Abstract
Introduction
Roxadustat is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that has demonstrated safety and efficacy versus placebo in phase III trials in patients with anemia of chronic kidney disease (CKD) who were not on dialysis (NDD).
Methods
This was a phase III, active-controlled, multicenter, partially randomized, open-label study in Japanese patients with NDD CKD. Patients who had used recombinant human erythropoietin or darbepoetin alfa (DA) before conversion were randomized to roxadustat or DA (comparative arms). Patients who had used epoetin beta pegol before conversion were allocated to roxadustat (reference arm). The primary endpoint was change in average hemoglobin (Hb) level from baseline during the evaluation period (Weeks 18–24). Longer term efficacy and safety were evaluated in roxadustat-treated patients over 52 weeks.
Results
In this study, 334 patients were randomized/allocated to receive treatment (n = 132, roxadustat [comparative]; n = 131, DA [comparative]; n = 71, roxadustat [reference]). The estimated difference between the roxadustat (comparative) and DA (comparative) groups in the least squares mean of change of average Hb levels of Weeks 18 to 24 from baseline was –0.07 g/dl, with the lower limit of 95% confidence interval of –0.23 g/dl, thereby confirming the noninferiority of roxadustat to DA. Common treatment-emergent adverse events (≥3% of patients in any treatment group) observed during the 24-week treatment period included nasopharyngitis, CKD, hyperkalemia, and hypertension.
Conclusion
Roxadustat maintained Hb within 10 to 12 g/dl in NDD CKD patients and was noninferior to DA. The safety profiles observed in this study are consistent with previous studies performed in this patient population.
Keywords: active comparator, anemia, chronic kidney disease, conversion, non–dialysis dependent, roxadustat
Graphical abstract
See Commentary on Page 1751
CKD is characterized by decreased synthesis of erythropoietin by the kidneys due to impaired oxygen sensing1,2 and an altered iron metabolism.3 As such, anemia often occurs concomitantly with CKD, especially in patients with advanced CKD.4 Although treatable, anemia of CKD is associated with poor outcomes.4,5 Currently, iron therapy (oral or i.v.) is the standard first-line treatment for CKD anemia, whereas traditional erythropoiesis-stimulating agents (ESAs) are available in the event that iron therapy cannot effectively correct CKD anemia.6 Despite their role in the current CKD anemia treatment landscape, studies have highlighted shortcomings related to the safety, efficacy, and mode of administration of iron therapy and ESAs in patients with non–dialysis-dependent (NDD) CKD.5,7, 8, 9, 10, 11 As such, patients may benefit from novel treatment options.
Hypoxia-inducible factor (HIF) is an oxygen-sensitive transcription factor that regulates erythropoiesis. In the presence of normal oxygen tension, HIF-α subunits are marked for degradation by the activity of HIF prolyl hydroxylase enzymes, whereas in the presence of reduced oxygen tension (hypoxia), the activity of these enzymes is suppressed, which allows HIF-α to dimerize with HIF-β and to accumulate, leading to increased transferrin receptor expression, iron absorption, and erythropoiesis.12 HIF prolyl hydroxylase inhibitors (HIF-PHIs) represent a new strategy to improve hemoglobin (Hb) levels via activation of the body’s natural response to hypoxia, independent of cellular oxygen levels.13, 14, 15
Roxadustat is an orally active HIF-PHI that has demonstrated safety and efficacy in non–comparator-controlled phase III trials in patients with CKD who are NDD.16,17 In correcting and maintaining Hb, roxadustat has been shown to be superior compared with placebo17 and noninferior compared with traditional ESAs.18,19 Roxadustat has also been shown to increase or stabilize transferrin levels and total iron-binding capacity (TIBC),16, 17, 18, 19 and to stabilize or decrease hepcidin.16, 17, 18, 19 Roxadustat is approved in China for the treatment of dialysis dependent (DD) and NDD CKD anemia and in Japan for the treatment of DD CKD anemia.
This phase II, partially randomized, active-comparator study was conducted at 71 sites in Japan. The study aimed to evaluate the efficacy and safety of roxadustat after conversion from recombinant human erythropoietin (rHuEPO) or darbepoetin alfa (DA) to roxadustat, as well as to evaluate the long-term efficacy and safety of roxadustat after conversion from epoetin beta pegol (EBP) to roxadustat, in Japanese NDD CKD patients with anemia of CKD; DA was used as an active comparator.
Methods
Study Design
This was a phase III, multicenter, partially randomized, DA-controlled, open-label study in patients with NDD CKD at the time of randomization (ClinicalTrials.gov identifier: NCT02988973). Patients who had used rHuEPO or DA before conversion were randomized to either the roxadustat treatment arm or the DA treatment arm (comparative arms; Figure 1). Patients who had used EBP before conversion were allocated to the roxadustat treatment arm (reference arm). Patients who received roxadustat started administration of roxadustat 3 times a week from the day of prescription. Patients randomized to the DA treatment arm started administration of DA once every 2 weeks from the day of prescription.
Figure 1.
Study design. Registration was conducted on the day when the most recent dose of erythropoiesis-stimulating agent was scheduled to be administered within 4 weeks, in principle, based on the assessments that confirmed that all the inclusion/exclusion criteria were satisfied. Conduct of registration was on the same day of the Week 0 visit.
For patients who received roxadustat, treatment was continued until Week 52. Treatment was ended at Week 24 for patients who were randomized to the DA treatment arm. While the primary endpoint of this study was the change in average Hb level from baseline during the assessment period (Weeks 18–24), a 52-week treatment period was used for the roxadustat treatment arm to provide findings regarding safety and efficacy following long-term administration of roxadustat in NDD CKD patients, as specified in the Guidelines for Clinical Evaluation of Therapeutic Drugs for Renal Anemia published by the Ministry of Health, Labour and Welfare of Japan.
The original study protocol (November 2016) was amended 5 times; these revisions consisted of administrative changes and a change in planned study duration. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines, and applicable laws and regulations. The protocol was approved by each institutional review board, and all subjects provided written informed consent.
Study Population
In this study, eligible patients were ≥20 years old, had been diagnosed with CKD (estimated glomerular filtration rate [eGFR] ≤89 ml/min/1.73 m2), and were not receiving dialysis. Patients had CKD anemia and had been receiving subcutaneous ESA, within the doses approved in Japan, for ≥8 weeks before prescreening assessments and were considered to have stable Hb levels. The mean of each patient’s 2 most recent Hb values before registration had to be between 10.0 and 12.0 g/dl; Hb values were assessed within ≥1 week of each other. Patients were excluded if they had undergone red blood cell transfusion or any surgical procedure considered to promote anemia within 4 weeks before their prescreening assessments or if they had received any prior treatment with roxadustat. A full list of eligibility criteria, as well as information regarding compliance requirements, can be found in the Supplementary Methods.
Study Drug Administration
Initial conversion doses and dose adjustments were regulated according to prespecified criteria in all randomized or registered patients, regardless of treatment arm; initial conversion doses were determined on the basis of the dose of ESA being taken immediately before registration (Supplementary Tables S1 and S2). The randomization for patients who had used rHuEPO or DA before conversion was conducted by a dynamic allocation method using a biased-coin minimization approach with the study site, mean of the 2 most recent Hb levels before registration (<11.0 vs. ≥11.0 g/dl), ESA dose before registration (rHuEPO: <4500 vs. ≥4500 IU/week; DA: <20 vs. ≥20 μg/week), previous or concurrent retinal vascular disorder (present vs. absent), and diabetes mellitus (present vs. absent) as allocation factors.
From Week 4, the doses of roxadustat and DA could be adjusted so that patients’ Hb levels were maintained within the target range (referred to as 10.0–12.0 g/dl unless stated otherwise) (Supplementary Tables S1 and S3). Generally speaking, roxadustat dosing could be adjusted every 4 weeks; a decision on whether to perform a dose adjustment was made at every scheduled visit (every 2 weeks) from Week 4 if both the Hb level in the relevant Week and the change in the Hb levels from 4 Weeks before the relevant Week (difference between the Hb levels in the relevant Week and 4 weeks earlier) met the criteria outlined in Supplementary Table S4.
Patients who were randomized or registered to the roxadustat treatment arm received roxadustat 3 times a week for up to 52 weeks. The first dose of roxadustat was taken on the day of prescription or the day after prescription. Patients took roxadustat at 2- or 3-day intervals (Monday–Wednesday–Friday, Tuesday–Thursday–Saturday, etc.) throughout the administration period. When coadministered with phosphate binders (bixalomer, precipitated calcium carbonate, lanthanum carbonate hydrate, ferric citrate hydrate, etc.), patients had to take roxadustat at least 1 hour before or 1 hour after taking the phosphate binder. Darbepoetin alfa was administered subcutaneously once every 2 weeks for up to 24 weeks, where the final dose was administered at the week 22 visit. Rescue therapy, such as red blood cell transfusions, were prohibited through the end of week 52 (week 24 in case of a DA treatment arm) or the time of discontinuation.
Study Outcomes and Assessments
Primary Endpoint
In this study, the primary endpoint was the change of average Hb level of Weeks 18 to 24 from baseline. Baseline Hb level was defined as the mean of 3 Hb values: the patient’s 2 most recent Hb levels before registration, and their Hb level at Week 0. Patients’ Hb levels were measured at each study site.
Secondary Endpoints to End of Week 24
Secondary endpoints included the average Hb levels of Weeks 18 to 24, maintenance rate of the target Hb level (proportion of patients who achieved the average Hb level of 10.0 to 12.0 g/dl during Weeks 18–24), the proportion of patients who achieved the target Hb level at each week, change of Hb levels from Week 0 to each week, and the proportion of measurement points that met the target Hb level from Weeks 18 to 24. The rate of rise in Hb levels (g/dl/week) from Week 0 to the earliest date of Week 4, time of discontinuation, and time of dose adjustment were also assessed. Secondary endpoints also included lab values such as serum iron (Fe), ferritin, transferrin, TIBC, soluble transferrin receptor (sTfR), and transferrin saturation (TSAT); these laboratory measure values were measured centrally at SRL, Inc. (Japan). Hepcidin levels were an exploratory endpoint.
Secondary Endpoints to End of Week 52
Similar to the endpoints assessed at the end of Week 24, secondary endpoints assessed at the end of Week 52 included the average Hb levels of Weeks 44 to 52 (calculated for patients who had ≥1 Hb value during Weeks 44–52) and the maintenance rate of the target Hb level (proportion of patients who achieved the average Hb level of 10.0 to 12.0 g/dl for Weeks 44 to 52). Secondary endpoints also included laboratory measures of values such as serum Fe, ferritin, transferrin, TIBC, sTfR, and TSAT. Hepcidin levels were an exploratory endpoint.
