Abstract
Loneliness is associated with increased morbidity and mortality. Deeper understanding of neurobiological mechanisms underlying loneliness is needed to identify potential intervention targets. We did not find any systematic review of neurobiology of loneliness. Using MEDLINE and PsycINFO online databases, we conducted a search for peer-reviewed publications examining loneliness and neurobiology. We identified 41 studies (n = 16,771 participants) that had employed various methods including computer tomography (CT), structural magnetic resonance imaging (MRI), functional MRI (fMRI), electroencephalography (EEG), diffusion tensor imaging (DTI), single-photon emission computed tomography (SPECT), positron emission tomography (PET), and post-mortem brain tissue RNA analysis or pathological analysis. Our synthesis of the published findings shows abnormal structure (gray matter volume or white matter integrity) and/or activity (response to pleasant versus stressful images in social versus nonsocial contexts) in the prefrontal cortex (especially medial and dorsolateral), insula (particularly anterior), amygdala, hippocampus, and posterior superior temporal cortex. The findings related to ventral striatum and cerebellum were mixed. fMRI studies reported links between loneliness and differential activation of attentional networks, visual networks, and default mode network. Loneliness was also related to biological markers associated with Alzheimer’s disease (e.g., amyloid and tau burden). Although the published investigations have limitations, this review suggests relationships of loneliness with altered structure and function in specific brain regions and networks. We found a notable overlap in the regions involved in loneliness and compassion, the two personality traits that are inversely correlated in previous studies. We have offered recommendations for future research studies of neurobiology of loneliness.
Subject terms: Physiology, Anatomy
Introduction
Loneliness is a critical determinant of well-being and also a grand challenge to society [1, 2]. Defined as distress due to perceived discrepancy between desired and existing social relationships, loneliness is associated with higher rates of cardiovascular disorders [3], dementia [4], anxiety, depression, suicidal ideation [5, 6], and 30% greater mortality [7–9]. Loneliness is distinct from objective social isolation or the lack of social relationships/contacts. The National Academies of Science, Engineering, and Medicine recently published a report on social isolation and loneliness among older adults, calling for more research of neurobiology and interventions [2]. During the COVID-19 pandemic, loneliness, which has been linked to physical distancing measures, is a growing concern for all age groups across the world.
Humans are a social species and have ingrained neural, hormonal, and genetic mechanisms to help navigate social connections. Absence of quality relationships threatens health and reproduction [10]. Cacioppo et al. posited loneliness evolved to improve survivability when socially isolated, through hypervigilance and increasing motivation to connect with others [10]. Animal models of social isolation have demonstrated alterations in neurotransmitters, receptor sensitivities, and levels of certain biomarkers [10, 11]. Few studies have examined the impact of social isolation on specific brain regions [11–13]. Furthermore, the subjective nature of loneliness as well as inter-species differences in social functioning and brain structure limit the applicability of the animal studies to the uniquely human state of loneliness [11].
Our recent investigations have found a strong and consistent inverse correlation between the personality traits of loneliness and wisdom, especially the empathy/compassion component of wisdom [14–17]. In contrast to loneliness, wisdom is associated with better mental and physical health [18–20]. The prefrontal cortex and limbic striatum reportedly play a major role in the neurobiology of empathy/compassion and wisdom [21]. Identifying neurobiological mechanisms underlying loneliness is critical for understanding how loneliness contributes to poor mental and physical health and for conceptualizing potential pharmacological and neurostimulation targets. Therefore, we conducted a systematic review to identify and synthesize published brain-based findings linked to loneliness.
Methods
Search strategy
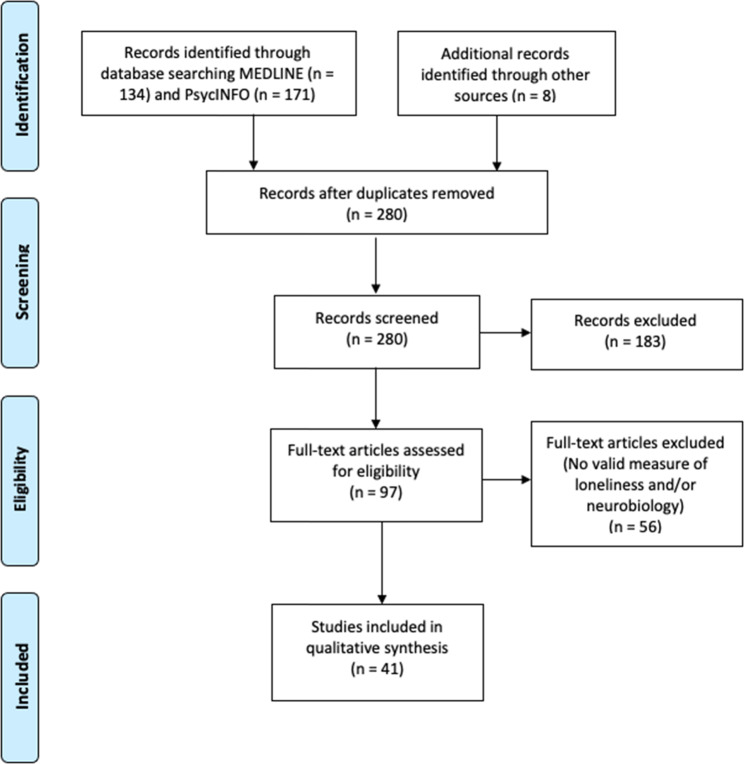
We conducted a literature search for peer-reviewed publications examining loneliness and neurobiology, outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram (Fig. 1). We surveyed MEDLINE and PsycINFO online databases on September 24, 2020, with the following inclusion criteria: (1) use of a validated scale for assessing loneliness and a measure of neurobiology, (2) published in English, (3) minimum of 10 human participants, and (4) statistical analysis examining the relationship of loneliness and neurobiology. We excluded animal studies and literature reviews.
Fig. 1.
PRISMA flow diagram for this systematic review that details the database searches, number of abstracts screened, and full-text articles evaluated for this literature review.
We defined validated measures of loneliness as scales or questions that measured feeling lonely, socially isolated, or disconnected. The most commonly used scale was the University of California Los Angeles Loneliness Scale (UCLA-LS [15, 17, 22]), although we also included validated, briefer multiple- or single-item questions [23]. Neurobiology measures included assessments of brain structure or function: computed tomography (CT), magnetic resonance imaging (MRI), functional MRI (fMRI), diffusion tensor imaging (DTI), positron emission tomography (PET), single-photon emission computed tomography (SPECT), or electroencephalography (EEG). We also included brain pathology studies and genetic investigations that extracted genetic materials from brain regions. We did not include studies with only cognitive measures or studies using cortisol or other peripheral biomarkers from blood or other tissues outside the brain. The specific search strategy is outlined in Supplementary Appendix A.
The search yielded 305 articles of interest. After removing duplicates and adding potentially relevant papers from bibliographies of the articles selected, each study title and abstract was screened for eligibility by at least two authors (JAL, ERM, KEY, MR). Articles with any uncertainties were discussed and resolved among all authors. Data from each of the final batch of 41 studies selected (Fig. 1) were extracted by the primary author and checked by at least one other coauthor. Sample sizes ranged from 19 to 10,129. Most of the studies (61%) included fewer than 100 individuals, 38% with 100–942 individuals, and one study with over 10,000 individuals.
To assess the quality of the studies, we used the Joanna Briggs Institute appraisal checklist for cross-sectional studies and cohort studies and Newcastle-Ottawa Scale for case-control studies (Supplementary Table 2).
Results
Study participant characteristics
Twenty-four studies focused on younger adults (mean age 18–60; [24–47]), 12 on older adults (mean age >60; [48–59]), two on adolescents [60, 61], and two across the lifespan [62, 63]. Most reports included healthy individuals while seven focused on clinical populations: four with depression [47, 49, 51, 57], and one each with traumatic brain injury (TBI) [58], schizophrenia [28], and severe hearing impairment [27]. Eighteen studies came from the US, 13 from China, three each from Germany and Taiwan, and two from the UK, and one each from Japan and the Netherlands. Of the 41 studies, 5 (12.2%) had hypothesis-driven analyses (e.g., region of interest focused), 21 (51.2%) had exploratory analyses (e.g., whole brain analyses), 10 (24.4%) had both, with 5 studies not fitting into any of the above categories.
Fifteen studies analyzed the relationship between loneliness and brain structures using CT [58] or MRI. Twelve were cross-sectional, with eight focusing on gray matter volume, [26, 31, 35, 36, 46, 57, 59, 63], and four on white matter features, employing DTI or diffusion MRI [29, 32, 38, 61]. The three longitudinal investigations included a randomized controlled trial (RCT) of effects of exercise on gray matter volume [54], a prospective cohort study of progression of white matter hyperintensities [53], and a study of TBIs localized to different brain areas [58]. These study findings are summarized in Table 1.
Table 1.
