Abstract
OBJECTIVE.
The objective of our study was to evaluate the utility of ferumoxytol-enhanced MR lymphography (MRL) in detection of metastatic lymph nodes (LNs) in patients with prostate, bladder, and kidney cancer.
SUBJECTS AND METHODS.
This phase 2 single-institution study enrolled patients with confirmed prostate (arm 1), bladder (arm 2), and kidney (arm 3) cancer and evidence of suspected LN involvement. Participants underwent ferumoxytol-enhanced MRL 24 and 48 hours after IV injection of 7.5 mg Fe/kg of ferumoxytol. A retrospective quantitative analysis was performed to determine the optimal timing for ferumoxytol-enhanced MRL using percentage change in normalized signal intensity (SI) from baseline to 24 and 48 hours after injection, which were estimated using the linear mixed-effects model in which time (24 vs 48 hours), diseases status, and time and disease status interaction were the fixed-effects independent variables. Differences in normalized SI values between subgroups of lesions were estimated by forming fixed-effects contrasts and tested by the Wald test.
RESULTS.
Thirty -nine patients (n = 30, arm 1; n = 6, arm 2; n = 3, arm 3) (median age, 65 years) with 145 LNs (metastatic, n = 100; benign, n = 45) were included. LN-based sensitivity, specificity, positive predictive value, and negative predictive value of ferumoxytol-enhanced MRL was 98.0%, 64.4%, 86.0%, and 93.5%, respectively. Sensitivity and specificity of ferumoxytol-enhanced MRL did not vary by LN size. Metastatic LNs showed a significantly higher percentage decrease of normalized SI on MRL at 24 hours after ferumoxytol injection than at 48 hours after ferumoxytol injection (p = 0.023), whereas the normalized SI values for nonmetastatic LNs were similar at both imaging time points (p = 0.260).
CONCLUSION.
Ferumoxytol-enhanced MRL shows high sensitivity in the detection of metastatic LNs in genitourinary cancers independent of LN size. The SI difference between benign and malignant LNs on ferumoxytol-enhanced MRL appears similar 24 and 48 hours after ferumoxytol injection, suggesting that imaging can be performed safely within 1 or 2 days of injection. Although ferumoxytol-enhanced MRL can be useful in settings without an available targeted PET agent, issues of iron overload and repeatability of ferumoxytol-en-hanced MRL remain concerns for this method.
Keywords: bladder cancer, ferumoxytol, kidney cancer, lymph node, prostate cancer
Accurate lymph node (LN) staging has a substantial impact on the management of patients with prostate, bladder, and kidney malignancies [1–3]. CT and MRI use morphologic assessments including size and shape features of LNs that are highly integrated into staging guidelines. Although their use is established and considered routine, CT and MRI suffer from low sensitivity and specificity in staging LN involvement [4–7].
Lymphotrophic ultrasmall superparamagnetic iron oxide (USPIO)-enhanced MR lymphography (MRL) is a novel method for noninvasive nodal staging in various cancer types including prostate and bladder malignancies. Several studies have reported the utility of USPIO-enhanced MRI for accurate staging of prostate cancer before surgery and radiotherapy planning, even in populations with normal-sized LNs [8–12]. However, the most commonly studied USPIO agent, ferumoxtran-10, has not been approved by the U.S. Food and Drug Administration (FDA) and is available only in The Netherlands [13]. Ferumoxytol, which is similar to ferumoxtran-10 but is not as highly lymphotrophic, has potential wide availability for MRL because it is FDA-approved for iron replacement therapy in patients with chronic kidney failure and, therefore, is more accessible than ferumoxtran-10. Ferumoxytol was reported to be useful for detection of nodal metastases in an early study [14]. However, no further studies followed that work for detection and characterization of nodal involvement in malignancies. Our group reported a phase 1 dosing study of ferumoxytol in 15 patients and documented an optimal dose of 7.5 mg/Fe/kg for ferumoxytol-enhanced MRL [15]. On the basis of that phase 1 dosing study, we initiated a phase 2 efficacy study in which we aimed to evaluate the utility of ferumoxytol-enhanced MRL in detection of metastatic LNs in patients with prostate, bladder, and kidney cancer. Herein, we report the results of this study.
Subjects and Methods
Study Design and Patient Population
This HIPAA-compliant prospective phase 2 single-institution study was approved by the institutional ethics committee (National Clinical Trials identifier 02141490 on ClinicalTrials.gov). The inclusion criteria were histologically confirmed prostate cancer (adenocarcinoma [arm 1]), bladder cancer (transitional cell carcinoma [arm 2]), or kidney cancer (all renal cell cancer types [arm 3]) and imaging evidence of LN involvement; LN involvement was defined as at least one LN in the chest, abdomen, or pelvis with a short-axis diameter of 1 cm or larger on conventional CT or MRI performed within 8 weeks of ferumoxytol-enhanced MRL. The exclusion criteria were as follows: a history of a known hypersensitivity or allergy to iron and iron compounds, iron overload, contraindications for MRI (e.g., severe claustrophobia unresponsive to oral anxiolytics, cardiac pacemaker, cerebral aneurysm clips, shrapnel injury), or abnormal serum liver function test results suggesting liver dysfunction (serum aspartate aminotransferase and alanine aminotransferase ≥ 3 times the upper limit of normal; total bilirubin ≥ 2 times the upper limit of normal or ≥ 3 mg/dL in patients with Gilbert syndrome).
