Abstract
The genetic hallmark of epithelioid hemangioendothelioma (EHE) is a recurrent WWTR1-CAMTA1 fusion, which is present in most cases bearing a conventional histology. A subset of cases is characterized by a distinct morphology and harbors instead a YAP1-TFE3 fusion. Nevertheless, isolated cases lack these canonical fusions and remain difficult to classify. Triggered by an index case of a left atrial mass in a 76-year-old female with morphologic features typical of EHE, but which showed an WWTR1-MAML2 fusion by targeted RNA sequencing, we searched our files for similar cases displaying alternative WWTR1 fusions. A total of 6 EHE cases were identified with variant WWTR1 fusions, four of them presenting within the heart. There were 3 females and 3 males, with a wide age range at diagnosis (21–76 years, mean 62, median 69). The 4 cardiac cases occurred in older adults (mean age of 72, equal gender distribution), three involved the left atrium and one the right ventricle. One case presented in the vertebral bone and one in pelvic soft tissue. Microscopically, all tumors had morphologic features within the spectrum of classic EHE; two of the cases appeared overtly malignant. All cases were tested by FISH and 4 were investigated by targeted RNA sequencing. Two tumors harbored WWTR1-MAML2 fusions, one WWTR1-ACTL6A, and in 3 cases no WWTR1 partner was identified. Of the 4 patients with follow-up, 2 died of disease, one was alive with lung metastases, and the only patient free of disease was s/p resection of a T11 vertebral mass. Our findings report on additional genetic variants involving WWTR1 rearrangements, with WWTR1-MAML2 being a recurrent event, in a small subset of EHE, which appears to have predilection for heart.
Keywords: Epithelioid hemangioendothelioma, WWTR1, heart
1. INTRODUCTION
Epithelioid hemangioendothelioma (EHE) is a malignant vascular neoplasm, characterized by significant heterogeneity in both clinical presentation and prognosis1. Its clinical behavior is intermediate in severity between the benign nature of hemangiomas and highly aggressive angiosarcomas2. Microscopically, conventional EHE is composed of nests and cords of epithelioid endothelial cells within a myxohyaline or sclerotic stroma; immunohistochemistry (IHC) usually demonstrates diffuse strong expression of ERG and CD31. The majority of EHE (>90%) harbor unique WWTR1-CAMTA1 fusions. This may be leveraged for diagnostic purposes by molecular methods, or surrogate IHC for CAMTA13–5. A small subset of EHE bearing a distinct morphology, but overlapping clinical features, harbor instead a YAP1-TFE3 fusion with concomitant nuclear TFE3 overexpression6. A minority of tumors with otherwise typical histologic features, however, lack these canonical fusions thereby complicating molecular classification. Prompted by an index case of a cardiac EHE with a variant WWTR1-MAML2 fusion identified by RNA sequencing, we studied five additional EHE cases from our molecular files with variant WWTR1 fusion partners.
2. MATERIAL AND METHODS
Clinical data, including age, gender, and anatomic site were retrieved from pathology reports. Hematoxylin and eosin-stained slides from biopsy and resection specimens were re-reviewed by two of us (AS, CRA). Pathologic criteria for aggressive clinical behavior (aka malignant EHE) included moderate to severe nuclear atypia (nuclear enlargement and hyperchromasia), easily discerned nucleoli, and mitotic activity. In all cases IHC for endothelial markers (CD31, ERG) was confirmed positive. Tumors were first tested by fluorescence in situ hybridization (FISH) for WWTR1-CAMTA1 and YAP1-TFE3 gene rearrangement, and if negative were subsequently tested by FISH for WWTR1-MAML2 (the fusion gene found in the index case), or targeted RNA sequencing for possible other gene abnormalities. Follow-up information was obtained from the electronic medical records or referring pathologists. The study was approved by the Institutional Review Board.
2.1. Fluorescence In Situ Hybridization
Custom probes made by bacterial artificial chromosomes (BAC) clones flanking the genes of interest according to UCSC genome browser (http://genome.ucsc.edu) and obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (Oakland, CA; https://bacpacresources.org/). DNA from each BAC was isolated according to the manufacturer’s instructions. The BAC clones were labeled with fluorochromes (fluorescent-labeled dUTPs, Enzo Life Sciences, New York, NY) by nick translation and validated on normal metaphase chromosomes. The 4 μm-thick FFPE slides were deparaffinized, pretreated, and hybridized with denatured probes. After overnight incubation, the slides were washed, stained with 4’,6-diamidino-2-phenylindole, mounted with an antifade solution, and then examined on a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany) controlled by Isis 5 software (Metasystems). Two hundred successive nuclei were examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems, Newton, MA). A positive score was interpreted when at least 20% of the nuclei showed a break-apart signal. Nuclei with incomplete set of signals were omitted from the score.