(A) Structural gray matter studies (structural MRI). (B) Structural white matter studies (DMRI/DTI). (C) Structural longitudinal/cohort studies (MRI or CT).
| (A) | ||||||
| Study, location | Participants | Mean age (SD; range) | % Female | Loneliness measure mean (SD) | Primary findings | Notes |
|
Kanai et al. United Kingdom [26] |
108 healthy adults |
23.5 (4.4; 18–32) |
57% |
UCLA-LS (20 items) NS |
Loneliness was negatively associated with GMV in the left pSTS. This relationship was mediated by social perception skills. Covariates: age, gender |
—Limited covariates —Exploratory whole brain analysis |
|
Kong et al. China [31] |
308 healthy adults |
19.9 (1.3; 18–27) |
54% | UCLA-LS (20 item): 41.70 (7.9) |
Loneliness was positively associated with rGMV of left dlPFC. This relationship was partially mediated by neuroticism and extraversion. Covariates: age, gender, and total gray matter volume |
—Limited covariates —Exploratory whole brain analysis |
|
Tian et al. China [36] |
130 total adults; 118 analyzed | 19.9 (1.0) for the 130 total | 54% for 130 total | UCLA-LS (20 item): 61.0 (42.2) for 130 total |
Loneliness mediates the relationship between left amygdala volume and social distress. Covariates: age, gender, and the intracranial volume |
—No primary analysis of loneliness and neurobiology. Only included loneliness as a covariate in their primary analysis of “social distress” and brain volumes |
|
Sin et al. Taiwan [57] |
52 Total 23 healthy control (HC) 19 single episode of depression (SE) 10 multiple episodes of depression (ME) |
67.92 (5.0) HC: 67.1 (4.8) SE: 67.1 (5.5) ME: 71.4 (2.6) |
63% HC: 61% SE: 74% ME: 50% |
UCLA-LS (20 item): 37.7 (10.9) HC: 33.2 (8.3) SE: 41.4 (12.5) ME: 41.0 (10.2) |
Loneliness was associated with GMV in the left striatum and was affected by the recurrence of depressive episodes. Covariates: age, gender |
—Limited covariates —Exploratory whole brain analysis |
|
Liu et al. Taiwan [35] |
405 healthy adults | 19.9 (1.2) | 54% | UCLA-LS (20 item): 41.1 (8.0) |
Loneliness partially mediates the negative correlation between the GMV of the left dlPFC and attitudes toward suicide. Covariates: gender, age, intelligence, tGMV |
—Exploratory whole brain analysis |
|
Düzel et al. Germany [59] |
319 healthy older adults; Berlin Aging Study | 70.1 (3.7; 61–82) | 38% | UCLA-LS (7 item): 1.6 (0.6) |
Loneliness was associated with smaller GMV in the left amygdala/anterior hippocampus, left posterior parahippocampus, and left cerebellum. Significant loneliness by age interactions were found for dlPFC, amygdala, hippocampus, and anterior cingulate cortex. Loneliness by depression interaction were revealed in dlPFC and insula. Covariates: age, gender, education, social network, size, depressive affect, openness, morbidity, total intracranial volume, time interval between MRI and cognitive/psychosocial assessment |
—Exploratory whole brain analysis and hypothesis-driven ROI analysis —ROI were selected based on prior studies exploring brain regions |
|
Wong et al. China [63] |
99 total 33 concordant [C; similar levels of loneliness (L) and social isolation (SI)] 33 robust (R; lower L, high SI) 33 susceptible (S; higher L, low SI) |
33.4 (18.6; 14–69) C: 32.9 (16.8) R: 38.7 (20.1) S: 28.6 (17.7) |
52% C: 61% R: 36% S: 58% |
UCLA-LS (20 item): 38.1 (8.7) C: 37.9 (8.1) R: 33.4 (7.7) S: 43.0 (7.8) |
Significant group differences (between C, R, and S) detected in vermis lobule VI and vermis crus II in the cerebellum GM. S group had more cerebellar GM than the C and R groups. Covariates: age, sex, education, depressive affect, openness, morbidity, time interval between the two sessions, number of confidants, and TIV |
—Hypothesis-driven ROI analysis —No primary analysis of loneliness and neurobiology. Instead, authors focused on susceptibility to loneliness. |
|
Kiesow et al. United Kingdom [46] |
10,129 individuals from UK Biobank | 55 (7.5; 40–69) | 52% | One-item loneliness measure | Greater volumetric deviations of the amygdala between lonely and non-lonely males compared to females and more volumetric deviations in vmPFC and visual sensory network in between lonely and non-lonely females compared to males. |
—Exploratory whole brain analysis —Probabilistic modeling strategy —Only presented data on sex differences |
| (B) | ||||||
| Study, location | Participants | Mean age (SD; range) | % Female | Loneliness measure: mean (SD) | Primary findings | Notes |
|
Tian et al. China [29] |
30 adults | 21.3 (2.4) | 0% | UCLA-LS (20 items): NS | DTI: Loneliness associated with poorer connectivity of white matter tracts linked to the nodes (IFG, AI, and TPJ) of the ventral attentional network. |
—Hypothesis-driven ROI analysis: IFG, TPJ, and AI, —All-male sample |
|
Nakagawa et al. Japan [32] |
776 healthy adults | 20.7 (1.8; 18–27) | 44% | UCLA-LS (20 item): 37.0 (9.2) |
DTI: Loneliness negatively correlated with regional white matter density in the bilateral IPL, right AI, left pSTS, left pTPJ, left dmPFC, and left rlPFC. Covariates: age, gender, general intelligence, and total intracranial volume |
—Exploratory whole brain analysis |
|
Meng et al. China [38] |
162 total 42 Val/Val Genotype (V/V) 90 Val/Met Genotype (V/M) 30 Met/Met Genotype (M/M) |
19.8 (1.3; 18–26) V/V −20.0 (1.2) V/M −19.6 (1.2) M/M −20.0 (1.4) |
59% V/V −57% V/M −59% M/M −60% |
UCLA-LS. (20 item): 41.6 (7.9) V/V −42.6 (8.3) V/M −40.7 (7.5) M/M −43.0 (8.5) |
DTI: The relationships between loneliness and diffusion measures were significantly different between the Val/Met group and the Val/Val group, particularly in the white matter of corpus callosum, bilateral posterior corona radiata, bilateral superior longitudinal fasciculus, and bilateral corona radiata, and right SLF. Covariates: age, gender, depressive symptoms, anxious symptoms |
—Exploratory whole brain analysis |
|
Wong et al. China [61] |
40 adolescents (20 same-sex sibling pairs) | 17.8 (1.2) | 55% | UCLA-LS (20 item): 39.5 (9.5) | DMRI: Loneliness associated with lower structural local efficiency in the posterior right cerebellum lobe, right hippocampus, left caudate nucleus, bilateral superior and inferior temporal lobe, calcarine fissure, and middle occipital gyrus. Structural network efficiency was found to mediate the association between negative affect scores and loneliness. | —Exploratory whole brain analysis |
| (C) | |||||||
| Authors | Participants | Mean age (SD; range) | % Female | Loneliness measure: mean (SD) | Study design | Primary findings | Notes |
|
Duan et al. China [53] |
219 total 83 non-empty nest elderly control group (C) 70 couples empty-nest group (CE) 66 single empty-nest group (SE) |
69.9 (5.5) C: 69.7 (5.8) CE: 70.5 (4.8) SE: 69.4 (5.8) |
51% C: 47% CE: 50% SE: 56% |
UCLA-LS (20 item) 37.0 (10.0) C: 29.6 (6.5) CE: 36.1 (7.0) SE: 47.2 (7.3) |
Cohort study that monitored progression of WMHs Average follow-up time of 5.2 years |
Loneliness was significantly and positively correlated with changes in total WMH, periventricular WMH, and deep WMH. Increase in volume of periventricular WMH and total WMH in CE and SE groups were greater than those in the C group. Covariates: Sex, age, smoking, alcohol consumption, education, BMI, HTN, DM, HTN medication, DM medication, BP, fasting lipids, and glucose levels |
—Large attrition rate (38%) from baseline to follow-up |
|
Ehlers et al. USA [54] |
247 healthy community-dwelling older adults who had low physical activity | 65.4 (4.6) | 68% |
UCLA-LS (20 item) Baseline: 37.1 (9.8) 6-month follow-up: 35.3 (8.91) |
Exercise intervention study (no control group). Three 1-h exercise sessions per week for 24 weeks |
At baseline, loneliness was correlated with higher baseline amygdala volume. Loneliness score reduction was associated with larger amygdala or PFC. |
—Inclusion criteria included low activity or inactivity —>30% percent of the sample had missing MRI data at baseline and/or post-intervention |
|
Cristofori et al. USA [58] |
167 total 132 Male Vietnam War veterans with penetrating traumatic brain injury (pTBI) 35 healthy men (HC) |
63.3 (3.1) pTBI: 63.3 (2.9) HC: 63.2 (3.7) |
pTBI: 0% HC: 0% |
UCLA-LS (20 item): 41.2 (11.1) pTBI: 40.0 (10.6) HC: 45.9 (11.9) |
Prospective long-term follow-up study of veterans with focal pTBI typically due to low velocity shrapnel wound |
pTBI patients reported lower UCLA-LS scores than HC group. pTBI patients with right AI and right PFC lesions reported significantly less loneliness than HC group. Covariates: social network size, self-reported quality of friendship, education, depression |
—Voxel-based lesion-symptom mapping analyses to investigate the causal role of focal brain lesions on self-report of loneliness. —Used CT (unlike above two studies) —All-male sample |
(A) C concordant, dlPFC dorsal lateral prefrontal cortex, GM gray matter, GMV gray matter volume, HC healthy control, IFG_tri inferior frontal gyrus triangular part, L loneliness, ME multiple episodes of depression, MTG middle temporal gyrus, MRI magnetic resonance imaging, NS not stated, pSTS posterior superior temporal sulcus, R robust, rGMV regional gray matter volume, ROI region of interest, S susceptible, SE single episode of depression, SI social isolation, tGMV total gray matter volume, TIV total intracranial volume, UCLA-LS University of California Los Angeles loneliness scale, vmPFC ventromedial prefrontal cortex.
(B) AI anterior insula, dmPFC dorsal medial prefrontal cortex, DMRI diffusion MRI, DTI diffusion tensor imaging, IFG inferior front gyrus, IPL inferior parietal lobule, M/M Met/Met genotype, NR not stated, pSTS posterior superior temporal sulcus, pTPJ posterior temporoparietal junction, rlPFC rostro lateral prefrontal cortex, ROIs regions of interest, rWMD regional white matter density, SLF superior longitudinal fasciculus, TPJ temporoparietal junction, UCLA-LS University of California Los Angeles Loneliness Scale, VAN ventral attentional network, V/M Val/Met genotype, V/V Val/Val genotype.