Planned accrual was 35, 10, and 10 participants for arm 1 (prostate cancer), arm 2 (bladder cancer) and arm 3 (kidney cancer) of the study, respectively. Between May 2014 and September 2017, a total of 44 patients (35 patients in arm 1, six in arm 2, three in arm 3) were enrolled in this study.
MRI Protocol
All MRI studies were performed on a 3-T magnet (Achieva-TX, Philips Healthcare) using a 32-channel sensitivity-encoding cardiac coil. The MRI protocol included axial T2-weighted turbo spin-echo and axial fat-saturated T2*-weighted pulse sequences as defined in our previous study [15]. Patients underwent MRI at baseline and 24 and 48 hours after ferumoxytol injection using the same scanner and image acquisition protocol.
Ferumoxytol injection
Ferumoxytol was manually injected through an IV line at a dose of 7.5 mg Fe/kg over 10–15 minutes followed by a flush of 15 mL of normal saline. This dose was previously established to achieve signal loss in normal nodes while having minimal effect on abnormal nodal tissue [15]. Vital signs were obtained before and 15, 30, 45, and 60 minutes after injection.
Ferumoxytol-Enhanced MR Lymphography Analysis
Imaging analysis was conducted in two parts. First, images were evaluated prospectively using a qualitative approach to determine nodal metastasis in visible nodes using a previously defined and validated USPIO-enhanced MRL evaluation method by a genitourinary radiologist (12 years of experience) [12, 16]. The qualitative evaluation included interpretation of MRI studies performed at baseline and 24 and 48 hours after injection on a commercial PACS (Carestream Health). In the second part, qualitatively evaluated visible LNs were retrospectively quantified with manually contoured ROIs on axial T2*-weighted MRI to obtain the mean signal intensity of the LNs (SInode). The signal intensity (SI) of the visible LNs was normalized using the mean SI of the adjacent muscle tissue on the same slice (SImuscle) The following equation was used to obtain the normalized SI from the LNs (SInormal):
This image-processing method was performed on MRL studies performed at baseline and 24 and 48 hours after injection to define the SI change differences between benign and malignant LNs and to determine the optimum timing (24 vs 48 hours after injection) for assessing these SI changes. The quantitative analysis was performed by a genitourinary radiology fellow using the same PACS platform.
Validation of Ferumoxytol-Enhanced MR Lymphography Findings
Ferumoxytol-enhanced MRL findings were validated using conventional imaging follow-up (CT or MRI) for a minimum duration of 12 months using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria or histopathology results (surgery or biopsy) when available [17].
Statistical Analysis
Both LN-based and patient-based sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of ferumoxytol-enhanced MRL were estimated; 95% CIs for the LN-based estimators were obtained from 2.5% and 97.5% percentiles of the bootstrap resampling distribution of 2000 bootstrap samples obtained by sampling patients with replacement. The Agresti-Coull method was used to calculate the 95% CIs for the patient-based estimators [18].
Percentage changes in normalized SI from baseline to 24 and 48 hours after injection were estimated using the linear mixed-effects model in which time (24 vs 48 hours), disease status (metastatic vs nonmetastatic), and time and disease status interaction were the fixed-effects independent variables, and random intercept at the patient level was used to account for intrapatient correlation of repeated measurements. Percentage changes in normalized SI from baseline were estimated by forming fixed-effects contrasts and differences in LN groups (metastatic vs benign) tested by the Wald test. All tests were two-sided, and p values < 0.05 were considered statistically significant. R software (version 3.5.0, The R Foundation) was used for statistical analysis.
Results
Among the 44 participants, five patients from arm 1 were excluded because there was no histopathology or imaging follow-up information available for validation. The final study population included 39 patients (median age, 65 years; mean age, 63 years; age range, 35–82 years): 30 patients with prostate cancer patients (arm 1; mean serum prostate-specific antigen [PSA], 26.16 ng/mL; median PSA, 4.19 ng/mL; PSA range, 0–270.2 ng/mL]), six patients with bladder cancer (arm 2), and three patients with kidney cancer (arm 3). Forty-two of 44 patients tolerated the ferumoxytol injections well without an allergic reaction or change in vital signs. Two patients experienced grade 1 adverse reactions related to ferumoxytol injections. One patient experienced abdominal discomfort and felt flushed during the injection. In this patient, the injection was stopped immediately, vital signs were checked and were found to be within normal limits, and the injection was started again. Once the injection was completed, the abdominal discomfort and flush feeling symptoms subsided. In another patient, a generalized erythematous rash was observed 1 day after the injection, and the rash resolved within 4 weeks after onset. In all participants, there was no evidence of iron overload during follow-up evaluations.
There were 145 evaluable LNs on feru- moxytol-enhanced MRL: 61 retroperitoneal, 27 external iliac, 20 common iliac, 18 internal iliac, 11 mesenteric, five inguinal, and three mediastinal. The mean long-axis diameter of the LNs was 1.5 cm (median, 1.5 cm; range, 0.5–3.6 cm), whereas the mean short-axis diameter was 1.0 cm (median, 1.0 cm; range, 0.1–2.6 cm). Validation of ferumoxy-tol-enhanced MRL findings was achieved using histopathology in nine patients (n = 43 LNs) (Figs. 1–3) and imaging follow-up in 30 patients (n = 102 LNs) (Fig. 4). Among the 145 LNs, 100 were classified as metastatic in 32 patients and 45 were classified benign in seven patients.
