Abstract
Background:
Early coronary artery calcium (CAC) screening and preventive therapy may reduce long term risk of a coronary heart disease (CHD) event. However, identifying younger individuals at increased risk remains a challenge. Genetic risk scores (GRS) for CHD are age independent and can stratify individuals on various risk trajectories.
Objectives:
The goal of this study was to assess the utility of a GRS in targeted CAC screening among young individuals.
Methods:
Using 142 variants associated with CHD events, we calculated a GRS in 1927 individuals in the CARDIA cohort (aged 32–47) and 6600 individuals in the MESA cohort (aged 44–87). We assessed GRS utility to predict CAC presence in the CARDIA cohort and stratify individuals of varying risk for CAC presence over the lifetime in both cohorts.
Results:
The GRS predicted CAC presence in CARDIA males. It was not predictive in CARDIA females, which had a CAC prevalence of 6.4%. In combined analysis of the CARDIA and MESA cohorts, the GRS was predictive of CAC in both males and females and was used to derive an equation for the age at which CAC probability crossed a predetermined threshold. When assessed in combination with traditional risk factors, the GRS further stratified individuals. For individuals with an equal number of traditional risk factors, probability of CAC reached 25% approximately 10 years earlier for those in the highest GRS quintile compared to the lowest.
Conclusions:
The GRS may be used to target high risk younger individuals for early CAC screening.
Keywords: Coronary heart disease, computed tomography, genetic risk score, primary prevention
Introduction
Coronary heart disease (CHD) is the worldwide leading cause of mortality (1). Studies suggest that long term exposure to lower LDL-c reduces CHD event risk over the lifetime (2, 3), so it is critical to identify high risk individuals early. Traditional screening tools estimate event risk over 10 years and are heavily influenced by age (4), making young individuals of increased risk challenging to distinguish. Coronary artery calcium (CAC) scanning improves risk prediction beyond traditional markers (5), and current ACC/AHA guidelines provide CAC with a Class IIa recommendation for individuals of intermediate (7.5–20%) 10-year risk when traditional tools leave treatment uncertain (6); the average non-Hispanic white male reaches intermediate risk at age 60 (4). Furthermore, CAC absence is associated with very low event rates over long term (≥10 year) follow up (7–10). CAC presence early in life has been shown to confer a 5-fold increase in CHD risk (11), and recent analysis highlights the potential utility of scanning young, high-risk individuals (12); therefore it would be beneficial for a young individual to know his or her CAC status. However, CAC incidence varies with age, and a screening program that targets all younger individuals will result in a very low detection rate.
In this emerging era of precision medicine, genetic risk, which is established at birth, widely available, and inexpensively measured, may be used to target high risk individuals to improve CAC as a screening strategy. Genome-wide association studies (GWAS) have identified single nucleotide polymorphisms (SNPs) associated with CHD (13–17), and the effects of these variants have been combined in genetic risk scores (GRS) to estimate an individual’s cumulative genetic risk for CHD. GRS for CHD have been associated with CHD events (18) and with subclinical atherosclerosis (19–22). We recently demonstrated for individuals older than 45 that a GRS can be used to calculate the age at which an individual’s probability for CAC presence reaches a predetermined threshold using data from the Multiethnic Study of Atherosclerosis (MESA) (23). However, it is clear that further understanding of the interplay between the GRS and CAC in a younger group of individuals would be valuable (24). Additionally, increased levels of traditional risk factors early in life have been associated with CAC presence in young adulthood (25–28), and the Society of Cardiovascular Computed Tomography (SCCT) endorses CAC scanning even in low risk individuals with risk enhancing factors (29). This prompts the question: how does the GRS affect risk prediction among young adults when incorporated with traditional risk factors?
Here, we provide a more comprehensive evaluation by incorporating data from the Coronary Artery Risk Development in Young Adults (CARDIA) study (30) which extends the range of data to include individuals 32 through 87 years of age. In addition, we assess the effect of traditional risk factors in combination with genetic risk on the probability of CAC over an individual’s lifetime.
Methods
Coronary Artery Risk Development in Young Adults
The CARDIA study was designed to examine cardiovascular disease beginning in young adulthood (30). 5115 healthy European American and African American participants 18–30 years old were enrolled between 1985 and 1986. Follow-up examinations occurred at years 2, 5, 7, 10, 15, 20, and 25. CAC was measured via CT at the year 15 exam (2000–2001).
GRS Calculation
CARDIA genotype information from the Affymetrix Genome-Wide Human SNP 6.0 was obtained from two sub-studies of the CARDIA cohort on dbGaP (Study Accession: phs000285.v3.p2): CARDIA Gene Environment Association Studies Initiative (CARDIA GENEVA) and CARDIA Candidate Gene Association Resource (CARDIA CARe). The CARDIA data was accessed and analyzed after IRB approval at UCSD, filtered using PLINK (31), and imputed using the Michigan Imputation Server (32) (Appendix 1). MESA genotype information was obtained for 6660 participants of European American, Chinese American, African American, and Hispanic American ancestry (33, 34). The GRS was calculated for each individual as previously described (23) and included 142 of the known CHD risk loci (17) that were genotyped or imputed with high quality (R2>0.6) in all three datasets (CARDIA GENEVA, CARDIA CARe, MESA) (Appendix 1, Online Table 1).