2.2. Targeted RNA Sequencing
Four cases were analyzed by targeted RNA sequencing, using RNA extracted from FFPE tissue with the Amsbio’s ExpressArt FFPE Clear RNA Ready kit (Amsbio LLC, Cambridge, MA). The fragment length was assessed with an RNA 6000 chip on an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA-seq libraries were prepared using 20 to 100 ng total RNA with the TruSight RNA Fusion Panel (Illumina, San Diego, CA). Targeted RNA sequencing was performed on an Illumina MiSeq platform. Reads were independently aligned with STAR (version 2.3) against the human reference genome (hg19) and analyzed by STAR-Fusion.
3. RESULTS
We retrieved 6 EHE with variant WWTR1 gene fusions. Their clinical and pathological features are summarized in Table 1. Of the 4 patients with available follow-up, 2 died of disease, at 9 and 15 months since diagnosis. One patient was alive with lung metastases, while one patient remained free of disease 70 months follow-up, s/p resection of a T11 vertebral mass.
Table 1.
Clinicopathological features of 6 EHE cases with variant WWTR1 gene rearrangements
| Case 1α | WWTR1-MAML2 | EHE | 76/F | Heart, left atrium | N/A |
| Case 2 | WWTR1-MAML2 | EHE | 21/M | Bone, vertebra T11 | NED, 70 months (s/p resection) |
| Case 3α | WWTR1-ACTL6A | Malignant EHE | 73/F | Heart, right ventricle | DOD, 9 months |
| Case 4α | WWTR1 rearrangement | EHE | 72/F | Heart, left atrium | DOD, 15 months (s/p chemo Adriamycin +DTIC), soft tissue metastases |
| Case 5α | WWTR1 rearrangement | EHE | 67/M | Heart, left atrium, | Lung metastases at diagnosis |
| Case 6 | WWTR1 rearrangement | Malignant EHE | 65/M | Pelvic mass | Recent case |
cases tested by targeted RNA sequencing (case 4 RNAseq failed, case 5 was negative); N/A, not available
3.1. Alternative WWTR1 fusions are associated with EHE presenting as cardiac tumors
Remarkably, 4/6 EHE with variant WWTR1 gene rearrangements originated as cardiac tumors. All 4 patients with cardiac EHE were elderly (range 67–73 years), including three females and one male. Three tumors were localized in the left atrium, while one patient presented with a right ventricle mass and developed lung metastases. The three atrial EHE showed classic histologic features of relatively large epithelioid cells with eosinophilic and vacuolated cytoplasm arranged in radiating cords or solid sheets, embedded in a variable amount of myxohyaline mesenchymal stroma. The cells lacked significant nuclear atypia and mitoses were sparse (Figure 1). In contrast, the right ventricular EHE had overt malignant features, with increased cellularity and limited fibrous stroma. This EHE had a nested or cord-like architecture with relatively large epithelioid cells showing significant nuclear atypia and brisk mitotic activity (Figure 2). By IHC, all four cases stained positive for CD31 and ERG.
Figure 1. Morphologic spectrum of cardiac atrial tumors with WWTR1 variant fusions.