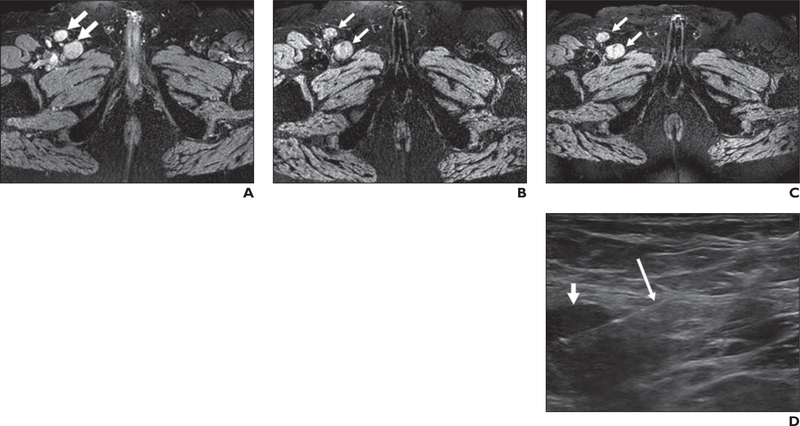
Fig. 1—
67-year-old man with prostate cancer (serum prostate-specific antigen level, 47.63 ng/mL).
A, Axial T2*-weighted MR image obtained before ferumoxytol injection shows right inguinal adenopathy (arrows).
B and C, T2*-weighted MR images obtained 24 (B) and 48 (C) hours after ferumoxytol injection show lack of ferumoxytol uptake within right inguinal adenopathy (arrows).
D, Sonogram obtained during sonography-guided biopsy shows right inguinal adenopathy (short arrow). Long arrow shows biopsy needle. Histopathology results revealed poorly differentiated prostate adenocarcinoma.
Fig. 3—
40-year-old woman with renal cell carcinoma.
A, Axial T2*-weighted MR image obtained before ferumoxytol injection shows right retroperitoneal adenopathy (arrow).
B and C, T2*-weighted MR images obtained 24 (B) and 48 (C) hours after ferumoxytol injection show lack of ferumoxytol uptake within right retroperitoneal adenopathy (arrows). Patient underwent surgery, and histopathology results were consistent with metastatic renal cell carcinoma, papillary type.
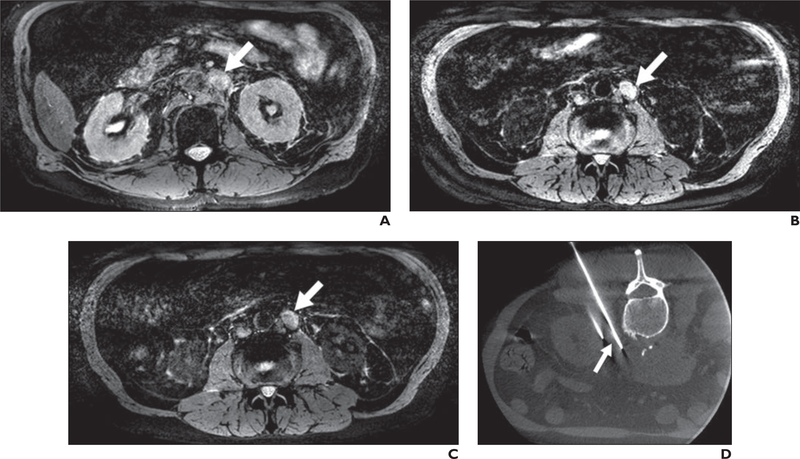
Fig. 4 —
67-year-old man with prostate cancer (serum prostate-specific antigen level, 56.76 ng/mL).
A, Axial T2*-weighted MR image obtained before ferumoxytol injection shows left iliac chain adenopathy (arrow).
B and C, T2*-weighted MR images obtained 24 (B) and 48 (C) hours after ferumoxytol injection show lack of ferumoxytol uptake within left iliac chain adenopathy (arrows). D, Follow-up CT image obtained 6 months after diagnosis and after androgen deprivation therapy shows significant decrease in size of left iliac chain adenopathy (arrow) to current size of 1 × 0.6 cm compared with size on baseline image (A) (1.8. x 1.3 cm). Findings are consistent with metastatic disease within this left iliac chain adenopathy.
Per LN-based sensitivity, specificity, PPV, and NPV of ferumoxytol-enhanced MRL was 98.0%, 64.4%, 86.0%, and 93.5%, respectively. Sensitivity and specificity of ferumoxytol-enhanced MRL did not vary the LN size (short-axis diameter), whereas PPV and NPV of ferumoxytol-enhanced MRL improved at the LN diameter cutoff values of 0.8 and 1.2 cm, respectively (Table 1). Patient-based sensitivity, specificity, PPV, and NPV of ferumoxytol-enhanced MRL was 96.9%, 42.9%, 88.6%, and 75.0%, respectively. The overall accuracy of ferumoxytol-enhanced MRL for LN metastases detection was 87.2% (Table 2).