Phenotype Data
Phenotype data for both studies was retrieved from dbGaP. CAC was assessed in both CARDIA and MESA according to the methods described in Carr et. al. (35), and calcium presence was defined as a nonzero mean Agatston score from two scans taken sequentially during the same imaging session. Family history reporting was not consistent between CARDIA and MESA. Family history in CARDIA, defined as history of heart attack in a mother, father, sister, or brother, was recorded at the exam 5 years prior to CAC measurement. Family history in MESA, defined as history of heart attack in a parent, sibling, or child, was recorded at the same exam as CAC measurement.
Statistical Analysis
Analysis was implemented in R v3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). 1927 participants in the CARDIA cohort had both GRS data and a CAC score. The association between the GRS and traditional risk factors was assessed using linear regression for continuous variables and logistic regression for binary variables. The rate of CAC presence was determined within the low, intermediate, and high genetic risk categories (GRS quintile 1, GRS quintiles 2–4, and GRS quintile 5, respectively) of the CARDIA cohort and within 10-year age groups of the MESA cohort. Logistic regression was used to calculate the odds ratio for CAC presence within each GRS quintile of the CARDIA cohort.
The GRS was assessed as a continuous variable to determine the odds ratio for CAC presence per standard deviation from the GRS population mean in the CARDIA and MESA cohorts combined (n=8587) with age and sex included as covariates. From this analysis, an equation was derived to calculate the age at which an individual’s probability of CAC presence crosses a predetermined threshold. Further analysis was done within each ancestral group separately.
Additionally, we assessed the effect of traditional risk factors in combination with the GRS on risk of CAC presence using a multivariable model that included age, sex, GRS quintile, and number of traditional risk factors (0–5). Traditional risk factors were defined as smoking, diabetes, hypertension (systolic blood pressure ≥130 or taking blood pressure medication), BMI ≥30, and LDL-c ≥130.
The added value of the GRS to predict CAC beyond traditional risk factors was assessed by comparing (1) a model that included age, sex, and number of traditional risk factors (0–5), with (2) a model that included age, sex, number of traditional risk factors, and continuous GRS. The models were compared using the likelihood ratio test (LRT) and area under the receiver operator characteristic curve (AUC). Continuous as well as 3-category net reclassification improvement (NRI) (36) were calculated. Prediction models were derived using all ages from both CARDIA and MESA. AUC and NRI were calculated over the sex-stratified 10-year age range in which most individuals cross a 25% probability threshold, males 40–50 and females 50–60 (3-cagegory NRI intermediate risk defined as 15–35% probability of CAC). AUC and NRI were also calculated in the youngest 10-year age category with CAC incidence >10%, males 34–44 and females 44–54 (3-cagegory NRI intermediate risk defined as 10–25% probability of CAC). Analysis was performed using the “pROC” (37) and “nricens” packages.
Results
Genotype and CAC information was available for 1927 individuals in the CARDIA cohort and 6600 individuals in the MESA cohort. Each of the 142 SNPs included in the GRS was genotyped directly on the Affymetrix 6.0 chip (GENEVA n=25, CARe n=29, MESA n=30) or imputed with high quality (R2>0.6) in all datasets (GENEVA n=117, CARe n=113, MESA n=112). The GRS was normally distributed in each ancestral group within each cohort (p>0.05, Shapiro-Wilk test). The mean GRS within each ancestral group (European American and African American) was not significantly different (European American p=0.33, African American p=0.73) between each cohort in a one-way analysis of variance test (Appendix 2, Online Fig. 1).
In the CARDIA cohort, when assessed within each ancestral group separately, regression results showed no association between the GRS and any traditional risk factor except for family history (p=0.017) (Table 1). The GRS was marginally predictive of LDL-c in CARDIA European Americans but did not achieve significance (slope=1.51mg/dl per standard deviation of GRS, p=0.088).
Table 1.
Traditional risk factors in the CARDIA cohort
| European American | African American | |||
|---|---|---|---|---|
| Mean | p (regression) | Mean | p (regression) | |
| Age (years) | 40.8 ± 3.3 | 0.64 | 39.6 ± 3.8 | 0.40 |
| Male (%) | 48.2 | 0.82 | 42.3 | 0.35 |
| BMI (kg/m^2) | 27.2 ± 5.5 | 0.70 | 30.2 ± 6.7 | 0.71 |
| Smoking (%) | 18.6 | 0.62 | 25.8 | 0.22 |
| Diabetes mellitus (%) | 4.9 | 0.19 | 6.4 | 0.64 |
| Systolic blood pressure | 109.9 ± 12.8 | 0.27 | 116.5 ± 16.5 | 0.48 |
| Antihypertensive therapy (%) | 4.1 | 0.16 | 13.5 | 0.87 |
| Total cholesterol (mg/dl) | 186.6 ± 34.0 | 0.28 | 182.7 ± 36.1 | 0.13 |
| LDL-c (mg/dl) | 114.5 ± 30.8 | 0.088 | 112.7 ± 33.0 | 0.18 |
| HDL-c (mg/dl) | 49.9 ± 14.7 | 0.28 | 50.9 ± 13.6 | 0.47 |
| Cholesterol medication (%) | 2.9 | 0.44 | 2.4 | 0.45 |
| Family history (%) | 22.6 | 0.017 | 18.9 | 0.81 |
Traditional risk factors in the CARDIA cohort. P-value calculated using a linear regression for continuous variables and logistic regression for binary variables.
