Abstract
BACKGROUND
The diagnosis of acute myocarditis typically requires either endomyocardial biopsy (which is invasive) or cardiovascular magnetic resonance imaging (which is not universally available). Additional approaches to diagnosis are desirable. We sought to identify a novel microRNA for the diagnosis of acute myocarditis.
METHODS
To identify a microRNA specific for myocarditis, we performed microRNA microarray analyses and quantitative polymerase-chain-reaction (qPCR) assays in sorted CD4+ T cells and type 17 helper T (Th17) cells after inducing experimental autoimmune myocarditis or myocardial infarction in mice. We also performed qPCR in samples from coxsackievirus-induced myocarditis in mice. We then identified the human homologue for this microRNA and compared its expression in plasma obtained from patients with acute myocarditis with the expression in various controls.
RESULTS
We confirmed that Th17 cells, which are characterized by the production of interleukin-17, are a characteristic feature of myocardial injury in the acute phase of myocarditis. The microRNA mmu-miR-721 was synthesized by Th17 cells and was present in the plasma of mice with acute autoimmune or viral myocarditis but not in those with acute myocardial infarction. The human homologue, designated hsa-miR-Chr8:96, was identified in four independent cohorts of patients with myocarditis. The area under the receiver-operating-characteristic curve for this novel microRNA for distinguishing patients with acute myocarditis from those with myocardial infarction was 0.927 (95% confidence interval, 0.879 to 0.975). The microRNA retained its diagnostic value in models after adjustment for age, sex, ejection fraction, and serum troponin level.
CONCLUSIONS
After identifying a novel microRNA in mice and humans with myocarditis, we found that the human homologue (hsa-miR-Chr8:96) could be used to distinguish patients with myocarditis from those with myocardial infarction. (Funded by the Spanish Ministry of Science and Innovation and others.)
MYOCARDITIS IS A DISEASE WITH MULtiple causes1,2 (e.g., infectious pathogens, toxins, drugs, and autoimmune disorders3,4) that may resolve spontaneously, cause sudden cardiac death, or evolve into dilated cardiomyopathy.5 The actual prevalence of the disease remains uncertain because of the difficulty of reaching a confirmatory diagnosis in many cases. Myocarditis is a frequent final diagnosis in patients who receive an initial diagnosis of acute myocardial infarction with nonobstructive coronary arteries (MINOCA),6 a clinical entity that occurs in approximately 10 to 20% of patients who meet the criteria for myocardial infarction.7,8 The diagnosis of myocarditis is usually established after ruling out coronary artery disease by means of coronary angiography or computed tomography and confirming the presence of Lake Louise criteria on cardiac magnetic resonance imaging (MRI).9 However, cardiac MRI is not available in all centers.10,11 The reference standard for diagnosis relies on endomyocardial biopsy, which is usually reserved for severe cases.1 Therefore, reliable and accessible diagnostic tools for the early diagnosis of acute myocarditis are an unmet clinical need.
The phenotypes of circulating T cells are altered in both myocarditis12 and myocardial infarction.13 Cardiac myosin-specific type 17 helper T (Th17) lymphocytes14 play a central role in the development of myocarditis and dilated cardiomyopathy15 and in the production of anti–myocardial antibodies in viral myocarditis.16 MicroRNAs (miRNAs) have emerged as innovative biomarkers for cardiovascular disease.17 We sought to identify a novel miRNA that could be used to distinguish acute myocarditis from myocardial infarction.
Methods
OVERVIEW OF STUDY STRATEGY
By analyzing circulating T cells obtained from mice and humans with myocarditis and myocardial infarction, we confirmed that Th17 cells are a characteristic feature of myocardial injury in the acute phase of myocarditis. Screening of miRNAs that are synthesized by Th17 cells with the use of microarrays and quantitative polymerase-chain-reaction (qPCR) assays showed that mmu-miR-721 was specific for Th17 cells and plasma in mice with autoimmune or viral myocarditis. Since the human homologue of mmu-miR-721 had not been described previously, we cloned and sequenced the putative human homologue in plasma obtained from patients with myocarditis. Coimmunoprecipitation and luciferase assays showed that the human homologue, which we designated hsa-miR-Chr8:96, is a new functional human miRNA. We validated hsa-miR-Chr8:96 as a suitable myocarditis detector in four independent cohorts using qPCR. The animal models and human study participants are described below; details regarding the study methods are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
This multicenter study was approved by the ethics committee of the Carlos III Institute of Health and the research ethics committee at each of the participating hospitals; a list of centers is provided in the Supplementary Appendix. All the participants provided written informed consent for the use of their samples and data.
MURINE MODELS OF AUTOIMMUNE MYOCARDITIS AND MYOCARDIAL INFARCTION
Wild-type BALB/c mice and CD69 knockout mice were bred at the National Center for Cardiovascular Research (CNIC) animal facility for use in our experimental autoimmune myocarditis model. Mice between the ages of 8 and 12 weeks were immunized subcutaneously on days 0 and 7 with 100 μg per 0.2 ml of cardiac α-myosin heavy-chain peptide (residues 614–629; Ac-RSLKLMATLFSTYASADR-OH), emulsified in a 1:1 ratio in complete Freund’s adjuvant. Severe experimental autoimmune myocarditis was induced in the CD69 knockout mice18; less severe (moderate) experimental autoimmune myocarditis was induced in the wild-type mice. Sex- and age-matched littermates were immunized with complete Freund’s adjuvant and saline as controls. In separate experiments, wild-type BALB/c mice between the ages of 8 and 12 weeks underwent permanent ligation of the left anterior descending coronary artery to induce myocardial infarction, with sham-operated mice used as controls, as described in the Supplementary Appendix.
Experimental autoimmune myocarditis was also induced in mice expressing antigen-specific T-cell receptors. We purchased C57BL/6-Tg (TcraTcrb) 425Cbn/J mice (called OT-II) expressing a T-cell receptor specific for peptide 323–339 of ovalbumin in the context of murine major histocompatibility complex class II alleles H-2 I-Ab from the Jackson Laboratory (stock number 004194). Additional OT-II mice were backcrossed with CD69 knockout mice, which are both from the same inbred strain (C57BL/6 background).