TABLE 1:
Per-Lymph Node (LN)–Based Sensitivity, Specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) of Ferumoxytol-Enhanced MR Lymphography
| Sensitivity |
Specificity |
Positive Predictive Value |
Negative Predictive Value |
No. of LNs |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LN Size (cm) | Value (%) | 95% CI (%) | p | Value (%) | 95% CI (%) | p | Value (%) | 95% CI (%) | p | Value (%) | 95% CI (%) | p | TP | FP | TN | FN | Total |
| < 0.5 | 100 | 100–100 | 0.145 | 75.0 | 0–100 | 0.663 | 66.7 | 50.0–100 | 0.320 | 100 | 100–100 | 0.157 | 2 | 1 | 3 | 0 | 6 |
| ≥ 0.5 | 98.0 | 94.9–100 | 63.4 | 40.5–83.3 | 86.5 | 76.1–94.5 | 92.9 | 80.8–100 | 96 | 15 | 26 | 2 | 139 | ||||
| < 0.6 | 100 | 100–100 | 0.145 | 57.1 | 0–100 | 0.766 | 40.0 | 0–100 | 0.048 | 100 | 100–100 | 0.164 | 2 | 3 | 4 | 0 | 9 |
| ≥ 0.6 | 98.0 | 94.9–100 | 65.8 | 40.6–85.7 | 88.1 | 78.8–95.5 | 92.6 | 80.0–100 | 96 | 13 | 25 | 2 | 136 | ||||
| < 0.7 | 90.0 | 77.8–100 | 0.243 | 66.7 | 25.0–100 | 0.896 | 69.2 | 40.0–100 | 0.229 | 88.9 | 72.7–100 | 0.536 | 9 | 4 | 8 | 1 | 22 |
| ≥ 0.7 | 98.9 | 96.1–100 | 63.6 | 38.5–84.6 | 88.1 | 79.3–95.5 | 95.5 | 80.0–100 | 89 | 12 | 21 | 1 | 123 | ||||
| < 0.8 | 94.1 | 85.7–100 | 0.329 | 64.0 | 40.0–87.1 | 0.948 | 64.0 | 41.2–88.9 | 0.014 | 94.1 | 84.0–100 | 0.896 | 16 | 9 | 16 | 1 | 42 |
| ≥ 0.8 | 98.8 | 95.7–100 | 65.0 | 36.4–90.0 | 92.1 | 85.3–97.9 | 92.9 | 72.7–100 | 82 | 7 | 13 | 1 | 103 | ||||
| < 0.9 | 95.7 | 88.5–100 | 0.441 | 67.7 | 46.9–86.8 | 0.533 | 68.8 | 47.8–89.4 | 0.018 | 95.5 | 86.7–100 | 0.611 | 22 | 10 | 21 | 1 | 54 |
| ≥ 0.9 | 98.7 | 95.4–100 | 57.1 | 25.0–90.9 | 92.7 | 86.0–98.7 | 88.9 | 60.0–100 | 76 | 6 | 8 | 1 | 91 | ||||
| < 1.0 | 97.1 | 91.4–100 | 0.675 | 63.9 | 41.7–84.4 | 0.914 | 72.3 | 52.8–89.6 | 0.028 | 95.8 | 88.2–100 | 0.495 | 34 | 13 | 23 | 1 | 71 |
| ≥ 1.0 | 98.5 | 94.5–100 | 66.7 | 25.0–100 | 95.5 | 88.2–100 | 85.7 | 50.0–100 | 64 | 3 | 6 | 1 | 74 | ||||
| < 1.1 | 97.8 | 93.1–100 | 0.913 | 66.7 | 44.4–86.0 | 0.623 | 77.6 | 61.1–91.9 | 0.076 | 96.3 | 88.9–100 | 0.406 | 45 | 13 | 26 | 1 | 85 |
| ≥ 1.1 | 98.1 | 93.3–100 | 50.0 | 0–100 | 94.6 | 86.2–100 | 75.0 | 0–100 | 53 | 3 | 3 | 1 | 60 | ||||
| < 1.2 | 98.4 | 95.5–100 | 0.717 | 64.4 | 42.9–83.3 | 79.7 | 66.7–91.4 | 0.001 | 96.7 | 90.0–100 | < 0.001 | 63 | 16 | 29 | 1 | 109 | |
| ≥ 1.2 | 97.2 | 90.0–100 | 100 | 100–100 | 0.0 | 0–0 | 35 | 0 | 0 | 1 | 36 | ||||||
| < 1.3 | 98.4 | 95.5–100 | 0.717 | 64.4 | 42.9–83.3 | 79.7 | 66.7–91.4 | 0.001 | 96.7 | 90.0–100 | < 0.001 | 63 | 16 | 29 | 1 | 109 | |
| ≥ 1.3 | 97.2 | 90.0–100 | 100 | 100–100 | 0.0 | 0–0 | 35 | 0 | 0 | 1 | 36 | ||||||
| < 1.4 | 98.6 | 95.8–100 | 0.611 | 64.4 | 42.9–83.3 | 81.4 | 68.8–92.2 | 0.002 | 96.7 | 90.0–100 | < 0.001 | 70 | 16 | 29 | 1 | 116 | |
| ≥ 1.4 | 96.6 | 87.5–100 | 100 | 100–100 | 0.0 | 0–0 | 28 | 0 | 0 | 1 | 29 | ||||||
| < 1.5 | 98.8 | 96.3–100 | 0.490 | 64.4 | 42.9–83.3 | 83.2 | 72.0–92.8 | 0.002 | 96.7 | 90.0–100 | < 0.001 | 79 | 16 | 29 | 1 | 125 | |
| ≥ 1.5 | 95.0 | 83.3–100 | 100 | 100–100 | 0.0 | 0–0 | 19 | 0 | 0 | 1 | 20 | ||||||
| < 1.6 | 98.8 | 96.5–100 | 0.454 | 64.4 | 42.9–83.3 | 84.2 | 73.4–93.2 | 0.002 | 96.7 | 90.0–100 | < 0.001 | 85 | 16 | 29 | 1 | 131 | |
| ≥ 1.6 | 92.9 | 75.0–100 | 100 | 100–100 | 0.0 | 0–0 | 13 | 0 | 0 | 1 | 14 | ||||||
| < 1.7 | 98.9 | 96.6–100 | 0.439 | 64.4 | 42.9–83.3 | 84.6 | 74.1–93.4 | 0.002 | 96.7 | 90.0–100 | < 0.001 | 88 | 16 | 29 | 1 | 134 | |
| ≥ 1.7 | 90.9 | 66.7–100 | 100 | 100–100 | 0.0 | 0–0 | 10 | 0 | 0 | 1 | 11 | ||||||
| < 1.8 | 98.9 | 96.6–100 | 0.439 | 64.4 | 42.9–83.3 | 84.6 | 74.1–93.4 | 0.002 | 96.7 | 90.0–100 | <0.001 | 88 | 16 | 29 | 1 | 134 | |