GRS = genetic risk score, BMI = body mass index, LDL-c = low density lipoprotein cholesterol, HDL = high density lipoprotein
In analysis of the total CARDIA population, CAC presence increased with increasing GRS group (Table 2). CAC presence was significantly larger in the CARDIA male subgroup (17.2%) compared to the CARDIA female subgroup (6.4%) (χ2 p = 1.3×10−13), and CAC prevalence did not increase with increasing GRS group in CARDIA females. CAC prevalence increased with increasing GRS group in CARDIA males and was nearly double (24.1 vs 12.1%) in the high GRS group compared to the low GRS group (Table 2). In the MESA cohort, CAC prevalence increased with increasing GRS group in each 10-year age category in both female and male populations (Appendix 2, Online Table 2). The odds ratio for calcium was assessed in each quintile of the entire CARDIA cohort (Appendix 2, Online Fig. 2 and 3).
Table 2.
Prevalence of non-zero CAC in CARDIA
| Population | Total | Female | Male |
|---|---|---|---|
| Total people | 1927 | 1038 | 889 |
| Total CAC>0 | 219 | 66 | 153 |
| Low GRS: CAC>0/# People (%) | 34/386 (8.8%) | 13/213 (6.1%) | 21/173 (12.1%) |
| Int GRS: CAC>0/# People (%) | 129/1156 (11.1%) | 42/627 (6.7%) | 87/529 (16.4%) |
| High GRS: CAC>0/# People (%) | 56/385 (14.5%) | 11/198 (5.6%) | 45/187 (24.1%) |
| Total CAC>0 rate | 11.4% | 6.4% | 17.2% |
| High/Low | 1.65 | 0.91 | 1.98 |
CAC prevalence in the CARDIA cohort (aged 32–47) stratified into low (GRS quintile 1), intermediate (GRS quintiles 2–4) and high (GRS quintile 5) genetic risk categories. High/Low indicates the ratio of the rate of CAC presence in the high genetic risk category to the rate of CAC presence in the low genetic risk category.
CAC = coronary artery calcium, GRS = genetic risk score
The GRS was assessed as a continuous variable in the CARDIA and MESA cohorts combined (n=8587) to include data that spans the age range of 32–87 years. The mean GRS was 3.31±0.183, and the odds ratio for CAC was 1.33 (1.26–1.40, p<2E-16) per GRS standard deviation from the population mean in a multivariable model including age and sex as covariates (Fig. 1) and was 1.28 (1.21–1.36, p<2E-16) in a multivariable model including age, sex, and other traditional clinical variables (diabetes status, smoking status, LDL-c, HDL-c, systolic blood pressure, antihypertensive therapy status, cholesterol medication status, self-identified race). Using the model developed with age and sex included as covariates, we derived an equation for the age at which the probability of CAC presence crosses a predetermined threshold:
| (Eq. 1) |
where r is the predetermined threshold for CAC, chosen by the healthcare provider or patient, GRSn is the patient’s GRS normalized to the population mean, and S is the patient’s sex (0 for females, 1 for males). For example, at a 25% nonzero CAC rate, this reduces to:
and
Figure 1. Probability of CAC presence as a function of age.
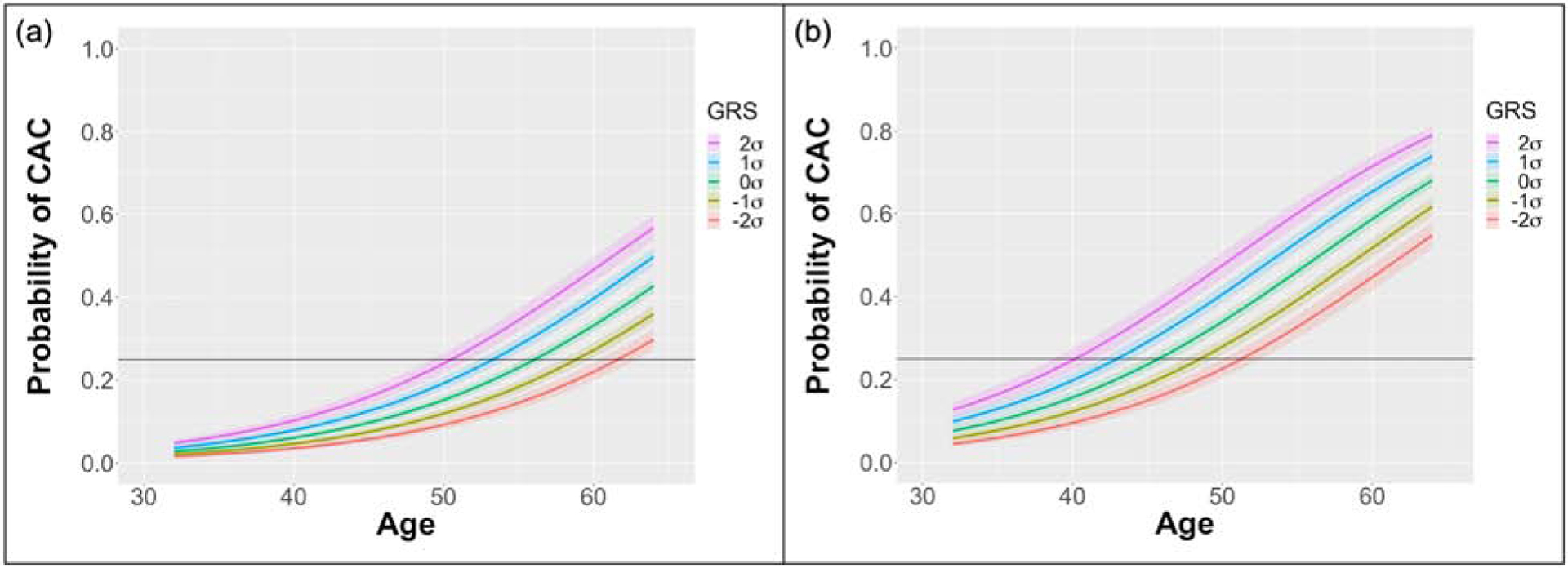
CAC probability vs age calculated using data from both the CARDIA and MESA cohorts (n=8587) under a multivariable model with age and sex included as covariates. Data shown for an (a) female and (b) male. Curves presented for normalized GRS 1 and 2 standard deviations (σ) above and below the population mean. Horizontal line represents a 25% probability of CAC presence.
