Abstract
Calcifying nested stromal epithelial tumor is a very rare primary liver tumor in children. To our knowledge, few cases have been reported in literature. We describe the imaging appearance and histopathologic features of this tumor incidentally detected in a 2-year-old girl. This tumor should be considered in the differential when a large heterogeneous liver tumor with central scar and coarse/chunky calcifications is identified at imaging in the absence of elevated alpha-fetoprotein in a child.
Introduction
Calcified nested stromal epithelial tumors of the liver are characterized by non-hepatocytic, non-biliary tumors with nests of epithelial and spindle cells with associated myofibroblastic stroma, and variable intra-lesional calcification and ossification [1]. Previous case reports of this tumor have been published under a variety of names including ossifying stromal epithelial tumor, desmoplastic nested spindle cell tumor and ossifying malignant mixed epithelial and stromal tumor.
Case report
A 2-year-old girl presented with an incidentally detected calcified liver lesion on a chest radiograph (Fig. 1) which was obtained for cough.
Fig. 1.
(A and B) AP and lateral chest radiograph demonstrating a 1.8 cm coarse calcification in the liver.
Patient subsequently underwent a computed tomography (CT) examination of the abdomen and was then referred to our institution for further care. Initial laboratory results were as follows: aspartate aminotransferase - 32 U/L [15-37 U/L], alanine aminotransferase - 24 U/L [30-65 U/L], alkaline phosphatase - 143 U/L [154-415 U/L], lactate dehydrogenase - 358 U/L [81-234 U/L], total bilirubin - 0.3 mg/dL [0.0-1.0 mg/dL], total protein - 7.4 g/dL [4.9-8.1 g/dL], beta human chorionic gonadotropin - less than 1 mIU/mL [0-6 mIU/mL], and alpha-fetoprotein (AFP) - less than 5 ng/mL [0-12 ng/mL]. CT demonstrated a 5.5-centimeter sized heterogeneous mass with large coarse calcifications (Figs. 2A and B).
Fig. 2.
(A and B) Computed tomography (CT) with contrast demonstrated a 5.5 centimeter heterogeneously enhancing mass (arrow in Fig B) in Coinaud segment VII of the liver with large coarse calcifications (arrowhead in Fig A) at the superior aspect of the mass.
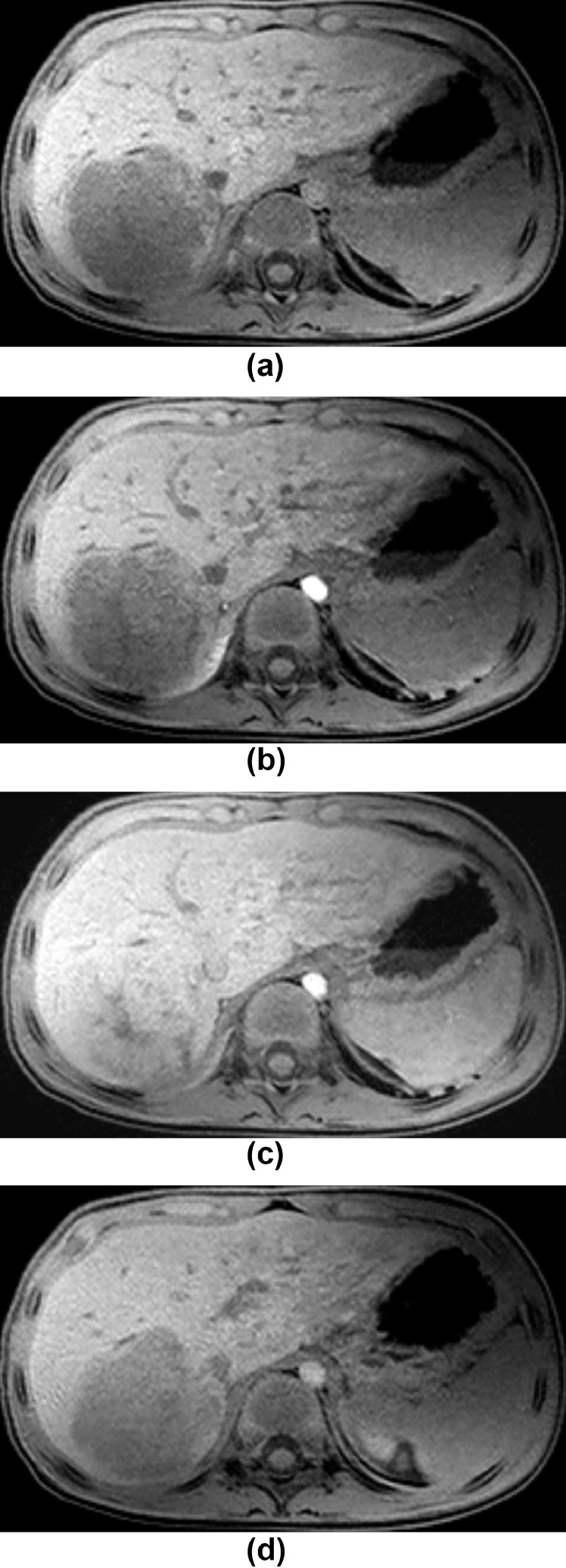
Subsequent magnetic resonance imaging (MRI) of the liver better demonstrated the margins of the lesion, which was predominantly hyper-intense on T2-weighted images. Large areas of signal void were seen in the superior aspect of the lesion, corresponding to the calcifications seen on CT (Figs. 3A and B). The mass restricted diffusion (Fig. 3C). Postcontrast, the lesion demonstrated enhancement in the portal venous phase with washout on the delayed phase.