(C) AI anterior insula, BMI body mass index, BP blood pressure, C non-empty nest elderly control group, CE couples empty-nest group, CT Computerized tomography, DM diabetes mellitus, HC healthy controls, HTN hypertension, MRI magnetic resonance imaging, PFC prefrontal cortex, pTBI penetrating traumatic brain injuries, SE single empty-nest group, UCLA-LS University of California Los Angeles Loneliness Scale, WMH white matter hyperintensities.
Eighteen reports analyzed the relationship between loneliness and brain function or connectivity using fMRI. Ten of these studies were task-based (n = 10; [24, 25, 28, 34, 44, 45, 51, 60, 62, 63]), and two were resting-state fMRI (n = 8; [37, 39–43, 47, 49]) (Table 2). One report appears in both Tables 1 and 2 [63]
Table 2.
(A) Task-based fMRI Studies. (B) Resting state fMRI.
| (A) | |||||||
| Study, location | Participants | Mean age (SD; range) | % Female | Loneliness measure: mean score (SD) | Task | Finding | Notes |
|
Eisenberger et al. USA [24] |
30 healthy adults | 20.7 (3.2) | 60% | Two-item scale assessing end-of-day social disconnection: NS | Cyberball task: online game that mimics ostracism during a game | Individuals with greater left hippocampal and left mPFC activity response also had a stronger correlation between momentary social distress and end-of-day social disconnection. |
—Exploratory whole brain analysis —Cyberball task may have limited external validity |
|
Cacioppo et al. USA [25] |
23 healthy undergraduate students | NS | 100% | UCLA-LS (20 item): NS | Displaying images with emotional (unpleasant or pleasant) and social (nonsocial or social) content |
Loneliness correlated to neural activity (negative for pleasant social images, positive for pleasant nonsocial images) in VS, left dmPFC, right medial frontal gyrus, left fusiform gyrus, and left anterior insula. Loneliness correlated to neural activity (negative for unpleasant social images, positive for unpleasant nonsocial images) in left primary visual cortex, right caudate, right inferior frontal gyrus, left superior temporal gyrus, right superior temporal gyrus, and right secondary visual cortex. |
—Exploratory whole brain analysis —All-female sample —Task may have limited external validity for experiencing pleasant and non-pleasant interactions in everyday social interactions. |
|
Lindner et al. Germany [28] |
76 total 36 patients with schizophrenia (SZ) 40 Control (NC) |
30.1(8.1) SZ: 30.8 (7.9; 18–51) NC: 29.5 (8.3; 19–49) |
36% SZ: 39% NC: 33% |
Multidimensional loneliness questionnaire: 28.5 (11.9) SZ: 36.8 (12.9) NC: 23.9 (6.6) |
Displaying sequences of facial expressions with fear, disgust, happiness, and neutral |
In SZ group, but not NC group, bilateral insula activation in response to disgust was positively correlated with loneliness (no significant correlation in NC group). Covariates: Age, gender, trait anxiety, depression severity |
—Exploratory whole brain analysis and hypothesis-driven ROI analysis —Task may have limited external validity for experiencing emotions of others in everyday social interactions. |
|
Inagaki et al. USA [34] |
31 healthy adults | 24.3 (7.6) | 48% | UCLA-LS (20 item): 44.2 (8.7) | Displaying images of either close relationships or gender-, race-, and age-matched strangers | Lonely individuals (≥1 SD above mean on UCLA-LS) had greater VS activity in response to images of close others vs. strangers; no such difference observed in non-lonely individuals (>1 SD below mean on UCLA-LS). |
—Hypothesis-driven ROI analysis —Task may have limited external validity for seeing others everyday social interactions. |
|
Wong et al. China [51] |
54 total 31 with late-life depression (LLD) 23 healthy control (NC) |
67.3 (5.1; >60) LLD: 67.5 (5.4) NC: 67.1 (4.8) |
57% LLD: 55% NC: 61% |
UCLA-LS (20 item): 37.1 (10.5) LLD: 40.0 (11.1) NC: 33.2 (8.3) |
Displaying sequence of positive, negative, and neutral human and non-human pictures |
Loneliness associated with weaker amygdala-SFG connection. Loneliness associated with increased functional connectivity within the DMN and corticostriatal network in LLD, but negatively correlated in NC when processing negative stimuli. Covariates: age, gender, MMSE, Depressive symptoms |
—Whole brain and ROI analysis —Task may have limited external validity for experiencing emotions of others in everyday social interactions. |
|
Wong et al. Chinaa [63] |
99 total community-dwelling adults 33 concordant [C; similar levels of loneliness (L) and social isolation (SI)] 33 robust (R; lower L despite high SI) 33 susceptible (S; higher L despite low SI) |
33.4 (18.6; 14–69) C: 32.9 (16.8) R: 38.7 (20.1) S: 28.6 (17.7) |
52% C: 61% R: 36% S: 58% |
UCLA-LS (20 item): 38.1 (8.7) C: 37.9 (8.1) R: 33.4 (7.7) S: 43.0 (7.8) |
Two-run modified emotion-word Stroop |
C group had a stronger and more negative association between loneliness and activity in the right posterior cerebellum, compared to R and S groups. During positive processing, loneliness positively predicted the right posterior cerebellum functional connectivity with the right visual cortex. |
—Hypothesis-driven ROI analysis —Task may have limited external validity as it only displayed words of emotions. —Task did not include tasks that explicitly assess social cognition or emotion. —No explicit assessment of the participants’ state or trait affect. |
|
D’Agostino et al. USA [62] |
99 total students and community-dwelling older adults 50 young (Y) 49 old (O) |
41.4 (21.8; 18–81) Y: 20.4 (2.0; 18–28) O: 62.9 (6.1; 55–81) |
57% Y: 52% O: 61% |
UCLA-LS (20 item): 40.6 (8.1) Y: 43.6 (8.9) O: 37.6 (7.9) |
Displaying pleasant and unpleasant social and nonsocial images | No significant association between measures of loneliness and any regions in the brain, including two ROIs—the VS and the amygdala —in both Y and O groups. |
—Exploratory whole brain and hypothesis-driven ROI analysis —Task may have limited external validity for experiencing pleasant and non-pleasant interactions in everyday social interactions. —Used different types of social images compared to Cacioppo et al. [25]. |
|
Golde et al. Germany [60] |
41 adolescent students | 15.2 (0.5; 14–16) | 69% | UCLA-LS (10 item): 2.25 (0.5) for 33 participants | Trait-judgment task: rating self, friends, teachers, and politicians with respect to 30 personality traits | Loneliness was related to lower vmPFC activation during self-processing in the adolescent brain. No significant associations during friends and teacher parts of the task. |
—Hypothesis-driven ROI analysis —Task may have limited external validity for seeing others everyday social interactions. |
|
Gao et al. Taiwan [45] |
45 total 21 adults with major depressive disorder (MDD) 24 controls (NC) |
50.2 (5.7) MDD: 52.0 (5.0) NC: 4158.7 (6.0) |
76% MDD: 71% NC: 79% |
UCLA-LS (20 item): 41.1 (12.6) MDD: 50.2 (11.2) NC: 33.2 (7.4) |
n-back (0- and 1-back) working memory task |
Loneliness was positively associated with IPC-rostral dmPFC connectivity in both MDD and NC groups. Loneliness was positively associated with SMA-caudal dmPFC connectivity in NC, but negatively associated in the MDD group. |
—Exploratory whole brain and hypothesis-driven ROI analysis —Limited external validity for task-based MRI |
|
Courtney et al. USA [44] |
50 recruited college students and community-dwelling adults. 43 analyzed |
20.2 (4.6; 18–47) | 60% | UCLA-LS: 41.8 (9.4) | Reflection task on self, close friends, acquaintances, and celebrities, others of varying degrees of closeness reflection task | Loneliness modulates activation to the self and others in mPFC and posterior cingulate cortex. Loneliness was also associated with less mPFC activation. |
—Exploratory whole brain and hypothesis-driven ROI analysis —Limited external validity for task-based MRI neural self-other overlap |
| (B) | ||||||
| Authors | Participants | Mean age (SD; range) | % Female | Loneliness measure mean (SD) | Finding | Notes |
|
Lan et al. Taiwan [49] |
85 Han Chinese veteran males | 80.3 (5.6; >65) | 0% | UCLA-LS (20 item): 29.6 (8.7) | Loneliness was positively correlated with short-range functional connectivity over the bilateral lingual gyrus. |
—Exploratory analysis —Used functional connectivity density mapping —All-male sample |
|
Layden et al. USA [37] |
55 healthy young adults | 23.7 (2.1; 20–29) | 56% | UCLA-LS (20 item): 40 (8.1) |
Loneliness associated with increased brain-wide functional connectivity in the right central operculum and right supramarginal gyrus, which corresponds to the cingulo-opercular network. Covariates: age, gender, objective social isolation, and depressive symptoms |
—Exploratory and hypothesis-driven analysis —Post-hoc secondary analysis. |
|
Tian et al. China [39] |
30 total 15 men with UCLA-LS score >45 15 men with UCLA-LS score <28 |
21.3 (2.4) | 0% | UCLA -LS: NS | Loneliness associated with decreased causal flow from the affective to the visual network, causal flow from dorsal attentional to ventral attentional network. |
—Exploratory and hypothesis-driven analysis —Did not control for objective social isolation or transient mood states —All-male sample |
|
Feng et al. China [41] |
75 healthy adults | 21.9 (3.0) | 17% | Revised UCLA-LS (20 items): NS |
Loneliness predicted key nodes that contributed to the prediction model comprised regions previously implicated in loneliness, including the dlPFC, lateral orbital frontal cortex, ventral mPFC, caudate, amygdala, and temporal regions. Covariates: age, gender, relationship status, and motion |
—Exploratory analysis —Did not completely examine the specificity of the predictive model |
|
Mwilambwe-Tshilobo et al. USA [43] |
942 healthy adults; Human Connectome Project | 28.0 (3.5; 23–37) | 54% | Loneliness survey from the NIH Toolbox on Emotion: 51.0 (8.5) | Loneliness associated with dense, lower modularity (increased integration) between default, frontoparietal, attention and perceptual networks. | —Exploratory analysis |
|
Yi et al. USA [40] |
92 healthy adults; Human Connectome Project | NS (22–35) | 57% | Loneliness survey from the NIH Toolbox on Emotion: 50.5 (8.6) |
Loneliness was positively related to the mean ALFF value within right ITG. The negative relation between emotional support and loneliness was explained by a decrease in the spontaneous neural activity within right ITG, but this pattern was not observed for instrumental support. |
—Exploratory analysis |
|
Liégeois et al. USA [42] |
419 unrelated adults; Human Connectome Project | NS (22–35) | NS | Loneliness survey from the NIH Toolbox on Emotion: NS | Static and dynamic FC explain loneliness equally well, while specifically dynamic FC encodes cognitive tasks like working memory. |
—Exploratory analysis —Limited analysis to percentage of variance explained by static vs. dynamic functional connectivity |
|
Saris et al. Netherlands [47] |
74 patients with major depressive disorder | 36.9 (11.9) | 66% | de Jong-Gierveld Loneliness questionnaire (11 items): 6.4 (3.4) | Higher social dysfunction was associated with decreased DMN connectivity, specifically within rostro mPFC and posterior superior frontal gyrus. |
—Exploratory and hypothesis-driven analysis —Focused on a composite index of social dysfunction (composite score including loneliness, higher social disability, and smaller social network) |
(A) aAlso featured in the structural MRI work; C concordant, DLPFC dorsal lateral prefrontal cortex, DMN default mode network, dmPFC dorsal medial prefrontal cortex, fMRI functional magnetic resonance imaging, GMV gray matter volume, L loneliness, LLD late-life depression, MDD major depressive disorder, mPFC medial prefrontal cortex, NS not stated, NC non-psychiatric control group, O old, R robust, ROI region of interest, S susceptible, SFG superior frontal gyrus, SI social isolation, SMA supplementary motor area, SZ individuals with schizophrenia, UCLA-LS University of California Los Angeles Loneliness Scale, VS ventral striatum, vmPFC ventromedial prefrontal cortex, Y young.