CAC = coronary artery calcium, GRS = genetic risk score
Therefore, for a female with a GRS 2 standard deviations above the population mean, the age of scan is 50.5 (49.2, 51.8) and for a female 2 standard deviations below the population mean, the age of scan is 61.6 (60.4, 62.9). Similarly, for males, the ages are 40.2 (38.7, 41.5) and 51.3 (50.0, 52.6), respectively. Different equations can be derived for different target rates, r, of nonzero CAC.
In separate analyses of each ancestry, the GRS remained predictive of CAC (Appendix 2, Online Fig. 4–5), and scanning recommendations were calculated for each group (Table 3). Analysis was repeated within sex-stratified groups, and results were similar (Appendix 2, Online Table 3). Finally, we investigated the risk of CAC presence across both varying GRS categories and number of traditional clinical risk factors (Central Illustration). Both increased genetic risk and number of traditional risk factors were associated with a higher risk. Among individuals of the same sex with the same number of traditional risk factors, CAC risk reached a 25% threshold approximately 10 years earlier for those in the highest quintile of genetic risk compared to the lowest. Due to inconsistent reporting of family history in the CARDIA and MESA cohorts, family history was not included as a traditional risk factor in analyses conducted across both datasets. However, the GRS remained predictive of CAC in analysis of the MESA cohort alone when family history was included in the model and when the cohort was stratified by family history (Appendix 2, Online Table 4).
Table 3.
Recommended age of scan as a function of GRS
| Cohort | N | GRS population mean±SD | Sex | Recommended age of scan | Scan Age High GRS (+2σ) | Scan Age Low GRS (−2σ) |
|---|---|---|---|---|---|---|
| All | 8587 | 3.31±0.18 | Female | Age = 55.9 – 2.76*GRSn | 50.5 (49.2, 51.8) | 61.6 (60.4, 62.9) |
| Male | Age = 45.6 – 2.76*GRSn | 40.2 (38.7, 41.5) | 51.3 (50.0, 52.6) | |||
| European American | 3898 | 3.36±0.17 | Female | Age = 55.2 – 2.26*GRSn | 50.7 (48.9, 52.4) | 59.8 (58.0, 61.5) |
| Male | Age = 43.8 – 2.26*GRSn | 39.3 (37.5, 41.1) | 48.4 (46.5, 50.1) | |||
| African American | 2510 | 3.22±0.16 | Female | Age = 57.2 – 1.85*GRSn | 53.5 (50.8, 56.0) | 60.9 (58.2, 63.5) |
| Male | Age = 48.3 – 1.85*GRSn | 44.5 (41.6, 47.2) | 51.9 (49.2, 54.5) |
Recommendation for females and males 2 standard deviations above the population mean and 2 standard deviations below the population mean at a 25% non-zero CAC discovery rate. Data was derived from analysis of the CARDIA and MESA cohorts combined.
GRS = genetic risk score, GRSn = genetic risk score normalized to the population mean
Central Illustration. CAC probability vs age for varying levels of genetic and traditional risk factors.
