Abstract
Background
/Objective: In recent years, prostheses have been widely used for limb reconstruction after pelvic tumour resection. However, prostheses are associated with problems leading to tumour recurrence, poor implant matching, defects after tumour resection, and easy implant looseness or failure. To achieve a precise preoperative design, complete tumour resection, and better anatomical structure matching and prosthesis stability, this study used three-dimensionally (3D)-printed osteotomy guides and personalised prostheses for reconstruction after pelvic tumour resection. This study aimed to explore the early clinical efficacy of 3D printed personalised prostheses for the reconstruction of bone defects after pelvic tumour resection.
Methods
A total of 20 patients (12 males, 8 females) with pelvic tumours surgically treated at our hospital between October 2014 and October 2019 were selected. There were 10 cases each of giant cell bone tumours and osteochondrosarcomas. According to Enneking zoning, there were 11 and 9 cases with tumours located in zones I and II, respectively. All cases were equally divided into conventional and 3D printing groups. For repair and reconstruction, a nail rod system or a steel plate was used in the conventional group while individualised 3D-printed prostheses were used in the 3D printing group. The surgical incision, duration of surgery, intraoperative blood loss, and the negative rate of resection margins in postoperative tumour specimens were examined. The follow-up focused on tumour recurrence, complications, and the Musculoskeletal Tumor Society (MSTS) score.
Results
All cases were followed-up for 6–24 months. The average incision length, duration of surgery, amount of intraoperative blood loss, and MSTS score of the 3D printing group were 10.0 ± 3.1 cm, 115.2 ± 25.3 min, 213.2 ± 104.6 mL, 23.8 ± 1.3, respectively, and those of the conventional group were 19.8 ± 8.4 cm, 156.8 ± 61.4 min, 361.4 ± 164.2 mL, and 18.3 ± 1.4, respectively. Histological tumour specimen examination showed nine and three cases with negative resection margins in the 3D printing group and the conventional group, respectively. The abovementioned indicators were significantly different between both groups (P < 0.05).
Conclusion
Applying 3D printed surgical guides and personalised prostheses for pelvic tumour resection, repair, and reconstruction, as well as preoperative planning and design, enables more accurate tumour resections and better prosthesis-patient matchings, possibly reducing surgical trauma, shortening the duration of surgery, and promoting the functional recovery of patients postoperatively.
The Translation Potential of this Article
Contrary to existing studies on 3D printed personalised prostheses, this study reports the clinical efficacy of the aforementioned technology in treating bone defects in a series of patients who underwent pelvic tumour resection. Moreover, it presents a comprehensive comparison of this technology with conventional procedures, thus strengthening its importance in treatment regimens for reconstructing bone defects.
Keywords: Pelvis, Neoplasm, Bone and bones, Prostheses and implants
1. Introduction
Bone tumours may occur at any site. When a bone tumour is located at a site with a complex anatomical structure, a stable and effective functional reconstruction after resection is essential [[1], [2], [3]]. The pelvis, featuring a complex anatomical structure, is adjacent to important vascular nerves and pelvic organs. Conventional pelvic tumour surgery may cause problems such as incomplete tumour resection and poor prosthesis-patient matching, resulting in a high incidence of complications, including tumour recurrence, aseptic prosthesis loosening, and poor surgical effects [[4], [5], [6], [7]]. To address these issues, stable, effective, and personalised treatment regimens are needed. Theoretically, implanted individualised and conventional prostheses may be stabilised and fixed by improving the anatomical matching between the prosthesis and the adjacent bony structure, thus restoring the function of the affected limb. However, conventional bone grafts, along with internal fixation or prosthetic implants, are often prone to fixation failure and poor postoperative function due to poor matching. With the emergence and application of three-dimensional (3D) printing technology in orthopaedics as well as computer-aided pelvic tumour resection, repair, and reconstruction, precise surgical planning and personalised porous prosthesis implantation may improve the accuracy of bone tumour surgical resection. Moreover, it may also improve the compatibility between prostheses and bone defects, and restore pelvic stability due to the conduciveness of the porous structure to bone ingrowth [8,9]. This study aimed to explore the application of 3D-printed personalised prostheses for treating bone defects after pelvic tumour resection and compare this technology with conventional surgical methods to evaluate its safety and effectiveness.