The mice were housed under specific pathogen-free conditions at the CNIC animal facility. All procedures involving the mice were approved by the Comunidad Autónoma de Madrid and conducted in accordance with Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010, on the protection of animals used for scientific purposes.
MURINE MODEL OF VIRAL MYOCARDITIS
Wild-type BALB/c or C57BL/6 mice were obtained from the Jackson Laboratory. Eight-week-old mice were inoculated intraperitoneally with 103 plaque-forming units of coxsackievirus B3 that had been isolated from a patient (Nancy strain) and passaged through Vero cells and then through the heart (i.e., heart-passaged) in saline, as described in the Supplementary Appendix. Control mice received intraperitoneal saline injections. Serum and hearts were collected 10 days after infection, as described previously.19 Mice were maintained under pathogen-free conditions in the animal facility at Mayo Clinic Jacksonville, and approval was obtained from the Animal Care and Use Committee of Mayo Clinic for all procedures.
STUDY PATIENTS
The main study cohort included 132 patients with initial clinical suspicion of acute myocarditis and myocardial injury, ventricular dysfunction, or both (Tables S1 and S2 in the Supplementary Appendix). Of these patients, 42 had a final diagnosis of myocarditis on the basis of cardiac MRI that met the typical Lake Louise diagnostic criteria; 90 patients were diagnosed with myocardial infarction and were included in the analysis as comparators. A total of 80 healthy participants with no abnormal findings on electrocardiography or echocardiography were included as additional controls.
We validated the use of hsa-miR-Chr8:96 for the diagnosis of human myocarditis using four additional patient cohorts. Validation cohort 1 was provided by the Partners Biobank (Boston) and included 34 patients with an established diagnosis of acute myocarditis and 11 patients with myocardial infarction as comparators (Table S3). Validation cohort 2, which has been described previously,20 was provided by the Center for Molecular Cardiology of the University Hospital Zurich (Switzerland). This cohort included 35 patients with an established diagnosis of acute myocarditis and 20 patients with MINOCA (Table S4). Validation cohort 3 included samples from 40 patients selected from a prospective cohort recruited at the University of Padua (Italy) with a biopsy-proven diagnosis of myocarditis. In addition, samples from 49 patients with acute myocardial infarction from the Biobank Regional Platform (Murcia, Spain) were analyzed in parallel as comparators (Table S5). Finally, as a control cohort, we included samples obtained from patients with Th17-related immunologic diseases without cardiac involvement (Table S6). A detailed description of the cohorts is provided in the Supplementary Appendix.
STATISTICAL ANALYSIS
We first evaluated the normality of the distributions using the D’Agostino–Pearson test. If distributions were normal, we used the unpaired Student’s t-test for two-group comparisons and one-way analysis of variance analysis with Tukey’s post hoc test when more than two groups were compared. If distributions were nonnormal, we used the Mann–Whitney U-test for the analysis of two groups and the Kruskal–Wallis test with Dunn’s post hoc test for multiple-group comparisons. In the kinetic experiments, one-way repeated-measures analysis of variance with Dunnett’s multiple comparison post hoc test or two-way repeated-measures analysis of variance with Sidak’s multiple comparison post hoc test were performed. To assess the association of hsa-miR-Chr8:96 with myocarditis, we performed logistic-regression analyses after adjustment for sex, age, troponin level (normalized value), and ejection fraction with or without the addition of hsa-miR-Chr8:96 as an independent variable. We performed analysis of the receiver-operating-characteristic (ROC) curves to evaluate diagnostic performance and DeLong’s test for comparisons. We estimated the best cutoff value for hsa-miR-Chr8:96 (expressed as a relative gene-expression value calculated by the delta–delta Ct [cycle threshold] method for the qPCR analysis) for achieving both a sensitivity and specificity of more than 90% or the value presenting the highest likelihood ratio. Data analyses were performed with GraphPad Prism software, version 7.0, and R software, version 3.6.2.
RESULTS
TH17-SPECIFIC RESPONSES IN ACUTE MYOCARDITIS AND MYOCARDIAL INFARCTION
We assessed myocardial injury and T-cell responses after inducing experimental autoimmune myocarditis or myocardial infarction in mice (Fig. S1). Substantial elevations in levels of cardiac troponin I, creatine kinase MB, and lactate dehydrogenase above the basal level suggested that myocardial injury peaked approximately 21 days after the induction of myocarditis and 3 days after the induction of myocardial infarction (Fig. 1A and Fig. S1A and S1B). The left ventricular ejection fraction dropped promptly after myocardial infarction (>50% reduction at day 3) and also declined in myocarditis (significant decrease at day 21) (Fig. 1B and Fig. S2A). An analysis of circulating T cells showed a significant increase in the number of Th17 cells 14 days after either myocarditis or myocardial infarction (Fig. 1C and Fig. S2B through S2F). The number of Th17 cells was not significantly increased at days 3 or 7 after myocardial infarction (the time of peak biomarker elevation). Therefore, Th17 cells appear to be characteristic of the acute phase of myocardial injury in myocarditis (on day 21) but not the acute phase of myocardial injury in myocardial infarction (on day 3) (Fig. 1C).
Figure 1 (facing page). Circulating Th17 Cells in Acute Myocarditis.