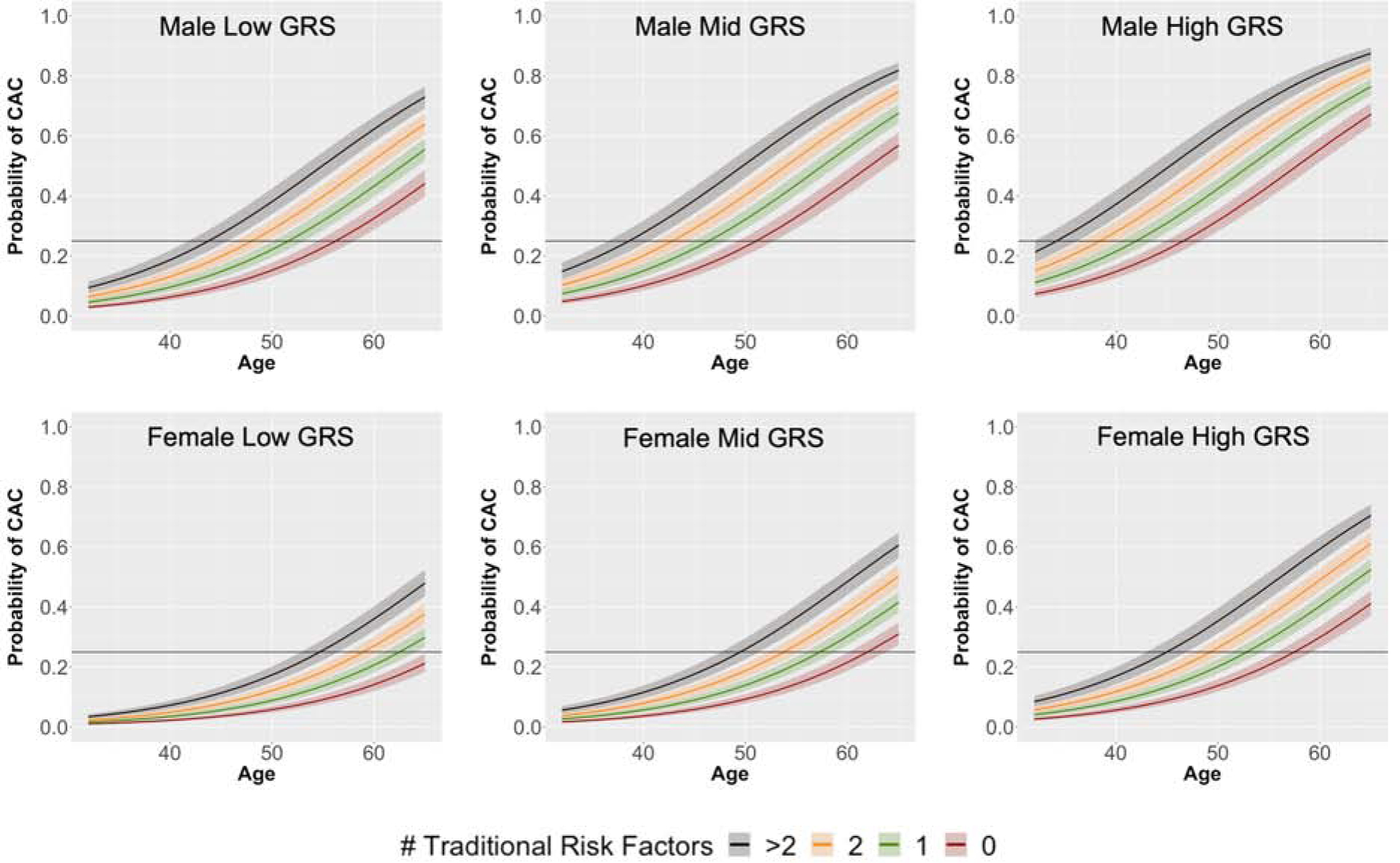
Probability of CAC presence as a function of age, presented for increasing number of traditional risk factors (0–5). Calculated using data from the CARDIA and MESA cohorts combined (n=8587). Traditional risk factors were defined as smoking, diabetes, hypertension (systolic blood pressure ≥130 or taking blood pressure medication), BMI ≥30, and LDL-c ≥130. Low GRS = quintile 1, Mid GRS = quintile 3, High GRS = quintile 5.
BMI = body mass index, CAC = coronary artery calcium, GRS = genetic risk score, LDL-c = low density lipoprotein cholesterol
The addition of the GRS to a model including age, sex, and traditional risk factors significantly improved model fit (LRT χ2(1)=145.56 p=1.62E-33). GRS addition also increased AUC, but the increase was not significant. 3-category NRI and continuous NRI for males 40–50 were 9.2% and 32.5%, respectively, and for females 50–60 were 10.9% and 36.3%, respectively. 3-category NRI and continuous NRI for males 34–44 were 14.3% and 26.3%, respectively, and for females 44–54 were 7.1% and 18.6% respectively (Table 4). Out of 106 males aged 34–44 and with CAC, 19 had >25% risk according to the model including age, sex, and traditional risk factors, whereas 31 had >25% risk according to the model that also included GRS (Appendix 2, Online Table 5).
Table 4.
AUC and NRI to predict CAC presence
| Sex Age | n | AUC Model 1 (95% CI) | AUC Model 2 (95% CI) | Categorical NRI Low Risk Cutoff | Categorical NRI High Risk Cutoff | Categorical NRI (95% CI) | Continuous NRI (95% CI) |
|---|---|---|---|---|---|---|---|
| Female 50–60 | 964 | 0.630 (0.588, 0.671) | 0.661 (0.6204, 0.701) | 0.15 | 0.35 | 0.109 (0.037, 0.185) | 0.363 (0.218, 0.512) |
| Male 40–50 | 931 | 0.673 (0.633, 0.712) | 0.690 (0.651, 0.729) | 0.15 | 0.35 | 0.092 (0.015, 0.175) | 0. 325 (0.187, 0.476) |
| Female 44–54 | 1062 | 0.680 (0.638, 0.723) | 0.688 (0.645, 0.732) | 0.10 | 0.25 | 0.071 (-0.008, 0.150) | 0.186 (0.004, 0.353) |
| Male 34–44 | 699 | 0.694 (0.639, 0.749) | 0.697 (0.645, 0.750) | 0.10 | 0.25 | 0.143 (0.047, 0.243) | 0.263 (0.049, 0.477) |
AUC and NRI for the addition of GRS to traditional risk factors to predict CAC presence. Model 1 included age, sex, and number of traditional risk factors as predictors. Model 2 included age, sex, number of traditional risk factors, and GRS as predictors.
