Abstract
Platelet distribution width has been recognized as risk predictors of idiopathic pulmonary arterial hypertension. This study aims to investigate whether in-hospital platelet distribution width would be useful to predict all-cause death in patients with severe pulmonary hypertension due to chronic lung diseases (CLD-PH). Early in-hospital platelet distribution width was measured in 67 severe CLD-PH patients who were confirmed by right heart catheterization and followed up. Event-free survival was estimated using the Kaplan–Meier method and analyzed with the log-rank test. Cox proportional hazards models were performed to determine the association between the platelet distribution width level and all-cause death. During median of 2.4 (2.5, 3.7) years of follow-up, 44 patients died. A significant association was noted between in-hospital platelet distribution width level and the adjusted risk of all-cause mortality (hazard ratio: 1.245; 95% confidence interval: 1.117–1.386, P < 0.001). Compared with those with platelet distribution width <16.1%, the hazard ratio for all-cause death increased by 5.278 (95% confidence interval: 2.711–10.276, P < 0.0001) among patients with platelet distribution width ≥16.1%. Higher levels of platelet distribution width were also associated with increased risk of all-cause death. In-hospital platelet distribution width was independently associated with all-cause death in patients with severe CLD-PH. This potentially could be used to estimate the severity of severe CLD-PH.
Keywords: all-cause death, chronic lung diseases, pulmonary hypertension, platelet distribution width, prognosis
Introduction
Pulmonary hypertension (PH) is a common complication of chronic lung diseases (CLD) and often progresses to right heart failure (RHF) and death. 1 , 2 According to hemodynamics, 2 , 3 PH in CLD is classified into mild PH (mean pulmonary artery pressure (mPAP) ≥25 mmHg) and severe PH (mPAP ≥35 mmHg or 25 mmHg < mPAP < 35 mmHg with cardiac index (CI) < 2.0 L/min/m2 or pulmonary vascular resistance (PVR) > 6 Wood units). Although accounting for only a minority of pulmonary hypertension due to chronic lung diseases (CLD-PH) cases, severe CLD-PH patients generally have progressive vascular remodeling accompanying parenchymal disease that develops independently from pulmonary functional impairment, and generally progress to RHF and death. 2 , 4 Currently, studies regarding severe CLD-PH survival are sparse. Thus, we explored the risk factors that may affect the prognosis of severe CLD-PH.
Recently, Mark R Looney et al. 5 identified that the lungs made substantial contributions as a primary site of terminal platelet production and an organ with considerable hematopoietic potential, accounting for approximately 50% of total platelet production or 10 million platelets per hour. Idiopathic pulmonary arterial hypertension (IPAH) patients with a lower platelet level before treatment had a higher mortality rate than those with a higher platelet level. 6 , 7 The platelet distribution width (PDW), in addition to serving as a marker of platelet activation, has been reported to increase in PAH associated with congenital heart diseases8–10 and IPAH, 11 and could help to predict disease severity as well.
Therefore, the objective of this study was to investigate the value of PDW in predicting survival in patients with severe CLD-PH.
Methods
Study design and patients
This is a retrospective study. We included patients according to the following criteria: (1) suspected PH associated with CLD; 12 (2) diagnosis of CLD confirmed by experienced specialists according to the appropriate guidelines; 13 , 14 (3) “severe PH” diagnosed by right heart catheterization (RHC); and (4) blood sample and RHC within seven days at a clinically stable stage. The established diagnosis of “severe PH” was in accordance with the following criteria: 2 , 3 mPAP ≥35 mmHg or 25 mmHg < mPAP < 35 mmHg with CI < 2.0 L/min/m2 or PVR ≥6 Wood units. Patients were excluded if they were (1) diagnosed with other types of PH as per the NICE criteria; 12 (2) on anticoagulant or antiplatelet drug therapy including aspirin on admission; and (3) they lacked a blood sample or RHC at a clinically stable stage. Finally, 67 patients with severe CLD-PH were enrolled in Shanghai Pulmonary Hospital from 2009 to 2014. Clinical data were obtained in the process of routine clinical care and collected from hospital records.
This study was conducted in accordance with the amended Declaration of Helsinki. The Institutional Ethics Committee of Shanghai Pulmonary Hospital approved the protocol (K08-015C), and written informed consent was obtained from all patients.