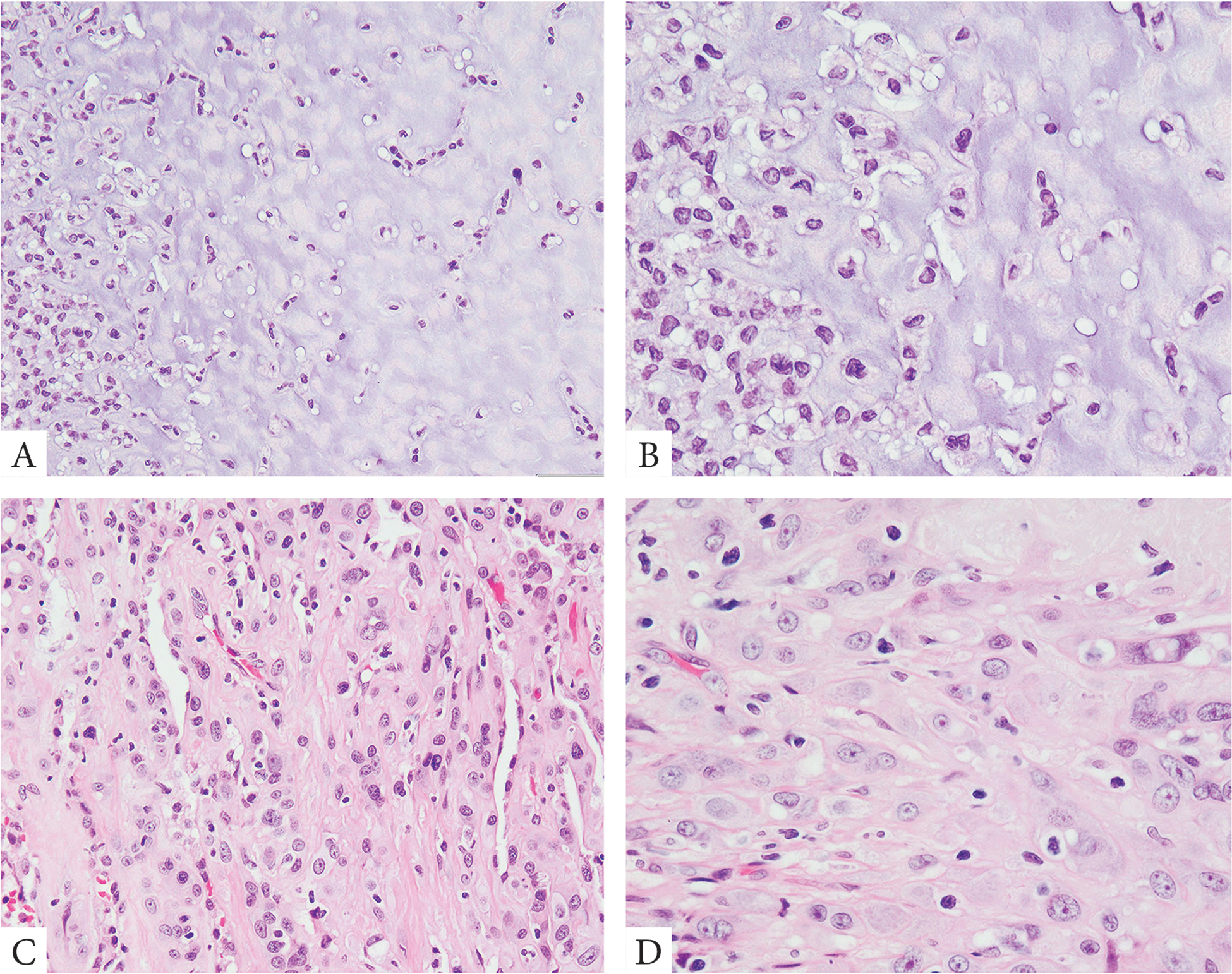
Bland epithelioid cells arranged in single files and cords within an abundant myxochondroid matrix (A, B, case 1). A more cellular lesion showing compact epithelioid cells with dense eosinophilic cytoplasm arranged in solid sheets with a less conspicuous fibrotic stromal component (C, D, case 5).
Figure 2.
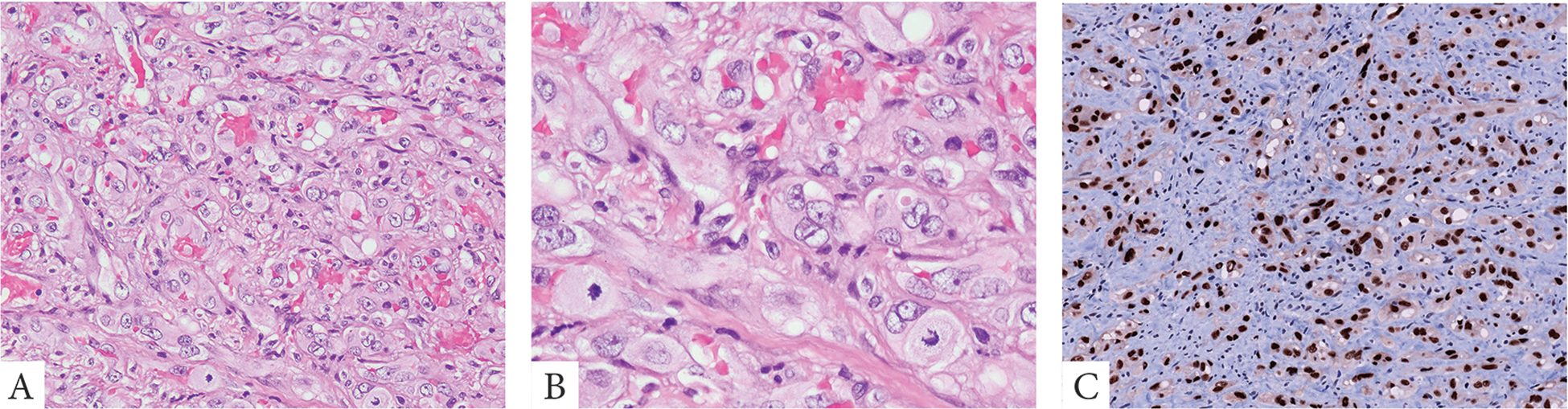
A malignant EHE presenting as a right ventricular mass with lung metastases. Tumor was composed of sheets of enlarged epithelioid cells with abundant pale eosinophilic cytoplasm and enlarged nuclei with moderate atypia and prominent nucleoli. Mitotic figures were abundant. Tumor showed strong immunoreactivity for ERG (A-C, case 3).