AUC = area under the receiver operator characteristic curve, NRI = net reclassification improvement, GRS = genetic risk score,
Discussion
In this study of over 8500 asymptomatic individuals, we assessed the utility of a GRS to improve CT-based CAC scanning as a CHD screening strategy. The GRS can be calculated via widely available, inexpensive consumer genetic testing. We show that a GRS composed of 142 CHD risk SNPs can be used to predict the risk of CAC presence over the lifetime (ages 32–87) and improves risk prediction when used in conjunction with traditional risk factors.
Though the GRS was developed from SNPs initially associated with CHD events, it was also predictive of CAC. In the CARDIA cohort, the GRS was not associated with any traditional risk factors except for family history. This implies the GRS is not linked to CAC through any single traditional marker other than family history, another measure of genetic predisposition for disease. Despite a marginal association of LDL-c with GRS in the European American population, prior report of the effect size of LDL-c on CAC presence (26) indicates the GRS is not predictive of CAC due to this small difference in LDL-c alone.
In previous analysis of individuals aged 44–54 in the MESA cohort, CAC incidence was more than double in the high GRS group compared to the low group. Within the younger CARDIA cohort, aged 32–47, CAC presence increased with increasing GRS group, and in males, it was nearly double in the high GRS group compared to the low. CAC did not increase with GRS in CARDIA females, where the low incidence of CAC (<7%) reduced statistical power, but analysis of the older MESA cohort demonstrated that CAC presence increased with GRS in females.
We additionally used the GRS to assess the probability of CAC as a function of age for varying levels of genetic risk. This expanded assessment of both the CARDIA and MESA cohorts combined allowed analysis across the entire spectrum of ages at which the probability crosses a 25% threshold. Interestingly, in this combined analysis, the estimated probability for individuals under 44 years old remained similar to that extrapolated from analysis of the MESA cohort alone. While our findings on the probability of CAC stratified by GRS were derived from combining CARDIA and MESA data (ages 32–87), they are especially applicable to young individuals because most men reach a 25% probability before age 50 and most women reach a 25% probability before age 60. When analysis was repeated for sex-stratified data, scanning equations and recommended ages remained similar. Thus, when all relevant ages are included in the model, the GRS appears equally predictive with the same added risk in both sexes.
We used this analysis of the CARDIA and MESA cohorts combined to derive an equation to calculate the age at which an individual’s probability of CAC crosses a predetermined threshold, given the age independent risk factors of genetic risk and sex. In this way, our analysis provides the flexibility for a healthcare provider or patient to choose any threshold by changing the value of r in Equation 1. We found a difference in over 10 years between the age at which an individual with a GRS 2 standard deviations above the population mean reaches the threshold compared to someone 2 standard deviations below. Analysis was repeated for each ancestral group separately. For the European American population, the scanning equation intercept (age of scan for normalized GRS=0) fell below that of the total population, as the European American population had higher rates of CAC at younger ages. Conversely, the African American population had a scanning equation intercept above that of the total population, due to the lower incidence of CAC. Analysis of the total population resulted in a larger regression coefficient for the normalized GRS compared to separate analysis of each ancestral group because the regression was primarily driven by the African American population at lower GRS values and by the European American population at higher GRS values.
When assessed with traditional clinical risk factors, the GRS remained predictive of CAC presence in combined analysis of the CARDIA and MESA cohorts. Indeed, the GRS can be used together with traditional risk factors to further stratify an individual’s risk of CAC. Individuals in the top GRS quintile with no traditional risk factors reached a 25% risk threshold at a similar age to those in the lowest quintile of genetic risk but with 2 traditional risk factors (47–48 years for males and 58–59 years for females). This finding is notably similar to the pattern Inouye et al found in men reaching a 10% probability of CAD events 20 years later in life (38) and highlights the utility of CAC as a preclinical marker of disease. Furthermore, NRI results indicate the GRS improves risk prediction over traditional risk factors specifically in the 10-year age range over which most individuals reach a 25% risk probability of CAC (males 40–50 and females 50–60).
It should be noted that the SCCT endorses CAC scanning even in low (<5% 10-year) risk adults aged 40–75 with other prominent risk markers such as family history of premature disease. While the GRS was associated with family history, it remained predictive of CAC in additional assessment of the MESA cohort alone when stratified by family history and when family history was included in the model, indicating the GRS is predictive of CAC independent of family history. This finding is consistent with prior research (39) and may be due to the fact that family history, unlike the GRS, is also determined by environmental factors, relies on self-reporting, and varies with age.