| ≥ 0.8 | 90.9 | 66.7–100 | 100 | 100–100 | 0.0 | 0–0 | 10 | 0 | 0 | 1 | 11 | ||||||
| < 1.9 | 98.9 | 96.6–100 | 0.406 | 64.4 | 42.9–83.3 | 85.0 | 74.7–93.7 | 0.002 | 96.7 | 90.0–100 | < 0.001 | 91 | 16 | 29 | 1 | 137 | |
| ≥ 1.9 | 87.5 | 55.6–100 | 100 | 100–100 | 0.0 | 0–0 | 7 | 0 | 0 | 1 | 8 | ||||||
| < 2.0 | 98.9 | 96.6–100 | 0.406 | 64.4 | 42.9–83.3 | 85.0 | 74.7–93.7 | 0.002 | 96.7 | 90.0–100 | < 0.001 | 91 | 16 | 29 | 1 | 137 | |
| ≥ 2.0 | 87.5 | 55.6–100 | 100 | 100–100 | 0.0 | 0–0 | 7 | 0 | 0 | 1 | 8 | ||||||
| All nodes | 98.0 | 95.0–100 | 64.4 | 42.9–83.3 | 86.0 | 75.9–94.1 | 93.5 | 82.6–100 | 98 | 16 | 29 | 2 | 145 | ||||
Note—TP = true-positive, FP = false-positive, TN = true-negative, FN = false-negative.
TABLE 2:
Patient-Based Sensitivity, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and Accuracy of Ferumoxytol-Enhanced MR Lymphography
| Performance Value | Value |
|---|---|
| Sensitivity (%) | 96.9 (31/32) [83–100] |
| Specificity (%) | 42.9 (3/7) [16–75] |
| PPV (%) | 88.6 (31/35) [73–96] |
| NPV (%) | 75.0 (3/4) [29–97] |
| Accuracy (%) | 87.2 (34/39) [73–95] |
Note—The prevalence of metastatic lymph nodes was 82.1% (32/39 patients). Data are presented as percentages with raw numbers of patients in parentheses and 95% CIs (%) in brackets.
The mean percentage changes in normalized SI in metastatic and nonmetastatic LNs on MRL 24 hours after ferumoxytol injec tion were −33.6% and −46.9%, respectively (p = 0.003). The mean percentage changes in normalized SI in metastatic and nonmetastatic LNs on MRL 48 hours after ferumoxytol injection were −24.0% and −40.0%, respectively (p = 0.020). Metastatic LNs showed a significantly higher percentage decrease in normalized SI 24 hours after ferumoxytol injection than 48 hours after ferumoxytol injection (p = 0.023), whereas for nonmetastatic LNs the normalized SI percentage changes were similar at both time points (p = 0.260) (Table 3). The percentage change in normalized SI within both nonmetastatic and metastatic LNs exhibited larger variability on MRL 48 hours after ferumoxytol injection than on MRL 24 hours after ferumoxytol injection (Fig. 5).
TABLE 3:
Comparison of Normalized Signal Intensity (SI) Percentage Changes From Preinjection (Baseline) MR Lymphography (MRL) to Postinjection MRL With Respect to Lymph Node (LN) Disease Status and Time After Ferumoxytol Injection
| 95% Confidence Limits |
|||||
|---|---|---|---|---|---|
| Postinjection Time Point, LNs (Subgroup) or LN Comparison (Subgroups Compared) | Value | SE | Lower | Upper | p |
| Normalized SI percentage change from preinjection MRL to postinjection MRL (%) | |||||
| 24 Hours after ferumoxytol injection | |||||
| Nonmetastatic LNs (A) | −46.9 | 4.0 | −54.8 | −39.0 | |
| Metastatic LNs (B) | −33.6 | 2.9 | −39.2 | −28.0 | |
| 48 Hours after ferumoxytol injection | |||||
| Nonmetastatic LNs (C) | −40.0 | 5.9 | −51.5 | −28.4 | |
| Metastatic LNs (D) | −24.0 | 4.2 | −32.2 | −15.8 | |
| Absolute difference between normalized SI percentage change from preinjection MRL to postinjection MRL (%) | |||||
| 24 Hours after ferumoxytol injection | |||||
| Nonmetastatic vs metastatic LNs (B – A) | 13.3 | 4.4 | 4.6 | 22.0 | 0.003 |
| 48 Hours after ferumoxytol injection | |||||
| Nonmetastatic vs metastatic LNs (D – C) | 16.0 | 6.8 | 2.5 | 29.5 | 0.020 |
| 24 vs 48 hours after ferumoxytol injection | |||||
| Nonmetastatic LNs (C – A) | 6.9 | 6.1 | −5.1 | 19.0 | 0.260 |
| Metastatic LNs (D – B) | 9.6 | 4.2 | 1.4 | 17.9 | 0.023 |
Note—SE = standard error.