Safety Assessment
The safety and tolerability of roxadustat and DA were also assessed by monitoring the occurrence of treatment-emergent adverse events (TEAEs). Severity of TEAEs was graded according to the Classification of Seriousness of Adverse Drug Reaction of Medicinal Products (Pharmaceutical Affairs Bureau Safety Division’s Notification No. 80 [1992]). Additional safety assessments included findings from laboratory tests, vital signs, physical examinations, and 12-lead electrocardiograms. Because the upregulation of the vascular endothelial growth factor (VEGF) via the HIF-1α pathway during hypoxia can increase retinal angiogenesis, which may be associated with an increased risk of certain retinal pathologies,20,21 it is important to ascertain whether roxadustat, by acting on the HIF pathway, may be associated with an increased risk of adverse ophthalmological events. As such, ophthalmological examinations (funduscopic photograph, optical coherence tomography [OCT], visual acuity) were conducted for patients who used rHuEPO or DA before conversion. Funduscopic photography and OCT were performed in the comparative groups (roxadustat and DA) at the end of Week 24 and were centrally assessed by 2 blinded independent graders.
Statistical Methods
Study Populations and Sample Size Analysis
In this study, the full analysis set (FAS) was defined as the population of patients who received ≥1 dose of the study drug and who had data of ≥1 efficacy variable measured after the start of the study treatment. The per protocol set (PPS) was defined as a subset of patients in the FAS who had received treatment for ≥18 weeks, had received ≥70% of treatment as designated, and had measurements of Hb at baseline and ≥2 points from Weeks 18 to 24. The safety analysis set (SAF) consisted of patients who received ≥1 dose of study drug.
In confirming the noninferiority of roxadustat to DA for the change of average Hb from baseline during the evaluation period (Weeks 18–24) as a primary endpoint, the power for the lower limit of the 95% confidence interval (CI) in the difference between roxadustat and DA exceeding the noninferiority margin of −0.75 g/dl was calculated with assumptions of a difference of –0.25 g/dl between groups and a standard deviation of 1.1 g/dl. A sample size of 103 for each group would provide 90% power to demonstrate the noninferiority of roxadustat to DA.
Assuming an approximate 20% dropout rate from the PPS, a total of 130 patients for each treatment arm was planned to be randomized for conversion to roxadustat or DA (referred to as “comparative” from this point forward). As a reference arm that was not included in the primary analysis set, 65 patients were planned to be registered for conversion from EBP to roxadustat (referred to as “reference” from this point forward). As per the Ministry of Health, Labour and Welfare Guidelines for Clinical Evaluation of Therapeutic Drugs for Renal Anemia, which required ≥300 patients to receive a drug for ≥6 months and ≥100 patients to receive the drug for ≥1 year, safety and efficacy results following long-term administration of roxadustat in NDD CKD patients can be obtained from this study.
Additional information regarding the assumption for sample size can be found in the Supplementary Methods
Statistical Analysis
Analysis of the primary endpoint was performed on the PPS as the primary analysis set. Efficacy of roxadustat was confirmed if the 95% CI of the average Hb level of Weeks 18 to 24 was within the target range of 10.0 to 12.0 g/dl in the roxadustat (comparative) treatment arm. Noninferiority of roxadustat to DA was also evaluated. The change of average Hb levels of Weeks 18 to 24 from baseline was analyzed by a mixed model of repeated measurements with an unstructured covariance matrix within patients to calculate the 95% CI in the difference between roxadustat (comparative) and DA (comparative). The model considered randomization arms, visit, baseline Hb, ESA dose before registration, previous or concurrent retinal vascular disorder, diabetes mellitus, and visit by randomization arm interaction as explanatory variables. Noninferiority of roxadustat to DA was confirmed if the lower limit of 95% CI for the difference was above the noninferiority margin of –0.75 g/dl. Analysis of covariance with multiple imputation and pattern-mixture model analyses, assuming multiple missing data mechanisms, were conducted as sensitivity analysis of the primary endpoint.
Analyses of secondary and exploratory endpoints were performed in the FAS; details regarding these analyses can be found in the Supplementary Methods. Subgroup analyses stratified by high-sensitivity C-reactive protein (hs-CRP; <28.57 or ≥28.57 nmol/l, where 28.57 nmol/l represents the upper limit of normal) and eGFR (<15 or ≥15 ml/min/1.73m2) were performed to examine possible trends in Hb maintenance and study drug dosing across treatment groups. Demographic and other baseline characteristics were summarized using descriptive statistics. Safety analyses were performed in the SAF. All data processing, summarization, and analyses were performed using SAS Version 9.4.
Results
Of the 431 patients who provided informed consent, 334 were registered/randomized to receive treatment (132 in the roxadustat [comparative] group, 131 in the DA [comparative] group, and 71 in the roxadustat [reference] group; Figure 2). A total of 97 patients discontinued before randomization/registration due to screen failures. Two patients (in the roxadustat [comparative and reference] groups) did not take the study drug and a total of 332 patients received the study drug and were analyzed.
Figure 2.
Patient disposition at the end of (a) Week 24 and (b) Week 52. aPatients who signed informed consent but discontinued before randomization/registration were screen failures. bPatients who did not receive the study drug were included. cPatients in the roxadustat (comparative) group and the roxadustat (reference) group continued to receive the study drug for up to 52 weeks.
At the end of Week 24, a total of 296 (88.6%) patients (109 [82.6%] in the roxadustat [comparative] group, 121 [92.4%] in the DA [comparative] group, and 66 [93.0%] in the roxadustat [reference] group) completed the 24-week treatment period (Figure 2a). At the end of Week 52, a total of 146 (71.9%) patients in the pooled roxadustat group (95 [72.0%] in the roxadustat [comparative] group and 51 [71.8%] in the roxadustat [reference] group) completed the 52-week treatment period (Figure 2b). Additional details regarding patients’ disposition can be found in the Supplementary Results.
In the FAS, patient demographics and baseline characteristics were similar between the 3 treatment groups (Table 1). The baseline values of efficacy variables were also similar between the 3 treatment groups.
Table 1.
Patient demographics and baseline characteristics (full analysis set)
| Parameter | Darbepoetin alfa (comparative) (n = 131) |
Roxadustat (comparative) (n = 131) |
Roxadustat (reference) (n = 70) |
Pooled roxadustat (n = 201) |
|---|---|---|---|---|
| Sex | ||||
| Male | 75 (57.3%) | 83 (63.4%) | 35 (50.0%) | 118 (58.7%) |
| Female | 56 (42.7%) | 48 (36.6%) | 35 (50.0%) | 83 (41.3%) |
| Age (years) (informed consent) | ||||
| Mean | 70.9 | 68.9 | 70.0 | 69.3 |
| SD | 10.2 | 11.6 | 10.5 | 11.2 |
| Age group (years) (informed consent) | ||||
| <65 | 35 (26.7%) | 36 (27.5%) | 17 (24.3%) | 53 (26.4%) |
| ≥65 | 96 (73.3%) | 95 (72.5%) | 53 (75.7%) | 148 (73.6%) |
| BMI (kg/m2) (prescreening) | ||||
| Mean | 23.95 | 23.58 | 23.73 | 23.63 |
| SD | 3.57 | 4.59 | 3.66 | 4.28 |
| eGFR (ml/min/1.73 m2) (prescreening) | ||||
| Mean | 18.2 | 17.9 | 17.2 | 17.7 |
| SD | 8.8 | 8.2 | 7.3 | 7.9 |
| Median | 15.0 | 16.0 | 16.5 | 16.0 |
| eGFR group (ml/min/1.73 m2) (prescreening) | ||||
| <15 | 56 (42.7%) | 46 (35.1%) | 31 (44.3%) | 77 (38.3%) |
| ≥15 | 75 (57.3%) | 85 (64.9%) | 39 (55.7%) | 124 (61.7%) |
| Duration of anemia associated with CKD (months) | ||||
| Mean | 33.95 | 28.39 | 31.15 | 29.33 |
| SD | 45.94 | 31.42 | 27.56 | 30.11 |
| Baseline Hb (g/dl) | ||||
| Mean | 10.96 | 10.98 | 11.14 | 11.03 |
| SD | 0.52 | 0.57 | 0.54 | 0.56 |
| Baseline Hb group (g/dl) | ||||
| <11.0 | 64 (48.9%) | 64 (48.9%) | 29 (41.4%) | 93 (46.3%) |
| ≥11.0 | 67 (51.1%) | 67 (51.1%) | 41 (58.6%) | 108 (53.7%) |
| Transferrin (g/l) | ||||
| Mean | 1.947 | 2.007 | 1.999 | 2.004 |
| SD | 0.346 | 0.384 | 0.323 | 0.363 |
| Iron (μmol/l) | ||||
| Mean | 15.0 | 16.0 | 16.1 | 16.0 |
| SD | 5.0 | 5.3 | 6.0 | 5.5 |
| Iron repletion, n (%) | ||||
| Ferritin ≥100 ng/ml and TSAT ≥20% | 66 (50.4%) | 69 (52.7%) | 37 (52.9%) | 106 (52.7%) |
| Ferritin <100 ng/mL and TSAT ≥20% | 52 (39.7%) | 51 (38.9%) | 26 (37.1%) | 77 (38.3%) |
| Ferritin ≥100 ng/ml and TSAT <20% | 9 (6.9%) | 6 (4.6%) | 2 (2.9%) | 8 (4.0%) |
| Ferritin <100 ng/ml and TSAT <20% | 4 (3.1%) | 5 (3.8%) | 5 (7.1%) | 10 (5.0%) |
| Iron groups by repletion status, n (%) | ||||
| Ferritin ≥100 ng/ml and TSAT ≥20% | 66 (50.4%) | 69 (52.7%) | 37 (52.9%) | 106 (52.7%) |
| Ferritin <100 ng/ml or TSAT <20% | 65 (49.6%) | 62 (47.3%) | 33 (47.1%) | 95 (47.3%) |
| Total iron binding capacity (μmol/l) | ||||
| Mean | 46.5 | 47.7 | 47.4 | 47.6 |
| SD | 7.4 | 7.8 | 6.8 | 7.5 |
| Soluble transferrin receptor (nmol/l) | ||||
| Mean | 23.33 | 22.80 | 24.75 | 23.48 |
| SD | 8.37 | 9.28 | 11.38 | 10.08 |
| Transferrin saturation (%) | ||||
| Mean | 32.72 | 34.00 | 34.26 | 34.09 |
| SD | 11.67 | 11.67 | 12.49 | 11.93 |
| hs-CRP (nmol/l) | ||||
| Mean | 25.869 | 16.402 | 16.311 | 16.370 |
| SD | 63.396 | 26.940 | 39.877 | 31.943 |
| hs-CRP (nmol/l), n (%) | ||||
| <28.57 | 106 (80.9%) | 108 (82.4%) | 60 (85.7%) | 168 (83.6%) |
| ≥28.57 | 25 (19.1%) | 23 (17.6%) | 10 (14.3%) | 33 (16.4%) |
| Previous or concurrent retinal vascular disorder,an (%) | ||||
| Absent | 68 (51.9%) | 66 (50.4%) | — | — |
| Present | 63 (48.1%) | 64 (48.9%) | — | — |
| Missing | — | 1 (0.7%) | — | — |
| Diabetes mellitus, n (%) | ||||
| Absent | 63 (48.1%) | 63 (48.1%) | 33 (47.1%) | 96 (47.8%) |
| Present | 68 (51.9%) | 68 (51.9%) | 37 (52.9%) | 105 (52.2%) |
BMI, body mass index; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; high-sensitivity C-reactive protein group; TSAT, transferrin saturation.