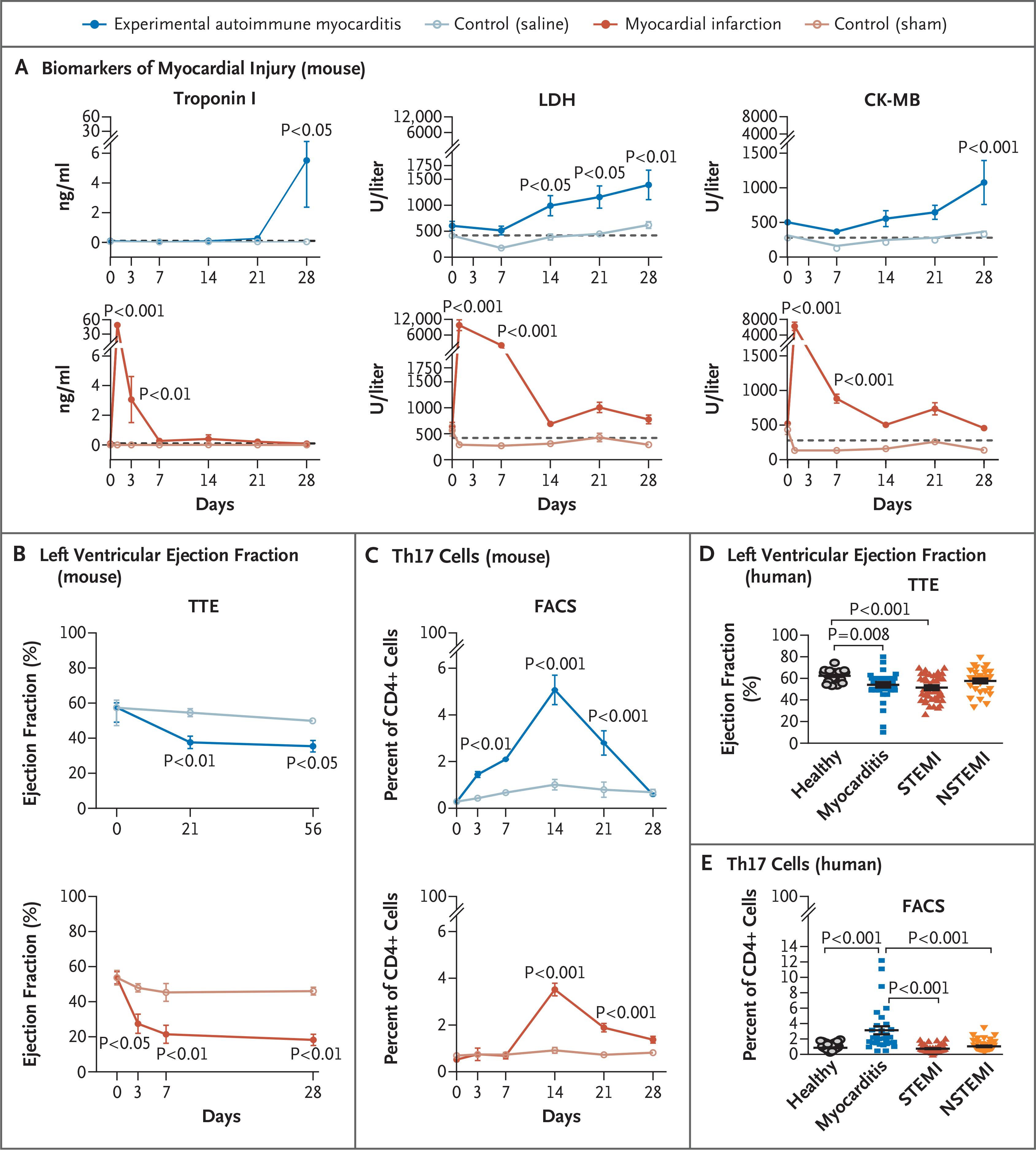
Panel A shows the kinetics of biomarkers of myocardial injury — troponin I, lactate dehydrogenase (LDH), and creatine kinase MB (CK-MB) — in the serum of BALB/c mice after induction of experimental autoimmune myocarditis (following immunization with cardiac α-myosin heavy-chain peptide) as compared with control mice (following immunization with phosphate-buffered saline). Also shown are the changes in the same biomarkers after the induction of myocardial infarction (following ligation of the left anterior descending coronary artery) as compared with control mice (following a sham operation). Dashed lines represent basal levels of each biomarker. P values, which are shown for the comparisons with controls, were calculated by two-way analysis of variance with Sidak’s multiple comparisons test. Data are representative of more than three independent experiments in 3 to 10 pools of two mice each. Panel B shows the time course of changes in the left ventricular ejection fraction after the induction of myocarditis or myocardial infarction, as quantified by transthoracic echocardiography (TTE), with 4 to 7 mice per group. Panel C shows the time course of changes in the percentage of type 17 helper T (Th17) cells in CD4+ T cells in the blood of BALB/c mice after myocarditis or myocardial infarction (and respective controls), according to the results of fluorescence-activated cell sorting (FACS) in 6 mice per group. P values were calculated by means of two-way repeated-measures analysis of variance (Sidak’s post hoc test). Data in Panels B and C are representative of more than three independent experiments. Panel D shows the left ventricular ejection fraction, as measured by TTE, in samples obtained from 43 patients with myocarditis, 43 patients with ST-segment elevation myocardial infarction (STEMI), 35 patients with non-STEMI (NSTEMI), and 23 healthy controls. Data were analyzed by means of the Kruskal–Wallis test with Dunn’s multiple comparisons test. Panel E shows the quantification of Th17 cells on FACS as a percentage of all CD4+ T cells in peripheral-blood samples obtained from patients with acute myocarditis, STEMI, or NSTEMI and from healthy controls. P values were calculated by the same method used in Panel D and were performed in the analysis of samples from 33 patients with myocarditis, 45 with STEMI, and 41 with NSTEMI, along with 62 healthy controls. In all five panels, the data are represented as means, with I bars representing standard errors.
We also assessed T-cell responses in 42 patients with acute myocarditis, in 45 patients with ST-segment elevation myocardial infarction (STEMI), in 45 patients with non–ST-segment elevation myocardial infarction (NSTEMI), and in 80 healthy controls. Demographic and clinical data are summarized in Tables S1 and S2. Patients with myocarditis and those with STEMI had significantly greater reductions in the ejection fraction than did healthy controls (Fig. 1D). T-cell subgroups that were analyzed in blood showed a significant increase in Th17 cells in patients with acute myocarditis (Fig. 1E and Fig. S3A). The prominent role of Th17 cells in patients with myocarditis is highlighted by increased ratios of Th17 cells to both Th1 cells and regulatory T cells (Fig. S3B) and by increased absolute numbers of Th17 cells (Fig. S3C).