(B) ACC anterior cingulate cortex, ALFF amplitude of low-frequency fluctuations, DMN default mode network, dlPFC dorsal lateral prefrontal cortex, FC functional connectivity, fMRI functional magnetic resonance imaging, ITG inferior temporal gyrus, mPFC medial prefrontal cortex, NIH National Institutes of Health, NS not stated, UCLA-LS University of California Los Angeles Loneliness Scale.
Three investigations used EEG to examine high-density event-related potentials (ERPs) during different tasks [30, 33, 64], two analyzed RNA expression of post-mortem brain tissue [52, 55], two employed PET to analyze amyloid and tau proteins [50, 56], one longitudinal cohort study examined the association between post-mortem brain tissue and Alzheimer’s disease [48], and one used SPECT to analyze dopamine release in the brain [27] (Table 3).
Table 3.
Other studies.
| Study | Methods/Task | Participants | Mean age (SD; range) | % Female | Loneliness measure: mean (SD) | Primary findings | Notes |
|---|---|---|---|---|---|---|---|
|
Cacioppo et al. USA [30] |
EEG task—Displaying sequences of positive and social, positive and nonsocial, negative and social, or negative and nonsocial words |
70 total 38 high loneliness (HL) 32 low loneliness (LL) |
23.6 (5.6) |
43% HL: 45% LL: 41% |
UCLA-LS. (20 item): 40.6 (10.1) HL: 48.0 (6.7) LL: 31.9 (5.1) |
Loneliness associated with differences in ERP waveform between negative (social, nonsocial) and positive (social, nonsocial) words. The differentiation of negative social from negative nonsocial words by brain microstates evoked in the Stroop task occurred earlier in lonely compared to non-lonely. |
—Exploratory whole brain analysis —Hypothesis-generating paper —CENA (Cacioppo, Weiss, et al.) used for analyzing high-density ERP waveforms over a 128-sensor space is non-standard EEG approach [88]. —Limited external validity for experimental block design |
|
Cacioppo et al. USA [33] |
EEG task—Displaying sequences of positive and social, positive and nonsocial, negative and social, or negative and nonsocial words |
27, 19 analyzed 10 high loneliness (HL) 9 low loneliness (LL) |
24.1 (18–44) | 53% |
UCLA-LS. (20 item): 42.3 (11.9) HL: 51.8 (6.6) LL: 31.7 (5.4) |
Loneliness associated with significant differences in the ERP waveform between type of stimulus (social threat, nonsocial threat) and loneliness. Social threat images were differentiated from nonsocial threat stimuli earlier in lonely individuals compared to non-lonely individuals. |
—Exploratory whole brain analysis —Hypothesis-generating paper —CENA (Cacioppo, Weiss, et al.) used for analyzing high-density ERP waveforms over a 128-sensor space is non-standard EEG approach [88]. |
|
Bocincova et al. USA [64] |
EEG task—Participants assigned to write about either a nostalgic event or ordinary experience |
60 Healthy adults 30 Nostalgic Group (NG) 30 Control Group (CG) |
NS | NS | UCLA-LS (10 item): 2.1 (0.5) | There were no significant main effects of loneliness or interactions between loneliness and experimental nostalgia condition on event-related negativity (negative deflection of ERP) amplitude. |
—Exploratory whole brain analysis —The experimental paradigm does not involve external stimuli relevant to social or positive/negative contexts, which are shown by other studies to be sensitive to loneliness. —The participants of the study were not uniformly distributed to include participants with high loneliness scores. |
|
Wilson et al. USA [48] |
Global AD pathology in post-mortem brain and clinical diagnosis of AD |
823 healthy controls from Rush Memory and Aging Project 90 with post-mortem pathology |
80.7 (7.1) at baseline | 76% | de Jong-Gierveld Loneliness Scale: 2.3 (0.6) |
Lonely individuals more likely to develop an AD-like dementia syndrome, even after controlling for level of social isolation. Loneliness was unrelated to summary measures of AD pathology or to cerebral infarction. |
—Post-mortem brain pathology available for only a small subset of participants |
|
Donovan et al. USA [50] |
PET scan | 79 Healthy adults from Harvard Aging Brain Study | 76.4 (6.2; 68–89) | 54% | UCLA-LS (3 item): 5.3 (1.4) |
Loneliness was significantly associated with greater amyloid burden; this association was stronger in APOEε4 carriers. Covariates: age, sex, APOEε4, socioeconomic status, depression, anxiety, and social network |
—Exploratory whole brain analysis |
|
d’Oleire et al. USA [56] |
PET scan | 117 Healthy adults from Harvard Aging Brain Study | 76.0 (6.2; 64–92) | 59% | UCLA-LS (3 item): 5.2 (2.0) |
Loneliness associated with higher tau pathology in the right entorhinal cortex. Covariates: age, sex, apolipoprotein E ε4, the Alzheimer’s disease genetic risk marker, socioeconomic status, social network, depression and anxiety scores, and memory performance |
—Hypothesis-driven ROI analysis on entorhinal cortex and inferior temporal cortex and whole-brain exploratory analysis |
|
Gevonden et al. Netherlands [27] |
SPECT scan pre- and post- amphetamine challenge |
38 total adults 19 severe hearing impairment (SHI) 19 Healthy Controls (HC) |
25.6 (3.0) SHI: 26.0 (3.0) HC: 25.1 (3.0) |
84% SHI: 84% HC: 84% |
UCLA-LS (20 item): 42.0 SHI: 39.6 (8.1) HC: 47.0 (7.1) |
SHI, which was associated with significantly higher loneliness than the HC group, which was associated with a hypersensitive dopamine system. Covariates: age, tobacco smoking Sensitivity analysis: cochlear implantation |
—Hypothesis-driven ROI analysis |
|
Canli et al. USA [52] |
Genome-wide RNA expression in post-mortem nucleus accumbens | 26 healthy adults from Rush Memory and Aging Project | 84.5 (6.6) at initial visit | 46% | Self-reported loneliness scores (5 items): 2.5 (0.8) at last visit before death |
Loneliness associated with 1710 differentially expressed transcripts (previously associated with behavioral processes, neurological disease, psychological disorders, cancer, organismal injury, and skeletal and muscular disorders.) Loneliness associated with AD genes. Study controlled for known AD diagnosis. |
—Exploratory analysis —Extraction of the tissue was conducted without the removal of blood cells and vessels. |
|
Canli et al. USA [55] |
Genome-wide RNA expression in post-mortem dlPFC | 181 healthy adults from Rush Memory and Aging Project | 89.5 (6.2) at death | 66% | Self-reported loneliness scores (5 items): 2.5 (0.7) at last visit before death | Loneliness was most associated with up- or down-regulation of genes associated with AD, cancer, and gene sets associated with the aging brain, behavior, and neuronal or synaptic processes. |
—Exploratory analysis —Tissue was processed without removal of blood leukocytes |
AD Alzheimer’s Disease, CENA Chicago Electrical Neuroimaging Analytics, CG control group, dlPFC dorsal lateral prefrontal cortex, EEG electroencephalogram, ERP event-related potential, HC healthy control, HL high loneliness, LL low loneliness, NG nostalgic group, NS not stated, PET positron emission tomography, SHI severe hearing impairment, SPECT single-photon emission computed tomography, RNA ribonucleic acid, UCLA-LS University of California Los Angeles Loneliness Scale.