Not recorded in reference arm.
In the SAF, all patients received previous ESA medication (Table 2). The previous ESA medications in the roxadustat (comparative) and DA (comparative) groups were DA (96.9% and 96.2%, respectively), rHuEPO (2.3% and 3.8%, respectively), and EBP (0.8% and 0%, respectively). One patient in the roxadustat (comparative) group who received prior EBP was excluded from the PPS. In the roxadustat (reference) group, all patients previously received EBP.
Table 2.
Detail of previous ESA medication (safety analysis set)
| Parameter | Darbepoetin alfa (comparative) (n = 131) |
Roxadustat (comparative) (n = 131) |
Roxadustat (reference) (n = 70) |
Pooled roxadustat (n = 201) |
|---|---|---|---|---|
| Previous ESA medication | ||||
| rHuEPO | 5 (3.8%) | 3 (2.3%) | 0 | 3 (1.5%) |
| Darbepoetin alfa | 126 (96.2%) | 127 (96.9%) | 0 | 127 (63.2%) |
| Epoetin beta pegol | 0 | 1 (0.8%)c | 70 (100.0%) | 71 (35.3%) |
| Dose level of previous ESA medication category | ||||
| Lowa | 95 (72.5%) | 95 (72.5%) | 57 (81.4%) | 152 (75.6%) |
| Highb | 36 (27.5%) | 36 (27.5%) | 13 (18.6%) | 49 (24.4%) |
ESA, erythropoiesis-stimulating agent; rHuEPO, recombinant human erythropoietin.
Dose level of rHuEPO category (IU/week) <4500 or dose level of darbepoetin alfa category (μg/week) <20 or dose level of epoetin beta pegol category (μg/4 weeks) ≤100.
Dose level of rHuEPO category (IU/week) ≥4500 or dose level of darbepoetin alfa category (μg/week) ≥20 or dose level of epoetin beta pegol category (μg/4 weeks) >100.
This patient used epoetin beta pegol as previous ESA medication; the patient was mistakenly registered as a patient whose treatment was converted from rHuEPO and was therefore randomized to the roxadustat (comparative) group.
Details regarding study drug exposure and treatment compliance are presented in the Supplementary Results. The mean (SD) allocated dose level per intake at Week 22 was 46.9 (20.2) mg in the roxadustat (comparative) group, 32.0 (27.0) μg in the DA (comparative) group, and 42.9 (21.6) mg in the roxadustat (reference) group (Figure 3a and b). In the SAF, the mean (SD) allocated dose level per intake at Week 48, which was calculated using a single dose per intake, was 44.2 (25.7) mg in the pooled roxadustat group (45.0 [27.4] mg in the roxadustat [comparative] group and 42.8 [22.9] mg in the roxadustat [reference] group) (Figure 3a).
Figure 3.
Mean and SD plot of (a) roxadustat and (b) darbepoetin alfa per intake over time (safety analysis set)
Primary Efficacy Endpoint (PPS)
The mean (SE) of average Hb levels of Weeks 18 to 24 in the roxadustat (comparative) group was 11.14 (0.07) g/dl with a 95% CI of 11.01 to 11.27 g/dl, which was within the target range of 10.0 to 12.0 g/dl. Thus, the efficacy of roxadustat was confirmed. The least squares (LS) mean (SE) of change of average Hb levels of Weeks 18 to 24 from baseline was 0.15 g/dl (0.06 g/dl) in the roxadustat (comparative) group and 0.22 g/dl (0.06 g/dl) in the DA (comparative) group. The estimated difference between the roxadustat (comparative) and DA (comparative) groups in the LS mean of change of average Hb levels of Weeks 18 to 24 from baseline was –0.07 g/dl with the lower limit of 95% CI of –0.23 g/dl, which was above the predefined noninferiority margin of –0.75 g/dl. Thus, the noninferiority of roxadustat to DA was confirmed (Table 3).
Table 3.
Change of average Hb levels of Weeks 18 to 24 from baseline (per protocol set)
| Parameter | Treatment group | LS mean (SE) (95% CI)a | Estimated treatment difference (SE) (95% CI)a | Noninferiority margin |
|---|---|---|---|---|
| Change of average Hb levels of Weeks 18–24 from baseline (g/dl) | Darbepoetin alfa | 0.22 (0.06) (0.10–0.33) |
— | — |
| (comparative) | ||||
| Roxadustat (comparative) | 0.15 (0.06) (0.03–0.27) |
–0.07 (0.08) (–0.23–0.10) |
–0.75 |
CI, confidence interval; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; LS, least square; MMRM, mixed model for repeated measures; SE, standard error.
Hb values in analysis visit windows at Weeks 18, 20, 22, and 24 were used for calculating the average of Weeks 18 to 24. Baseline Hb was defined as the mean of 3 Hb values: 2 latest Hb values before registration and 1 Hb value at Week 0. When Hb value at Week 0 was the same value and on the same date as the latest Hb value before registration, baseline Hb was defined as the mean of 2 latest Hb values before registration.
MMRM with an unstructured covariance matrix within patients was used. The model considered randomization arms (roxadustat [comparative] or darbepoetin alfa [comparative]), visit, baseline Hb, ESA dose just before registration, previous or concurrent retinal vascular disorder, diabetes mellitus, and visit by randomization arm interaction as explanatory variables.
The results of secondary (FAS) and sensitivity analyses (PPS) showed that the lower limits of the 95% CI of the difference between the roxadustat (comparative) and DA (comparative) groups in the LS mean of change of average Hb levels of Weeks 18 to 24 from baseline were above –0.75 g/dl, which confirmed the robustness of the results of the primary analysis (Table 4).
Table 4.
Change of average Hb levels of Weeks 18 to 24 from baseline—secondary (FAS) and sensitivity (PPS) analysis
| LS mean (SE) (95% CI)a | Estimated treatment difference (SE) (95% CI)a | Model | |
|---|---|---|---|
| Secondary analysis (FAS) treatment group | |||
| Darbepoetin alfa (comparative) | 0.23 (0.06) (0.11–0.34) |
— | MMRM |
| Roxadustat (comparative) | 0.11 (0.06) (–0.01–0.24) |
–0.11 (0.09) (–0.29–0.06) |
|
| Sensitivity analysis (PPS) treatment group | |||
| Darbepoetin alfa (comparative) | 0.22 (0.06) (0.11–0.33) |
— | ANCOVA with MI |
| Roxadustat (comparative) | 0.15 (0.06) (0.03–0.27) |
–0.07 (0.08) (–0.24 to 0.09) |
|
| Darbepoetin alfa (comparative) | 0.22 (0.06) (0.10–0.33) |
— | PMM (last mean carried forward) |
| Roxadustat (comparative) | 0.15 (0.06) (0.03–0.27) |
–0.07 (0.08) (–0.24 to 0.09) |
|
| Darbepoetin alfa (comparative) | 0.22 (0.06) (0.10–0.33) |
— | PMM (last mean carried forward for roxadustat and randomized arm MAR for darbepoetin alfa) |
| Roxadustat (comparative) | 0.15 (0.06) (0.03–0.27) |
–0.07 (0.08) (–0.24–0.09) |
ANCOVA, analysis of covariance; CI, confidence interval; FAS, full analysis set; Hb, hemoglobin; LS, least square; MAR, missing at random; MI, multiple imputation; MMRM, mixed model for repeated measures; PMM, pattern-mixture model; PPS, per protocol set; SE, standard error.
A mixed model for repeated measures with an unstructured covariance matrix within patients was used. The model considered randomization arms (roxadustat [comparative] or darbepoetin alfa [comparative]), visit, baseline hemoglobin, erythropoietin analogue dose before registration, previous or concurrent retinal vascular disorder, diabetes mellitus, and visit by randomization arm interaction as explanatory variables.
Secondary and Exploratory Endpoints Through Week 24 (Full Analysis Set)
The mean (SD) of average Hb levels of Weeks 18 to 24 was 11.11 g/dl (0.81 g/dl) in the roxadustat (comparative) group, 11.23 g/dl (0.65 g/dl) in the DA (comparative) group, and 11.08 g/dl (0.66 g/dl) in the roxadustat (reference) group. The difference (95% CI) between the roxadustat (comparative) and DA (comparative) groups in the average Hb levels of Weeks 18 to 24 was –0.13 g/dl (–0.31 to 0.06 g/dl).
The maintenance rate (95% CI) of the target Hb level (10.0–12.0 g/dl) during Weeks 18 to 24 was 77.1% (68.9%–84.0%; 101/131 patients) in the roxadustat (comparative) group, 85.5% (78.3%–91.0%; 112/131 patients) in the DA (comparative) group, and 84.3% (73.6%–91.9%; 59/70 patients) in the roxadustat (reference) group. The difference (95% CI) between the roxadustat (comparative) and DA (comparative) groups in the maintenance rate of the target Hb level was –8.4% (–18.5% to 1.8%). In the patients with ≥1 Hb value during Weeks 18 to 24, the maintenance rate (95% CI) of the target Hb level during Weeks 18 to 24 was 87.8% (80.4%–93.2%; 101/115 patients) in the roxadustat (comparative) group, 88.9% (82.1%–93.8%; 112/126 patients) in the DA (comparative) group, and 89.4% (79.4%–95.6%) (59/66 patients) in the roxadustat (reference) group. The difference (95% CI) between the roxadustat (comparative) and DA (comparative) groups in the maintenance rate of the target Hb level was –1.1% (–10.0% to 7.9%).