SYNTHESIS OF MMU-MIR-721 BY TH17 CELLS IN AUTOIMMUNE MYOCARDITIS
We performed miRNA screening of T-cell subgroups obtained from the peripheral blood of mice that had experimental autoimmune myocarditis. The T-cell subgroups that were analyzed included CD4+ T cells, polyclonal Th17 cells, and cultures enriched with ovalbumin antigen-specific Th17 (Th17ag-sp) cells or Th17ag-sp sorted cells (Fig. S4A). Microarray analysis identified 27 miRNAs that were up-regulated in both Th17ag-sp cultures and Th17ag-sp sorted cells (Fig. 2A, Fig. S4B, and Table S7). MiRNA profiling of Th17ag-sp sorted cells obtained from CD69 knockout mice (in which severe myocarditis develops18) as compared with wild-type mice (in which moderate myocarditis develops) showed mmu-miR-721 and mmu-miR-483–5p as the most strongly up-regulated miRNAs (Table S8). Analysis by qPCR showed that mmu-miR-721 expression was specific for Th17ag-sp cells (Fig. 2B and Fig. S4C). Pathway analysis software (Ingenuity) identified roles for the selected miRNAs in T-cell homeostasis and differentiation (Fig. S4D) and identified validated target genes related to Th17 cells, such as Pparγ, Nos2, Stat3, Tgfβ, and Cd69 (Fig. S4E).
Figure 2 (facing page). Synthesis of miRNAs by Circulating Th17 Cells in Myocarditis.
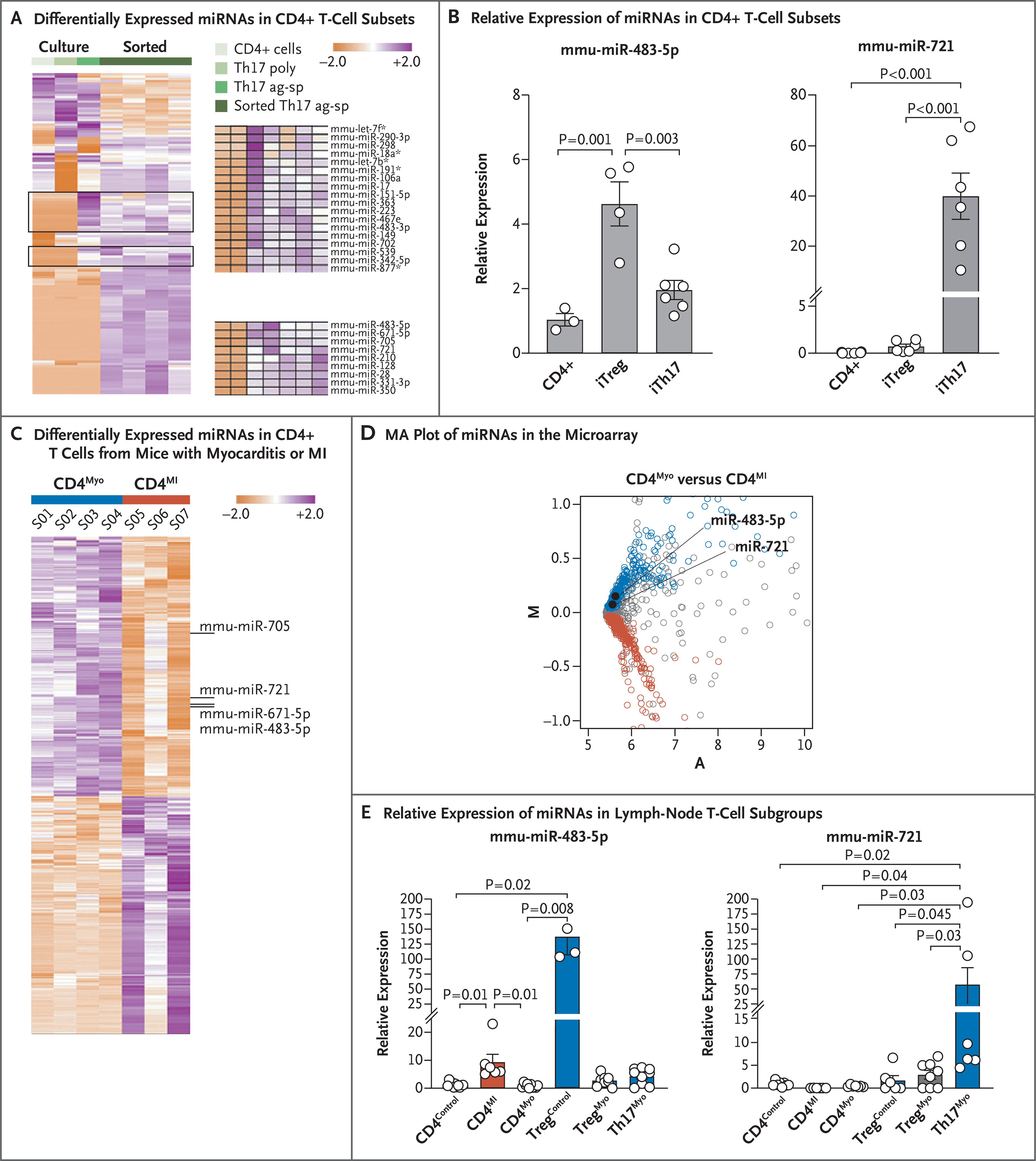
Panel A shows a heat map representing unsupervised hierarchical clustering for the murine microRNAs (miRNAs) expressed in common among the cell types displayed, as analyzed by means of miRNA microarray. Lymph node CD4+ T cells were activated for 48 hours with monoclonal antibodies against T-cell activation molecules CD3 and CD28 or underwent polyclonal differentiation into Th17 (Th17 poly) cells or ovalbumin antigen-specific Th17 (Th17ag-sp) cells in vitro, before sorting or in parallel with sorted interleukin-17+ cells (sorted Th17ag-sp in 4 samples). All data have been normalized by the median of each miRNA. The color scale indicates the relative expression level of each miRNA, with white indicating 0 expression, purple indicating an expression of more than 0, and orange indicating an expression of less than 0. The two gates include 27 miRNAs that were differentially expressed by Th17ag-sp cells (magnified on the right). Panel B shows the relative expression of mmu-miR-483–5p and mmu-miR-721 in freshly isolated CD4+ T cells and in vitro differentiated Th17ag-sp (iTh17) and regulatory T (Treg) ag-sp (iTreg) cultures analyzed by quantitative polymerase-chain-reaction (qPCR) assay (3 to 6 experiments for each cell type). Data are represented as means (±SE), and P values were calculated by one-way analysis of variance with Tukey’s post hoc test. Panel C shows a heat map for the differentially expressed miRNAs with significant expression variance between sorted CD4+ T cells obtained from axillary lymph nodes 6 days after the induction of experimental autoimmune myocarditis (CD4Myo, in 4 mice) and from mediastinal lymph nodes obtained 3 days after myocardial infarction (MI) following coronary-artery ligation (CD4MI, 3 pools of 2 mice each) in BALB/c mice, after normalization by the median of each miRNA. Individual