2. Materials and methods
2.1. Study patients
A total of 20 patients (12 men and 8 women) with pelvic tumours who underwent surgery at our hospital between October 2014 and October 2019 were included in this study. This study was approved by the ethics committee of XXX (approval number: XXX) and written informed consent was obtained from all patients. According to the Enneking pelvic zoning system [10], tumours were located in zones I and II in 11 and 9 patients, respectively. The average age of patients was 30.3 ± 8.4 (range, 18–45) years, and all patients underwent biopsy for pathological diagnosis preoperatively to confirm the nature of bone tumours.
In the conventional group, there were 10 patients, comprising 6 males and 4 females, with an average age of 31.2 ± 9.6 (range, 19–45) years. Among these patients, five had a giant cell bone tumour, while the other five had osteochondrosarcoma. Tumours were in zones I and II in six and four cases, respectively. Disease courses were 3–24 months, with an average of 11.5 ± 6.5 months (Table 1).
Table 1.
Clinical characteristics of patients in the conventional group.
| Case | Age (years) | Gender | Disease course (months) | Tumour nature | Tumour zone | Bone defect volume (cm3) | Time to last follow-up (months) |
|---|---|---|---|---|---|---|---|
| 1 | 29 | Male | 12 | Giant cell bone tumour | Ⅱ | 53.2 | 18 |
| 2 | 32 | Female | 11 | Osteochondrosarcoma | Ⅰ | 48.4 | 6 |
| 3 | 38 | Female | 21 | Osteochondrosarcoma | Ⅰ | 42.1 | 12 |
| 4 | 21 | Male | 10 | Giant cell bone tumour | Ⅰ | 87.9 | 20 |
| 5 | 37 | Female | 6 | Giant cell bone tumour | Ⅱ | 53.9 | 24 |
| 6 | 45 | Male | 24 | Giant cell bone tumour | Ⅰ | 48.1 | 7 |
| 7 | 44 | Female | 12 | Osteochondrosarcoma | Ⅰ | 91.2 | 18 |
| 8 | 27 | Male | 3 | Osteochondrosarcoma | Ⅱ | 37.8 | 13 |
| 9 | 20 | Female | 7 | Giant cell bone tumour | Ⅰ | 48.4 | 7 |
| 10 | 19 | Male | 9 | Osteochondrosarcoma | Ⅱ | 52.3 | 8 |
In the 3D printing group, there were 10 cases, comprising 6 males and 4 females, with an average age of 29.4 ± 7.5 (range, 20–44 years). Among these patients, five had a giant cell bone tumour, while the other five had osteochondrosarcoma. Tumours were in zone I and zone II in five cases, each. Disease courses were 4–18 months, with an average of 9.7 ± 3.8 months (Table 2).
Table 2.
Clinical characteristics of patients in the three-dimensionally printing group.
| Case | Age (years) | Gender | Disease course (months) | Tumour nature | Tumour zone | Bone defect volume (cm3) | Time to last follow-up (months) |
|---|---|---|---|---|---|---|---|
| 1 | 20 | Female | 11 | Giant cell bone tumour | Ⅰ | 60.4 | 8 |
| 2 | 26 | Male | 9 | Giant cell bone tumour | Ⅰ | 42.1 | 24 |
| 3 | 36 | Female | 18 | Osteochondrosarcoma | Ⅱ | 38.0 | 12 |
| 4 | 23 | Male | 8 | Giant cell bone tumour | Ⅱ | 97.0 | 16 |
| 5 | 44 | Male | 12 | Osteochondrosarcoma | Ⅱ | 43.9 | 9 |
| 6 | 35 | Male | 4 | Giant cell bone tumour | Ⅰ | 58.1 | 7 |
| 7 | 30 | Female | 11 | Giant cell bone tumour | Ⅰ | 78.2 | 18 |
| 8 | 31 | Female | 6 | Osteochondrosarcoma | Ⅱ | 39.5 | 10 |
| 9 | 28 | Male | 10 | Osteochondrosarcoma | Ⅰ | 49.4 | 6 |
| 10 | 21 | Male | 8 | Osteochondrosarcoma | Ⅱ | 52.3 | 9 |
2.2. Surgical method
All patients underwent tracheal intubation and surgery under general anaesthesia. According to the position of the pelvic tumour, patients were in a supine or a floating lateral position. Skin was routinely disinfected, and sterile drapes were whisked. According to the location of the bone tumour and the preoperative surgical plan, the anterior, posterior, or anterior and posterior approach was used, and the skin and subcutaneous tissue were cut layer by layer to expose the lesion. As the tumour should be completely resected first and functional reconstruction should be considered subsequently during pelvic tumour resection and reconstruction, osteotomy levels should be > 3–5 cm from both ends of the tumour; this can be determined by radiography, computed tomography (CT), and magnetic resonance imaging. Normal tissues, such as those of muscles, with a thickness of about 1 cm should be preserved around the tumour. Intra-articular resection can be used if the tumour has not invaded the joint; otherwise, extra-articular resection can be performed.