The proportion (95% CI) of patients who achieved the target Hb level (10.0–12.0 g/dl) at the end of Week 24 was 69.5% (60.8%–77.2%) in the roxadustat (comparative) group, 75.6% (67.3%–82.7%) in the DA (comparative) group, and 72.9% (60.9% to 82.8%) in the roxadustat (reference) group. The difference (95% CI) between the roxadustat (comparative) and DA (comparative) groups in the proportion of patients who achieved the target Hb level at the end of Week 24 was –6.1% (–17.7% to 5.4%) and was mainly driven by the higher proportion of roxadustat-treated patients with Hb <10.0 g/dl.
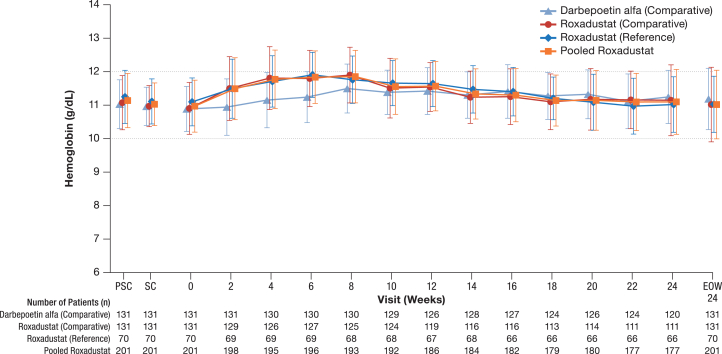
Mean Hb values were maintained within the target Hb level (10.0–12.0 g/dl) in all 3 treatment groups through Week 24 and/or the end of Week 24 (Figure 4). The mean Hb values in the roxadustat groups slightly increased through Week 8, although no remarkable changes were observed in the DA (comparative) group. The difference between the roxadustat (comparative) and DA (comparative) groups in the change of Hb levels from Week 0 ranged from 0.39 to 0.64 g/dl from Week 2 through Week 8 because of the temporal increase of Hb levels from Week 0 in the roxadustat (comparative) group; these differences were smaller (–0.18–0.11 g/dl) through Week 24 and/or the end of Week 24.
Figure 4.
Mean and SD plot of hemoglobin (g/dl) through Week 24 or end of Week 24 for all treatment arms (full analysis set). EOW24, end of Week 24; PSC, prescreening; SC, screening.
The mean (SD) proportion of measurement points that met the target Hb level from Weeks 18 to 24 was 77.75% (27.08%) in the roxadustat (comparative) group, 80.89% (27.57%) in the DA (comparative) group, and 81.82% (25.02%) in the roxadustat (reference) group. The difference (95% CI) between the roxadustat (comparative) and DA (comparative) groups in the proportion of measurement points that met the target Hb level was –3.13% (–10.08%–3.81%).
The mean (SD) rate of rise in Hb levels was 0.302 g/dl/week (0.252 g/dl/week) in the roxadustat (comparative) group, 0.206 g/dl/week (0.273 g/dl/week) in the roxadustat (reference) group, and 0.268 g/dl/week (0.263 g/dl/week) in the pooled roxadustat group. The proportion of patients whose rate of rise in Hb levels exceeded 0.5 g/dl/week was 14.8% (19/128 patients) in the roxadustat (comparative) group and 11.4% (8/70 patients) in the roxadustat (reference) group.
No remarkable changes in mean iron values were observed in either the roxadustat (comparative and reference) or DA (comparative) group at each visit through Week 24 and/or the end of Week 24 (Table 5, Supplementary Figure S1A).
Table 5.
Iron parameters and hepcidin at Week 0, the end of Week 24, and the end of treatment (full analysis set)
| Parameter | Week 0 | End of Week 24 | End of treatment |
|---|---|---|---|
| Darbepoetin alfa—comparative | n = 131 | n = 131 | n = NA |
| Iron, μmol/l | 15.0 (5.0), 14.0 | 13.8 (4.6), 14.0 | NA |
| Ferritin, ng/ml | 150.42 (150.40), 117.00 | 117.26 (137.77), 80.10 | NA |
| Transferrin, g/l | 1.947 (0.346), 1.920 | 1.995 (0.389), 1.940 | NA |
| TIBC, μmol/l | 46.5 (7.4), 46.0 | 47.1 (8.3), 46.0 | NA |
| sTfR, nmol/l | 23.33 (8.37), 22.10 | 28.76 (13.80), 26.10 | NA |
| TSAT, % | 32.72 (11.67), 31.20 | 29.82 (10.40), 29.50 | NA |
| Hepcidin, ng/ml | 39.745 (24.898), 35.700 | 29.792 (25.251), 23.500 | NA |
| Roxadustat—comparative | n = 131 | n = 131 | n = 131 |
| Iron, μmol/l | 16.0 (5.3), 15.0 | 15.2 (6.5), 15.0 | 13.9 (5.6), 13.0 |
| Ferritin, ng/ml | 138.03 (101.22), 113.00 | 114.66 (93.98), 90.70 | 108.21 (82.77), 84.80 |
| Transferrin, g/l | 2.007 (0.384), 1.950 | 2.304 (0.489), 2.280 | 2.274 (0.494), 2.260 |
| TIBC, μmol/l | 47.7 (7.8), 47.0 | 53.2 (10.1), 52.0 | 52.6 (10.4), 52.0 |
| sTfR, nmol/l | 22.80 (9.28), 20.20 | 25.96 (15.19), 23.00 | 25.41 (14.86), 23.00 |
| TSAT, % | 34.00 (11.67), 32.80 | 29.07 (13.44), 28.50 | 26.84 (11.31), 25.50 |
| Hepcidin, ng/ml | 38.100 (24.499), 33.300 | 25.773 (22.322), 20.800 | 28.223 (24.270), 20.700 |
| Roxadustat—reference | n = 70 | n = 70 | n = 70 |
| Iron, μmol/l | 16.1 (6.0), 16.0 | 15.4 (6.2), 16.0 | 13.7 (5.3), 13.0 |
| Ferritin, ng/ml | 151.22 (132.65), 111.00 | 137.32 (139.75), 97.80 | 117.18 (140.68), 69.70 |
| Transferrin, g/l | 1.999 (0.323), 2.000 | 2.316 (0.465), 2.250 | 2.349 (0.490), 2.350 |
| TIBC, μmol/L | 47.4 (6.8), 47.5 | 53.5 (9.6), 53.0 | 54.2 (10.4), 53.5 |
| sTfR, nmol/l | 24.75 (11.38), 20.55 | 24.41 (12.74), 20.80 | 26.03 (12.63), 21.10 |
| TSAT, % | 34.26 (12.49), 33.80 | 29.83 (12.96), 28.80 | 26.44 (11.08), 25.45 |
| Hepcidin, ng/ml | 41.648 (29.042), 36.950 | 23.889 (21.665), 18.900 | 25.075 (24.694), 16.700 |
| Roxadustat—pooled | n = 201 | n = 201 | n = 201 |
| Iron, μmol/l | 16.0 (5.5), 15.0 | 15.3 (6.4), 15.0 | 13.8 (5.5), 13.0 |
| Ferritin, ng/ml | 142.62 (113.00), 113.00 | 122.55 (112.23), 93.60 | 111.33 (106.30), 82.10 |
| Transferrin, g/l | 2.004 (0.363), 1.980 | 2.308 (0.479), 2.260 | 2.300 (0.492), 2.300 |
| TIBC, μmol/l | 47.6 (7.5), 47.0 | 53.3 (9.9), 52.0 | 53.1 (10.4), 53.0 |
| sTfR, nmol/l | 23.48 (10.08), 20.20 | 25.42 (14.37), 22.80 | 25.63 (14.09), 22.70 |
| TSAT, % | 34.09 (11.93), 33.10 | 29.33 (13.25), 28.50 | 26.70 (11.20), 25.50 |
| Hepcidin, ng/ml | 39.335 (26.153), 34.500 | 25.117 (22.059), 20.200 | 27.127 (24.403), 19.100 |
NA, not available; sTfR, soluble transferrin receptor; TIBC, total iron binding capacity; TSAT, transferrin saturation.
Data are presented as mean (SD), median.
The mean ferritin values decreased from Week 0 through Week 2 and then became stable through Week 24 and/or the end of Week 24 in both the roxadustat (comparative and reference) and DA (comparative) groups (Table 5, Supplementary Figure 2A).
The mean transferrin values in the roxadustat (comparative and reference) groups increased from Week 0 through Week 4, then decreased through Week 12, and then became stable through Week 24 and/or the end of Week 24 at higher levels than Week 0. In the DA (comparative) group, no remarkable changes in the mean transferrin values were observed at each visit through Week 24 and/or the end of Week 24 (Table 5, Supplementary Figure S3A).
Mean TIBC values in the roxadustat (comparative and reference) groups increased from Week 0 through Week 4, then decreased through Week 12, and then became stable through Week 24 and/or the end of Week 24 at higher levels than Week 0. In the DA (comparative) group, no remarkable changes in the mean TIBC values were observed at each visit through Week 24 and/or the end of Week 24 (Table 5, Supplementary Figure S4A).
The mean sTfR values in the roxadustat (comparative and reference) groups increased from Week 0 through Week 4, then decreased through Week 12, and then became stable through Week 24 and/or the end of Week 24. In the DA (comparative) group, the mean sTfR values slightly increased from Week 0 through Week 4 and then became stable through Week 24 and/or the end of Week 24 (Table 5, Supplementary Figure S5A).
Mean TSAT values in the roxadustat (comparative and reference) groups decreased from Week 0 through Week 2, then increased from Week 4 through Week 8, and then became stable through Week 24 and/or the end of Week 24. In the DA (comparative) group, no remarkable changes in the mean TSAT values were observed at each visit through Week 24 and/or the end of Week 24 (Table 5, Supplementary Figure S6A).