miRNAs of interest are identified to the right of the heat map. Panel D shows an “MA” plot indicating the average expression (A) versus the log2 of the mean difference (M) of miRNAs detected in the microarray. In the plot, miRNAs that are significantly overrepresented (P<0.1 after adjustment) in CD4Myo cells (blue) and CD4MI cells (red) are indicated. In both these cell populations, miRNAs that are indistinctly represented (P>0.1 after adjustment) are indicated in gray. Panel E shows the relative expression of mmu-miR-483–5p and mmu-miR-721 by qPCR in T-cell subgroups isolated from mediastinal or axillary lymph nodes normalized to CD4+ control cells; CD4Control corresponds to CD4+ T cells isolated from axillary lymph nodes from saline-injected control mice, CD4MI to CD4+ T cells isolated from mediastinal lymph nodes after myocardial infarction, CD4Myo to CD4+ T cells isolated from axillary lymph nodes after the induction of experimental autoimmune myocarditis, TregControl to CD4+Foxp3+ cells isolated from axillary lymph nodes from saline-injected control mice, TregMyo to CD4+Foxp3+ cells isolated from axillary lymph nodes after the induction of experimental autoimmune myocarditis, and Th17Myo to CD4+ interleukin-17 cells sorted from axillary lymph nodes after the induction of experimental autoimmune myocarditis. Histograms indicate the mean relative expression of each miRNA in cells pooled from three independent experiments; I bars indicate standard errors. P values were calculated by one-way analysis of variance with Tukey’s post hoc test (in 5 to 7 pools of 2 mice each for mmu-miR-721) and by the Kruskal–Wallis test with Dunn’s post hoc test (in 3 to 7 pools of 2 mice each for mmu-miR-483–5p).
MiRNA profiling in sorted CD4+ T cells from draining lymph nodes 6 days after the induction of myocarditis or 3 days after coronary artery ligation in mice (Fig. S4F) revealed that mmumiR-721 and mmu-miR-483–5p were overexpressed in myocarditis-associated CD4+ T cells as compared with myocardial infarction-associated CD4+ T cells (Fig. 2C and 2D and Table S9). Analysis of sorted T cells by qPCR (Fig. S4G) confirmed that mmu-miR-721 was synthesized by Th17ag-sp cells in myocarditis (Fig. 2E).
DETECTION OF MMU-MIR-721 IN PLASMA FROM MICE WITH AUTOIMMUNE AND VIRAL MYOCARDITIS
We detected expression of mmu-miR-721 in plasma from mice with experimental autoimmune myocarditis and expression of mmu-miR-483–5p in plasma from mice with acute myocardial infarction (Fig. 3A). The relative expression of mmu-miR-721 increased in CD69 knockout mice (Fig. 3B). During the development of experimental autoimmune myocarditis, the time course of the rise and fall of circulating Th17 cells was similar to that of circulating mmu-miR-721 (Fig. 3C). In a murine model of viral myocarditis caused by coxsackievirus B3 infection (Fig. S5), circulating mmu-miR-721 expression at 10 days was significantly higher in C57BL/6 mice, in which severe heart inflammation developed, than in BALB/c mice, in which less severe inflammation developed (Fig. 3D).
Figure 3 (facing page). Circulating miRNAs in Mice with Autoimmune or Viral Myocarditis.