The abridged general view is as follows (Fig. 1):
Fig. 1.
The course of preoperative planning and surgery.
2.3. Conventional group
For patients with tumours in zone I, tumour lesions were cut and scraped. Allograft bones and two to three segments of the fibula were selected according to defect size and were implanted between the L4, L5, and ilium or between the sacrum and ilium using a nail-rod system or steel plate and screw for fixation. For cases with tumours in zone II, tumour lesions were also cut and scraped, and acetabular roof reconstruction with titanium mesh cups, bone grafting or bone cement filling, and artificial hip replacement were used to restore hip joint function. According to the intraoperative situation, the soft tissue attachment was reconstructed. Postoperatively, a drainage tube was placed, and the incision was sutured layer by layer.
2.4. 3D printing group
2.4.1. Preoperative planning and design
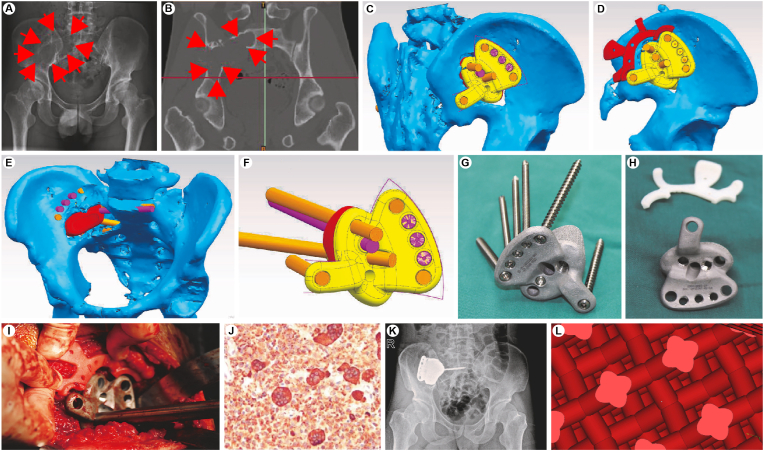
The 3D-printed prostheses used in this study were designed by the authors and manufactured by Shanghai Shengshi Medical Instrument Co., Ltd. (Shanghai, China). First, a thin-slice CT (layer thickness, 2 mm) of the pelvis was performed. Next, the corresponding CT data were imported into Mimics 19.0 software (Materialise, Leuven, Belgium) to build a 3D model of the affected sites including the tumours (Fig. 2C–E). According to the size and shape of the bone defect after virtual tumour resection, a preliminary model of a personalised filling prosthesis and surgical guide was obtained. The 3D-printed surgical guide was made of photosensitive resin. Prostheses matched the size of the bone defect; the upper edge of the surgical guide matched the direction of the posterior (or anterior) iliac superior spine, and the lower edge matched the upper edge of prostheses (Fig. 2C–J). The interface between the prosthesis and the bone was designed as a 3D porous structure to facilitate bone ingrowth (Fig. 2K), and immediate stability was achieved by screw fixation. Finally, personalised prostheses (Fig. 2G and H) were printed using an electron beam melting 3D printer (Arcam, Mölndal, Sweden). The printing material was titanium alloy (Ti6AI4V) that was sterilised under high temperature and pressure. The size of the porous structure was 600–800 μm (Fig. 2L). The interval between surgical regimen confirmation and complete prosthesis construction was approximately 8 (range, 5–14) days.
Fig. 2.