The mean hepcidin values in the roxadustat (comparative and reference) groups decreased from Week 0 at Week 4, then increased at Week 12, and then became stable at Week 24 and/or the end of Week 24 at lower levels than Week 0. In the DA (comparative) group, the mean hepcidin values slightly decreased from Week 0 at Week 4 and then became stable through Week 24 and/or the end of Week 24 at a lower level than Week 0 (Table 5, Supplementary Figure S7A).
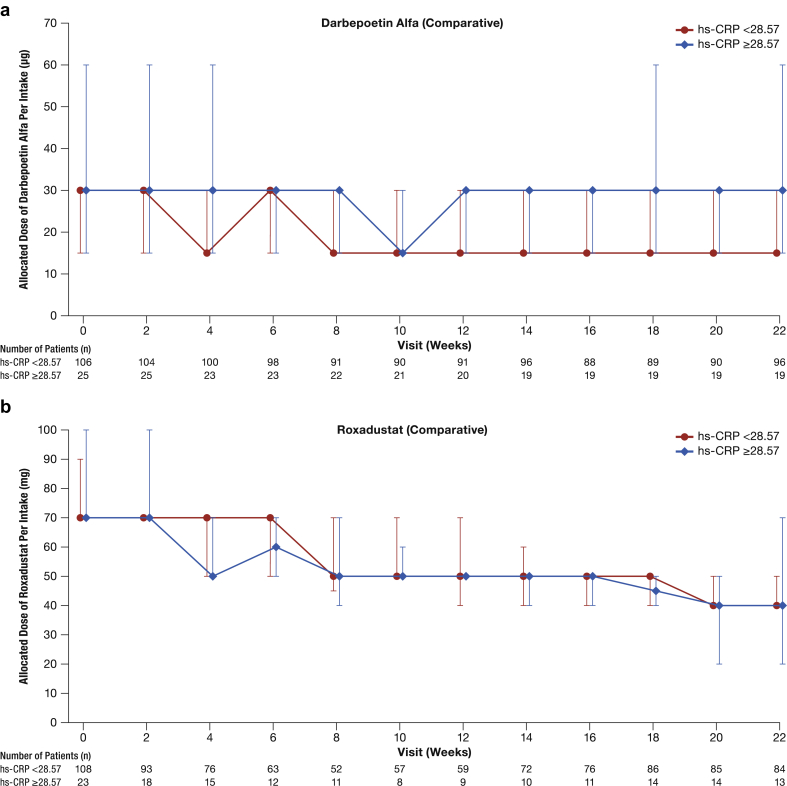
In a subgroup analysis stratified by hs-CRP levels, the mean (SD) change of average Hb levels of Weeks 18 to 24 from baseline in the roxadustat (comparative), DA (comparative), and roxadustat (reference) groups were 0.09 (0.76), 0.28 (0.73), and –0.03 (0.81) g/dl in the hs-CRP <28.57 nmol/L subgroup, and 0.19 (0.91), 0.08 (0.58), and –0.17 (0.72) g/dl in the hs-CRP ≥28.57 nmol/L subgroup, respectively. Median allocated dose levels over time for each treatment group can be seen in Figure 5. Roxadustat doses required to maintain target Hb levels did not appear to be influenced by hs-CRP levels, whereas DA doses appeared to be higher in DA-treated patients with high hs-CRP.
Figure 5.
Median (interquartile range) of allocated dose of study drug by high-sensitivity C-reactive protein (hs-CRP): (a) darbepoetin alfa (comparative), (b) roxadustat (comparative), (c) roxadustat (reference), (d) roxadustat (pooled).
In a subgroup analysis stratified by eGFR, the mean (SD) change of average Hb levels of Weeks 18 to 24 from baseline in the roxadustat (comparative), DA (comparative), and roxadustat (reference) groups were 0.00 (0.83), 0.14 (0.68), and –0.19 (0.75) g/dl in the eGFR <15 ml/min/1.73 m2 subgroup, and 0.15 (0.76), 0.32 (0.72), and 0.07 (0.82) g/dl in the eGFR ≥15 ml/min/1.73 m2 subgroup, respectively. Up to Week 22, there did not appear to be any relationship between allocated roxadustat dosing and eGFR (<15 vs. ≥15 ml/min/1.73 m2) (Supplementary Table S5).
Secondary and Exploratory Endpoints Through Week 52 (Full Analysis Set)
Mean (SD) of average Hb levels of Weeks 44 to 52 was 10.99 (0.66) g/dl in the pooled roxadustat group (11.05 [0.68] g/dl in the roxadustat [comparative] group and 10.87 [0.61] g/dl in the roxadustat [reference] group) (Figure 6).
Figure 6.
Mean and SD plot of hemoglobin (g/dl) through Week 52 or end of treatment for all treatment arms (full analysis set). EOT, end of treatment; FAS, full analysis set; PSC, prescreening; SC, screening.
The mean (SD) change of average Hb levels of Weeks 44 to 52 from baseline was –0.08 (0.71) g/dl in the pooled roxadustat group (0.03 [0.74] g/dl in the roxadustat [comparative] group and –0.26 [0.63] g/dl in the roxadustat [reference] group).
The maintenance rate (95% CI) of the target Hb level (10.0–12.0 g/dl) during Weeks 44 to 52 was 68.2% (61.2%–74.5%; 137/201 patients) in the pooled roxadustat group (64.9% [56.1%–73.0%]; 85/13 patients, in the roxadustat [comparative] group and 74.3% [62.4%–84.0%], 52/70 patients, in the roxadustat [reference] group). In the patients with ≥1 Hb value during Weeks 44 to 52, the maintenance rate (95% CI) of the target Hb level during Weeks 44 to 52 was 90.7 (84.9%–94.8%, 137/151 patients) in the pooled roxadustat group (89.5% [81.5%–94.8%, 85/95 patients] in the roxadustat [comparative] group and 92.9% [82.7%–98.0%, 52/56 patients] in the roxadustat [reference] group). After the end of Week 24, the mean Hb values were maintained within the target Hb level (10.0–12.0 g/dl) in the pooled roxadustat group through Week 52 and/or the end of treatment. Mean values for iron, ferritin, transferrin, TIBC, sTfR, and TSAT remained stable through Week 52 and/or the end of treatment in roxadustat-treated patients (Table 5, Supplementary Figures S1B–S6B). Mean hepcidin values in the pooled roxadustat group remained stable through Week 52 and/or the end of treatment at lower levels than Week 0 (Table 5, Supplementary Figure S7B).
Safety—Roxadustat and Darbepoetin Alfa Through Week 24 or End of Week 24
In the SAF, the incidence of TEAEs was comparable across treatment arms. The incidence of TEAEs was 78.6% (103/131 patients) in the roxadustat (comparative) group, 70.2% (92/131 patients) in the DA (comparative) group, and 77.1% (54/70 patients) in the roxadustat (reference) group (Table 6).
Table 6.
Overview of TEAEs through Week 24 (safety analysis set)
| Parameter | Darbepoetin alfa |
Roxadustat |
Roxadustat |
Pooled roxadustat |
|---|---|---|---|---|
| (comparative) (n = 131) |
(comparative) (n = 131) |
(reference) (n = 70) |
(n = 201) |
|
| n (%) | n (%) | n (%) | n (%) | |
| TEAE | 92 (70.2%) | 103 (78.6%) | 54 (77.1%) | 157 (78.1%) |
| Serious TEAE | 17 (13.0%) | 23 (17.6%) | 9 (12.9%) | 32 (15.9%) |
| TEAE leading to withdrawal of treatment | 7 (5.3%) | 15 (11.5%) | 4 (5.7%) | 19 (9.5%) |
| Death | 1 (0.8%) | 0 | 2 (2.9%) | 2 (1.0%) |
TEAE, treatment-emergent adverse event.
The incidence of serious TEAEs (TESAEs) was 17.6% (23/131 patients) in the roxadustat (comparative) group, 13.0% (17/131 patients) in the DA (comparative) group, and 12.9% (9/70 patients) in the roxadustat (reference) group (Supplementary Table 6). CKD was the only TESAE reported in ≥2 patients in the roxadustat (comparative and reference) groups (5.3% [7/131 patients] and 4.3% [3/70 patients], respectively). In the DA (comparative) group, TESAEs observed in ≥2 patients were CKD (3.1% [4/131 patients]) and pneumonia (2.3% [3/131 patients]). The incidence of TEAEs leading to withdrawal of treatment was 11.5% (15/131 patients) in the roxadustat (comparative) group, 5.3% (7/131 patients) in the DA (comparative) group, and 5.7% (4/70 patients) in the roxadustat (reference) group. At the end of Week 24, in the SAF, CKD was the only TEAE leading to withdrawal of treatment reported in ≥2 patients in the roxadustat (comparative) and DA (comparative) groups (4.6% [6/131 patients] and 3.1% [4/131 patients], respectively). No TEAEs leading to withdrawal of treatment occurred in ≥2 patients in the roxadustat (referential) group. No deaths were reported in the roxadustat (comparative) group, 1 patient (0.8%) died in the DA (comparative) group, and 2 died (2.9%) in the roxadustat (reference) group; the death in the DA group was due to gastrointestinal necrosis, and the deaths in the roxadustat reference group were due to pulmonary embolism and myocardial ischemia (n = 1 each) (see Supplementary Results).
The most common TEAEs (occurring in ≥3% of patients in any treatment group) included nasopharyngitis, CKD, hyperkalemia, and hypertension (Table 7). The incidence of all the events in the roxadustat (comparative) group was similar to or lower than that in the DA (comparative) group.
Table 7.