The quality of the included cross-sectional studies varied primarily on detailed descriptions of the study sample and setting, identification of confounding factors, and use of appropriate statistical strategies for confounders (Supplementary Table 2). The quality of cohort studies varied primarily on representative case sampling and controlling for confounders. There was only one identified cohort study.
Brain regions
Supplementary Table 2 lists publications sorted by brain regions studied.
Prefrontal cortex or PFC (N = 14 studies)
Two articles focused on overall PFC. In one, male veterans with TBI to the right PFC had lower levels of loneliness compared to healthy controls [58]. An RCT examining effects of exercise on loneliness in older adults found a greater reduction in stress and loneliness in participants with a larger baseline PFC volume, although PFC volume did not change over the 6-month intervention [54].
In seven studies of medial PFC (mPFC), loneliness was associated with greater mPFC activation in task-based fMRI during a social exclusion paradigm [24], less similarity between self-representation and other-representation in mPFC activation [44], lower dorsomedial PFC (dmPFC) white matter density [32], lower left dmPFC response when looking at pleasant social images and greater left dmPFC response when examining nonsocial images [25], increased functional connectivity between dmPFC and inferior parietal cortex during a working memory task [45], reduced vmPFC activation when looking at images of themselves [60], and nonsignificantly greater gray matter volumetric deviations of the vmPFC in females compared to males [46].
In five reports on dorsolateral PFC (dlPFC), loneliness was associated with increased gray matter in left dlPFC [31], partially mediated the negative association between gray matter volume in left dlPFC and attitudes toward suicide [35], and was associated with lower gray matter volume in dlPFC, especially in subjects aged 69–82 compared to those 61–69 years old and in individuals with depression compared to nondepressed subjects [59]. A resting-state fMRI study found the dlPFC as a node in the predictive model of loneliness [41]. Another dlPFC RNA study is discussed below [55].
Insula (N = 6)
Investigators reported an association of loneliness with a lesion in the right insula [58], lower gray matter volume, which was even lower in individuals with depression [59], lower regional white matter density in anterior insula [32], and poorer white matter tract connectivity with the nodes in ventral attentional network [29]. fMRI paradigms showed that among lonely individuals, activation of insula (especially anterior insula) was greater among adults looking at pleasant social (than pleasant nonsocial) images, while ventral striatum activation was greater among non-lonely individuals [25]. Among persons with schizophrenia, insula responsiveness was positively correlated with levels of loneliness, while overall insula activation with faces expressing disgust was decreased [28].
Amygdala (N = 6)
One investigation reported a positive correlation between left amygdala gray matter volume and social distress score, which was mediated by loneliness [36]. Another found that loneliness was associated with lower gray matter volume in left amygdala, especially in subjects aged 61–70 (compared to ages 70–82) [59]. In an RCT of group exercise to improve loneliness among older adults, participants with larger baseline amygdala volumes experienced greater reductions in loneliness [54]. Kiesow et al. found nonsignificantly greater gray matter volumetric deviations of amygdala between lonely and non-lonely males compared to females. [46]. An fMRI region of interest (ROI) analysis failed to find significant differences in amygdala response to social stimuli in young or old adults [62], while another fMRI study found loneliness was associated with a weaker amygdala to superior frontal gyrus connectivity [51].
Ventral striatum/nucleus accumbens (N = 5)
Studies of ventral striatum response to images with task-based fMRI paradigms among lonely individuals (vs. non-lonely individuals) reported different results: reduced response to pleasant social (compared to pleasant nonsocial) images [25]; greater response to images of close others (compared to strangers) [34]; and no significant differences in response to pleasant and non-pleasant social and nonsocial images [62]. One report on association of loneliness with gray matter volume in left striatum among older adults with late-life depression found a positive correlation in single depressive episode individuals and negative association in multiple depressive episode individuals [57]. Another nucleus accumbens RNA study is discussed below [52].
Posterior superior temporal cortex (N = 4)
We defined this region as including both posterior superior sulcus (temporal-parietal junction or TPJ) and the region immediately below it, superior temporal gyrus. Studies reported an association of loneliness with lower white matter regional density [32], less gray matter volume in left posterior superior sulcus [26], lower structural local efficiency in the bilateral superior temporal gyrus [61], and lower bilateral superior temporal gyrus response when looking at unpleasant social images and greater response when looking at unpleasant nonsocial images [25].
Hippocampus (N = 3)
Investigators reported an association of loneliness with reduced anterior hippocampus gray matter volume, especially in older adults [59], lower white matter local structural efficiency (i.e., shorter weighted paths between local nodes) [61], and greater hippocampal response during a social exclusion task [24].
Cerebellum (N = 3)
One study reported that loneliness was associated with lower left cerebellar gray matter volume [59]; however, another report found no main effects of loneliness on cerebellar ROIs [63]. Loneliness was associated with higher connectivity between cerebellum and visual cortex during an fMRI Stroop task using positive words [63], and lower local structural efficiency in the white matter of the posterior cerebellum on structural MRI [61].
Networks
Visual systems (N = 5)
Investigators demonstrated that loneliness was associated with increased activation of left primary visual cortex and right secondary visual cortex when presented with unpleasant social (compared to unpleasant nonsocial) images [25], differences in the connection of visual network (fusiform gyrus, calcarine fissure, lingual gyrus, middle occipital gyrus, cuneus, superior occipital gyrus, and inferior occipital gyrus) to other networks, with decreased causal flow from affective to visual networks [39], and greater right visual cortex functional connectivity to posterior cerebellum when presented positive words in task-based fMRI [63]. Examining sex differences, the volume of visual sensory network (comprised of fusiform gyrus, posterior superior temporal sulcus, and middle temporal V5 area) deviated between lonely and non-lonely women but not men [46].
Attentional systems (N = 4)
Investigations reported an association of loneliness with poorer connectivity of white matter tracts between the nodes of ventral attentional network [29] as well as differential activation of TPJ, a node in ventral attentional network. Loneliness was also linked to a weaker relationship between dorsal and ventral attentional networks indicating decreased ability to filter less relevant stimuli [39], as well as increased functional connectivity in brain regions associated with cingulo-opercular network [37].
Default mode network (DMN) (N = 3)
One study found that higher social dysfunction (defined by loneliness, higher social disability, and smaller social network) was associated with decreased DMN connectivity, specifically in anterior mPFC and posterior superior frontal gyrus [47], while another report showed that loneliness was associated with reduced DMN functional connectivity in older healthy individuals compared to those with late-life depression [51]. Using network analyses, one study found that among lonely individuals, overall resting-state network structures had increased integration (lower modularity) between attentional, visual, and default mode networks [43].
Other studies
Alzheimer’s disease (AD) pathology (N = 4)
A prospective longitudinal study demonstrated that increases in loneliness were correlated with increases in white matter hyperintensities among non-demented older adults [53]. Two cross-sectional studies using PET imaging found a significant relationship between loneliness and higher amyloid burden, especially in APOEε4 carriers [50, 56], and greater tau pathology in right entorhinal cortex and right fusiform gyrus [56]). Another cohort study reported that the risk of the development of AD was significantly higher in lonely (than non-lonely) individuals; however, global AD pathology (β-amyloid plaques, neurofibrillary tangles, or cerebral infarction) in post-mortem brains (n = 90) showed no significant relationship to loneliness [48].
EEG (N = 3)
In two separate publications using a Stroop task, loneliness was associated with faster ERPs with negative social (compared to negative nonsocial) words and threatening social (compared to threatening nonsocial) images [30, 33]. However, another report found no significant main effect of loneliness on error-related negativity (a component of ERP) when writing about a nostalgic event versus an ordinary experience [64].
Brain RNA expression (N = 2)
Two studies of RNA expression in post-mortem brain tissue in nucleus accumbens and dlPFC [52, 55] identified hundreds of differentially expressed transcripts and genes among lonely compared to non-lonely individuals, especially genes associated with AD [52, 55]. The relationships between loneliness and white matter structures were significantly different between BDNF genotypes [38].
Other investigations (N = 3)
One investigation reported loneliness was explained equally well by whole brain static and dynamic functional connectivity, in contrast to traits like cognitive functioning, which were explained better by dynamic connectivity [42]. Another study found loneliness was linked to altered brain activity in right inferior temporal gyrus on MRI and that the neural activity mediated the relationship between loneliness and emotional support [40]. A longitudinal study reported that individuals with severe hearing impairment were lonelier and had a hypersensitive dopamine system in a SPECT scan pre- and post- amphetamine challenge, compared to people without hearing impairment [27].
Discussion
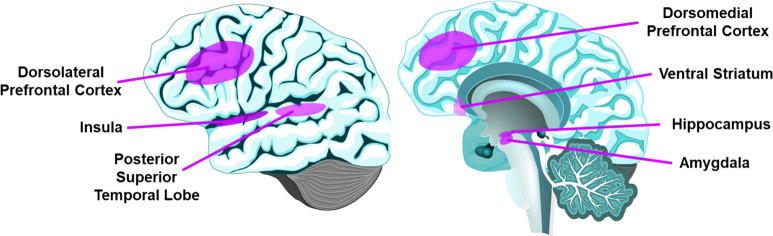
To our knowledge, this is the first systemic review of neurobiology of loneliness. The 41 publications meeting our criteria show that, despite some mixed evidence, loneliness is associated with structural and functional differences in PFC, insula, hippocampus, amygdala, and posterior superior temporal cortex (Fig. 2, Supplementary Table 1), as well as attentional and visual networks and DMN. Drawing overall conclusions from this review is limited by the high heterogeneity of study methodologies and cohorts.