Three-dimensionally-printed personalised prosthesis for bone defect repair and reconstruction following giant cell right iliac bone tumour resection. A. Preoperative pelvic frontal radiography film suggested a right iliac bone tumour; B. preoperative computed tomography image; C–F. a personalised prosthesis and surgical guide were designed preoperatively for bone defect repair and reconstruction following right iliac bone tumour resection; G-H. three-dimensionally-printed personalised prosthesis and surgical guide; I. intraoperative image; J. postoperative pathological results suggested giant cell bone tumour; K. postoperative pelvic frontal radiography film; L. the size of the porous structure.
2.5. Surgical process
According to the aforementioned preoperative simulation, the surgical guide was placed, and the tumor lesions were cut and scraped. The sclerotic bone invaded by the tumor was ground with a high-speed drill until normal bone tissue was reached. Approximately 3–5 mm of normal cancellous bone tissue was also ground. Bone grafting was performed first with a small amount of autologous iliac bone alone or combined with the allograft bone. A 3D-printed personalised filling prosthesis was implanted, and according to the preoperative design, screws were implanted to fix the prosthesis and reconstruct the soft tissue attachment. Postoperatively, a drainage tube was placed, and the incision was sutured layer by layer.
2.6. Postoperative treatment, follow-up, and evaluation indicators
Antibiotics were routinely administered intravenously at one day postoperatively. On the first postoperative day, patients started undergoing exercises for the affected limb and walked without weight bearing on crutches. The weight bearing time was determined for each patient according to the implant condition. Two to three days postoperatively, the drainage tube was removed after the drainage volume reached <30 mL. Patients were followed-up at 1, 3, 6, and 12 months postoperatively and once a year thereafter. The limb function score, subjective satisfaction, and presence of complications or tumour recurrence were recorded at follow-ups. The incision length, duration of surgery, intraoperative blood loss, and the negative rate of resection margins corresponding to the postoperative tumour specimens, which were revealed by histologically examining both groups, were recorded and compared between groups. The Musculoskeletal Tumor Society (MSTS) scoring system was used to evaluate postoperative limb function [11].
2.7. Statistical methods
SPSS software version 26.0 (IBM, Armonk, New York) was used for all statistical analyses. Continuous data were analysed using a t-test with independent samples and expressed as ¯x ± s; enumeration data were compared by a chi-squared test. A P-value <0.05 was considered statistically significant.
3. Results
All cases were followed up for 6–24 months. The average incision length, duration of surgery, amount of intraoperative blood loss, and MSTS score of the 3D printing group were 10.0 ± 3.1 cm, 115.2 ± 25.3 min, 213.2 ± 104.6 mL, and 23.8 ± 1.3, respectively, and those of the conventional group were 19.8 ± 8.4 cm, 156.8 ± 61.4 min, 361.4 ± 164.2 mL, and 18.3 ± 1.4, respectively. Histological postoperative tumour specimen examination showed that resection margins were negative in nine and three cases in the 3D printing and conventional groups, respectively. These aforementioned indicators were statistically different between groups (P < 0.05) (Table 3).
Table 3.
Intraoperative conditions and postoperative follow-up results.
| Group | Number of cases | Surgery duration (min) | Blood loss (mL) | Surgical incision length (cm) | Number of cases with negative resection margins shown by histological examination | MSTS score at last follow-up |
|---|---|---|---|---|---|---|
| 3D printing group | 10 | 115.2 ± 25.3 | 213.2 ± 104.6 | 10.0 ± 3.1 | 9 | 23.8 ± 1.3 |
| Conventional group | 10 | 156.8 ± 61.4 | 361.4 ± 164.2 | 19.8 ± 8.4 | 3 | 18.3 ± 1.4 |
| aT/X2 value | 2.868 | 3.489 | 6.053 | 3.132 | ||
| P value | 0.006 | 0.001 | 0.000 | 0.02 | 0.029 |
MSTS, Musculoskeletal Tumor Society
The comparison of the above indicators between the two groups showed a statistically significant difference (P < 0.05)
The conventional group was followed-up for 13.3 months. Postoperatively, there was one case each of tumour recurrence with internal fixation loosening, internal fixation loosening, and wound infection. The 3D printing group was followed-up for 11.9 ± 5.7 months, with one case of incision infection postoperatively. This infection was relieved after anti-infective treatment, and there were no cases of prosthetic implant loosening or breakage.