Assessment of patients
Demographic variables such as sex, age, body mass index, pulmonary function test (PFT), echocardiography, and RHC parameters were obtained at baseline. RHC was performed as described previously. 15 The baseline hemodynamic variables evaluated included mPAP, right arterial pressure, pulmonary artery wedge pressure, cardiac output, CI, and PVR. Transthoracic echocardiography was performed as described previously. 16
Blood samples
Blood samples were obtained in the nonfasting state on the second day after admission to the hospital. The blood sample for PDW measurement was collected in 2-mL dipotassium EDTA tubes. Whole blood samples were processed via an automatic blood counter (ACL top 700; Beckman Corporation, USA). A technician who was blinded to the patients’ data performed the blood test within 30 min. The reference values for PDW ranged between 9.0% and 17.0%.
Outcomes
The main outcome was the time from the date of blood sampling to the occurrence of all-cause death. All patients were followed up until death, or through 30 April 2019, whichever occurred first. We had an established PH databased in our center. Data were obtained during follow-up or by telephone interview. Specific events were confirmed through medical records, death certificates, or confirmation provided by immediate family members.
Statistical analysis
The results are expressed as the mean with standard deviation or median (and interquartile range) for continuous variables and as absolute numbers for categorical variables. The receiver-operating characteristic (ROC) method was used to assess the cutoff of the PDW level in predicting all-cause death. Comparisons were performed using independent-sample t-test or Mann–Whitney U tests for continuous variables and chi-square tests for categorical variables among patients grouped by the cutoff PDW value. Pearson or Spearman correlation tests were performed to assess correlations between variables of interest and the PDW level. The multivariable-adjusted hazard ratios (HRs) and 95% CI for all-cause mortality were used in a Cox proportional hazards regression analysis after adjustments for covariates. In a multivariate model, PDW was adjusted for CI. Survival curves were plotted using the Kaplan–Meier method and analyzed with the log-rank test. The Bonferroni method for correcting the significance level for multiple comparisons was applied. All tests were two-tailed.
All statistical methods were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.04 software (GraphPad Software, Inc., San Diego, CA).
Results
Characteristics of participants
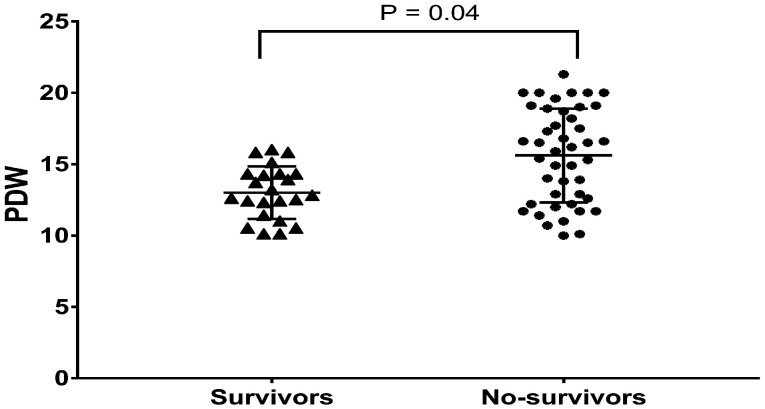
A total of 67 patients with severe CLD-PH matched the inclusion criteria. The mean age at diagnosis was 58.0 ± 1.6 years, with a male preponderance of 56.7% (Table 1). PDW ranged from 10.0% to 21.3% with a median of 14.3%, and 22 (32.8%) had a PDW level ≥16.1%. The median follow-up was 2.4 (2.5, 3.7) years with a maximal duration of 7.1 years. During follow-up, 44 (65.7%) patients died, and no patients underwent lung transplantation. No patient was lost to follow-up, giving us a 100% follow-up rate. In-hospital PDW was significantly higher in nonsurvivors (n = 44) than in survivors (n = 23) (15.6 ± 3.3% vs. 13.0 ± 1.8%, P = 0.004; Fig. 1). Based on the cutoff value of the ROC curve, all the patients were divided into two groups: PDW ≥16.1% group (n = 22) and the PDW < 16.1% group (n = 45). Table 1 presents the demographic, PFT, hemodynamic and echocardiographic data of the two groups. In our study, 25 variables initially were compared between the PDW ≥16.1% group and the PDW < 16.1% group; therefore, when Bonferroni’s correction of the significance level (P < 0.05) was applied, the adjusted significance level was 0.002. Consequently, all of the results of these comparisons became statistically insignificant. No statistically significant differences were observed in the demographics, etiology, hemodynamics, PFT, or echocardiography between the two groups (Table 1).