Fig. 4.
(A-D) Axial pre contrast, 30 seconds, 70 seconds and 4 minutes post contrast e-thrive images demonstrate heterogeneous enhancement of the lesion at 70 sec/portal venous phase (Fig. 4C) with washout on the delayed phase (Fig. 4D).
Fig. 3.
(A and B) Coronal and axial T2 weighted MR images. There is a lobulated lesion involving Coinaud segment VII demonstrating high T2 signal. Region of signal loss in the superior portion (arrowhead in Fig 3A) corresponds with the calcification seen on radiographs and CT. There was no vascular invasion although the lesion was near the right hepatic vein and inferior vena cava (arrows in Fig. 3B). Fig. 3C Diffusion weighted image demonstrating restricted diffusion.
Initial diagnosis based on imaging findings and patient's age was hepatoblastoma. However, serum AFP was normal, which is unusual with hepatoblastoma. Although the imaging findings and patients age suggested hepatoblastoma, the normal AFP levels did not favor hepatoblastoma.
Patient subsequently underwent an open right hepatic lobe liver biopsy. An intraoperative frozen section consultation yielded a diagnosis of “spindle cell neoplasm." F18- fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) was subsequently obtained to evaluate for metastasis, and increased FDG uptake was seen within the primary hepatic lesion, maximum standard unit value of 3.5, with no evidence of FDG-avid metastatic disease (Fig. 5A and 5B).
Fig. 5.
(A and B) Coronal maximal intensity projection and fused axial image: demonstrate moderately increased FDG avidity in the liver lesion with SUV max of 3.5. No FDG avid metastatic disease was noted. Areas of mild in right upper lung uptake with SUV max of 2.5 were suggestive of an infectious process.
Based on the histoarchitectural features of the biopsy specimen, immuno-profile and negative molecular studies, a diagnosis of calcifying nested stromal-epithelial tumor (CNSET)of the liver was rendered. After a multidisciplinary discussion, the patient subsequently underwent right hepatectomy with cholecystectomy. Gross pathology specimen demonstrated the unencapsulated, lobulated features of the tumor with chunky intra-lesional calcification (Fig. 6).
Fig. 6.
Coronal section of right hepatectomy specimen demonstrating the unencapsulated, lobulated features of this tumor. Note the proximity to the right hepatic vein (large arrow) and the area of intralesional calcification (small arrow).