Fig. 2. Summary of the brain structures consistently implicated in loneliness.
Left panel shows the lateral view of the brain with the relevant brain regions highlighted and labeled, while the right panel shows the sagittal view of the brain regions.
While there is no literature on loneliness in non-human animals, neurobiological correlates of social isolation have been examined in several animal studies and a few human studies. There are a few reviews focused on integrating animal social isolation and human research [11–13]. However, the social isolation literature in animal models focus more on changes in endocrinology, neurotransmitters, and oxidative stress, rather than neuroanatomical or functional brain differences [11]. These differing methodologies and paradigms make direct comparison challenging, though there are likely overlapping risk and protective factors for social isolation and loneliness.
The PFC mediates higher-order behaviors like emotional regulation and inhibitory control [65, 66]. The dlPFC is implicated in working memory and executive function [67], and mPFC is implicated in self-referential processes such as self-criticism in social situations [68]. All 14 imaging studies examining PFC found associations of loneliness with structural (gray matter volume and white matter integrity) or functional components (activation with social vs. nonsocial images, and functional connectivity). These results are consistent with a previous review of animal studies of social isolation implicating PFC [13], and support loneliness as a complex socioemotional trait.
The insula somatic marker hypothesis states that insula receives and integrates information to create a “global emotional moment” [69]. Anterior insula plays a role in various behaviors including emotions, pain, and self-awareness [70]. In studies of gray matter, white matter connectivity, task-based activation, insula was reportedly associated with loneliness [25, 28, 29, 32, 58, 59]. It has been proposed that social rejection activates similar regions as physical pain, as supported by bilateral anterior insula activation with feelings of loneliness [70, 71], although a more recent social rejection meta-analysis of fMRI studies did not find anterior insula involvement [72].
The amygdala is implicated in fear detection, positive stimuli processing, and emotional memories [73]. Four studies of amygdala reported some association between gray matter volume or task-based activation and loneliness, with possible age- and sex-interactions [36, 46, 54, 59]. These findings are consistent with loneliness activating brain regions that support experiencing emotions. However, an fMRI study found no relationship with amygdala response to social stimuli [62].
The ventral striatum, which includes nucleus accumbens, plays a central role in reward reinforcement [74]. Three studies of ventral striatum response to social images produced divergent results [25, 34, 62]. A recently published fMRI study (published past our cutoff date) reported similar activation in substantia nigra/ventral tegmental area (SN/VTA) among young adults undergoing either 10 h of social isolation or fasting from food [75], supporting loneliness as a state that motivates one to seek social interaction, much like hunger motivates one to seek food. Interestingly, lonely participants had less activation in the SN/VTA. Research based on social isolation in rodent models and social rejection in human experiments indicates that social isolation may alter social approach motivation [13], consistent with the findings that loneliness differentially alters ventral striatum and structures related to reward pathway.
The posterior superior temporal cortex, implicated in social cognition [76], was associated with loneliness in four studies [25, 26, 32, 61].
The hippocampus, known for its role in memory [77], and the cerebellum, known for sensorimotor coordination as well as cognitive and affective processes [78, 79] each had three papers that associated their function or structure with loneliness.
Attentional networks are responsible for effortful versus environmental, stimulus-driven control of attention, and are localized to distinct anatomical areas with specific cognitive functions [80]. Four publications reported that loneliness was associated with differences in ventral attentional (including TPJ), dorsal attentional, and cingulo-opercular networks, in terms of functional and effective connectivity [29, 37, 39, 43]. Attentional networks may be linked to hypervigilance and stress reactivity that are putatively involved in loneliness.
Visual systems are responsible for processing visual information. Five studies reported associations of loneliness with differences in primary and secondary visual cortex in terms of volume [46], functional connectivity [43], causal flow [39], or activation with social images [25, 63], supporting Cacioppo et al.’s hypervigilance theory of loneliness [6].
The DMN is active when the human brain is at rest and is implicated in mental representations of self across time and space, theory of mind, and pro-social behaviors [81]. Three studies showed an association between loneliness with DMN functional connectivity [43, 47, 51]. One report noted more dense, less modular connections between attentional, visual, and DM networks in lonely persons. A recent large (n = ~40,000), multi-modal study (published after our cutoff date for inclusion in this review) reported increased volume and white matter structural connectivity as well as increased functional connectivity of the DMN in lonely individuals [82]. Together, these results suggest that higher-order brain regions localized to PFC and DMN may play a critical role in loneliness. The DMN may be differentially activated when we are thinking about others; however, dysregulated activity in DMN may contribute to rumination and negative feelings associated with loneliness.
Two EEG studies showed that lonely individuals had faster ERPs to negative or threatening stimuli [30, 33], consistent with the hypervigilance hypothesis of loneliness [6], while another report found no difference in ERPs with a nostalgia-related task [64].
Regarding AD markers, two PET studies reported greater amyloid and tau burden [50, 56], and one MRI study reported progressive increase in white matter hyperintensities among lonely older adults [53]. However, one post-mortem study found no such differences in plaques, tangles, or infarcts [48]. Two studies extracting RNA from the brain identified differential AD-related gene expression in lonely individuals [52, 55]. The overall findings related to AD align with meta-analytic evidence linking loneliness to increased risk of AD [83].
The brain regions highlighted in this review of loneliness may also have roles in other related constructs. For example, we have found a strong inverse correlation between loneliness and wisdom, especially its compassion component [15–17]. An overview of the neurobiology of wisdom has highlighted the major roles of PFC, especially dlPFC, vmPFC, anterior cingulate, and insula as well as amygdala [21, 84]. One MRI study reported that loneliness and empathy were inversely associated with white matter density in lateral PFC, insula, and TPJ [32], while another MRI study found no links with gray matter density [26]. A recent EEG study demonstrated that loneliness and wisdom/compassion were related to contrasting modulations of cognitive processes, invoking similar (TPJ) and distinct (superior parietal vs. insula, respectively) neural circuits in specific emotional contexts [85]. These relationships are correlational and warrant further study employing neurobiological perturbations.
Limitations
This review article as well as the included studies have limitations. It is possible that, despite our best efforts, we missed a few relevant papers. Also, we did not include articles in non-English languages, and 73% (30/41) of the reports came from the USA or China, thereby limiting the generalizability to other countries. Most investigations were cross-sectional, preventing causal inferences. There may be confounding factors that are driving these relationships. There is risk of gender bias in self-reported assessments of loneliness. While there are no agreed upon objective measures of loneliness, indirect partial objective measures may include sedentary behavior assessed with wearable activity trackers, life space using GPS data, and sleep disturbances using wearable sensors. The studies included are limited by varied methodologies and analysis techniques in the rapidly evolving field of social neuroscience. For example, EEG has remarkable temporal resolution, but poor spatial resolution while the reverse is true with fMRI [86]. Many studies were hypothesis-generating and used single neurobiological modalities. Though one study included over 10,000 participants from the UK Biobank registry study [46], the majority (25/41; 61%) of the studies had fewer than 100 participants. Thus, most of the individual study findings are limited by small sample sizes, and overall generalizability may be low. Subject samples varied widely in sociodemographic characteristics, outcome measures, analysis protocols, and statistical methods, thereby precluding a metanalysis. It is not always clear if some brain regions not mentioned in the results had not been examined or were examined but not found to be significantly associated with loneliness.
Most studies only assessed and controlled for a small number of covariates such as demographic variables including age and sex. However, the complex psychosocial nature of loneliness extends beyond these basic demographic factors. Objective health status, environmental characteristics, stress, mental health, and personality traits are important confounders that were not included in many of the analyses. Only two studies had samples that could example the relationship of age (across the adult lifespan) with the loneliness-neurobiology associations [62, 63]. Wong et al. reported reduced cerebellar gray matter with older age, while D’Agostino et al.reported no age-related findings. While Düzel et al. presented age-related findings, they were restricted to older adults 61–82 years [59]. Several, but not all, studies have examined depression as a confounder [38, 58, 59], and three case-control studies specifically examined the effect of loneliness for the neurobiological differences between people with depression and healthy controls [45, 51, 57]. However, despite their potential impact, other constructs including grief, prolonged grief disorder, mild cognitive impairment, substance use disorders, and various stress-related conditions were not assessed and analyzed in most studies of loneliness.
Future directions
This systemic review of neurobiology of loneliness identified how loneliness is linked to specific brain regions and networks, including PFC, insula, amygdala, hippocampus, attentional networks, and DMN, and a strong relationship with AD. However, researchers will need to replicate and expand the quantity and quality of these studies to understand the brain processes underlying loneliness. Moving forward, task-based neurocircuitry fMRI studies and multi-modal imaging studies have promise, due to the complexity of social cognition and functioning. These approaches would be well-suited to loneliness interventions to identify associated changes in connectivity. Future studies should also include large and diverse samples of well-characterized subjects followed longitudinally, with hypothesis-based approaches and appropriate multivariate statistical analyses, to examine the role of age and other relevant factors.
Studies should examine how the neurobiological findings are linked to other behaviors associated with loneliness—including sleep disturbances, sedentary behaviors, and limited life space. Assessments should include multi-modal assessments of social functioning—including use of social media, GPS-derived life space data, speech data, sleep, and ecological momentary assessments that examine loneliness as a state rather than a trait [87]. Neurobiological assessments that examine structural and functional integrity or harness neuromodulation techniques such as transcranial magnetic stimulation can also provide novel insights into brain alterations associated with loneliness. Furthermore, RCTs of novel loneliness interventions and associated neurobiological changes are warranted. Such research will pave the way for the development of therapeutic and preventive interventions to manage the behavioral pandemic of loneliness.