Table 1.
Demographic characteristics, pulmonary function test results, hemodynamics, and echocardiography parameters of patients.
| All patients | PDW ≥ 16.1% | PDW < 16.1% | ||
|---|---|---|---|---|
| N = 67 | N = 22 | N = 45 | P a | |
| Age (years) | 58.0 ± 12.9 | 58.1 ± 13.2 | 58.0 ± 12.9 | 0.963 |
| Male (n, %) | 38 (56.7) | 13 (81.3) | 25 (49.0) | 0.189 |
| BSA (m2) | 1.58 ± 0.20 | 1.58 ± 0.20 | 1.59 ± 0.20 | 0.989 |
| COPD | 14 (63.6) | 12 (75.0) | 23 (51.1) | 0.337 |
| Bronchiectasis | 12 (16.1.9) | 4 (18.2) | 8 (17.8) | 0.968 |
| ILD | 6 (9.0) | 2 (9.1) | 4 (8.9) | 0.978 |
| Others | 12 (20.9) | 2 (9.1) | 10 (22.2) | 0.191 |
| Pulmonary function test | ||||
| FEV1% predicted | 32.4 (24.0, 44.0) | 35.0 (25.8, 43.7) | 31.5 (24.0, 47.9) | 0.828 |
| FEV1/FVC % predicted | 47.4 (42.8, 56.1) | 43.3 (36.3, 51.5) | 48.8 (43.7, 59.7) | 0.063 |
| RV % predicted | 204.1 ± 80.4 | 230.5 ± 99.3 | 197.1 ± 74.8 | 0.272 |
| TLC % predicted | 113.6 ± 29.3 | 126.6 ± 31.0 | 110.1 ± 28.3 | 0.136 |
| DLCO % predicted | 40.9 (27.9, 56.2) | 26.0 (23.9, 48.7) | 42.0 (30.0, 58.5) | 0.090 |
| Hemodynamics | ||||
| mPAP (mmHg) | 46.0 (42.0, 55.0) | 49.0 (42.0, 58.3) | 46.0 (41.5, 54.0) | 0.411 |
| PAWP (mmHg) | 9.8 ± 4.0 | 10.0 ± 3.9 | 9.7 ± 4.1 | 0.818 |
| CO (L/min) | 4.6 (4.0, 5.7) | 4.2 (3.9, 5.4) | 4.7 (4.3, 6.2) | 0.063 |
| CI (L/min/m2) | 3.1 (2.6, 3.7) | 2.8 (2.5, 3.6) | 3.2 (2.7, 3.7) | 0.159 |
| PVR, Wood units | 8.0 (6.3, 10.2) | 9.1 (6.5, 10.7) | 7.5 (5.9, 9.5) | 0.149 |
| Echocardiography | ||||
| LVEF (%) | 70.9 ± 9.0 | 70.6 ± 9.9 | 70.8 ± 8.8 | 0.923 |
| RATD (cm) | 4.8 (4.3, 5.5) | 5.3 (4.6, 5.9) | 4.7 (4.3, 5.5) | 0.112 |
| RALD (cm) | 5.2 (4.3, 5.9) | 5.5 (5.0, 6.2) | 5.1 (4.3, 5.9) | 0.131 |
| RVEDTD (cm) | 4.3 (3.8, 5.0) | 4.4 (4.0, 5.3) | 4.2 (3.8, 4.9) | 0.319 |
| RVEDLD (cm) | 6.6 ± 0.9 | 6.8 ± 0.6 | 6.5 ± 1.0 | 0.197 |
| PASP (mmHg) | 76.2 ± 22.6 | 80.6 ± 26.5 | 74.0 ± 20.4 | 0.265 |
| TAPSE (cm) | 1.7 (1.5, 1.9) | 1.7 (1.4, 1.9) | 1.8 (1.6, 2.0) | 0.220 |
| ENDSEI | 1.3 (1.0, 1.5) | 1.4 (1.1, 1.8) | 1.2 (1.0, 1.4) | 0.079 |
Values are mean ± standard deviation, median (interquartile interval) or n (%). CO: cardiac output; COPD: chronic obstructive pulmonary diseases; ILD: interstitial lung disease; mPAP: mean pulmonary artery pressure; RAP: right atrial pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; RV: residual volume; TLC: total lung capacity; DLCO: diffusion capacity for carbon monoxide of the lung; PDW: platelet distribution width. Others included pulmonary lobectomy, chest deformity, lung destruction due to tuberculosis, pneumoconiosis, combined pulmonary fibrosis and emphysema. RVEDTD: right ventricular end-diastolic transverse dimension; RVEDLD: right ventricular end-diastolic longitudinal dimension; RATD: right atrial transverse dimension; RALD: right atrial longitudinal dimension; PASP: pulmonary arterial systolic pressure; ENDSEI: end-systolic stage eccentricity index; TAPSE: tricuspid annular plane systolic excursion; LVEF: left ventricular ejection fraction.