Fig. 5—
Box plot shows percentage changes in normalized signal intensity (SInormal) in nonmetastatic and metastatic lymph nodes (LNs) on ferumoxytol- enhanced MR lymphography at 24 and 48 hours after ferumoxytol injection. Thick middle line shows median. Box shows 25th (Q1) to 75th (Q3) percentiles. Lower whisker is Q1 – 1.5x interquartile range (IQR), where IQR = Q3 - Q1. Upper whisker is Q3 + 1.5 x IQR. Circles show outliers.
In the subgroup analysis based on the three different arms of the study (prostate, bladder, and kidney cancers), percentage changes in normalized SI at 24- and 48-hour imaging in metastatic LNs were not different by cancer type (p = 0.384 and 0.404, respectively) (Table 4).
TABLE 4:
Comparison of Normalized Signal Intensity (SI) Percentage Changes in Metastatic Lymph Nodes (LNs) on MR Lymphography (MRL) Performed Before and After Ferumoxytol Injection by Postinjection Time Point and Cancer Type
| 95% Confidance Limits |
||||
|---|---|---|---|---|
| Postinjection MRL Time Point, Cancer Type | Value | SE | Lower | Upper |
| Normalized SI percentage change in metastatic LNs from preinjection MRL to postinjection MRL (%) | ||||
| 24 Hours after ferumoxytol injection | ||||
| Prostate cancer | −36.4 | 3.1 | −42.5 | −30.4 |
| Renal cancer | −26.7 | 7.6 | −41.5 | −11.9 |
| Bladder cancer | −21.1 | 9.7 | −40.1 | −2.1 |
| 48 Hours after ferumoxytol injection | ||||
| Prostate cancer | −29.0 | 5.1 | −39.0 | −19.1 |
| Renal cancer | −26.4 | 13.5 | −52.8 | 0.0 |
| Bladder cancer | −10.3 | 16.6 | −42.8 | 22.3 |
Note—SE = standard error.
Discussion
The results of our study indicate that ferumoxytol-enhanced MRL can be used to detect metastatic LNs in patients with genitourinary cancers including prostate, bladder, and kidney cancers with high sensitivity. The high sensitivities in LN-based (98.0%) and patient-based (96.9%) analyses are comparable with previous studies using ferumoxy-tol-enhanced MRL. Harisinghani et al. [10] investigated ferumoxtran-10 in 80 patients with prostate cancer and reported per-node and patient-based sensitivity values of 90.5% and 100%, respectively. In a multicenter trial, Heesakkers et al. [12] used ferumoxtran-10 in 375 patients with prostate cancer and reported a patient-based sensitivity of 82%. In a study of 75 patients with prostate or bladder cancer, Birkhäuser et al. [9] reported patient-based sensitivity of ferumoxtran-10-enhanced MRL to range between 65% and 75% among three readers. However, ferumoxtran-10 is not widely available and is not FDA-approved and requires an Investigational New Drug designation for use in patients. Our study differs from previous studies in that it uses ferumoxytol instead of ferumoxtran-10 because ferumoxytol is FDA approved and, therefore, is more widely available.
Ferumoxytol is similar to ferumoxtran-10 in that it is composed of an iron oxide core, but ferumoxytol has a carbohydrate coating and ferumoxtran-10 has a dextran-based coating that decreases aggregation. Both agents were developed by the same pharmaceutical company (AMAG Pharmaceuticals), and the metabolic pathway through which both compounds are taken up and metabolized within the reticuloendothelial system is similar [15, 19]. However, it is recognized that ferumoxytol is not taken up by macrophages as avidly as ferumoxtran-10, likely related to differences in macrophage affinity for the different coatings (carbohydrate vs dextran) of the iron oxide. Prior studies, including a phase 1 study from our group, indicate that a higher dose of ferumoxytol (7.5 mg Fe/kg) is needed for MRL applications than the dose for ferumoxtran-10-enhanced MRL (2.6 mg Fe/kg) [15]. The results of our current study show that ferumoxytol-en-hanced MRL can not only yield sensitivity values comparable to ferumoxtran-10 enhanced MRL at both per-node and patient- based levels but also reveal that the sensitivity of LN metastases detection is independent of LN size. Among 71 LNs less than 1 cm in the short axis (n = 35 metastatic), ferumoxytol-enhanced MRL achieved sensitivity (97.1%) nearly identical to its performance for larger LNs (98.5%). Similar results have been reported with ferumoxtran-10 [10], which confirms that the two agents act similarly, albeit at different iron doses.
Previous studies on ferumoxtran-10-en-hanced MRL report quite high specificity for metastases ranging from 93% to 95% [20]. However, both the LN-based and patient-based specificity results (64.4% and 42.9%, respectively) in our study are lower than the reported experience with ferumoxtran-10 in the literature. The lower specificity values can be explained by the relatively weaker T2* signal suppression of ferumoxytol compared with ferumoxtran-10, which was reported by Debats et al. [19] in a study of 44 patients, four of whom underwent both ferumoxtran-10- and ferumoxytol-enhanced MRL. In that study, iron oxide-induced signal suppression in normal LNs was significantly weaker for ferumoxytol than for ferumoxtran-10, and therefore ferumoxytol’s discriminative performance was likely to be lower resulting in more false-positive results [19]. Although this can explain the low specificities in our study, another reason could be our validation method that, in the majority of patients, was conventional imaging follow-up; in three of the four patients in our study with false-positive MRL results, the validation method was conventional imaging, and possible microscopic metastatic disease would be easily missed on conventional imaging. In the remaining one patient with a false-positive MRL result, the LN that appeared suspicious for metastases on ferumoxy-tol-enhanced MRL was in the inguinal region, where fatty replacement and fibrosis may have resulted in the false-positive MRL finding [15].