Supplementary information
Acknowledgements
The authors acknowledge the help of Dr. Dilip Jeste in providing administrative support for the research effort as well as Ms. Paula Smith in preparing and submitting the manuscript.
Author contributions
EEL and JAL contributed to the conception and design of the study. JAL wrote the first draft of the manuscript. JAL, ERM, KEY, MR were involved in the systematic review and data extraction. TTN, JM, BM, MT were involved in the data interpretation. JAL and EEL wrote sections of the manuscript and were involved in data interpretation. All authors contributed to manuscript revision, read, and approved the submitted version. JAL and EEL had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding and disclosures
This study was supported, in part, by the National Institute of Mental Health [NIMH R01MH094151 (PI: Dilip V. Jeste), NIMH K23MH119375-01 (PI: EEL), NIMH K23 MH118435 (PI: TTN)], by the VA San Diego Healthcare System, by the Stein Institute for Research on Aging (Director: Dilip V. Jeste, MD) at the University of California San Diego, and by IBM Research AI through the AI Horizons Network. The authors have no conflicts of interest with the work described.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01058-7.
References
- 1.Klinenberg E. “Is loneliness a health epidemic.” New York Times. 9 Feb. 2018, https://www.nytimes.com/2018/02/09/opinion/sunday/loneliness-health.html?smid=em-share. Accessed 20 Mar 2020.
- 2.National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: The National Academies Press; 2020. 10.17226/25663. [PubMed]
- 3.Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102:1009–16. doi: 10.1136/heartjnl-2015-308790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper JS, Zuidersma M, Voshaar RCO, Zuidema SU, van den Heuvel ER, Stolk RP, et al. Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2015;22:39–57. doi: 10.1016/j.arr.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Beutel ME, Klein EM, Brähler E, Reiner I, Jünger C, Michal M, et al. Loneliness in the general population: prevalence, determinants and relations to mental health. BMC Psychiatry. 2017;17:1–7. doi: 10.1186/s12888-017-1262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40:218–27. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227–37. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- 8.Rico-Uribe LA, Caballero FF, Martín-María N, Cabello M, Ayuso-Mateos JL, Miret M. Association of loneliness with all-cause mortality: a meta-analysis. PloS one. 2018;13:e0190033. doi: 10.1371/journal.pone.0190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci. 2013;110:5797–801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cacioppo JT, Cacioppo S, Boomsma DI. Evolutionary mechanisms for loneliness. Cogn Emot. 2014;28:3–21. doi: 10.1080/02699931.2013.837379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mumtaz F, Khan MI, Zubair M, Dehpour AR. Neurobiology and consequences of social isolation stress in animal model—A comprehensive review. Biomed Pharmacother. 2018;105:1205–22. doi: 10.1016/j.biopha.2018.05.086. [DOI] [PubMed] [Google Scholar]
- 12.Tomova L, Tye K, Saxe R. The neuroscience of unmet social needs. Soc Neurosci. 2021;16:221–31. [DOI] [PubMed]
- 13.Cacioppo S, Capitanio JP, Cacioppo JT. Toward a neurology of loneliness. Psychol Bull. 2014;140:1464. doi: 10.1037/a0037618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee EE, Depp C, Palmer BW, Glorioso D, Daly R, Liu J, et al. High prevalence and adverse health effects of loneliness in community-dwelling adults across the lifespan: role of wisdom as a protective factor. Int Psychogeriatr. 2019;31:1447–62. doi: 10.1017/S1041610218002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeste DV, Di Somma S, Lee EE, Nguyen TT, Scalcione M, Biaggi A, et al. Study of loneliness and wisdom in 482 middle-aged and oldest-old adults: a comparison between people in Cilento, Italy and San Diego, USA. Aging Ment Health. 2020;1–11. [DOI] [PMC free article] [PubMed]
- 16.Jeste DV, Thomas ML, Liu J, Daly RE, Tu XM, Treichler EB, et al. Is spirituality a component of wisdom? Study of 1,786 adults using expanded San Diego Wisdom Scale (Jeste-Thomas Wisdom Index) J Psychiatr Res. 2021;132:174–81. doi: 10.1016/j.jpsychires.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TT, Lee EE, Daly RE, Wu TC, Tang Y, Tu X, et al. Predictors of loneliness by age decade: study of psychological and environmental factors in 2,843 community-dwelling americans aged 20–69 years. J Clin Psychiatry. 2020;81. [DOI] [PMC free article] [PubMed]
- 18.Ardelt M, Jeste DV. Wisdom and hard Times: The ameliorating effect of wisdom on the negative association between adverse life events and well-being. J Gerontol. 2018;73:1374–83. doi: 10.1093/geronb/gbw137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zacher H, Staudinger U.M. Wisdom and well-being. In Diener E, Oishi S & Tay L (Eds), Handbook of well-being. Salt Lake City, UT: DEF Publishers; 2018. https://nobascholar.com.
- 20.Judge TA, Ilies R, Dimotakis N. Are health and happiness the product of wisdom? The relationship of general mental ability to educational and occupational attainment, health, and well-being. J Appl Psychol. 2010;95:454. doi: 10.1037/a0019084. [DOI] [PubMed] [Google Scholar]
- 21.Meeks TW, Jeste DV. Neurobiology of wisdom: a literature overview. Arch Gen Psychiatry. 2009;66:355–65. doi: 10.1001/archgenpsychiatry.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell D, Peplau LA, Ferguson ML. Developing a measure of loneliness. J Pres Assess. 1978;42:290–94. doi: 10.1207/s15327752jpa4203_11. [DOI] [PubMed] [Google Scholar]
- 23.Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging. 2004;26:655–72. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–54. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- 25.Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci. 2009;13:447–54. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanai R, Bahrami B, Duchaine B, Janik A, Banissy MJ, Rees G. Brain structure links loneliness to social perception. Curr Bio. 2012;22:1975–9. doi: 10.1016/j.cub.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gevonden M, Booij J, van den Brink W, Heijtel D, van Os J, Selten JP. Increased release of dopamine in the striata of young adults with hearing impairment and its relevance for the social defeat hypothesis of schizophrenia. JAMA Psychiatry. 2014;71:1364–72. doi: 10.1001/jamapsychiatry.2014.1325. [DOI] [PubMed] [Google Scholar]
- 28.Lindner C, Dannlowski U, Walhöfer K, Rödiger M, Maisch B, Bauer J, et al. Social alienation in schizophrenia patients: association with insula responsiveness to facial expressions of disgust. PLoS One. 2014;9:e85014. doi: 10.1371/journal.pone.0085014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian Y, Liang S, Yuan Z, Chen S, Xu P, Yao D. White matter structure in loneliness: preliminary findings from diffusion tensor imaging. Neuroreport. 2014;25:843–47. doi: 10.1097/WNR.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 30.Cacioppo S, Balogh S, Cacioppo JT. Implicit attention to negative social, in contrast to nonsocial, words in the Stroop task differs between individuals high and low in loneliness: Evidence from event-related brain microstates. Cortex. 2015;70:213–33. doi: 10.1016/j.cortex.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 31.Kong X, Wei D, Li W, Cun L, Xue S, Zhang Q, et al. Neuroticism and extraversion mediate the association between loneliness and the dorsolateral prefrontal cortex. Exp Brain Res. 2015;233:157–64. doi: 10.1007/s00221-014-4097-4. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa S, Takeuchi H, Taki Y, Nouchi R, Sekiguchi A, Kotozaki Y, et al. White matter structures associated with loneliness in young adults. Sci Rep. 2015;5:17001. doi: 10.1038/srep17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cacioppo S, Bangee M, Balogh S, Cardenas-Iniguez C, Qualter P, Cacioppo JT. Loneliness and implicit attention to social threat: a high-performance electrical neuroimaging study. Cogn Neurosci. 2016;7:138–59. doi: 10.1080/17588928.2015.1070136. [DOI] [PubMed] [Google Scholar]
- 34.Inagaki TK, Muscatell KA, Moieni M, Dutcher JM, Jevtic I, Irwin MR, et al. Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Soc Cogn Affect Neurosci. 2016;11:1096–101. doi: 10.1093/scan/nsv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Wang Y, Liu W, Wei D, Yang J, Du X, et al. Neuroanatomical correlates of attitudes toward suicide in a large healthy sample: a voxel-based morphometric analysis. Neuropsychologia. 2016;80:185–93. doi: 10.1016/j.neuropsychologia.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Tian X, Hou X, Wang K, Wei D, Qiu J. Neuroanatomical correlates of individual differences in social anxiety in a non-clinical population. Soc Neurosci. 2016;11:424–37. doi: 10.1080/17470919.2015.1091037. [DOI] [PubMed] [Google Scholar]
- 37.Layden EA, Cacioppo JT, Cacioppo S, Cappa SF, Dodich A, Falini A, et al. Perceived social isolation is associated with altered functional connectivity in neural networks associated with tonic alertness and executive control. Neuroimage. 2017;145:58–73. doi: 10.1016/j.neuroimage.2016.09.050. [DOI] [PubMed] [Google Scholar]