aWhen the Bonferroni method was employed for correcting for the significance level for 25 comparisons made in this study, the adjusted significant level was 0.002.
Fig. 1.
The box and whiskers of baseline PDW values between survivors and nonsurvivors. PDW: platelet distribution width.
Correlation between the in-hospital PDW level and PFT, hemodynamics, echocardiographic parameters, and all-cause death
Despite of the statistical trend, the right atrial transverse dimension showed no significant correlation with PDW in severe CLD-PH patients after Bonferroni correction (P < 0.002) (Supplementary Table). Univariate Cox regression analysis revealed that in-hospital PDW (HR = 1.245, 95% CI: 1.121–1.384, P < 0.001), CI (HR = 0.659, 95% CI: 0.454–0.958, P = 0.029), and COPD (HR = 2.357, 95% CI: 1.258–4.416, P = 0.007) were found to predictors of the risk of all-cause death in patients with severe CLD-PH. In multivariate Cox regression analysis, PDW (HR = 1.245, 95% CI: 1.117–1.386, P < 0.001) and CI (HR = 0.667, 95% CI: 0.455–0.978, P = 0.038) were independently associated with all-cause death (Table 2). Adjusted by CI, in comparison with patients with a PDW level < 16.1%, the HR for all-cause death among patients with a PDW above ≥16.1% increased by 5.278 (95% CI: 2.711–10.276, P < 0.0001) (Table 3).
Table 2.
Cox proportional hazards regression analysis for all-cause death in patients with severe CLD-PH.
| Univariate analysis |
Multivariate-adjusted analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender | 0.696 (0.381–1.271) | 0.239 | ||
| COPD | 2.357 (1.258–4.416) | 0.007 | ||
| Red blood cell (×1012/L) | 1.187 (0.829–1.689) | 0.350 | ||
| White blood cell (×109/L) | 0.932 (0.840–1.033) | 0.179 | ||
| Platelet (×109/L) | 0.996 (0.991–1.001) | 0.089 | ||
| Red blood cell distribution Width (%) | 1.027 (0.979–1.076) | 0.273 | ||
| PDW (%) | 1.245 (1.121–1.384) | <0.001 | 1.245 (1.117–1.386) | <0.001 |
| Platelet crit (%) | 0.006 (0.000–0.881) | 0.044 | ||
| Mean platelet volume (fl) | 0.992 (0.934–1.053) | 0.786 | ||
| FEV1/FVC % predicted | 0.973 (0.935–1.012) | 0.717 | ||
| DLCO % predicted | 0.999 (0.982–1.016) | 0.892 | ||
| mPAP (mmHg) | 1.016 (0.986–1.047) | 0.239 | ||
| PVR (Wood units) | 1.065 (0.994–1.141) | 0.074 | ||
| CI (L/min/m2) | 0.659 (0.454–0.958) | 0.029 | 0.667 (0.455–0.978) | 0.038 |
| RATD (cm) | 1.102 (0.837–1.405) | 0.491 | ||
HR: hazard ratio; CI: confidence interval; mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; CI: cardiac index; CLD-PH: pulmonary hypertension due to chronic lung diseases; PDW: platelet distribution width; PCT: platelet crit; MPV: mean platelet volume; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; DLCO: diffusion capacity for carbon monoxide of the lung; RATD: right atrial transverse dimension; COPD: chronic obstructive pulmonary diseases.
Table 3.