3.2. EHE with variant WWTR1 rearrangements may occasionally occur at other locations
One case originated in bone, and another in soft tissue. The first case, harboring a WWTR1-MAML2 fusion, represented a bone tumor involving the 11th thoracic vertebra of a 21-year-old male. A bone needle biopsy revealed a cellular but otherwise classic EHE composed of randomly arranged epithelioid cells without nuclear atypia or increased mitotic activity. The tumor cells stained positive for endothelial markers. The second case concerned a large pelvic mass in a 64-year-old female. This EHE consisted of small trabeculae with malignant epithelioid tumor cells, showing nuclear and nucleolar pleomorphism and easily discerned mitoses. Tumor cells were surrounded by scant fibrous stroma which contained many neutrophilic and eosinophilic granulocytes (Figure 3). In this case, a diagnosis of malignant EHE was established by IHC with expression of ERG and CD31. Moreover, FISH revealed rearrangement of WWTR1, but the tumor lacked rearrangement of both MAML2 and CAMTA1 genes. NGS in this case failed to identify any fusion gene candidate.
Figure 3.
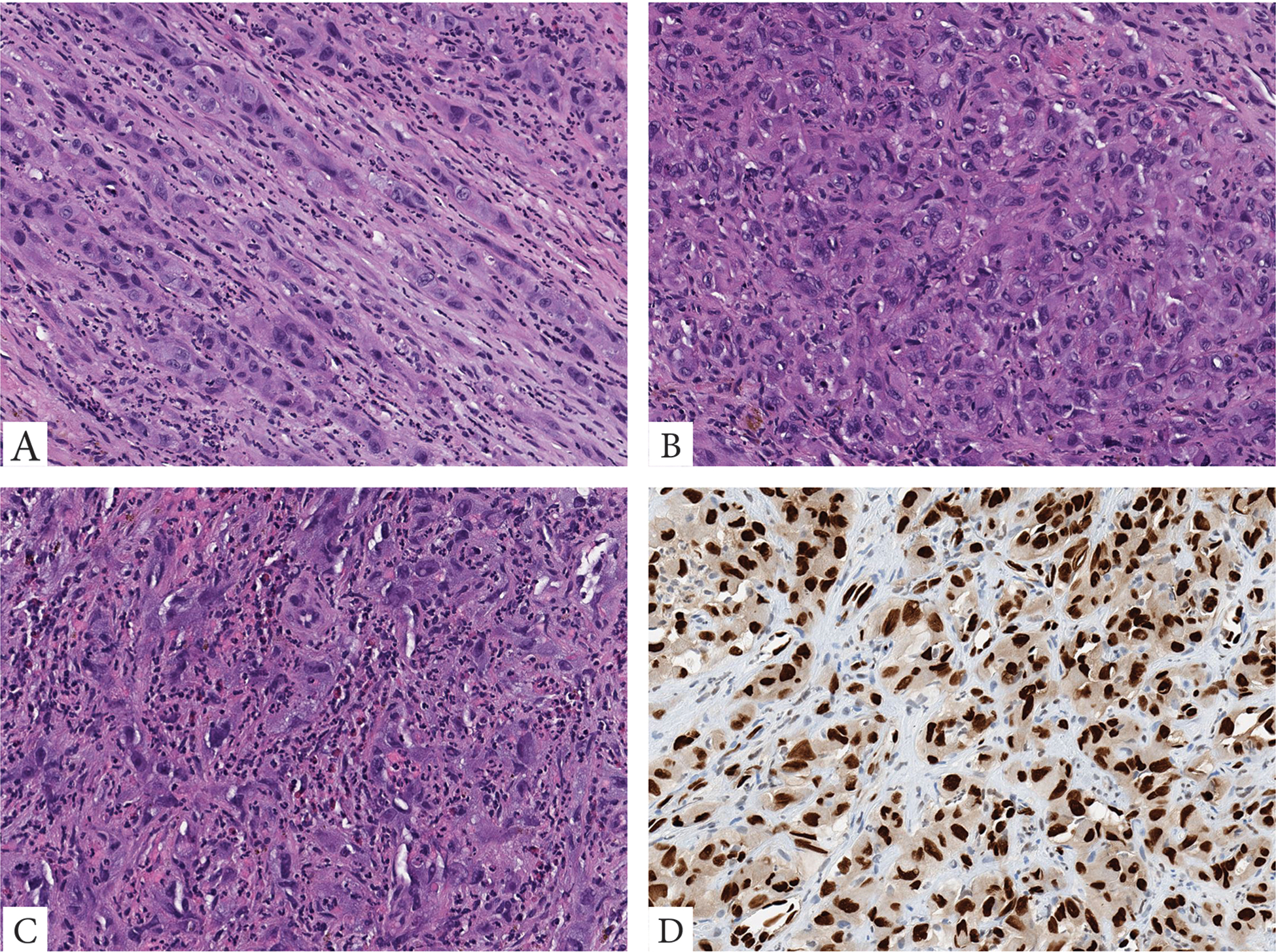
A malignant EHE with WWTR1 rearrangement only, presenting as a large pelvic mass in a 65-year-old male. The tumor is composed of large epithelioid to plump ovoid cells with abundant eosinophilic cytoplasm, arranged in cords (A), solid sheets (B), with variable nuclear pleomorphism, prominent nucleoli and intermixed inflammatory cells including eosinophils (C). Tumor cells show strong nuclear reactivity for ERG (D).