Common (≥3% in any treatment group) treatment-emergent adverse events through Week 24 or end of Week 24 (safety analysis set.)
| MedDRA version 19.0 System Organ Class Preferred Term | Darbepoetin alfa |
Roxadustat |
Roxadustat |
Pooled roxadustat |
|---|---|---|---|---|
| (comparative) (n = 131) | (comparative) (n = 131) | (reference) (n = 70) | (n = 201) | |
| Overall | 92 (70.2%) | 103 (78.6%) | 54 (77.1%) | 157 (78.1%) |
| Gastrointestinal disorders | 19 (14.5%) | 32 (24.4%) | 12 (17.1%) | 44 (21.9%) |
| Constipation | 4 (3.1%) | 5 (3.8%) | 2 (2.9%) | 7 (3.5%) |
| Diarrhea | 5 (3.8%) | 3 (2.3%) | 2 (2.9%) | 5 (2.5%) |
| Dental caries | 1 (0.8%) | 3 (2.3%) | 3 (4.3%) | 6 (3.0%) |
| Nausea | 1 (0.8%) | 5 (3.8%) | 1 (1.4%) | 6 (3.0%) |
| General disorders and administration site conditions | 13 (9.9%) | 13 (9.9%) | 5 (7.1%) | 18 (9.0%) |
| Edema peripheral | 4 (3.1%) | 5 (3.8%) | 1 (1.4%) | 6 (3.0%) |
| Pyrexia | 4 (3.1%) | 5 (3.8%) | 0 | 5 (2.5%) |
| Infections and infestations | 48 (36.6%) | 42 (32.1%) | 30 (42.9%) | 72 (35.8%) |
| Nasopharyngitis | 34 (26.0%) | 25 (19.1%) | 19 (27.1%) | 44 (21.9%) |
| Pneumonia | 4 (3.1%) | 4 (3.1%) | 0 | 4 (2.0%) |
| Gastroenteritis | 1 (0.8%) | 5 (3.8%) | 1 (1.4%) | 6 (3.0%) |
| Cystitis | 2 (1.5%) | 0 | 3 (4.3%) | 3 (1.5%) |
| Pharyngitis | 1 (0.8%) | 0 | 3 (4.3%) | 3 (1.5%) |
| Injury, poisoning and procedural complications | 10 (7.6%) | 15 (11.5%) | 9 (12.9%) | 24 (11.9%) |
| Contusion | 2 (1.5%) | 4 (3.1%) | 3 (4.3%) | 7 (3.5%) |
| Investigations | 6 (4.6%) | 8 (6.1%) | 5 (7.1%) | 13 (6.5%) |
| Blood potassium increased | 4 (3.1%) | 0 | 1 (1.4%) | 1 (0.5%) |
| Metabolism and nutrition disorders | 16 (12.2%) | 15 (11.5%) | 9 (12.9%) | 24 (11.9%) |
| Hyperkalemia | 5 (3.8%) | 5 (3.8%) | 4 (5.7%) | 9 (4.5%) |
| Hypoglycemia | 0 | 1 (0.8%) | 3 (4.3%) | 4 (2.0%) |
| Musculoskeletal and connective tissue disorders | 13 (9.9%) | 12 (9.2%) | 6 (8.6%) | 18 (9.0%) |
| Back pain | 5 (3.8%) | 4 (3.1%) | 1 (1.4%) | 5 (2.5%) |
| Nervous system disorders | 9 (6.9%) | 16 (12.2%) | 1 (1.4%) | 17 (8.5%) |
| Headache | 4 (3.1%) | 3 (2.3%) | 0 | 3 (1.5%) |
| Renal and urinary disorders | 13 (9.9%) | 15 (11.5%) | 5 (7.1%) | 20 (10.0%) |
| CKD | 9 (6.9%) | 9 (6.9%) | 4 (5.7%) | 13 (6.5%) |
| Skin and subcutaneous tissue disorders | 11 (8.4%) | 7 (5.3%) | 9 (12.9%) | 16 (8.0%) |
| Eczema | 3 (2.3%) | 1 (0.8%) | 3 (4.3%) | 4 (2.0%) |
| Vascular disorders | 6 (4.6%) | 5 (3.8%) | 6 (8.6%) | 11 (5.5%) |
| Hypertension | 5 (3.8%) | 3 (2.3%) | 4 (5.7%) | 7 (3.5%) |
Regarding ophthalmological exams, per investigator’s judgment, and following central grading by an independent expert panel, no clinically relevant findings were reported in this study. No clinically meaningful mean changes from Week 0 in the total number of retinal hemorrhages were found in either the roxadustat (comparative) or DA (comparative) group at each visit through Week 24 and/or the end of Week 24. The proportion of patients with new or worsening retinal hemorrhages in the roxadustat (comparative) group was slightly lower than that in the DA group (Table 8). In a subgroup analysis of those patients with no retinal hemorrhage at baseline, the proportion of patients with new retinal hemorrhages during the treatment period was 12.9% (8/62 patients) in the roxadustat (comparative) group and 25.0% (18/72 patients) in the DA (comparative) group. In a subgroup analysis of those patients with ≥1 retinal hemorrhage at baseline, the proportion of patients with new or worsening retinal hemorrhages during the treatment period was 50.8% (30/59 patients) in the roxadustat (comparative) group and 58.9% (33/56 patients) in the DA (comparative) group (Table 8). No clinically meaningful changes in retinal thickness were observed by OCT, as assessed by independent blinded central reviewers, from Week 0 through Week 24 and/or the end of Week 24 in either of the treatment groups.
Table 8.
New or worsening retinal hemorrhage by subgroup (total number of retinal hemorrhages at baseline) (safety analysis set at the end of Week 24)
| Subgroup | Analysis visit | Darbepoetin alfa |
Roxadustat |
|---|---|---|---|
| (comparative) (n = 131) | (comparative) (n = 131) | ||
| Total | Treatment period | 51/128 (39.8%) | 38/121 (31.4%) |
| Week 12 compared with baseline | 32/124 (25.8%) | 27/113 (23.9%) | |
| Week 24 compared with baseline | 41/121 (33.9%) | 21/104 (20.2%) | |
| End of Week 24 | 44/128 (34.4%) | 26/121 (21.5%) | |
| No retinal hemorrhages at baseline | Treatment period | 18/72 (25.0%) | 8/62 (12.9%) |
| Week 12 compared with baseline | 8/69 (11.6%) | 6/59 (10.2%) | |
| Week 24 compared with baseline | 13/67 (19.4%) | 3/56 (5.4%) | |
| End of Week 24 | 15/72 (20.8%) | 4/62 (6.5%) | |
| One or more retinal hemorrhage at baseline | Treatment period | 33/56 (58.9%) | 30/59 (50.8%) |
| Week 12 compared with baseline | 24/55 (43.6%) | 21/54 (38.9%) | |
| Week 24 compared with baseline | 28/54 (51.9%) | 18/48 (37.5%) | |
| End of Week 24 | 29/56 (51.8%) | 22/59 (37.3%) |
Treatment period = Detected throughout the entire 24-week treatment period.
No clinically significant changes were observed in the clinical laboratory evaluations, vital signs, or 12-lead electrocardiograms.
Safety—Roxadustat Through Week 52 or End of Study
In the SAF, the incidence of TEAEs was 88.6% (178/201 patients) in the pooled roxadustat group (Table 9). TEAEs occurring in ≥3% of patients in either roxadustat treatment group included (but were not limited to) nasopharyngitis, CKD, hyperkalemia, constipation, diarrhea, back pain, nausea, edema peripheral, contusion, and hypertension (Table 10). The incidence of TESAEs was 26.0% (34/131 patients) in the roxadustat (comparative) group and 27.1% (19/70 patients) in the roxadustat (reference) group (Supplementary Table S7). At the end of Week 52, in the SAF, TEAEs leading to withdrawal of treatment observed in two or more patients in the pooled roxadustat group were CKD (10.4% [21/201 patients]), and renal impairment and ESRD (1.0% each [2/201 patients]). No clinically significant changes were observed in the clinical laboratory evaluations, vital signs, or 12-lead electrocardiograms.
Table 9.
Overview of TEAEs—roxadustat through Week 52 or end of study (safety analysis set)
| Parameter | Roxadustat |
Roxadustat |
Pooled roxadustat |
|---|---|---|---|
| (comparative) (n = 131) |
(reference) (n = 70) |
(n = 201) |
|
| n (%) | n (%) | n (%) | |
| TEAE | 115 (87.8%) | 63 (90.0%) | 178 (88.6%) |
| Serious TEAE | 34 (26.0%) | 19 (27.1%) | 53 (26.4%) |
| TEAE leading to withdrawal of treatment | 26 (19.8%) | 14 (20.0%) | 40 (19.9%) |
| Death | 0 | 2 (2.9%) | 2 (1.0%) |
TEAE, treatment-emergent adverse event.
Table 10.