Multivariate-adjusted hazard ratios for the association between PDW and all-cause death.
| PDW category | Unadjusted |
Cardiac index adjusted |
|||||
|---|---|---|---|---|---|---|---|
| No. of events | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| PDW < 16.1% | 22/45 | Ref. | Ref. | ||||
| PDW ≥ 16.1% | 22/22 | 5.464 | 2.829–10.553 | <0.0001 | 5.278 | 2.711–10.276 | <0.0001 |
PDW: platelet distribution width; CI: confidence interval.
PDW difference in survival assessment
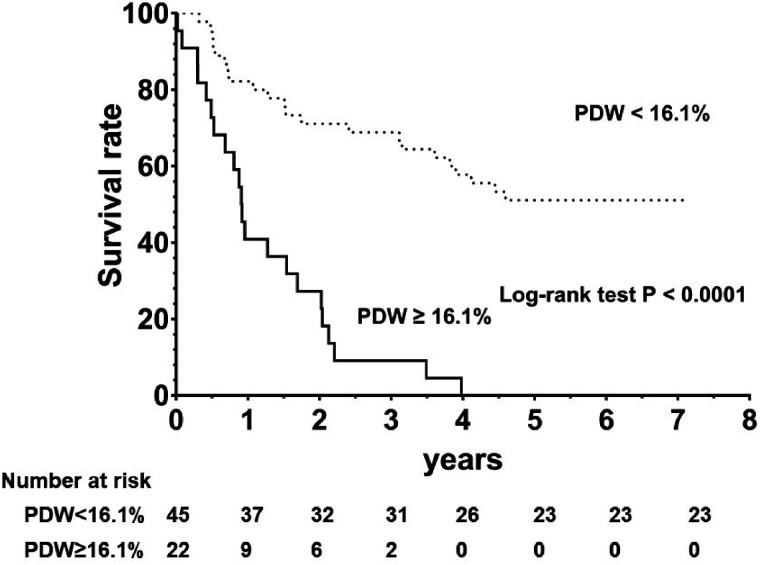
There were 44 all-cause death events during a median follow-up period of 2.4 (2.5, 3.7) years. The overall event-free survival rate was 82.2% and 40.9% for the first year; 71.1% and 27.3% during the second year; and 68.9% and 9.1% for the third year for the PDW < 16.1% and PDW ≥16.1% subgroups, respectively.
The overall event-free survival rate was significantly different between the two groups, with a log-ranked P-value ≤0.001 (Fig. 2).
Fig. 2.
Kaplan–Meier all-cause mortality survival plots by the cutoff value of ROC curve.
Discussion
PDW reflects the variability in the size of circulating platelets and is routinely reported by automated laboratory equipment. We found an independent association between the early in-hospital PDW value and the risk of all-cause death in patients with severe PH-CLD, most of whom had PDW levels within the normal range. Adjustment for multiple potential confounders did not eliminate the association between higher PDW levels and all-cause death. These findings are notable given that PDW is widely available to clinicians as part of the complete blood count.
PDW is a convenient indicator for assessing platelet function that reflects the platelet production rate and activation. Activation of platelets causes morphological changes, including pseudopodia formation. Progressively activated platelets with pseudopodia formation have heterogeneous sizes and may increase PDW accordingly. 16 , 17
The association of PDW with survival may be hypothetically linked with hypercoagulation, which plays a significant role in conditions associated with survival in COPD 18 and acute myocardial infarction. 19 , 20 Previously, PDW was found to increase in tumors (cervical and hepatocellular carcinoma, 21 , 22 breast and gastric cancer 23 , 24 ) and inflammatory disorders (sepsis 25 and acute pancreatitis 26 ) and was identified as a predictor for survival. Similarly, PDW has also been reported to be elevated in IPAH patients. 11 However, instead of predicting prognosis in pulmonary arterial hypertension (PAH), it could partially reflect disease severity. Conversely, in the present study, PDW predicted prognosis in patients with severe CLD-PH but did not correlate with CI, mPAP, or PVR. This could be explained by the heterogeneity between severe CLD-PH and IPAH. 4 , 27 To the best of our knowledge, this is the first demonstration that a higher PDW could predict mortality in patients with severe CLD-PH.