3.3. Molecular Findings
In the index case (case 1), targeted RNA sequencing revealed a WWTR1-MAML2 fusion gene, composed of the first 5 exons of WWTR1 fused to MAML2 exons 2–5 (Figure 4). In case 3, a malignant EHE in the right ventricle, targeted RNA sequencing revealed a novel WWTR1-ACTL6A gene fusion, composed of the first 4 exons of WWTR1 fused to most of the ACTL6A gene (exons 2–14) (Figure 4). Moreover, two tumors showed WWTR1 rearrangements by FISH (without MAML2 and CAMTA1 abnormalities).
Figure 4. Diagrammatic representation of 2 WWTR1 fusion variants identified on targeted RNA sequencing.
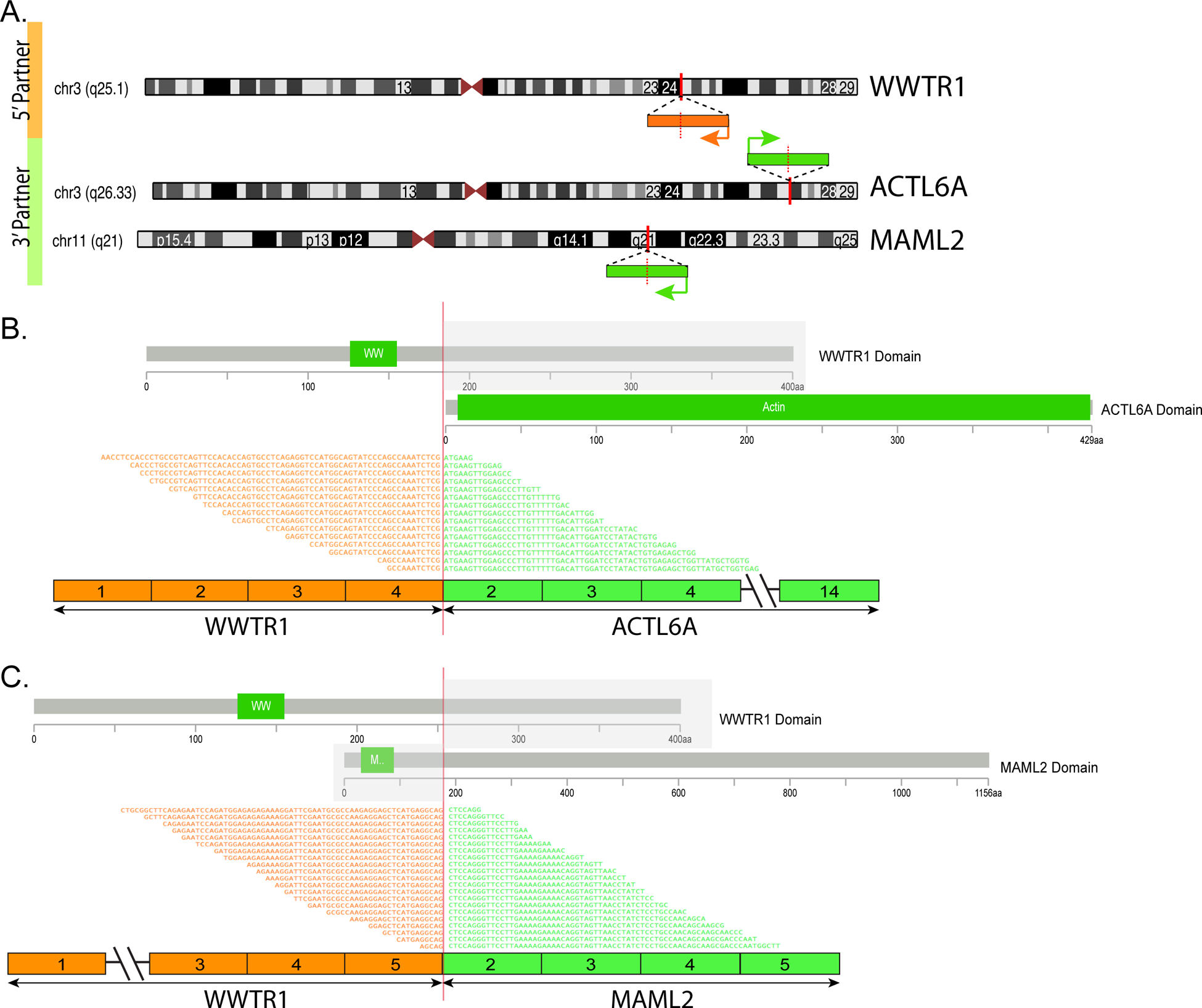
(A) Chromosomal location of WWTR1 gene locus in 3q25.1, ACTL6A in 3q26.33 and MAML2 in 11q21; red vertical lines depict the genomic breakpoint locus. Arrows show the direction of transcription of each gene. (B) WWTR1-ACTL6A transcript is composed of the first 4 exons of WWTR1 fused to most of ACTL6A (exons 2–14). (C) WWTR1-MAML2 transcript composed of the first 5 exons of WWTR1 gene fused to most of MAML2 gene (exons 2–5). The protein domains of each of the genes involved is also schematically depicted.
4. DISCUSSION