4. Discussion
Contrary to existing studies on 3D-printed personalized prostheses, this study reports the clinical efficacy of this technology in treating bone defects in a series of patients who underwent pelvic tumor resection. Moreover, this study presents a comprehensive comparison of this technology with conventional procedures, thus strengthening its importance in treatment regimens for reconstructing bone defects [16]. In the 1980s, limb salvage surgery for bone tumours began to emerge. This surgery included tumour resection and limb reconstruction [12]. However, when the tumour is located in the pelvis, the complexity of the anatomical structure makes it difficult for conventional surgical techniques to achieve desired outcome. The following difficulties are usually encountered during diagnosis and treatment processes [[13], [14], [15]]. First, it is difficult to identify and use tumour margins for tumour resection planning using only two-dimensional images. Second, it is difficult to accurately remove the tumour; as the anatomical structure of the pelvis is complex, the technical requirements for the removal of enough tumour mass without sacrificing the normal bone structures are demanding, and it is difficult to ensure tumour resection accuracy. Moreover, during resection, inaccurate osteotomy will affect repair and reconstruction. Third, pelvis repair and reconstruction is difficult. Universal prostheses are not well-matched with bone defects after pelvic tumour resection; therefore, it is difficult to meet the requirements for ideal repair and reconstruction. Fourth, traditional custom-made prostheses are manufactured mostly through processes such as machining or casting, which have disadvantages such as large material loss, high processing difficulty, long preparation cycle, and high cost.
The emergence of 3D printing technology provides innovative ideas to solve the abovementioned problems and design individualised and precise treatment strategies for bone tumours [8,9,16]. Moreover, 3D printing technology creates a simulation model of complex bone tumours, which can improve the cognition of tumours and surrounding anatomical structures of doctors and help formulate surgical plans and simulate surgical operations preoperatively [16]. Surgical guides designed and manufactured using 3D printing technology can be used to aid bone tumour resection or assist nail placement perioperatively. This may reduce surgical trauma, shorten surgery duration, and improve surgical accuracy [16]. Additionally, individualised prostheses and internal implants are designed and customised through 3D printing technology for repairing and reconstructing bone defects after bone tumour resection to ensure that the prosthesis and implant perfectly match the bone defect site, thereby improving postoperative effects and the service life of the prosthesis and implant [16,17].
However, studies on the clinical applications of 3D printing technology in pelvic tumour resection, repair, and reconstruction have mostly been case reports, and there is a lack of clinical case studies comparing 3D printing technology with conventional methods. Chen et al. [18] produced a 3D-printed model for the preoperative surgical planning of a patient with a bone tumour in pelvic zone I, designed and 3D-printed an iliac bone prosthesis, and used a computer navigation system to assist in tumour resection and prosthesis installation perioperatively. The postoperative functional recovery of the corresponding patient was satisfactory. Wong et al. [19] used a 3D printing model to plan a surgical regimen for a patient with a chondrosarcoma in pelvic zone II and designed and made osteotomy and nail placement guides for intraoperative tumour resection and prosthesis installation to repair and reconstruct bone defects after tumour resection. Histological postoperative tumour specimen examination showed negative resection margins, and the error of the tumour resection scope and prosthesis implantation position did not exceed 4 mm. Follow-up was performed at 11 months postoperatively and revealed that the patient's hip joint movement function was good and that there was no tumour recurrence or prosthesis loosening. Blakeney et al. [20] used 3D printing technology to perform surgery on a patient with chondrosarcoma in pelvic zone III, made a 3D printing model preoperatively, and designed and produced a 3D printed osteotomy guide based on the aforementioned model to aid intraoperative tumour resection. Total hip replacement was performed to restore limb function. Postoperative histological tumor specimen examination showed negative resection margins. The patient recovered well, was able to walk independently without pain, and showed no signs of tumour recurrence 6 months postoperatively. Wei et al. [21] used a 3D-printed prosthesis to repair and reconstruct a bone defect in a patient with chordoma in pelvic zone IV after en bloc sacrum resection. One year postoperatively, the patient was able to walk with crutches without pain, and there were no tumour recurrences or symptoms, such as pelvic instability.