Iron oxide-enhanced MRL is generally offered as a 2-day test in clinical practice. Patients usually undergo a baseline MRI examination and another MRI examination 24–36 hours after injection [8–12, 14, 16, 19]. However, no formal research has been conducted to date about the timing of the postinjection MRL examination. Our quantitative analysis findings indicate that both on 24- and 48-hour postinjection studies, normal and metastatic LNs show comparable SI changes. Both imaging times reveal normalized SI was significantly different between metastatic and nonmetastatic LNs. However, the percentage change in normalized SI within metastatic LNs exhibited a larger variability on ferumoxytol-enhanced MRL at 48 hours after injection than at the 24 hours after injection, which could result in further false-positive diagnoses for metastases detection. Although the impact of this increased variation at 48 hours was not formally explored in our study, it is a potential limitation of scanning at the later time point.
Ferumoxytol injections were well tolerated by most patients in our study; no grade 3 or 4 adverse events were reported. Although we injected a higher dose (7.5 mg Fe/kg, slightly > 1 vial in most of patients) than the clinically recommended dose (510 mg Fe = 1 vial), there was no evidence of iron overload in our cohort. In previous clinical studies, patients with kidney failure underwent multiple doses of ferumoxytol and the majority tolerated the injections well [21–23]. However, patients with kidney failure are prone to chronic anemia and require iron supplementation, whereas most patients with cancer have normal amounts of iron stores. Consistent with the policy at the time of this study, we injected ferumoxytol slowly over 10–15 minutes. Toward the end of our study, the FDA issued a warning stating that ferumoxytol should be administered diluted as an IV infusion over a minimum of 15 minutes and should not be given as an undiluted IV injection [24], We did not accrue any participants after that statement, but this change will further increase the safety profile of ferumoxy- tol injections.
Early detection of metastatic disease is crucial in genitourinary cancers. However, in addition to ferumoxytol-enhanced MRL there are now new alternatives for nodal staging. For prostate cancer, new PET agents such as fluciclovine (Axumin, Blue Earth Diagnostics) [25–27] and prostate-specific membrane antigen-targeting radiotracers are offering unprecedented sensitivity and specificity for detecting recurrent cancer in LNs after surgery or radiation [28–31]. Ferumoxytol-enhanced MRL is likely to be less sensitive and is certainly less specific than targeted PET tracers. Moreover, ferumoxytol-enhanced MRL is limited in its availability and requires the patient to return for imaging at least 24 hours after initial injection, whereas PET tracers provide whole-body coverage the same day as injection. Ferumoxytol-enhanced MRL should not be performed in patients with iron overload. Moreover, the repeatability of ferumoxytol-enhanced MRL is in question because it is not known how much iron is retained in patients without anemia. However, for patients with other genitourinary malignancies, such as renal or bladder cancer, there is no readily available targeted PET radiotracer and, in properly selected patients, ferumoxytol-enhanced MRL can aid in the diagnosis of LN metastases in these patient populations. In our cohort, ferumoxytol-enhanced MRL correctly depicted LN metastases in all nine patients with renal or bladder cancer.
Our study has several limitations. First, our study includes only 39 participants, the majority of whom had prostate cancer. It was not possible to accrue more patients with renal or bladder cancer because this 3-day study was an extra burden to the medical management schedule of those patients. The multiday nature of this examination will limit its applicability simply on practical terms. Second, in the majority of patients, biopsy was not feasible and annual imaging follow-up had to be used as a validation method. RECIST 1.1 was used for this purpose because it is a widely accepted method of follow-up with validation in large clinical trials.
In conclusion, ferumoxytol-enhanced MRL can yield a high sensitivity in the detection of metastatic LNs in genitourinary cancers independent of LN size. It could be an alternative imaging method for nodal staging in non-prostate cancer patients for whom a suitable targeted PET agent is not available. However, it is possible that the logistical challenges may interfere with the wide use of ferumoxytol-enhanced MRL in clinical practice. The SI difference on T2*-weighted MRL between benign and malignant LNs appears similar on ferumoxy-tol-enhanced MRL 24 and 48 hours after ferumoxytol injection, which suggests that imaging can be performed safely within 1 or 2 days of injection. Ferumoxytol-en-hanced MRL may be a viable option for nodal staging in well-selected patients with genitourinary malignancies.
Fig. 2—
76-year-old man with bladder cancer.
A, Axial T2*-weighted MR image obtained before ferumoxytol injection shows biopsy needle used to sample left retroperitoneal adenopathy (arrow).
B and C, T2*-weighted MR images obtained 24 (B) and 48 (C) hours after ferumoxytol injection show lack of ferumoxytol uptake within left retroperitoneal adenopathy (arrows).
D, CT image obtained during CT-guided biopsy shows biopsy needle used to sample left retroperitoneal adenopathy (arrow). Histopathology results revealed poorly differentiated urothelial carcinoma.