In the past decade, several molecular genetic discoveries have contributed significantly to the pathologic subclassification of epithelioid vascular neoplasms7. In this respect, 2 molecular subsets of epithelioid hemangioendothelioma (EHE) have been defined, characterized by recurrent gene fusions WWTR1-CAMTA1 (90%) and YAP1-TFE3 (10%), sharing overlapping clinical presentations but associated with distinct morphologic appearances3,4,6. EHE is a low grade malignant vascular tumor with clinical and morphologic features intermediate between benign epithelioid hemangioma and high-grade epithelioid angiosarcoma3,4,6,8. Triggered by a handful of cases lacking these cannonical genetic abnormalities, we investigated a small cohort of EHE harboring alternative WWTR1 fusions. By RNA sequencing, we identified two novel gene fusions, showing that WWTR1 may partner with either MAML2 or ACTL6A in EHE. To date, these fusion genes have not been reported in other neoplasms.
WWTR1 and its paralogue YAP1 encode the proteins TAZ and YAP, respectively, which are downstream effectors of the Hippo tumor suppressor pathway and function as regulators of TEAD-dependent transcription. In WWTR1-CAMTA1 fusion-positive EHE, CAMTA1 binding transports the chimeric TAZ-CAMTA1 protein from the cytoplasm to the nucleus, resulting in uncoupling of the Hippo pathway and activation of a TAZ-like transcriptional program, contributing to oncogenic transformation9.