According to current guidelines, the individuals who are most appropriate for preventive statin therapy are those with any CAC. Thus, given our analysis, an individual with high genetic risk may choose to undergo a first CAC scan earlier in life than recommended currently. If the individual has a positive CAC scan, he or she would likely benefit from early initiation of statin therapy. For example, the average non-Hispanic white male reaches intermediate risk (7.5% 10-year event risk) at age 60 (4). If this man underwent a CAC scan at 60 and the scan was positive, he could choose to begin statin therapy. For a reduction of 38.7 mg/dl (1 mmol/L) in LDL-c over 10 years, he could expect a 28% reduction in CHD risk by age 70 (3). Alternatively, the man could choose the age of his first CAC scan based on his probability of having non-zero CAC. If the man had a high genetic risk score and >2 traditional risk factors, he would have a 25% probability of non-zero CAC at age 34. If he started statin therapy at 34, he could expect a 51% reduction in CHD risk by age 70. Likewise, an individual with low genetic risk may avoid CAC scanning that is unlikely to result in initiation of statin therapy until later in life under the current guidelines.
Study limitations.
First, since calcification increases with age, CAC rates were lower in the CARDIA cohort compared to the MESA cohort. This resulted in an underpowered analysis of the CARDIA female subpopulation that warrants further study of larger young cohorts.
Second, while genetic risk and sex are age independent, other clinical risk factors change over time. In this study, risk markers were assessed at a single time point without follow up. Longitudinal studies may improve understanding of the effect of risk factor changes over time.
Third, though CAC scanning is a well-established technique for identifying subclinical atherosclerosis, not all plaques contain enough calcium to be detected via non-contrast CT. Atherosclerosis begins as early as childhood (40), and plaque calcification is a two stage process, beginning with microcalcifications <50μm in diameter that aggregate over time into macrocalcifications (41). Advanced CT technology that can detect smaller calcifications will enable earlier CAC detection and will likely improve GRS utility in younger populations.
Finally, the GRS used in this analysis included 142 variants reaching genome-wide significance for CHD in populations composed of primarily European ancestry. Increased diversity of discovery populations is necessary to discover SNPs more prevalent among other ancestral groups and improve GRS performance in non-European cohorts. Moreover, the GRS consisted of only variants that were in linkage equilibrium and reached a stringent genome-wide significance threshold. Advanced methods for SNP selection and weighting have enabled construction of GRS using millions of variants to improve prediction of CHD events (38, 42) and may improve GRS utility in a selective screening strategy for CAC.
Conclusion
A GRS composed of CHD risk variants is associated with CAC presence in a young cohort and can be used to identify high risk individuals with increased probability of CAC early in life. The GRS improves risk stratification for CAC presence over the lifetime when incorporated with traditional risk factors.
Perspectives
Competency in Medical Knowledge: Early initiation of preventive therapy reduces long term CHD event risk. CAC screening identifies individuals most likely to benefit from preventive therapy; however, determining when a young individual should undergo a first CAC scan remains a challenge. A GRS for CHD is independent of age and can be used to calculate the risk of CAC presence over the lifetime for a targeted CAC screening strategy.
Supplementary Material
Translational Outlook 1:
Widely available, inexpensive consumer genetic testing can provide novel information about both CHD and CAC risk over the lifetime.
Translational Outlook 2:
Additional study of cohorts with increased size and ancestral diversity are needed to validate GRS utility to predict CAC in young females as well as to identify CHD risk variants prevalent in non-European populations.
Acknowledgements:
The authors would like to thank the investigators and participants in the CARDIA (dbGaP study accession: phs000285.v3.p2) and MESA (dbGaP study accession: phs000420.v6.p3) studies for their valuable contributions (Appendix 3).
Support: This work is supported by NIH grants F31HL151081, T32HL105373, R01HL144678 and the CIFAR Azriele Global Scholar Award.
Dr. McVeigh holds founder shares in Clearpoint Neuro Inc. Dr. McVeigh receives research funding from GE Healthcare, Tendyne Holdings Inc., Pacesetter Inc., and Cardiowise. Dr. Contijoch receives funding from GE Healthcare. The remaining authors have nothing to disclose.