Footnotes
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as representing the views of the National Cancer Institute or the National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Gandaglia G, Soligo M, Battaglia A, et al. Which patients with clinically node-positive prostate cancer should be considered for radical prostatectomy as part of multimodal treatment? The impact of nodal burden on long-term outcomes. Eur Urol 2019; 75:817–825 [DOI] [PubMed] [Google Scholar]
- 2.Mazzone E, Preisser F, Nazzani S, et al. More extensive lymph node dissection improves survival benefit of radical cystectomy in metastatic urothelial carcinoma of the bladder. Clin Genitourin Cancer 2019; 17:105–113.e2 [DOI] [PubMed] [Google Scholar]
- 3.Nazzani S, Mazzone E, Preisser F, et al. Rates of lymph node invasion and their impact on cancer specific mortality in upper urinary tract urothelial carcinoma. Eur J Surg Oncol 2019; 45:1238–1245 [DOI] [PubMed] [Google Scholar]
- 4.Wolf JS Jr, Cher M, Dall’era M, Presti JC Jr, Hricak H, Carroll PR. The use and accuracy of cross-sectional imaging and fine needle aspiration cytology for detection of pelvic lymph node metastases before radical prostatectomy. J Urol 1995; 153:993–999 [PubMed] [Google Scholar]
- 5.Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 2008; 63:387–395 [DOI] [PubMed] [Google Scholar]
- 6.Jager GJ, Barentsz JO, Oosterhof GO, Witjes JA, Ruijs SJ. Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional TI-weighted magnetization-prepared-rapid gradient-echo sequence. AJR 1996; 167:1503–1507 [DOI] [PubMed] [Google Scholar]
- 7.Tiguert R, Gheiler EL, Tefilli MV, et al. Lymph node size does not correlate with the presence of prostate cancer metastasis. Urology 1999; 53:367–371 [DOI] [PubMed] [Google Scholar]
- 8.Thoeny HC, Triantafyllou M, Birkhaeuser FD, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol 2009; 55:761–769 [DOI] [PubMed] [Google Scholar]
- 9.Birkhäuser FD, Studer UE, Froehlich JM, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol 2013; 64:953–960 [DOI] [PubMed] [Google Scholar]
- 10.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003; 348:2491–2499 [DOI] [PubMed] [Google Scholar]
- 11.Heesakkers RA, Jager GJ, Hövels AM, et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumox-tran-10-enhanced MR imaging. Radiology 2009; 251:408–414 [DOI] [PubMed] [Google Scholar]
- 12.Heesakkers RA, Hövels AM, Jager GJ, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol 2008; 9:850–856 [DOI] [PubMed] [Google Scholar]
- 13.Fortuin AS, Brüggemann R, van der Linden J, et al. Ultra-small superparamagnetic iron oxides for metastatic lymph node detection: back on the block. Wiley Interdiscip Rev Nanomed Nanobio-technol 2018; 10:e1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harisinghani M, Ross RW, Guimaraes AR, Weissleder R. Utility of a new bolus-injectable nanoparticle for clinical cancer staging. Neoplasia 2007; 9:1160–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turkbey B, Agarwal HK, Shih J, et al. A phase I dosing study of ferumoxytol for MR lymphography at 3 T in patients with prostate cancer. AJR 2015; 205:64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desemo WM, Harisinghani MG, Taupitz M, et al. Urinary bladder cancer: preoperative nodal staging with ferumoxtran-10-enhanced MR imaging. Radiology 2004; 233:449–456 [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–247 [DOI] [PubMed] [Google Scholar]
- 18.Agresti A, Coull B. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 1998; 52:119–126 [Google Scholar]
- 19.Débats OA, Fortuin AS, Meijer HJ, et al. Intranodal signal suppression in pelvic MR lymphography of prostate cancer patients: a quantitative comparison of ferumoxtran-10 and ferumoxytol. Peer J 2016; 4:e2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoeny HC, Barbiéri S, Froehlich JM, Turkbey B, Choyke PL. Functional and targeted lymph node imaging in prostate cancer: current status and future challenges. Radiology 2017; 285:728–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auerbach M, Strauss W, Auerbach S, Rineer S, Bahrain H. Safety and efficacy of total dose infusion of 1,020 mg of ferumoxytol administered over 15 min. Am J Hematol 2013; 88:944–947 [DOI] [PubMed] [Google Scholar]
- 22.Spinowitz BS, Schwenk MH, Jacobs PM, et al. The safety and efficacy of ferumoxytol therapy in anemic chronic kidney disease patients. Kidney Int 2005; 68:1801–1807 [DOI] [PubMed] [Google Scholar]
- 23.Vasanawala SS, Nguyen KL, Hope MD, et al. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med 2016; 75:2107–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FDA drug safety communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). U.S. Food and Drug Administration website. www.fda.gov/drugs/drugsafety/ucm440138.htm. Published March 30, 2015. Accessed September 3, 2019
- 25.Odewole OA, Tade FI, Nieh PT, et al. Recurrent prostate cancer detection with anti-3 - [(18)F] FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 2016; 43:1773–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akin-Akintayo OO, Jani AB, Odewole O, et al. Change in salvage radiotherapy management based on guidance with FACBC (Fluciclovine) PET/CT in postprostatectomy recurrent prostate cancer. Clin Nucl Med 2017; 42:e22–e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selnæs KM, Krüger-Stokke B, Elschot M, et al. 18F-Fluciclovine PET/MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur Radiol 2018; 28:3151–3159 [DOI] [PubMed] [Google Scholar]
- 28.Roach PJ, Francis R, Emmett L, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian Prospective Multicenter Study. J Nucl Med 2018; 59:82–88 [DOI] [PubMed] [Google Scholar]
- 29.Giesel FL, Knorr K, Spohn F, et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 2019; 60:362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe SP, Macura KJ, Mena E, et al. PSMA-based [(18)F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol 2016; 18:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR. Metaanalysis of 68Ga- PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med 2019; 60:786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]