The cause of enhanced PDW in pulmonary diseases is not well understood. The chronic coagulation activation state, partly due to being platelets exposed to microtrauma in the pulmonary vasculature or yet unknown factors in CLD-PH, leads to an increase in PDW. Additionally, hypoxic pulmonary vasoconstriction with platelet activation, which causes morphological changes, including changes in platelet size, further provokes micro trauma and platelet activation. Whether any component within these morphologically altered platelets, particularly thromboxane-like compounds which are known vasoconstrictors, plays a provocative role in increasing PDW may be a point of debate and is just speculation on our part, as it is not a main point of study of this paper. The mechanism of PAH is complex and has multiple etiologies. Platelets are involved in thrombotic pulmonary vascular lesions, chronic vasoconstriction, and pulmonary vascular remodeling in PH. Inflammatory processes are increasingly recognized as major pathogenic components of pulmonary vascular remodeling. 28 Systemic inflammation, thrombotic microangiopathies, and immune dysfunction which were reported in patients with PAH might also cause platelet activation. 29 However, Looney et al. identify the lungs as a primary site of terminal platelet production and an organ with considerable hematopoietic potential. 5 Despite the chicken or the egg analogy, the exact cause of increased PDW in CLD-PH remains an interesting clinical debate.
Study limitations
Some potential limitations should be acknowledged. First, this was a clinical study, and thus, the underlying pathophysiological mechanisms might only be speculative. Second, we did not investigate the causes of the increased PDW values. Third, the study population is small. Further large-scale prospective studies are warranted to clarify the potential role of PDW in predicting all-cause death events.
Conclusion
We found an independent relation between high levels of PDW and the risk of all-cause death in severe CLD-PH patients. As it can be simply and rapidly measured from routine blood examination, should our results be confirmed in larger samples, PDW may prove to be a widely available, inexpensive, and repeatable prognostic marker.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_20458940211026484 for Survival in severe pulmonary hypertension due to chronic lung disease: influence of in-hospital platelet distribution width by Lan Wang, Li Shen, Ya-Lin Zhao, Bigyan Pudasaini, Qin-Hua Zhao, Su-Gang Gong, Rui Zhang, Ping Yuan, Jing He, Ci-Jun Luo, Hong-Ling Qiu, Jin-Ming Liu and Rong Jiang in Pulmonary Circulation
Acknowledgement
None.
Conflict of interest: The author(s) declare that there is no conflict of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Ethics approval and consent for publication: All clinical, radiological, and laboratory data were collected after obtaining approval from the Institutional Ethics Committee of Shanghai Pulmonary Hospital approved the protocol (K08-015C) and according to the international standards of good clinical practice. All medical data used in this study were irreversibly anonymized.
Consent for publication: Not applicable.
Authors’ contribution: Conception by R.J., B.P., L.W., Y-L.Z., Q-H.Z. and J.H. analyzed clinical data. Clinical management performed by P.Y., S-G.G., H-L.Q., R.Z., C-J.L., and J-M.L. Manuscript organization, writing, and editing by B.P., L.S., Y-L.Z., and L.W. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding: The work was funded by the Program of National Natural Science Foundation of China (81700045, 81870042, 82000059), the Program Supported by the Fundamental Research Funds for the Central University (22120180539), the National Key Research and Development Project (2018YFC1313603) and Program of Shanghai Pulmonary Hospital (FKLY 20005).
Guarantor: Rong Jiang.
ORCID iD: Rong Jiang https://orcid.org/0000-0003-4062-5550
Supplemental material: Supplemental material for this article is available online.