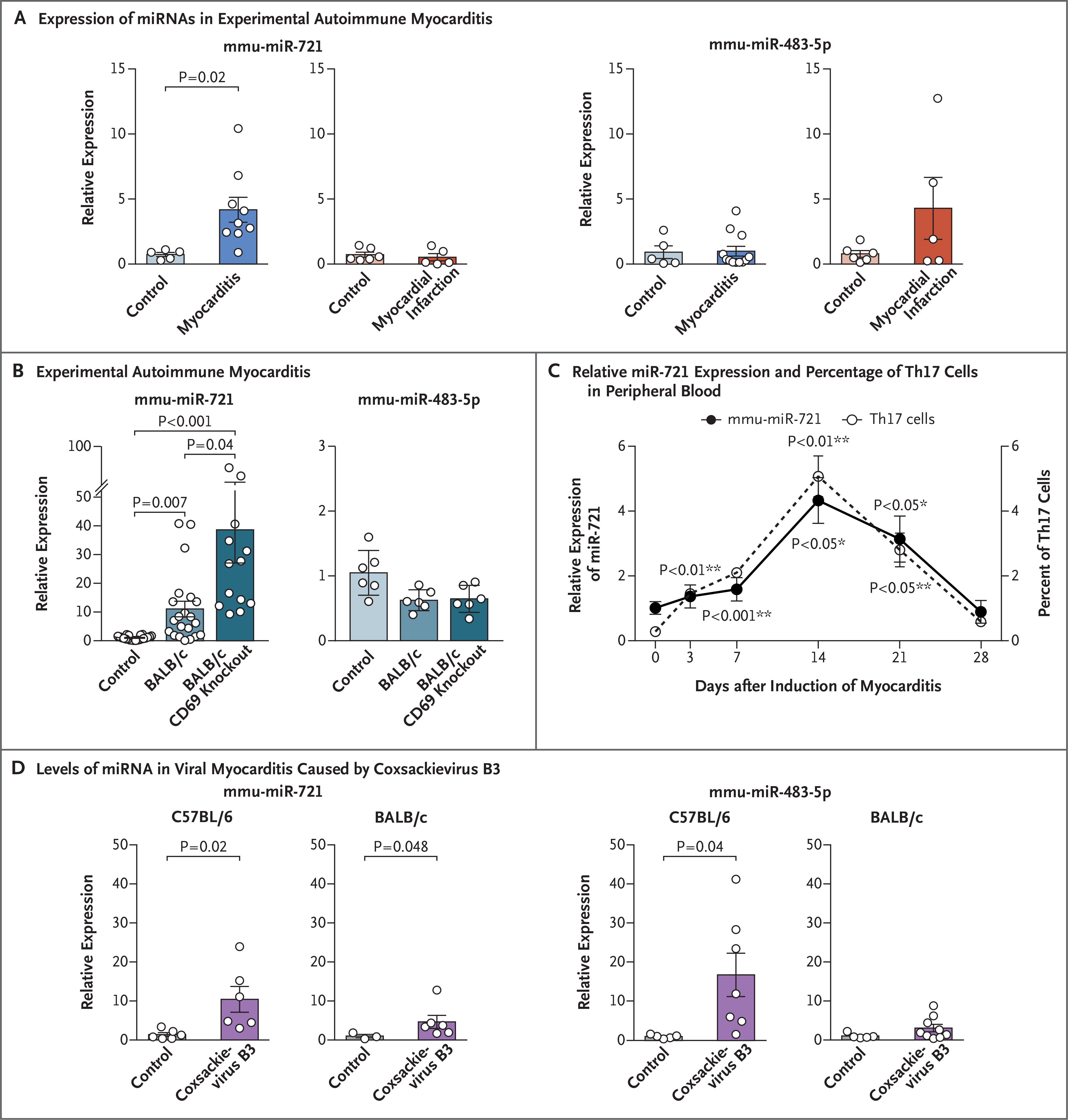
Panel A shows the expression of mmu-miR-721 and mmu-miR-483–5p in an analysis performed by qPCR in plasma obtained from BALB/c mice 21 days after the induction of experimental autoimmune myocarditis (following immunization with cardiac α-myosin heavy-chain peptide) or control mice (following immunization with phosphate-buffered saline), or 3 days after the induction of myocardial infarction (following ligation of the left anterior descending coronary artery) or control mice (following sham operation). Data are represented as means (±SE) from one representative among three independent experiments involving 5 to 9 mice per group. The P value was calculated by the unpaired t-test. Panel B shows the relative expression of mmu-miR-721 and mmu-miR-483–5p in serum obtained from mice with moderate myocarditis (BALB/c background) or severe myocarditis (BALB/c CD69 knockout background), and control mice immunized with saline (with 6 to 21 mice per group), analyzed by qPCR. Histogram values are means (±SE), with calculations performed by one-way analysis of variance with Tukey’s post hoc test. Panel C shows the relative expression of mmu-miR-721 (analyzed by qPCR) and the percentage of Th17 cells in peripheral blood (analyzed by flow cytometry) in samples obtained from mice at different time points after the induction of experimental autoimmune myocarditis in 6 mice. I bars represent standard errors. P values were determined by one-way repeated-measures analysis of variance. Dunnett’s multiple-comparison test was performed between values at day 3 to day 28 as compared with day 0 after induction of myocarditis for mmu-miR-721 relative expression (*) and for the percentage of Th17 cells (**) individually. Panel D shows levels of circulating miRNA that were assessed by qPCR during viral myocarditis at 10 days after infection with coxsackievirus B3. The expression in BALB/c and C57BL/6 mouse strains was analyzed in parallel with uninfected control groups (5 to 7 mice per group). Data that are reported as means (±SE) were analyzed by means of the unpaired t-test (in C57BL/6 mice) and the Mann–Whitney U test (in BALB/c mice).
IDENTIFICATION, CLONING, AND VALIDATION OF THE MMU-MIR-721 HUMAN HOMOLOGUE
The human homologue of mmu-miR-721 had not been identified,21 so we screened the genome of human and other mammalian species for homologous sequences (Fig. S6A). The yield of amplification product with the murine probe for mmumiR-721 (Fig. 4A and Fig. S6B) and cloning efficiency (Fig. 4B) were higher with plasma from patients with myocarditis than with plasma from patients with myocardial infarction or healthy controls. Screened colonies yielded an 18-nucleotide sequence identical to mmu-miR-721 (Fig. 4B), which was highly conserved across mammalian species (Fig. S6A) and located on chromosome 8 in the human genome (genomic sequence, NC_018919.2) (Fig. S6C). We postulated that the 3 nucleotides (TCT) at the 5′ end complete the putative mature human miR-721 homologue (TCTTGCAATTAAAAGGGGGAA), which we called hsa-miR-Chr8:96.
Figure 4 (facing page). Cloning and Validation of hsa-miR-Chr8:96.