Microscopic examination of the permanent sections revealed a tumor composed of nests of epithelial and spindle cells within a myofibroblastic stroma with occasional foci of bone and calcification (Fig. 7A and 7B). Focal proliferating bile ductules were present at the periphery of the nests and the grossly recognized hepatic parenchyma at the edge of the specimen revealed hepatocytes with mild steatosis. Mitotic activity within the lesional cells was low. A battery of immunohistochemical stains was applied and the epithelioid and spindle cells were weakly positive for desmin, cytokeratin (CK) AE1/AE3, CD56 and S100. The nest cells were strongly positive for CD117, b-catenin, vimentin (Fig. 7C), Wilms tumor 1 (WT-1: nuclear label–Fig. 7D), glypican 3 (nuclear label) and CAM5.2.
Fig. 7.
(A) Section of tumor displaying nests of spindle and epithelioid cells with intervening desmoplastic stroma with focal calcification (H&E x 4); (B) Higher magnification of same image demonstrating both epithelioid and spindle cells within a nest with associated calcification (H&E x 10); (C) Vimentin with diffuse immunoreactive staining of spindle and desmoplastic stromal cells with focal staining of epithelioid nest cells (x20); (D) WT-1 with positive nuclear staining of spindle cells and focal, weak nuclear staining of epithelioid cells (x20).
There has been no recurrent tumor seen during 2.5 years of ultrasound follow up examinations.
Discussion
CNSET is a recently described and exceedingly rare hepatic lesion. There are now approximately 40 reported cases in the literature. First reported by Ishak et al. in a series involving 3 patients, the authors proposed the term “ossifying stromal-epithelial tumor” of the liver [1]. Subsequent case reports described this new entity as “ossifying malignant mixed epithelial and stromal tumor,” “desmoplastic nested spindle cell tumor of liver," “nested stromal-epithelial tumor of the liver,” and “calcifying nested stromal-epithelial tumor of the liver” [2,3]. In 2009, Makhlouf et al. reinforced that this tumor should be classified as CNSET [4]. CNSETs mainly affect the pediatric and young adult population with an age range of 2-34 years with a predilection towards females (76%). In most of the reported cases, the tumors were discovered incidentally [5]. A few reported cases presented with Cushing/Cushing-like syndrome sequelae resultant from adrenocorticotropic hormone production by the tumor which resolved following surgical excision [3,5]. To date, 5 cases of locally recurring CNSETs, 1 case with lymph node metastasis and 1 case with pulmonary metastasis have been reported [6,7]]. Therefore, CNSETs may be considered a low-grade malignancy needing continued surveillance. Currently, the standard treatment is surgical excision, and liver transplantation if excision is not possible.
Imaging characteristics in previous case reports of these tumors are similar to that seen in our patient. These tumors are usually seen as large well circumscribed lesions with a macro-lobulated margin with dense calcifications. On CT, it has been described as heterogeneously, but avidly enhancing. On MRI, the lesion is predominantly T1 hypointense and T2 hyper-intense. Dense calcification creates a susceptibility signal void with low T2 signal and blooming on gradient echo sequences. Enhancement on MRI is similarly heterogeneous as on CT, more robust in later phases of contrast [7].
On histology, this neoplasm is a non-hepatocytic and non-biliary tumor of the liver and is characterized by well-demarcated nests of spindle and epithelioid cells in a densely desmoplastic stroma with variable amounts of calcification or ossification. Hepatoblastoma with bone or osteoid formation may pose a diagnostic dilemma, however significantly elevated serum alpha fetal protein in hepatoblastoma should aid in the distinction from CNSETs. The origin of CNSETs remains unknown. One proposal is that the tumor arises from hepatic progenitor cells which can differentiate into hepatocytes and bile duct cells [5]. Makhlouf et al [4] proposed CNSETs may begin as a small calcified lesion and subsequently progress into a large space occupying lesion with an indolent course. Assman et al. [8] suggest that CNSETs may be regarded as a variant of classic hepatoblastoma with a defective mesenchymal-epithelial transition. With the limited number of cases, further investigation is warranted to elucidate the histogenesis of this tumor.