- 38.Meng J, Hao L, Wei D, Sun J, Li Y, Qiu J. BDNF Val66Met polymorphism modulates the effect of loneliness on white matter microstructure in young adults. Biol Psychol. 2017;130:41–9. doi: 10.1016/j.biopsycho.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Tian Y, Yang L, Chen S, Guo D, Ding Z, Tam KY, et al. Causal interactions in resting-state networks predict perceived loneliness. PloS one. 2017;12:e0177443. doi: 10.1371/journal.pone.0177443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi Y, Li LMW, Xiao Y, Ma J, Fan L, Dai Z. Brain activity mediates the relation between emotional but not instrumental support and trait loneliness. Soc Cogn Affect Neurosci. 2018;13:995–1002. doi: 10.1093/scan/nsy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng C, Wang L, Li T, Xu P. Connectome-based individualized prediction of loneliness. Soc Cogn Affect Neurosci. 2019;14:353–65. doi: 10.1093/scan/nsz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liégeois R, Li J, Kong R, Orban C, Van De Ville D, Ge T, et al. Resting brain dynamics at different timescales capture distinct aspects of human behavior. Nat Commun. 2019;10:2317. doi: 10.1038/s41467-019-10317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mwilambwe-Tshilobo L, Ge T, Chong M, Ferguson MA, Misic B, Burrow AL, et al. Loneliness and meaning in life are reflected in the intrinsic network architecture of the brain. Soc Cogn Affect Neurosci. 2019;14:423–33. doi: 10.1093/scan/nsz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Courtney AL, Meyer ML. Self-other representation in the social brain reflects social connection. J Neurosci. 2020;40:5616–27. doi: 10.1523/JNEUROSCI.2826-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao M, Shao R, Huang CM, Liu HL, Chen YL, Lee SH, et al. The relationship between loneliness and working-memory-related frontoparietal network connectivity in people with major depressive disorder. Behav Brain Res. 2020;393:112776. doi: 10.1016/j.bbr.2020.112776. [DOI] [PubMed] [Google Scholar]
- 46.Kiesow H, Dunbar R, Kable J, Kalenscher T, Vogeley K, Schilbach L, et al. 10,000 social brains: sex differentiation in human brain anatomy. Sci Adv. 2020;6:eaaz1170. [DOI] [PMC free article] [PubMed]
- 47.Saris IMJ, Penninx B, Dinga R, van Tol MJ, Veltman DJ, van der Wee NJA, et al. Default mode network connectivity and social dysfunction in major depressive disorder. Sci Rep. 2020;10:194. doi: 10.1038/s41598-019-57033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64:234–40. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 49.Lan CC, Tsai SJ, Huang CC, Wang YH, Chen TR, Yeh HL, et al. Functional connectivity density mapping of depressive symptoms and loneliness in non-demented elderly male. Front Aging Neurosci. 2015;7:251. doi: 10.3389/fnagi.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donovan NJ, Okereke OI, Vannini P, Amariglio RE, Rentz DM, Marshall GA, et al. Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry. 2016;73:1230–37. doi: 10.1001/jamapsychiatry.2016.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong NM, Liu HL, Lin C, Huang CM, Wai YY, Lee SH, et al. Loneliness in late-life depression: structural and functional connectivity during affective processing. Psychol Med. 2016;46:2485–99. doi: 10.1017/S0033291716001033. [DOI] [PubMed] [Google Scholar]
- 52.Canli T, Wen R, Wang X, Mikhailik A, Yu L, Fleischman D, et al. Differential transcriptome expression in human nucleus accumbens as a function of loneliness. Mol Psychiatry. 2017;22:1069–78. doi: 10.1038/mp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan D, Dong Y, Zhang H, Zhao Y, Diao Y, Cui Y, et al. Empty-nest-related psychological distress is associated with progression of brain white matter lesions and cognitive impairment in the elderly. Sci Rep. 2017;7:43816. doi: 10.1038/srep43816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehlers DK, Daugherty AM, Burzynska AZ, Fanning J, Awick EA, Chaddock-Heyman L, et al. Regional brain volumes moderate, but do not mediate, the effects of group-based exercise training on reductions in loneliness in older adults. Front Aging Neurosci. 2017;9:110. doi: 10.3389/fnagi.2017.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canli T, Yu L, Yu X, Zhao H, Fleischman D, Wilson RS, et al. Loneliness 5 years ante-mortem is associated with disease-related differential gene expression in postmortem dorsolateral prefrontal cortex. Transl Psychiatry. 2018;8:2. doi: 10.1038/s41398-017-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.d’Oleire Uquillas F, Jacobs HIL, Biddle KD, Properzi M, Hanseeuw B, Schultz AP, et al. Regional tau pathology and loneliness in cognitively normal older adults. Transl Psychiatry. 2018;8:282. doi: 10.1038/s41398-018-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sin ELL, Liu HL, Lee SH, Huang CM, Wai YY, Chen YL, et al. The relationships between brain structural changes and perceived loneliness in older adults suffering from late-life depression. Int J Geriatr Psychiatry. 2018;33:606–12. doi: 10.1002/gps.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cristofori I, Pal S, Zhong W, Gordon B, Krueger F, Grafman J. The lonely brain: evidence from studying patients with penetrating brain injury. Soc Neurosci. 2019;14:663–75. doi: 10.1080/17470919.2018.1553798. [DOI] [PubMed] [Google Scholar]
- 59.Düzel S, Drewelies J, Gerstorf D, Demuth I, Steinhagen-Thiessen E, Lindenberger U, et al. Structural brain correlates of loneliness among older adults. Sci Rep. 2019;9:13569. doi: 10.1038/s41598-019-49888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Golde S, Romund L, Lorenz RC, Pelz P, Gleich T, Beck A, et al. Loneliness and adolescents’ neural processing of Sself, friends, and teachers: Consequences for the school self-concept. J Res Adolesc. 2019;29:938–52. doi: 10.1111/jora.12433. [DOI] [PubMed] [Google Scholar]
- 61.Wong NML, Shao R, Yeung PPS, Khong PL, Hui ES, Schooling CM, et al. Negative affect shared with siblings is associated with structural brain network efficiency and loneliness in adolescents. Neuroscience. 2019;421:39–47. doi: 10.1016/j.neuroscience.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 62.D’Agostino AE, Kattan D, Canli T. An fMRI study of loneliness in younger and older adults. Soc Neurosci. 2019;14:136–48. doi: 10.1080/17470919.2018.1445027. [DOI] [PubMed] [Google Scholar]
- 63.Wong NML, Shao R, Wu J, Tao J, Chen L, Lee TMC. Cerebellar neural markers of susceptibility to social isolation and positive affective processing. Brain Struc Funct. 2019;224:3339–51. doi: 10.1007/s00429-019-01965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bocincova A, Nelson T, Johnson J, Routledge C. Experimentally induced nostalgia reduces the amplitude of the event-related negativity. Soc Neurosci. 2019;14:631–4. doi: 10.1080/17470919.2019.1580612. [DOI] [PubMed] [Google Scholar]
- 65.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 66.O’Reilly RC. The what and how of prefrontal cortical organization. Trends Neurosci. 2010;33:355–61. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49:1195–205. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19:935–44. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- 69.Craig AD, Craig A. How do you feel−now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. [DOI] [PubMed]
- 70.Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–34. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- 71.Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT. A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep. 2013;3:1–3. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vijayakumar N, Cheng TW, Pfeifer JH. Neural correlates of social exclusion across ages: a coordinate-based meta-analysis of functional MRI studies. NeuroImage. 2017;153:359–68. doi: 10.1016/j.neuroimage.2017.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Lucas-Neto L, Neto D, Oliveira E, Martins H, Mourato B, Correia F, et al. Three dimensional anatomy of the human nucleus accumbens. Acta Neurochir. 2013;155:2389–98. doi: 10.1007/s00701-013-1820-z. [DOI] [PubMed] [Google Scholar]
- 75.Tomova L, Wang KL, Thompson T, Matthews GA, Takahashi A, Tye KM, et al. Acute social isolation evokes midbrain craving responses similar to hunger. Nat Neurosci. 2020;23:1597–605. doi: 10.1038/s41593-020-00742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31:217–38. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- 77.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–41. doi: 10.1016/S0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 78.Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Smet HJ, Paquier P, Verhoeven J, Mariën P. The cerebellum: its role in language and related cognitive and affective functions. Brain Lang. 2013;127:334–42. doi: 10.1016/j.bandl.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 80.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N. Y Acad Sci. 2014;1316:29. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spreng RN, Dimas E, Mwilambwe-Tshilobo L, Dagher A, Koellinger P, Nave G, et al. The default network of the human brain is associated with perceived social isolation. Nat Commun. 2020;11:6393. doi: 10.1038/s41467-020-20039-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lara E, Martín-María N, De la Torre-Luque A, Koyanagi A, Vancampfort D, Izquierdo A, et al. Does loneliness contribute to mild cognitive impairment and dementia? A systematic review and meta-analysis of longitudinal studies. Ageing Res Rev. 2019;52:7–16. doi: 10.1016/j.arr.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 84.Lee EE, Jeste DV. Neurobiology of wisdom. In: Sternberg RJ & Glück J, editors. The Cambridge handbook of wisdom. Cambridge: Cambridge University Press; 2019. p. 69–93.
- 85.Grennan G, Balasubramani PP, Alim F, Zafar-Khan M, Lee EE, Jeste DV, et al. Cognitive and Neural Correlates of Loneliness and Wisdom during Emotional Bias. Cereb Cortex. 2021;31:3311–22. [DOI] [PMC free article] [PubMed]
- 86.Harmon-Jones E, Beer J. Introduction to social and personality neuroscience methods. in: Harmon-Jones E, Beer JS, Schultheiss OC, Johnstone T, Stanton SJ, editors. Methods in Social Neuroscience. Guilford Press: New York, NY; 2009. p. 1–9.
- 87.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 88.Cacioppo S, Weiss RM, Runesha HB, Cacioppo JT. Dynamic spatiotemporal brain analyses using high performance electrical neuroimaging: theoretical framework and validation. J Neurosci Methods. 2014;238:11–34. doi: 10.1016/j.jneumeth.2014.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.