This study retrospectively analysed 10 cases each in which conventional and 3D printing techniques were used for pelvic tumour resection, repair, and reconstruction in the past 5 years and compared the effects of both methods in the surgical treatment of pelvic tumours. In this study, the average incision length, duration of surgery, and amount of intraoperative blood loss were significantly less in the 3D printing group than in the conventional group (P < 0.05), indicating the potential of 3D printing technology for reducing surgery duration and surgical trauma. Postoperative histological tumour specimen examination showed negative resection margins in nine and three cases in the 3D printing and conventional groups, respectively. The rate of negative resection margins was significantly higher in the 3D printing group than in the conventional group (P < 0.05), indicating that using digital medicine and 3D printing technology to formulate surgical plans preoperatively and using 3D printed surgical guides to aid the resection of bone tumours perioperatively allow more accurate and complete tumour resections. One patient had an osteochondrosarcoma in zone I in the 3D printing group. Postoperative histological tumour specimen examination showed positive resection margins, and positive areas were located at the sacroiliac joint. Considering that the tumour grew close to the sacrum in this patient, tumour cells might have invaded the sacroiliac joint to grow towards the sacrum; however, our surgical guide designing and planning only covered the sacroiliac joint and did not involve the sacrum. Regular follow-up was performed postoperatively. The MSTS score at the last follow-up was better in the 3D printing than in the conventional group (P < 0.05), indicating that the 3D printing surgical guide and personalised prosthesis used for pelvic tumour resection, repair, and reconstruction may improve postoperative functional recovery.
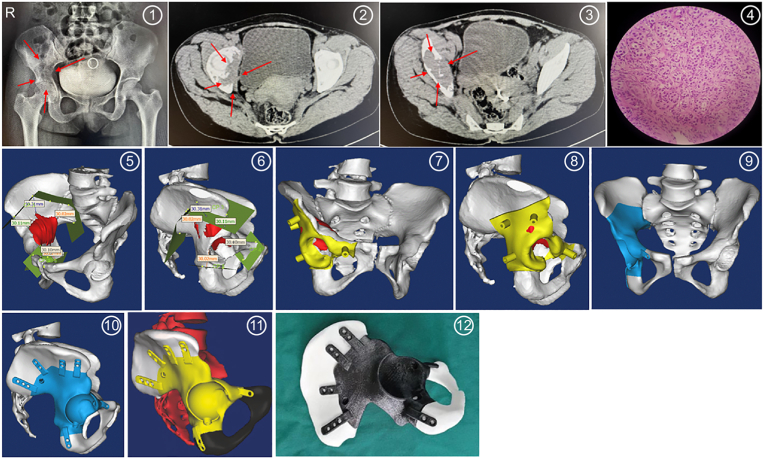
When applying 3D-printed personalised prostheses for the repair and reconstruction of bone defects after pelvic tumour resection, the following observations were made. First, in cases of bone tumour resection, repair, and reconstruction in pelvic zone I, it should be considered that the bone tumour is located in the ilium, adjacent to the sacrum. The bone defect after resection will affect pelvic ring continuity and normal load conduction. For such bone defects, an effective, matched, and firm repair and reconstruction enable patients to move early postoperatively, avoid spine and lower limb deformities, and restore good lower limb functions. The 3D-printed iliac bone prosthesis is designed according to the shape and size of the bone defect and is highly matched with it. The aforementioned iliac bone reserves one to three sacral nail channels and multiple iliac bone nail channels, allowing the firm fixation of the prosthesis and the reconstruction of the continuity of the pelvic ring and normal load conduction. Furthermore, the 3D-printed iliac bone reserves holes on the edges of the prosthesis, allowing muscle reattachment. The interface between the prosthesis and the bone must be designed as a porous 3D structure that allows bone ingrowth (Fig. 2 CG). Second, during bone tumour resection, repair, and reconstruction in pelvic zone II, bone tumours were in the acetabulum and surrounding areas. Tumour resection in this area often causes acetabular bone defects and requires the reconstruction of a stable and functional hip joint. Conventional treatment for such bone defects uses a titanium mesh cup for acetabular roof reconstruction and bone graft or bone cement filling, and artificial hip replacement to restore hip joint function; moreover, prostheses are not well-matched with bone defects using conventional treatment. The shape and size of 3D-printed prostheses in this area are highly matched with bone defects. The acetabular cup contains fixed anteversion and abduction angles. The proximal part of the acetabular cup is close to the iliac osteotomy surface, and 1–2 sacral nail channels and/or multiple iliac nail channels are reserved, while the distal part of the acetabular cup is a hook-like structure fixed to the obturator at the end proximal to the acetabulum; multiple nail channels of pubic/ischial branches may be reserved in the distal part. These designs are conducive to stabilizing and fixing the prosthesis (Fig. 3 ①-⑫). The interface between the prosthesis and the bone needs to be designed as a 3D porous structure to facilitate bone ingrowth. After the 3D-printed prosthesis is stabilised and fixed, the femoral stem is implanted, the artificial hip joint is installed, and the soft tissue attachment is reconstructed according to the total hip replacement procedure.