References
- 1.Mourani PM, Sontag MK, Younoszai A, et al. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 2008; 121: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: D109–D116. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Andreas S, Bastian A, et al. Pulmonary hypertension due to chronic lung disease: updated Recommendations of the Cologne Consensus Conference 2011. Int J Cardiol 2011; 154: S45–S53. [DOI] [PubMed] [Google Scholar]
- 4.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefrancais E, Ortiz-Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2016; 544: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi H, Kataoka M, Yanagisawa R, et al. Platelet level as a new prognostic factor for idiopathic pulmonary arterial hypertension in the era of combination therapy. Circ J 2012; 76: 1494–1500. [DOI] [PubMed] [Google Scholar]
- 7.Aytekin M, Aulak KS, Haserodt S, et al. Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: role of nitric oxide. Am J Physiol Lung Cell Mol Physiol 2012; 302: L512–L520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mese T, Guven B, Yilmazer MM, et al. Platelet activation markers in children with congenital heart disease associated with pulmonary arterial hypertension. Congenit Heart Dis 2018; 13: 506–511. [DOI] [PubMed] [Google Scholar]
- 9.Remkova A, Simkova I, Valkovicova T, et al. Platelet abnormalities in adults with severe pulmonary arterial hypertension related to congenital heart defects (Eisenmenger syndrome). Blood Coagul Fibrinolysis 2016; 27: 925–929. [DOI] [PubMed] [Google Scholar]
- 10.Arslan D, Cimen D, Guvenc O, et al. Platelet distribution width and mean platelet volume in children with pulmonary arterial hypertension secondary to congenital heart disease with left-to-right shunt: new indices of severity? Pediatr Cardiol 2013; 34: 1013–1016. [DOI] [PubMed] [Google Scholar]
- 11.Zheng YG, Yang T, Xiong CM, et al. Platelet distribution width and mean platelet volume in idiopathic pulmonary arterial hypertension. Heart Lung Circ 2015; 24: 566–572. [DOI] [PubMed] [Google Scholar]
- 12.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 13.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 14.Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012; 185: 1004–1014. [DOI] [PubMed] [Google Scholar]
- 15.Jiang R, Ai ZS, Jiang X, et al. Intravenous fasudil improves in-hospital mortality of patients with right heart failure in severe pulmonary hypertension. Hypertens Res 2015; 38: 539–544. [DOI] [PubMed] [Google Scholar]
- 16.Jiang R, Wu C, Pudasaini B, et al. A novel scoring index by Doppler echocardiography for predicting severe pulmonary hypertension due to chronic lung diseases: a cross-sectional diagnostic accuracy study. Int J Chron Obstruct Pulmon Dis 2016; 12: 1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae MH, Lee JH, Yang DH, et al. White blood cell, hemoglobin and platelet distribution width as short-term prognostic markers in patients with acute myocardial infarction. J Korean Med Sci 2014; 29: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bialas AJ, Pedone C, Piotrowski WJ, et al. Platelet distribution width as a prognostic factor in patients with COPD – pilot study. Int J Chron Obstruct Pulmon Dis 2016; 12: 2261–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huczek Z, Kochman J, Filipiak KJ, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2005; 46: 284–290. [DOI] [PubMed] [Google Scholar]
- 20.Rechcinski T, Jasinska A, Forys J, et al . Prognostic value of platelet indices after acute myocardial infarction treated with primary percutaneous coronary intervention. Cardiol J 2013; 20: 491–498. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Zhang FB, Li BJ, et al. Combination of preoperative D-dimer and platelet distribution width predicts postoperative deep venous thrombosis in patients with cervical carcinoma. Asian Pac J Cancer Prev 2019; 20: 1025–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo X, Kong W, Feng L, et al. Elevated platelet distribution width predicts poor prognosis in hepatocellular carcinoma. Cancer Biomark 2019; 24: 307–313. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Cui MM, Huang YX, et al. Preoperative platelet distribution width predicts breast cancer survival. Cancer Biomark 2018; 23: 205–211. [DOI] [PubMed] [Google Scholar]
- 24.Cheng S, Han F, Wang Y, et al. The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer. BMC Gastroenterol 2016; 16: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orak M, Karakoc Y, Ustundag M, et al. An investigation of the effects of the mean platelet volume, platelet distribution width, platelet/lymphocyte ratio, and platelet counts on mortality in patents with sepsis who applied to the emergency department. Niger J Clin Pract 2018; 21: 667–671. [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Meng Z, Li S, et al. Platelet distribution width levels can be a predictor in the diagnosis of persistent organ failure in acute pancreatitis. Gastroenterol Res Pract 2016; 2016: 8374215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 28.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009; 54: S10–S19. [DOI] [PubMed] [Google Scholar]
- 29.Rabinovitch M, Guignabert C, Humbert M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014; 115: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_20458940211026484 for Survival in severe pulmonary hypertension due to chronic lung disease: influence of in-hospital platelet distribution width by Lan Wang, Li Shen, Ya-Lin Zhao, Bigyan Pudasaini, Qin-Hua Zhao, Su-Gang Gong, Rui Zhang, Ping Yuan, Jing He, Ci-Jun Luo, Hong-Ling Qiu, Jin-Ming Liu and Rong Jiang in Pulmonary Circulation