Panel A shows the results of analyses with the use of polyacrylamide gel electrophoresis (at left) indicating the qPCR product (black arrowhead) in human plasma samples obtained from patients with acute myocarditis or acute myocardial infarction or from healthy controls with the mmu-miR-721 probe. Transfected mmu-miR-721 in human cells was used as a positive control (C+). MW denotes molecular-weight markers. Quantification of the average intensity of the qPCR product (stained with methylene blue and quantified with Image Studio Lite, version 4.0) is shown at right. Data are pooled from two independent gels. Histograms represent the mean values (±SE) and were analyzed by one-way analysis of variance with Tukey’s post hoc test (with 5 patients per group). Panel B shows quantification of pGEM-T cloning efficiency of the qPCR products from human plasma samples. The product obtained from sequencing the colonies included 18 nucleotides (nt) (black) and a polyadenine tail (gray) from the reverse primer poly dT (a short sequence of deoxythymidine nucleotide) used for qPCR, inserted into the vector sequence (green). Panel C shows a diagram of pCDH-CMV-MCS-EF1-copGFP–based lentiviral vector used to express the sequence of Chr8:96405654–96406003, containing 350 nucleotides including the putative primary miRNA pri-hsa-miR-Chr8:96 (at top). Also shown is representative blotting with Argonaute-2 antibody of the RNA–Argonaute-2–IgG1 coimmunoprecipitation fraction (middle graph) and the expression of the indicated miRNAs in the coimmunoprecipitation fractions evaluated by qPCR (lower graph). To quantify the expression of hsa-miR-Chr8:96, a custom-made probe was used to amplify the putative mature hsa-miR-Chr8:96 (TCT TGC AAT TAA AAG GGG GAA). RNU1A1, a small nuclear RNA that is processed independently of Argonaute-2, was used as an endogenous control for miRNA relative expression (1 of 4 independent experiments). Panel D shows a diagram of psiCHECK2-RC-hsa-miR-Chr8:96 luciferase dual reporter vector (at top). The 2-mer insert consists of a tandem repeat of the reverse complementary (RC) sequence to the mature hsa-miR-Chr8:96. Also shown are peripheral-blood leukocytes obtained from patients with acute myocarditis, from those with acute myocardial infarction, and from healthy controls that were cultured overnight, followed by collection of supernatants (bottom graph). Renilla and firefly dual luciferase reporter assays were performed after transiently transfecting HEK293T cells with an empty plasmid (psiCHECK2 Ø) or psiCHECK2-RC-hsa-miR-Chr8:96–expressing plasmid, by adding the supernatant of the different human samples. Firefly luciferase and renilla signals were analyzed (5 supernatants from 5 different study participants from each group). Data are means (±SE) and analyzed by two-way analysis of variance with Sidak’s post hoc test. CMV denotes cytomegalovirus promoter, CopGFP superbright green fluorescent protein, EF1 elongation factor 1 promoter, hLUC+ synthetic firefly luciferase gene, hRLUC synthetic renilla luciferase reporter gene, HSV-TK herpes simplex virus-1 thymidine kinase promoter, MCS multicloning site, Ø empty plasmid, pCDH complementary DNA cloning and expression lentivector, and T7 T7 promoter.
MiRNAs are defined by their length and their association with the Argonaute protein family.22 We cloned the pre-miRNA (Fig. 4C) and performed coimmunoprecipitation of RNA with Argonaute-2 or IgG subclass 1, followed by qPCR analysis, which showed that the cloned fragment was processed as a mature miRNA. For functional validation,23 we cloned the reverse-complementary sequence of hsa-miR-Chr8:96 (Fig. 4D) in a luciferase dual reporter vector. Supernatants from peripheral-blood leukocytes obtained from patients with myocarditis inhibited the luciferase activity, which supports the conclusion that hsa-miR-Chr8:96 is a mature and functionally active miRNA.
PRESENCE OF HSA-MIR-CHR8:96 IN ACUTE MYOCARDITIS
We performed qPCR to assay plasma samples from the main cohort for miRNA profiling (Table S1). We analyzed hsa-miR-483–5p and hsa-miR-21, which are abundant after myocardial injury,24 and hsa-miR-132–3p and hsa-miR-212–3p, which promote Th17-cell differentiation,25 together with hsa-miR-Chr8:96. The expression of the novel miRNA in plasma was greater in patients with myocarditis than in either patients with myocardial infarction or healthy controls (Fig. 5A). The novel miRNA was specifically synthesized by Th17 cells obtained from patients with myocarditis (Fig. S6D and S6E). ROC curves were generated to determine the diagnostic characteristics of hsa-miR-Chr8:96 expression measured in plasma. Among the patients with myocarditis, the area under the ROC curve was 0.927 (95% confidence interval [CI], 0.879 to 0.975) as compared with patients with myocardial infarction and 0.988 (95% CI, 0.970 to 1.006) as compared with healthy controls (Fig. 5B and Table S10). We analyzed additional validation cohorts with different comparators, which confirmed that the novel miRNA was specifically expressed in plasma from patients with myocarditis, as compared with those with myocardial infarction (Fig. 5C and Table S3) or MINOCA (Fig. 5D and Table S4). These data were also validated in a cohort of patients with biopsy-proven myocarditis (Fig. 5E and Table S5), along with patients who had other Th17-related diseases (rheumatoid arthritis, spondyloarthritis, psoriasis, and multiple sclerosis) (Table S6 and Fig. S6F). The ability of the novel miRNA to distinguish acute myocarditis from myocardial infarction remained significant after adjustment for age, sex, ejection fraction, and serum troponin level (Fig. 5F and 5G and Fig. S6G).
Figure 5 (facing page). Analysis of Circulating hsa-miR-Chr8:96 for the Detection of Acute Myocarditis.