Interestingly, recurrent YAP1-MAML2 fusion genes were recently described in a large group of skin adnexal tumors (poromas)10 and in uncommon biphasic thymic tumors (metaplastic thymomas)10; consistently retaining the N-terminal TEAD-binding domains of YAP1 within the chimeric oncoprotein. Furthermore, YAP1 fusion products accumulate in the nuclei of immortalized human epidermal keratinocytes, where they activate TEAD-dependent transcription, and promoted cell growth of mouse embryonic fibroblasts10. ACTL6A is a subunit of the SWI/SNF (BAF) complex and a regulator of various oncogenes, including the Hippo pathway regulator WWC1. Moreover, as observed in human glioma cells, ACTL6A enhances the transcriptional activity of nuclear YAP/TAZ by inhibition of YAP proteosomal protein degradation.11
In soft tissue locations, EHE has a broad anatomic distribution with a predilection for the extremities. Approximately half of cases are angiocentric and appear to arise from a large caliber blood vessel. EHE affects all age groups but is most common in the second decade of life. There is an almost equal gender distribution. Soft tissue EHE larger than 3 cm and with increased mitotic rate (> 3 mitoses in 50 HPF) are considered high-risk tumors apt to develop metastases in up to 40% of cases, whereas smaller EHE with low nuclear grade and scant mitoses are considered to be low-risk tumors.12 In a large cohort of 93 molecularly confirmed EHE involving different sites, it was found that most soft tissue EHE are solitary.1 In addition to tumor grade, multifocal disease, pleural involvement, and lymph node or distant metastases had significant power in predicting prognosis. Moreover, EHE with WWTR1-CAMTA1 fusions had a less favorable clinical outcome than EHE with YAP1-TFE3, with a five-year survival rate of 59% and 86%, respectively.1
In this modest series of EHE with alternative WWTR1 abnormalities, the majority (4/6) of cases represented cardiac tumors presenting in elderly patients. This is a remarkable observation, since most EHE originate in soft tissue and bone locations, and other visceral organs (in particular lung/pleura and liver), and present in adult patients with a wide age range. Of the 4 cardiac EHE, three occurred in the atria and showed classic histology with cords and nests of endothelial cells surrounded by variable myxohyaline stroma. This architectural pattern was highlighted by immunostaining for the endothelial markers ERG and CD31. In the 3 atrial EHE, the tumor cells had round nuclei and moderate amounts of eosinophilic cytoplasm, occasionally with intracytoplasmic vacuoles. The fourth cardiac EHE originated in the right ventricle and this tumor had malignant histologic features with aggressive clinical behavior, with development of lung deposits.
Primary cardiac tumors are uncommon. Among benign cardiac tumors, cardiac myxoma is most prevalent and frequently (in about 85% of cases) attached to the left atrial septum. The large majority of cardiac myxomas are sporadic tumors, but around 5% are a manifestation of the Carney complex13. Both manifestations are associated with PRKAR1A gene mutations14. Histologically, cardiac myxoma may be mistaken for EHE, since both neoplasms consist of cords of epithelioid tumor cells suspended in myxohyaline matrix. However, in cardiac myxoma the tumor cells usually form a perivascular reticular network, a histological growth pattern that becomes clearly visible with IHC staining for calretinin15.
Intimal sarcoma and angiosarcoma are the most frequent malignant cardiac tumors. Whereas intimal sarcoma may present in all heart chambers, cardiac angiosarcoma nearly always arises in the right heart. Morphologically, intimal sarcoma displays a variegated phenotype, but at the low-grade end of the spectrum, tumors contain myxoid areas of low cellularity. However, most intimal sarcomas are characterized by primarily spindle-shaped cells with variable nuclear pleomorphism and hyperchromasia. Moreover, at the molecular level, MDM2 and CDK4 gene amplification or overexpression may help in this distinction16. Cardiac angiosarcoma represents a clinically aggressive high grade sarcoma usually composed of slit-like channels or cellular fascicles of eosinophilic spindle cells, often associated with necrosis, increased mitotic activity and hemorrhagic stroma. In contrast to EHE, angiosarcomas have a complex molecular signature17 and lack recurrent WWTR1 gene rearrangements7.
The differential diagnosis of EHE mainly includes other epithelioid vascular tumors with IHC expression of ERG and CD31, in particular epithelioid hemangioma (EH) and pseudomyogenic hemangioendothelioma (PHE). Classic EH of soft tissue and bone has a wide age range, no gender predilection, and variable anatomic distribution. Occurrence in visceral sites is extremely uncommon. Morphologically, EH of soft tissue and bone has as lobular growth pattern, with overt vascular channel formation lined by epithelioid endothelial cells and surrounded by SMA-positive pericytes. However, the morphology of a subset of EH with cellular and atypical features may closely resemble EHE. In these challenging cases, molecular genetic studies may be of great help, as virtually all EHE harbor either WWTR1-CAMTA1 (90%) or YAP1-TFE3 (10%) fusion products, whereas about half of cellular/atypical EH harbor FOS or FOSB fusions with different partners18. PHE is another distinctive but rare epithelioid endothelial neoplasm of soft tissue and bone19. Most often, PHE presents as multicentric nodules in superficial and deep soft tissue of the extremities in young adult patients. PHE has a high recurrence rate and distant metastases may occur, albeit infrequently (around 5%). PHE is composed of fascicles of large spindle or epithelioid tumor cells with eosinophilic cytoplasm resembling myoid cells. By molecular genetics more than half of PHE harbor FOSB fusions with ACTB or SERPINE1 or WWTR120–22.