Common (≥3% in any treatment group) treatment-emergent adverse events through Week 52 or end of study (safety analysis set)
| MedDRA version 19.0 System Organ Class Preferred Term | Roxadustat |
Roxadustat |
Pooled roxadustat |
|---|---|---|---|
| (comparative) (n = 131) | (reference) (n = 70) | (n = 201) | |
| Overall | 115 (87.8%) | 63 (90.0%) | 178 (88.6%) |
| Cardiac disorders | 11 (8.4%) | 5 (7.1%) | 16 (8.0%) |
| Cardiac failure congestive | 4 (3.1%) | 1 (1.4%) | 5 (2.5%) |
| Gastrointestinal disorders | 50 (38.2%) | 18 (25.7%) | 68 (33.8%) |
| Constipation | 11 (8.4%) | 3 (4.3%) | 14 (7.0%) |
| Diarrhea | 10 (7.6%) | 3 (4.3%) | 13 (6.5%) |
| Nausea | 7 (5.3%) | 3 (4.3%) | 10 (5.0%) |
| Dental caries | 4 (3.1%) | 5 (7.1%) | 9 (4.5%) |
| Chronic gastritis | 4 (3.1%) | 0 | 4 (2.0%) |
| General disorders and administration site conditions | 18 (13.7%) | 9 (12.9%) | 27 (13.4%) |
| Edema peripheral | 7 (5.3%) | 3 (4.3%) | 10 (5.0%) |
| Edema due to renal disease | 4 (3.1%) | 3 (4.3%) | 7 (3.5%) |
| Pyrexia | 5 (3.8%) | 0 | 5 (2.5%) |
| Infections and infestations | 60 (45.8%) | 44 (62.9%) | 104 (51.7%) |
| Nasopharyngitis | 34 (26.0%) | 25 (35.7%) | 59 (29.4%) |
| Bronchitis | 5 (3.8%) | 2 (2.9%) | 7 (3.5%) |
| Gastroenteritis | 6 (4.6%) | 1 (1.4%) | 7 (3.5%) |
| Pneumonia | 6 (4.6%) | 0 | 6 (3.0%) |
| Cystitis | 0 | 5 (7.1%) | 5 (2.5%) |
| Influenza | 4 (3.1%) | 1 (1.4%) | 5 (2.5%) |
| Pharyngitis | 0 | 3 (4.3%) | 3 (1.5%) |
| Injury, poisoning and procedural complications | 20 (15.3%) | 11 (15.7%) | 31 (15.4%) |
| Contusion | 6 (4.6%) | 4 (5.7%) | 10 (5.0%) |
| Skin abrasion | 4 (3.1%) | 0 | 4 (2.0%) |
| Metabolism and nutrition disorders | 27 (20.6%) | 12 (17.1%) | 39 (19.4%) |
| Hyperkalemia | 10 (7.6%) | 6 (8.6%) | 16 (8.0%) |
| Hypoglycemia | 3 (2.3%) | 5 (7.1%) | 8 (4.0%) |
| Metabolic acidosis | 5 (3.8%) | 2 (2.9%) | 7 (3.5%) |
| Hyperphosphataemia | 4 (3.1%) | 2 (2.9%) | 6 (3.0%) |
| Musculoskeletal and connective tissue disorders | 20 (15.3%) | 12 (17.1%) | 32 (15.9%) |
| Back pain | 6 (4.6%) | 5 (7.1%) | 11 (5.5%) |
| Osteoarthritis | 0 | 3 (4.3%) | 3 (1.5%) |
| Nervous system disorders | 18 (13.7%) | 4 (5.7%) | 22 (10.9%) |
| Headache | 4 (3.1%) | 1 (1.4%) | 5 (2.5%) |
| Psychiatric disorders | 6 (4.6%) | 1 (1.4%) | 7 (3.5%) |
| Insomnia | 4 (3.1%) | 1 (1.4%) | 5 (2.5%) |
| Renal and urinary disorders | 27 (20.6%) | 14 (20.0%) | 41 (20.4%) |
| CKD | 15 (11.5%) | 11 (15.7%) | 26 (12.9%) |
| Renal impairment | 4 (3.1%) | 2 (2.9%) | 6 (3.0%) |
| Respiratory, thoracic and mediastinal disorders | 10 (7.6%) | 9 (12.9%) | 19 (9.5%) |
| Cough | 0 | 3 (4.3%) | 3 (1.5%) |
| Skin and subcutaneous tissue disorders | 17 (13.0%) | 17 (24.3%) | 34 (16.9%) |
| Pruritus | 3 (2.3%) | 4 (5.7%) | 7 (3.5%) |
| Eczema | 3 (2.3%) | 3 (4.3%) | 6 (3.0%) |
| Rash | 2 (1.5%) | 3 (4.3%) | 5 (2.5%) |
| Vascular disorders | 7 (5.3%) | 9 (12.9%) | 16 (8.0%) |
| Hypertension | 4 (3.1%) | 6 (8.6%) | 10 (5.0%) |
Discussion
This was a phase III, multicenter, partially randomized, DA-controlled, open-label study in Japanese patients with CKD who were not on dialysis at the time of randomization. Patients who had used rHuEPO or DA before conversion were randomized to either the roxadustat or the DA treatment arms, and patients who had used EBP before conversion were allocated to the roxadustat treatment arm. In the PPS, the mean (SE) of average Hb levels of Weeks 18 to 24 in the roxadustat (comparative) group was 11.14 (0.07) g/dl with a 95% CI of 11.01 to 11.27 g/dl, thereby confirming the efficacy of roxadustat. The estimated difference between the roxadustat (comparative) and DA (comparative) groups in the LS mean of change of average Hb levels of Weeks 18 to 24 from baseline was –0.07 g/dl with the lower limit of 95% CI of –0.23 g/dl, thereby confirming the noninferiority of roxadustat to DA. Through Week 24, the incidence of TEAEs was comparable across treatment arms.
To date, this is the only study evaluating efficacy and safety of roxadustat in NDD CKD patients converted from ESA treatment. In the current study, demographic and baseline characteristics were in line with those expected in this population (NDD CKD). One difference that should be noted is that patient age in the current study (mean: ∼69 years) is similar to the patient age in a report by Akizawa et al.16 (mean: ∼69 years) but slightly older than that in a report by Chen et al.17 (mean: ∼54 years), both of which examined patients with NDD CKD.
Mean Hb values at Weeks 18 through 24 in the current study were similar to those seen in ESA-naïve NDD patients.16 Mean Hb at the end of the current study was similar to Chen et al.,17 but their treatment period was shorter (9 weeks), relative to the current study. The current Hb results are also similar to those reported in both ESA-naïve and ESA-converted roxadustat-treated DD patients.18,22,23 Similar to the current study, roxadustat was shown to be noninferior to DA after 24 weeks of treatment in DD CKD patients18 and noninferior to epoetin alfa after 26 weeks in DD CKD patients.19 Taken together, accumulating evidence demonstrates that roxadustat is an effective alternative to ESAs in patients with anemia of CKD regardless of whether patients are on or off dialysis and whether roxadustat treatment is initiated in ESA-untreated or ESA-pretreated patients.
Regarding markers of iron utilization, no remarkable changes were observed in serum Fe through Week 24 in any group. This is similar to previous findings in roxadustat-treated NDD CKD16,17 patients where iron levels tended to remain stable throughout the study despite significantly increased Hb. Likewise, similar results have also been reported in roxadustat-treated DD CKD patients.19 Similar patterns emerged for other markers of iron utilization, where early changes may be seen, followed by stabilized values by Week 24. Results pertaining to ferritin, transferrin, TIBC, sTfR, and TSAT are similar between the current study and ESA-naïve NDD16 and NDD patients.17 Again, the current results are also in line with results seen in roxadustat-treated DD CKD patients.19 In the current study, hepcidin decreased early in the study period, followed by stable values by Week 24. These results align with data reported in roxadustat-treated ESA-naïve NDD patients16 and NDD patients.17 These values were also stable through Week 52 in roxadustat-treated patients in the current study, suggesting that long-term treatment with roxadustat is associated with stable iron utilization.
In a subgroup analysis stratified by hs-CRP levels, the mean change of average Hb levels of Weeks 18 to 24 from baseline in the roxadustat (comparative), DA (comparative), and roxadustat (reference) groups were 0.09, 0.28, and –0.03 g/dl in the hs-CRP <28.57 nmol/l (low) subgroup and 0.19, 0.08, and –0.17 g/dl in the hs-CRP ≥28.57 nmol/l (high) subgroup, respectively. Similar to previous findings,18,24 the roxadustat doses required to maintain target Hb levels did not appear to be influenced by hs-CRP levels, whereas DA doses appeared to be higher in DA-treated patients with high hs-CRP. These findings add to emerging evidence that suggests roxadustat may be more efficacious, relative to traditional ESAs, in the presence of inflammation. The effect of roxadustat in patients with inflammation has been shown in 1 previous phase II study24 and 1 phase III study.18 In a study comparing epoetin alfa with roxadustat, Provenzano et al. reported that hs-CRP levels were correlated with higher preenrollment doses of epoetin alfa, whereas in the same patients after 19 weeks of treatment, no association between the roxadustat dose required to maintain target Hb levels and hs-CRP levels was observed in the last 7 weeks of treatment.24 Akizawa et al. reported that roxadustat doses required to maintain target Hb levels did not appear to be influenced by hs-CRP levels, whereas DA doses appeared to be higher in DA-treated patients with high hs-CRP.18 Although firm conclusions cannot be made, accumulating evidence suggests that roxadustat may be beneficial in patients with extant inflammation.
The safety profile outlined in the current study is in line with previous studies and is expected in this patient cohort. The current safety results are similar to those reported in roxadustat-treated ESA-naïve NDD CKD patients16 and ESA-converted DD CKD patients.23 In a phase III study, Chen et al. reported lower overall rates of TEAEs, relative to the current study, but the patients assessed in Chen et al.’s report were not previously treated with ESAs, so strict comparisons cannot be made.17 Indeed, the patients in Chen et al.’s report were considerably younger (∼55 years old) than those in the current study.17 In the current study, the proportion of patients with new or worsening retinal hemorrhages in the roxadustat (comparative) group (31.4%; 38/121 patients) was slightly lower than the proportion in the DA group (39.8%; 51/128 patients). In the roxadustat (comparative) group, no patients died during the study. At the end of Week 24, deaths were reported in 1 patient (due to gastrointestinal necrosis) in the DA (comparative) group and 2 patients (due to pulmonary embolism and myocardial ischemia) in the roxadustat (reference) group.
As with any study, limitations in the current study may exist. This study used an open-label design, which could introduce bias. Furthermore, DA was not taken for 52 weeks, so 52-week data with roxadustat cannot be compared to DA. Lastly, considering this study was performed in Japanese patients, it is unknown whether these results are fully applicable to other populations.
In conclusion, in Japanese patients with NDD CKD, roxadustat was shown to be efficacious and noninferior to DA in terms of the change of average Hb levels of Weeks 18 to 24 from baseline. The safety profiles outlined in the current study are in line with previous studies performed in patients with NDD CKD. Roxadustat represents an effective and well-tolerated alternative to DA that can be orally administered.
Disclosure
This study was funded by Astellas Pharma, Inc. TO and YY are employees of Astellas Pharma, Inc. MR is an employee of Astellas Pharma Europe B.V. TA has received personal fees from Astellas, Bayer, Chugai, Fuso, GSK, JT Pharmaceuticals, KKC, Kissei, Nipro Corporation, Ono, Otsuka, Torii, and Sanwa Chemical Industrial Co., Ltd. All other authors have nothing to disclose.