Fig. 3.
Design of a personalised prosthesis for bone defect repair and reconstruction following right hip bone tumour resection. 1, 2, 3) Preoperative pelvic frontal radiography and computed tomography findings suggested right hip bone tumour; 2) postoperative pathological results suggested giant cell bone tumour; 4) postoperative pathological results suggested giant cell bone tumour; 5–9) A personalised prosthesis and surgical guide were designed preoperatively for bone defect repair and reconstruction following right iliac bone tumour resection. 10, 11) A personalised prosthesis and surgical guide were designed preoperatively for bone defect repair and reconstruction following right iliac bone tumour resection; 12) Bone defect following right hip bone tumour resection and three-dimensional printing of personalised prosthesis model.
This study has certain limitations. First, as this study was retrospective with few clinical cases, selection bias may exist. Second, the cases included in this study only involved pelvic tumours in pelvic zones I and II; however, the bone tumours in zones III, IV, and mixed zones were not included; furthermore, the follow-up time was short. Third, the time of the clinical use of 3D-printed metal prostheses was short, and most studies on the clinical use of 3D-printed prostheses are exploratory thus far. The cost, manpower, and time expenditure are higher than those of standard prostheses and are not suitable for emergency surgery. Further clinical studies including more bone tumour cases and involving various pelvis regions are warranted.
5. Conclusion
Applying 3D-printed surgical guides and personalised prostheses in patients undergoing pelvic tumour resection, repair, and reconstruction and preoperative planning and design allows more accurate tumour resection and better prosthesis-patient matching, potentially reducing surgical trauma, shortening surgery duration, and promoting postoperative functional recovery.
Unblinded ethical approval
The study was approved by Ethics Committee of Guigang People's Hospital (Approval no. GYLL–201401).
Funding
This work was supported by the National Key Research and Development Program [grant number 2017YFC1103400]; the Guangdong Province Science and Technology Program Project [grant number 2016B090917001]; Sanming Project of Medicine in Shenzhen [SZSM201612019]; the Guangxi Science and Technology Program Project [grant numbers AD17195042 and AD17129017]; and the Guigang City Science and Technology Development Program Project [grant number 183402]. The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Declarations of interest
None.
Acknowledgements
The authors would like to thank Dr. Tsung-Yuan Tsai for his kind pre-review, editorial and statistical support for this manuscript.
Contributor Information
Haitao Tan, Email: tanhaitao99@hotmail.com.
Wenhua Huang, Email: huangwenhua2009@139.com.