In conclusion, with the advent of targeted RNA sequencing the molecular subclassification of vascular tumors, including EHE, continues to evolve. Our results demonstrate that a subset of cases contain novel WWTR1 fusion partners, including MAML2 and ACTL6A, along with potentially other gene partners, which showed a striking preference for cardiac presentation. This anatomic location is very rarely encountered among EHE cases with the more typical fusions. Clearly, larger series of EHE with variant WWTR1 fusions are needed for more definitive clinicopathological correlations, including prognostication.
Disclosures:
Supported in part by: CA 140146 (CRA), CA217694 (CRA), CA008748, Cycle for Survival (CRA), Kristin Ann Carr Foundation (CRA)
Footnotes
Conflicts of Interests: none
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Rosenbaum E, Jadeja B, Xu B, et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher C, Bridge JA, Hogendoorn PC, et al. WHO Classification of Tumours of Soft Tissue and Bone. 4th Edition: . IARC: Lyon; 2013. [Google Scholar]
- 3.Errani C, Zhang L, Sung YS, et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanas MR, Sboner A, Oliveira AM, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Science Translational Medicine. 2011;3:98ra82. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LA, Fletcher CD, Hornick JL. Nuclear Expression of CAMTA1 Distinguishes Epithelioid Hemangioendothelioma From Histologic Mimics. Am J Surg Pathol. 2016;40:94–102. [DOI] [PubMed] [Google Scholar]
- 6.Antonescu CR, Le Loarer F, Mosquera JM, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. 2013;52:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonescu C Malignant vascular tumors--an update. Mod Pathol. 2014;27 Suppl 1:S30–38. [DOI] [PubMed] [Google Scholar]
- 8.Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, et al. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanas MR, Ma S, Jadaan FO, et al. Mechanism of action of a WWTR1(TAZ)-CAMTA1 fusion oncoprotein. Oncogene. 2016;35:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekine S, Kiyono T, Ryo E, et al. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. The Journal of Clinical Investigation. 2019;130:3827–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji J, Xu R, Zhang X, et al. Actin like-6A promotes glioma progression through stabilization of transcriptional regulators YAP/TAZ. Cell Death Dis. 2018;9:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deyrup AT, Tighiouart M, Montag AG, et al. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol. 2008;32:924–927. [DOI] [PubMed] [Google Scholar]
- 13.Burke A, Tavora F. The 2015 WHO Classification of Tumors of the Heart and Pericardium. J Thorac Oncol. 2016;11:441–452. [DOI] [PubMed] [Google Scholar]
- 14.Maleszewski JJ, Larsen BT, Kip NS, et al. PRKAR1A in the development of cardiac myxoma: a study of 110 cases including isolated and syndromic tumors. Am J Surg Pathol. 2014;38:1079–1087. [DOI] [PubMed] [Google Scholar]
- 15.Terracciano LM, Mhawech P, Suess K, et al. Calretinin as a marker for cardiac myxoma. Diagnostic and histogenetic considerations. American Journal of Clinical Pathology. 2000;114:754–759. [DOI] [PubMed] [Google Scholar]
- 16.Neuville A, Collin F, Bruneval P, et al. Intimal sarcoma is the most frequent primary cardiac sarcoma: clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. Am J Surg Pathol. 2014;38:461–469. [DOI] [PubMed] [Google Scholar]
- 17.Leduc C, Jenkins SM, Sukov WR, et al. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Hum Pathol. 2017;60:199–207. [DOI] [PubMed] [Google Scholar]
- 18.Huang SC, Zhang L, Sung YS, et al. Frequent FOS Gene Rearrangements in Epithelioid Hemangioma: A Molecular Study of 58 Cases With Morphologic Reappraisal. Am J Surg Pathol. 2015;39:1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35:190–201. [DOI] [PubMed] [Google Scholar]
- 20.Walther C, Tayebwa J, Lilljebjorn H, et al. A novel SERPINE1-FOSB fusion gene results in transcriptional up-regulation of FOSB in pseudomyogenic haemangioendothelioma. J Pathol. 2014;232:534–540. [DOI] [PubMed] [Google Scholar]
- 21.Agaram NP, Zhang L, Cotzia P, et al. Expanding the Spectrum of Genetic Alterations in Pseudomyogenic Hemangioendothelioma With Recurrent Novel ACTB-FOSB Gene Fusions. Am J Surg Pathol. 2018;42:1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panagopoulos I, Lobmaier I, Gorunova L, et al. Fusion of the Genes WWTR1 and FOSB in Pseudomyogenic Hemangioendothelioma. Cancer Genomics Proteomics. 2019;16:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]