Acknowledgments
Medical writing and editorial support were provided by Patrick Tucker, PhD, and Elizabeth Hermans, PhD (OPEN Health Medical Communications, Chicago, IL) and funded by the study sponsor. The authors acknowledge this study’s investigators: Kunihiro Ishioka, Shonan Kamakura General Hospital; Keiichiro Mishima, Gunmaken Saiseikai Maebashi Hospital; Takayuki Fujii, Seirei Sakura Citizen Hospital; Yuichiro Makita, Koshigaya City Hospital; Toshifumi Sakaguchi, Rinku General Medical Center; Ryoichi Ando, Musashino Red Cross Hospital; Yasuhiro Onodera, Sapporo Tokushukai Hospital; Hidetoshi Kanai, Kokura Memorial Hospital; Yosuke Saka, Kasugai Municipal Hospital; Chikako Nose, Toyama Prefectural Central Hospital; Shigeki Ando, Tohoku Medical and Pharmaceutical University Wakabayashi Hospital; Taihei Yanagida, Steel Memorial Yawata Hospital; Hideo Araki, Fukui Prefectural Hospital; Kazuhiko Funabiki, Juntendo University Juntendo Tokyo Koto Geriatric Medical Center; Hajime Fujisawa, Yokohama City Minato Red Cross Hospital; Hiroaki Kobayashi, Ibaraki Prefectural Central Hospital; Teruki Kondo, Nagano Chuo Hospital; Yoshitaka Maeda, JA Toride Medical Center; Tetsuro Takeda, Dokkyo Medical University Koshigaya Hospital; Taishi Yamakawa, Toyohashi Municipal Hospital; Hiroya Takeoka, Hyogo Prefectural Amagasaki General Medical Center; Tadashi Iitsuka, Ibaraki Seinan Medical Center Hospital; Kenjiro Kimura, Japan Community Healthcare Organization Tokyo Takanawa Hospital; Hideki Tanaka, Seiwa Clinic; Hisaki Shimada, Shinrakuen Hospital; Yasushi Asano, Japanese Red Cross Koga Hospital; Kenichiro Kojima, Ageo Central General Hospital; Hirotake Kasuga, Nagoya Kyouritsu Hospital; Jun Soma, Iwate Prefectural Central Hospital; Yukio Yokoyama, Hiroshima Red Cross Hospital & Atomic-bomb Survivors Hospital; Masaru Nakayama, National Hospital Organization Kyushu Medical Center; Nobuyoshi Nasu, National Hospital Organization Oita Medical Center; Kiyoki Kitagawa, National Hospital Organization Kanazawa Medical Center; Yasufumi Takahashi, Shinshu Ueda Medical Center; Tetsuji Arakawa, Hiroshima General Hospital; Takashi Sekikawa, Matsuyama Shimin Hospital; Takashi Kihara, Hiroshima City Hiroshima Citizens Hospital; Yasuhisa Tamura, Kyushu Hospital; Yutaka Sugiyama, Gifu Prefectural Tajimi Hospital; Harumichi Higashi, St. Mary's Hospital; Hitoshi Suzuki, Juntendo University Hospital; Shigeaki Nishimura, Ehime Prefectural Central Hospital; Noriyuki Iehara, Kyoto City Hospital; Takayuki Toda, Tsuchiura Kyodo General Hospital; Yasuo Tokita, Fujisawa City Hospital; Kei Matsushita, National Hospital Organization Yokohama Medical Center; Hironori Kobayashi, Japanese Red Cross Asahikawa Hospital; Shunsuke Takahashi, National Hospital Organization Kure Medical Center; Hideaki Shimizu, Daido Clinic; Atsushi Ueda, Hitachi General Hospital; Sumiko Hasumi, Nishiyamado Keiwa Hospital; Kosuke Ota, National Hospital Organization Okayama Medical Center; Tatsuo Tsukamoto, Kitano Hospital; Chikao Yasunaga, Saiseikai Yahata General Hospital; Masahito Imanishi, Ishikiriseiki Hospital; Makoto Watanabe, Makita General Hospital; Yoshio Konishi, Osaka City General Hospital; Yu Igarashi, Federation of National Public Service Personnel Mutual Aid Associations Tachikawa Hospital; Hideki Takano, Tokyo Teishin Hospital; Hideaki Yoshida, JR Sapporo Hospital; Kiyoshi Ikeda, Ikeda Vascular Access Dialysis and Internal Medicine Clinic; Naohiko Fujii, Hyogo Prefectural Nishinomiya Hospital; Takashi Araki, Hino Municipal Hospital; Katsuhiko Tamura, Shinonoi General Hospital; Hisako Mori, Yao Tokushukai General Hospital.
Author Contributions
Conception and design: MR, TA, and TO. Acquisition of data: TO, TA, and MI. Analysis and interpretation of the data: TA, MI, YY, TO, and MR. Drafting and critical revision of the article for important intellectual content: TA, MI, TO, YY, and MR.
DATA SHARING STATEMENT
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at http://www.clinicalstudydatarequest.com.
For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Footnotes
Supplementary Methods
Supplementary Results
Table S1. Initial Dose Conversion to Roxadustat, Based on ESA Use
Table S2. Initial Dose Conversion to Darbepoetin Alfa, Based on ESA Use
Table S3. Dose Adjustment of the Study Drug (Roxadustat)
Table S4. Dose Increase/Reduction Rules for Roxadustat
Table S5. Allocated Dose Level to Week 22 by eGFR (SAF)
Table S6. Treatment-Emergent Serious Adverse Events (SAF at the End of Week 24)
Table S7. Treatment-Emergent Serious Adverse Events (SAF at the End of Week 52)
Figure S1. Mean and SD Plot of Iron (μmol/l) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S2. Mean and SD Plot of Ferritin (ng/ml) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S3. Mean and SD Plot of Transferrin (g/l) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S4. Mean and SD Plot of Total Iron Binding Capacity (μmol/l) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S5. Mean and SD Plot of Soluble Transferrin Receptor (nmol/l) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S6. Mean and SD Plot of Transferrin Saturation (%) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S7. Mean and SD Plot of Hepcidin (ng/ml) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
CONSORT Checklist.
ClinicalTrials.gov Identifier:
Supplementary Material
Supplementary Methods
Supplementary Results
Table S1. Initial Dose Conversion to Roxadustat, Based on ESA Use
Table S2. Initial Dose Conversion to Darbepoetin Alfa, Based on ESA Use
Table S3. Dose Adjustment of the Study Drug (Roxadustat)
Table S4. Dose Increase/Reduction Rules for Roxadustat
Table S5. Allocated Dose Level to Week 22 by eGFR (SAF)
Table S6. Treatment-Emergent Serious Adverse Events (SAF at the End of Week 24)
Table S7. Treatment-Emergent Serious Adverse Events (SAF at the End of Week 52)
Figure S1. Mean and SD Plot of Iron (μmol/l) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S2. Mean and SD Plot of Ferritin (ng/ml) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S3. Mean and SD Plot of Transferrin (g/l) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S4. Mean and SD Plot of Total Iron Binding Capacity (μmol/l) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S5. Mean and SD Plot of Soluble Transferrin Receptor (nmol/l) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S6. Mean and SD Plot of Transferrin Saturation (%) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
Figure S7. Mean and SD Plot of Hepcidin (ng/ml) at the End of Week 24 (A) and Week 52 (B) (Full Analysis Set)
CONSORT Checklist.
References
- 1.Shih H.M., Wu C.J., Lin S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J Formos Med Assoc. 2018;117:955–963. doi: 10.1016/j.jfma.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Haase V.H. Therapeutic targeting of the HIF oxygen-sensing pathway: lessons learned from clinical studies. Exp Cell Res. 2017;356:160–165. doi: 10.1016/j.yexcr.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babitt J.L., Lin H.Y. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishbane S., Spinowitz B. Update on anemia in ESRD and earlier stages of CKD: core curriculum 2018. Am J Kidney Dis. 2018;71:423–435. doi: 10.1053/j.ajkd.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Iimori S., Naito S., Noda Y. Anaemia management and mortality risk in newly visiting patients with chronic kidney disease in Japan: the CKD-ROUTE study. Nephrology (Carlton, Vic) 2015;20:601–608. doi: 10.1111/nep.12493. [DOI] [PubMed] [Google Scholar]
- 6.Andrassy K.M. Comments on “KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.”. Kidney Int. 2013;84:622–623. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 7.Seliger S.L., Zhang A.D., Weir M.R. Erythropoiesis-stimulating agents increase the risk of acute stroke in patients with chronic kidney disease. Kidney Int. 2011;80:288–294. doi: 10.1038/ki.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeffer M.A., Burdmann E.A., Chen C.Y. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R., Kusek J.W., Pappas M.K. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015;88:905–914. doi: 10.1038/ki.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriguchi R., Taniguchi M., Ninomiya T. Hyporesponsiveness to erythropoiesis-stimulating agent as a prognostic factor in Japanese hemodialysis patients: the Q-Cohort study. J Nephrol. 2015;28:217–225. doi: 10.1007/s40620-014-0121-9. [DOI] [PubMed] [Google Scholar]
- 11.Luo J., Jensen D.E., Maroni B.J., Brunelli S.M. Spectrum and burden of erythropoiesis-stimulating agent hyporesponsiveness among contemporary hemodialysis patients. Am J Kidney Dis. 2016;68:763–771. doi: 10.1053/j.ajkd.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Gupta N., Wish J.B. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;69:815–826. doi: 10.1053/j.ajkd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan J.M., Sharma N., Dikdan S. Hypoxia-inducible factor and its role in the management of anemia in chronic kidney disease. Int J Molec Sci. 2018;19:389. doi: 10.3390/ijms19020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locatelli F., Fishbane S., Block G.A., Macdougall I.C. Targeting hypoxia-inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol. 2017;45:187–199. doi: 10.1159/000455166. [DOI] [PubMed] [Google Scholar]
- 15.Joharapurkar A.A., Pandya V.B., Patel V.J. Prolyl hydroxylase inhibitors: a breakthrough in the therapy of anemia associated with chronic diseases. J Med Chem. 2018;61:6964–6982. doi: 10.1021/acs.jmedchem.7b01686. [DOI] [PubMed] [Google Scholar]
- 16.Akizawa T., Yamaguchi Y., Otsuka T., Reusch M. A phase 3, multicenter, randomized, two-arm, open-label study of intermittent oral dosing of roxadustat for the treatment of anemia in Japanese erythropoiesis-stimulating agent-naive chronic kidney disease patients not on dialysis. Nephron. 2020;144:372–382. doi: 10.1159/000508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Hao C., Peng X. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 18.Akizawa T., Iwasaki M., Yamaguchi Y. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol. 2020;31:1628–1639. doi: 10.1681/ASN.2019060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen N., Hao C., Liu B.C. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381:1011–1022. doi: 10.1056/NEJMoa1901713. [DOI] [PubMed] [Google Scholar]
- 20.Aiello L.P., Avery R.L., Arrigg P.G. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D., Lv F.L., Wang G.H. Effects of HIF-1alpha on diabetic retinopathy angiogenesis and VEGF expression. Eur Rev Med Pharmacol Sci. 2018;22:5071–5076. doi: 10.26355/eurrev_201808_15699. [DOI] [PubMed] [Google Scholar]
- 22.Akizawa T., Otsuka T., Reusch M., Ueno M. Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: a randomized, phase 3, multicenter, open-label study. Ther Apher Dial. 2020;24:115–125. doi: 10.1111/1744-9987.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akizawa T., Ueno M., Shiga T., Reusch M. Oral roxadustat three times weekly in ESA-naive and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: results from two phase 3 studies. Ther Apher Dial. 2019 doi: 10.1111/1744-9987.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzano R., Besarab A., Wright S. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67:912–924. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.