References
- 1.Steel H.H. Partial or complete resection of the hemipelvis. An alternative to hindquarter amputation for periacetabular chondrosarcoma of the pelvis. J Bone Joint Surg Am. 1978;60:719–730. [PubMed] [Google Scholar]
- 2.Tan M.C., Yoon S.S. Surgical management of retroperitoneal and pelvic sarcomas. J Surg Oncol. 2015;111:553–561. doi: 10.1002/jso.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vissers G., Van Houtven L., Corthouts J., Snoeckx A., Luijks M., Thiessen F. Ewing's sarcoma of the sternum necessitating complex resection and reconstruction. Case Rep Plast Surg Hand Surg. 2019;6:125–130. doi: 10.1080/23320885.2019.1598867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connor M.I., Sim F.H. Salvage of the limb in the treatment of malignant pelvic tumors. J Bone Joint Surg Am. 1989;71:481–494. [PubMed] [Google Scholar]
- 5.Gebert C., Wessling M., Hoffmann C., Roedl R., Winkelmann W., Gosheger G. Hip transposition as a limb salvage procedure following the resection of periacetabular tumors. J Surg Oncol. 2011;103:269–275. doi: 10.1002/jso.21820. [DOI] [PubMed] [Google Scholar]
- 6.Chan L.W., Imanishi J., Ngan S.Y., Chander S., Chu J., Thorson R. Extracorporeal irradiation and reimplantation with total hip arthroplasty for periacetabular pelvic resections: a review of 9 cases. Sarcoma. 2016;2016:2549616. doi: 10.1155/2016/2549616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson E.R., Groundland J.S., Pala E., Dennis J.A., Wooten R., Cheong D. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 8.Liu X., Liu Y., Lu W., Liao S., Du Q., Deng Z. Combined application of modified three-dimensional printed anatomic templates and customized cutting blocks in pelvic reconstruction after pelvic tumor resection. J Arthroplasty. 2019;34:338–345. doi: 10.1016/j.arth.2018.10.001. e331. [DOI] [PubMed] [Google Scholar]
- 9.Han Q., Zhang K., Zhang Y., Wang C., Yang K., Zou Y. Individual resection and reconstruction of pelvic tumor with three-dimensional printed customized hemi-pelvic prosthesis: a case report. Medicine (Baltim) 2019;98 doi: 10.1097/MD.0000000000016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enneking W.F., Dunham W.K. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60:731–746. [PubMed] [Google Scholar]
- 11.Enneking W.F., Dunham W., Gebhardt M.C., Malawar M., Pritchard D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993:241–246. [PubMed] [Google Scholar]
- 12.Verma N.N., Kuo K.N., Gitelis S. Acetabular osteoarticular allograft after Ewing's sarcoma resection. Clin Orthop Relat Res. 2004:149–154. doi: 10.1097/00003086-200402000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Angelini A., Calabro T., Pala E., Trovarelli G., Maraldi M., Ruggieri P. Resection and reconstruction of pelvic bone tumors. Orthopedics. 2015;38:87–93. doi: 10.3928/01477447-20150204-51. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z.J., Jia J., Zhang Y.G., Tian W., Jin X., Hu Y.C. Internal fixation of complicated acetabular fractures directed by preoperative surgery with 3D printing models. Orthop Surg. 2017;9:257–260. doi: 10.1111/os.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung C.C., Li Y.T., Chou Y.C., Chen J.E., Wu C.C., Shen H.C. Conventional plate fixation method versus pre-operative virtual simulation and three-dimensional printing-assisted contoured plate fixation method in the treatment of anterior pelvic ring fracture. Int Orthop. 2019;43:425–431. doi: 10.1007/s00264-018-3963-2. [DOI] [PubMed] [Google Scholar]
- 16.Park J.W., Kang H.G., Kim J.H., Kim H.S. The application of 3D-printing technology in pelvic bone tumor surgery. J Orthop Sci. 2020;26:276–283. doi: 10.1016/j.jos.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Han Q., Zhao X., Wang C., Chen B., Wang X., Zhang Z. Individualized reconstruction for severe periprosthetic fractures around the tumor prosthesis of knee under assistance of 3D printing technology: a case report. Medicine (Baltim) 2018;97 doi: 10.1097/MD.0000000000012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X., Xu L., Wang Y., Hao Y., Wang L. Image-guided installation of 3D-printed patient-specific implant and its application in pelvic tumor resection and reconstruction surgery. Comput Methods Progr Biomed. 2016;125:66–78. doi: 10.1016/j.cmpb.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Wong K.C., Kumta S.M., Geel N.V., Demol J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput Aided Surg. 2015;20:14–23. doi: 10.3109/10929088.2015.1076039. [DOI] [PubMed] [Google Scholar]
- 20.Blakeney W.G., Day R., Cusick L., Smith R.L. Custom osteotomy guides for resection of a pelvic chondrosarcoma. Acta Orthop. 2014;85:438–441. doi: 10.3109/17453674.2014.920988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei R., Guo W., Ji T., Zhang Y., Liang One-step reconstruction with a 3D-printed, custom-made prosthesis after total en bloc sacrectomy: a technical note. Eur Spine J. 2017;26:1902–1909. doi: 10.1007/s00586-016-4871-z. [DOI] [PubMed] [Google Scholar]