Abbreviations:
- CAC
coronary artery calcium
- CHD
coronary heart disease
- GRS
genetic risk score
- GWAS
genome wide association study
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020;141:139–596. [DOI] [PubMed] [Google Scholar]
- 2.Ference BA, Yoo W, Alesh I, et al. Effect of Long-Term Exposure to Lower Low-Density Lipoprotein Cholesterol Beginning Early in Life on the Risk of Coronary Heart Disease. J. Am. Coll. Cardiol 2012;60:2631–2639. [DOI] [PubMed] [Google Scholar]
- 3.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmali KN, Goff DC, Ning H, Lloyd-Jones DM. A Systematic Examination of the 2013 ACC/AHA Pooled Cohort Risk Assessment Tool for Atherosclerotic Cardiovascular Disease. J. Am. Coll. Cardiol 2014;64:959–968. [DOI] [PubMed] [Google Scholar]
- 5.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of Novel Risk Markers for Improvement in Cardiovascular Risk Assessment in Intermediate-Risk Individuals. JAMA 2012;308:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:E1082–E1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenti V Ó Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: A prospective follow-up of 9,715 individuals. JACC Cardiovasc. Imaging 2015;8:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur. Heart J 2018;39:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grandhi GR, Mirbolouk M, Dardari ZA, et al. Interplay of Coronary Artery Calcium and Risk Factors for Predicting CVD/CHD Mortality. JACC Cardiovasc. Imaging 2019. [DOI] [PubMed] [Google Scholar]
- 10.Blaha MJ, Cainzos-Achirica M, Dardari Z, et al. All-cause and cause-specific mortality in individuals with zero and minimal coronary artery calcium: A long-term, competing risk analysis in the Coronary Artery Calcium Consortium. Atherosclerosis 2020;294:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr JJ, Jacobs DR, Terry JG, et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol. 2017;2:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miedema MD, Dardari ZA, Nasir K, et al. Association of Coronary Artery Calcium With Long-term, Cause-Specific Mortality Among Young Adults. JAMA Netw. Open 2019;2:e197440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samani NJ, Erdmann J, Hall AS, et al. Genomewide Association Analysis of Coronary Artery Disease. N. Engl. J. Med 2007;357:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helgadottir A, Thorleifsson G, Manolescu A, et al. A Common Variant on Chromosome 9p21 Affects the Risk of Myocardial Infarction. Science (80-;.). 2007;316:1491–1493. [DOI] [PubMed] [Google Scholar]
- 15.McPherson R, Pertsemlidis A, Kavaslar N, et al. A Common Allele on Chromosome 9 Associated with Coronary Heart Disease. Science (80-.). 2007;316:1488–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikpay M, Goel A, Won H-H, et al. A comprehensive 1000 Genomes–based genome-wide association meta-analysis of coronary artery disease. Nat. Genet 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Harst P, Verweij N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ. Res 2018;122:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aragam KG, Natarajan P. Polygenic Scores to Assess Atherosclerotic Cardiovascular Disease Risk: Clinical Perspectives and Basic Implications. Circ Res 2020;126:1159–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thanassoulis G, Peloso GM, Pencina MJ, et al. A Genetic Risk Score Is Associated With Incident Cardiovascular Disease and Coronary Artery Calcium. Circ. Cardiovasc. Genet 2012;5:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natarajan P, Young R, Stitziel NO, et al. Polygenic Risk Score Identifies Subgroup With Higher Burden of Atherosclerosis and Greater Relative Benefit From Statin Therapy in the Primary Prevention Setting. Circulation 2017;135:2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salfati E, Nandkeolyar S, Fortmann SP, et al. Susceptibility Loci for Clinical Coronary Artery Disease and Subclinical Coronary Atherosclerosis Throughout the Life-Course. Circ. Cardiovasc. Genet 2015;8:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khera AV, Emdin CA, Drake I, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N. Engl. J. Med 2016;375:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Severance LM, Contijoch FJ, Carter H, et al. Using a genetic risk score to calculate the optimal age for an individual to undergo coronary artery calcium screening. J. Cardiovasc. Comput. Tomogr 2019;13:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cainzos-Achirica M, Mortensen MB, Blaha MJ. Exploring the intersection between genetic risk scores and coronary artery calcium – Mutually exclusive or complementary? J. Cardiovasc. Comput. Tomogr 2019;13:172–173. [DOI] [PubMed] [Google Scholar]
- 25.Mahoney LT, Burns TL, Stanford W, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: The muscatine study. J. Am. Coll. Cardiol 1996;27:277–284. [DOI] [PubMed] [Google Scholar]
- 26.Loria CM, Liu K, Lewis CE, et al. Early Adult Risk Factor Levels and Subsequent Coronary Artery Calcification. J. Am. Coll. Cardiol 2007;49:2013–2020. [DOI] [PubMed] [Google Scholar]
- 27.Hartiala O, Kajander S, Knuuti J, et al. Life-course risk factor levels and coronary artery calcification. The Cardiovascular Risk in Young Finns Study. Int. J. Cardiol 2016;225:23–29. [DOI] [PubMed] [Google Scholar]
- 28.Osei AD, Uddin SMI, Dzaye O, et al. Predictors of coronary artery calcium among 20–30-year-olds: The Coronary Artery Calcium Consortium. Atherosclerosis 2020;301:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr 2017;11:157–168. [DOI] [PubMed] [Google Scholar]
- 30.Friedman GD, Cutter GR, Donahue RP, et al. Cardia: study design, recruitment, and some characteristics of the examined subjects. J. Clin. Epidemiol 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 31.Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015;4:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das S, Forer L, Schönherr S, et al. Next-generation genotype imputation service and methods. Nat. Genet 2016;48:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am. J. Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 34.Burke G, Lima J, Wong ND, Narula J. The Multiethnic Study of Atherosclerosis. Glob. Heart 2016;11:267. [DOI] [PubMed] [Google Scholar]
- 35.Carr JJ, Nelson JC, Wong ND, et al. Calcified Coronary Artery Plaque Measurement with Cardiac CT in Population-based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 36.Pencina MJ, Steyerberg EW, D’Agostino RB. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inouye M, Abraham G, Nelson CP, et al. Genomic Risk Prediction of Coronary Artery Disease in 480,000 Adults. J. Am. Coll. Cardiol 2018;72:1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tada H, Melander O, Louie JZ, et al. Risk prediction by genetic risk scores for coronary heart disease is independent of self-reported family history. Eur. Heart J 2016;37:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berenson GS, Wattigney WA, Tracy RE, et al. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (the Bogalusa Heart Study). Am. J. Cardiol 1992;70:851–858. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Osborne MT, Tung B, Li M, Li Y. Imaging Cardiovascular Calcification. J. Am. Heart Assoc 2018;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet 2018;50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