CNSET should be considered in the differential when a large heterogeneous liver tumor with coarse/ chunky calcifications is identified at imaging in the absence of elevated serum AFP in a child. Currently the standard treatment in complete surgical excision and liver transplantation if excision is not possible.
Patient consent
Specific informed consent for case report publication was not directly obtained from the patient or parents at time of diagnosis. Case data was acquired from the medical record before I took over the case. Attempting to find the patient data would require accessing their medical record again. The situation was presented to our IRB which requires case reports be checked by them to prove that they do not meet their definition of research. This was confirmed for this case. Also, in the general admission policies when the patient was admitted, permission for deidentified images and deidentified data to be used for educational and research purposes is included.
The statement should probably read something like, "We declare that under the 'Recordings' section in the Conditions of Admission document signed at admission, images and laboratory data may be used for certain scientific and research activities' and no identifiable information was recorded or published."
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2021.04.087.
Appendix. Supplementary materials
References
- 1.Benedict M, Zhang X. Calcifying nested stromal-epithelial tumor of the liver: an update and literature review. Arch Pathol Lab Med. 2019;143(2):264–268. doi: 10.5858/arpa.2017-0346-RS. PMID: 30354275. [DOI] [PubMed] [Google Scholar]
- 2.Hill DA, Swanson PE, Anderson K, Covinsky MH, Finn LS, Ruchelli ED. Desmoplastic nested spindle cell tumor of liver: report of four cases of a proposed new entity. Am J Surg Pathol. 2005;29(1):1–9. doi: 10.1097/00000478-200501000-00001. PMID: 15613851. [DOI] [PubMed] [Google Scholar]
- 3.Heerema-McKenney A, Leuschner I, Smith N, Sennesh J, Finegold MJ. Nested stromal epithelial tumor of the liver: six cases of a distinctive pediatric neoplasm with frequent calcifications and association with Cushing syndrome. Am J Surg Pathol. 2005;29(1):10–20. doi: 10.1097/01.pas.0000147398.03015.9e. PMID: 15613852. [DOI] [PubMed] [Google Scholar]
- 4.Makhlouf HR, Abdul-Al HM, Wang G, Goodman ZD. Calcifying nested stromal epithelial tumors of the liver: a clinicopathologic, immunohistochemical, and molecular genetic study of 9 cases with a long-term follow-up. Am J Surg Pathol. 2009;33(7):976–983. doi: 10.1097/PAS.0b013e31819c1ab3. PMID: 19363442. [DOI] [PubMed] [Google Scholar]
- 5.Brodsky SV, Sandoval C, Sharma N, Yusuf Y, Facciuto ME, Humphrey M. Recurrent nested stromal epithelial tumor of the liver with extrahepatic metastasis: case report and review of literature. Pediatr Dev Pathol. 2008;11(6):469–473. doi: 10.2350/07-12-0391.1. Epub 2008 Feb 25. PMID: 18338937. [DOI] [PubMed] [Google Scholar]
- 6.Hommann M, Kaemmerer D, Da_ner W, Prasad V, Baum RP, Petrovitch A. Nested stromal epithelial tumor of the liver - liver transplantation and follow-up. J Gastrointest Cancer. 2011;42(4):292–295. doi: 10.1007/s12029-010-9248-7. PMID: 21221846. [DOI] [PubMed] [Google Scholar]
- 7.Jo BJ, Yoon SW, Ahn HJ, Kwon SW. Imaging findings of calcifying fibrous tumour of the liver. Br J Radiol. 2011;84(998):e31–e34. doi: 10.1259/bjr/30585776. PMID: 21257832; PMCID: PMC3473855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assman G, Kappler R, Zeindl-Eberhart E, Schmid I, Häberle B, Graeb C. β-Catenin mutations in 2 nested stromal epithelial tumors of the liver - a neoplasia with defective mesenchymal-epithelial transition. Hum Pathol. 2012;43(11):1815–1827. doi: 10.1016/j.humpath.2012.03.018. Epub 2012 Jun 29. PMID: 22749188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.