [DOI] [PubMed] [Google Scholar]
- 11.Gouva C., Nikolopoulos P., Ioannidis J.P. Treating anemia early in renal failure patients shows the decline of renal function: a randomized controlled trial. Kidney Int. 2004;66:753–760. doi: 10.1111/j.1523-1755.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 12.Collister D., Komenda P., Hiebert B. The effect of erythropoietin-stimulating agents on health –related quality of life in anemia of chronic kidney disease: a systemic review and meta-analysis. Ann Intern Med. 2016;164:472–478. doi: 10.7326/M15-1839. [DOI] [PubMed] [Google Scholar]
- 13.Singh A.K., Szczech L., Tang K.L. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer M.A., Burdmann E.A., Chen C.Y. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 15.Palmer S.C., Navaneethan S.D., Craig J.C. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153:23–33. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]
- 16.Szczech L.A., Barnhart H.X., Inrig J.K. Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int. 2008;74:791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullough P.A., Barnhart H.X., Inrig J.K. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol. 2013;37:549–558. doi: 10.1159/000351175. [DOI] [PubMed] [Google Scholar]
- 18.Elliott S., Tomita D., Endre Z. Erythropoiesis stimulating agents and reno-protection: a meta-analysis. BMC Nephrol. 2017;18:14. doi: 10.1186/s12882-017-0438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locattelli F., Fishbane S., Block G.A. Targeting hypoxia-inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol. 2017;45:187–199. doi: 10.1159/000455166. [DOI] [PubMed] [Google Scholar]
- 20.Minamishima Y.A., Kaelin W.G., Jr. Reactivation of hepatic EPO synthesis in mice after PHD loss. Science. 2010;329:407. doi: 10.1126/science.1192811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nangaku M., Eckardt K.U. Hypoxia and the HIF system in kidney disease. J Mol Med. 2007;85:1325–1330. doi: 10.1007/s00109-007-0278-y. [DOI] [PubMed] [Google Scholar]
- 22.Wang G.L., Jiang B.H., Rue E.A. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukui K., Shinozaki Y., Kobayashi H. JTZ-951 (enarodustat), a hypoxia-inducible factor prolyl hydroxylase inhibitor, stabilizes HIF-α protein and induces erythropoiesis without effects on the function of vascular endothelial growth factor. Eur J Pharmacol. 2019;859:172532. doi: 10.1016/j.ejphar.2019.172532. [DOI] [PubMed] [Google Scholar]
- 24.Akizawa T., Nangaku M., Yamaguchi T. A placebo-controlled, randomized trial of enarodustat in patients with chronic kidney disease followed by long-term trial. Am J Nephrol. 2019;49:165–174. doi: 10.1159/000496929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akizawa T., Nangaku M., Yamaguchi T. Enarodustat, conversion and maintenance therapy for anemia in hemodialysis patients: a randomized, placebo-controlled phase 2b trial followed by long-term trial. Nephron. 2019;143:77–85. doi: 10.1159/000500487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi T., Wada A., Soma J. Randomized therapeutic equivalence study of darbepoetin alfa with epoetin alfa for anemia treatment in chronic kidney disease patients not on dialysis (in Japanese) Kidney Dial. 2010;68:931–945. [Google Scholar]
- 27.Kaulz L., Jung G., Valore E.V. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Cox S.R., Morita T. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5' enhancer. Circ Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 29.Goel H.L., Mercurio A.M. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krock B.L., Skuli N., Simon M.C. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong C.W., Wong T.Y., Cheng C.Y. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. 2014;85:1290–1302. doi: 10.1038/ki.2013.491. [DOI] [PubMed] [Google Scholar]
- 32.Deva R., Alias M.A., Colville D. Vision-threatening retinal abnormalities in chronic kidney disease stage 3 to 5. Clin J Am Soc Nephrol. 2011;6:1866–1871. doi: 10.2215/CJN.10321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunwald J.E., Alexander J., Maguire M. Prevalence of ocular fundus pathology in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:867–873. doi: 10.2215/CJN.08271109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito M., Tanaka T., Ishii T. Prolyl hydroxylase inhibition protects the kidneys from ischemia via upregulation of glycogen storage. Kidney Int. 2020;97:687–701. doi: 10.1016/j.kint.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 35.Sugahara M., Tanaka S., Tanaka T. Prolyl hydroxylase domain inhibitor protects against metabolic disorders and associated kidney disease in obese type 2 diabetic mice. J Am Soc Nephrol. 2020;31:560–577. doi: 10.1681/ASN.2019060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa S., Tanaka T., Saito T. The oral hypoxia-inducible factor prolyl hydroxylase inhibitor enarodustat counteracts alterations in renal energy metabolism in the early stages of diabetic kidney disease. Kidney Int. 2020;97:934–950. doi: 10.1016/j.kint.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Uchida L., Tanaka T., Saito H. Effects of a prolyl hydroxylase inhibitor on kidney and cardiovascular complications in a rat model of chronic kidney disease. Am J Physiol Renal Physiol. 2020;318:F388–F401. doi: 10.1152/ajprenal.00419.2019. [DOI] [PubMed] [Google Scholar]
- 38.Wakashima T., Tanaka T., Fukui K. JTZ-951, an HIF prolyl hydroxylase inhibitor, suppresses renal interstitial fibroblast transformation and expression of fibrosis-related factors. Am J Physiol Renal Physiol. 2020;318:F14–F24. doi: 10.1152/ajprenal.00323.2019. [DOI] [PubMed] [Google Scholar]
- 39.Inaguma D., Imai E., Takeuchi A. Risk factors for CKD progression in Japanese patients: findings from the Chronic Kidney Disease Japan Cohort (CKD-JAC) study. Clin Exp Nephrol. 2017;21:446–456. doi: 10.1007/s10157-016-1309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.