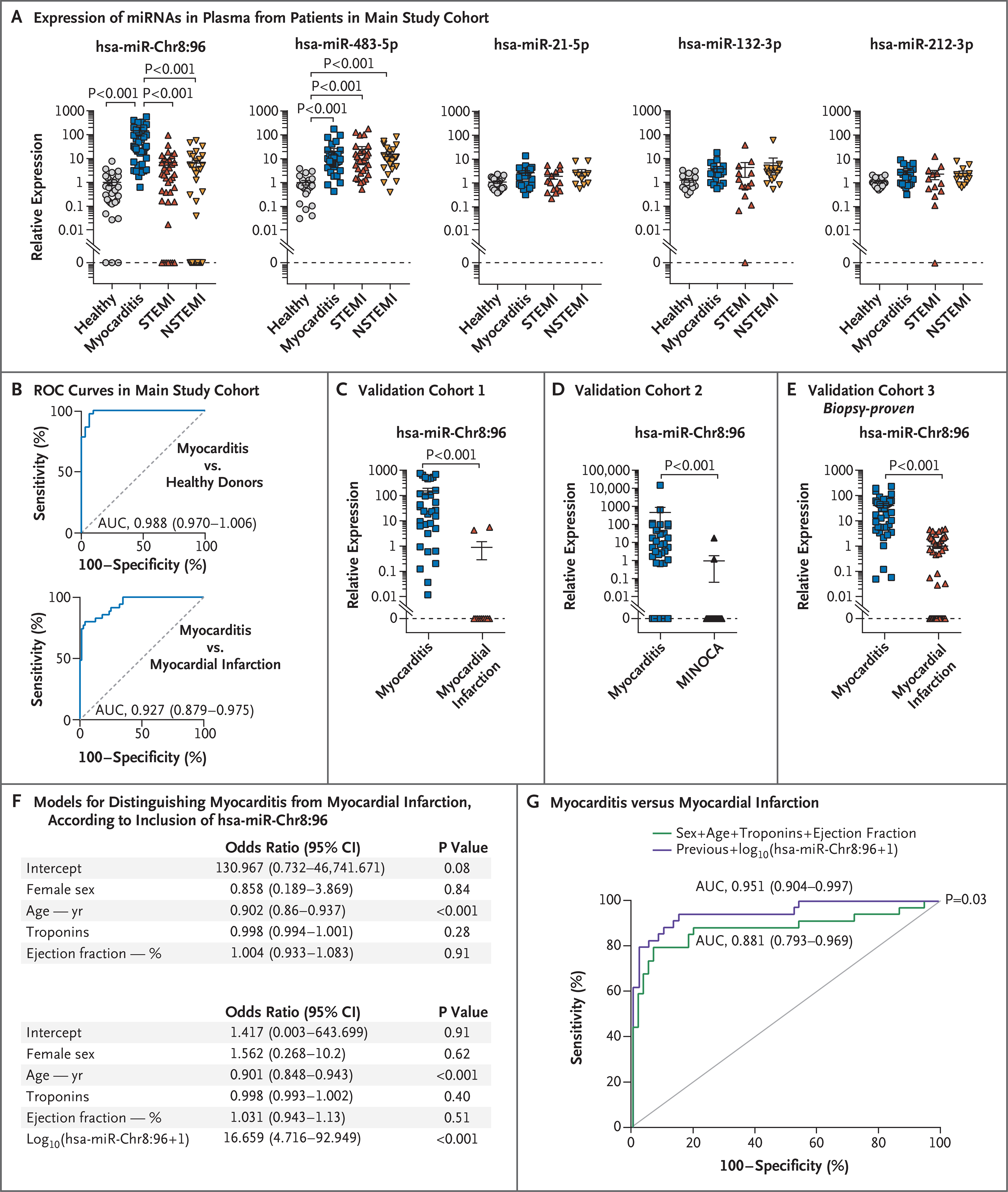
Panel A shows quantification of miRNAs by qPCR in circulating plasma obtained from 39 patients with acute myocarditis, 39 patients with STEMI, and 38 patients with NSTEMI, along with plasma from 31 healthy controls in the main study cohort in Spain. Data are represented as log10 of miRNA relative expression in plasma. I bars represent standard errors. Data were analyzed by Kruskal–Wallis with Dunn’s post hoc test. Panel B shows receiver-operating-characteristic (ROC) curves of hsa-miR-Chr8:96 determinations in plasma, based on the relative gene-expression values calculated by the delta–delta Ct (cycle threshold) method for the qPCR analysis, which were generated to distinguish patients with acute myocarditis from those with acute myocardial infarction and from healthy controls. Also indicated are the area under the ROC curve (AUC), 95% confidence interval (CI), and P value for each comparison. Panel C shows validation cohort 1 of patients with myocarditis or acute myocardial infarction from the Partners Biobank (Boston) (AUC, 0.952; 95% CI, 0.883 to 1.000). Panel D shows validation cohort 2 of patients with myocarditis, as compared with those with acute myocardial infarction with nonobstructive coronary arteries (MINOCA), from University Hospital Zurich (AUC, 0.831; 95% CI, 0.722 to 0.941). Panel E shows validation cohort 3 of patients with biopsy-proven acute myocarditis from the University of Padua and patients with acute myocardial infarction from the Biobank Regional Platform (Murcia, Spain) (AUC, 0.938; 95% CI, 0.889 to 0.988). In Panels C, D, and E, circulating miRNA levels are represented as means (±SE) and were analyzed by the Mann–Whitney U test. Panel F shows multivariable logistic-regression models with or without inclusion of hsa-miR-Chr8:96 after adjustment for potential confounders (age, sex, serum troponin levels, and left ventricular ejection fraction) to distinguish patients with myocarditis from those with myocardial infarction. Panel G shows ROC curves for the multivariable logistic-regression models with or without hsa-miR-Chr8:96 and the variables of sex, age, serum troponin levels, and left ventricular ejection fraction. DeLong’s test was used for the comparison of the AUC.
DISCUSSION
In this study, we identified a novel miRNA, mmu-miR-721, as a marker of myocarditis in murine models (including experimental autoimmune myocarditis and viral myocarditis) and its human homologue, which we designated has-miR-Chr8:96. The diagnostic ability of the detection of hsa-miR-Chr8:96 in plasma to discriminate myocarditis from other conditions was confirmed in several patient cohorts with different comparators, including myocardial infarction, MINOCA, and autoimmune diseases, as compared with healthy volunteers.
Previous studies of miRNA expression in the heart during autoimmune or viral myocarditis26,27 and of circulating miRNAs in patients with heart failure28–30 suggest that the discovery strategies that were used in those studies would not identify candidate miRNAs that are differentially expressed among different causes of cardiac injury. In comparison, we have investigated the expression of novel miRNAs by Th17 cells, which play an important role in both myocarditis15 and myocardial infarction,31 and detected an miRNA that could be used to differentiate between these conditions.
In clinical practice, the diagnosis of myocarditis remains a challenge because of the lack of easily accessible diagnostic methods that are both sensitive and specific.32 Endomyocardial biopsy remains the reference standard, but it is not routinely performed owing to its associated risks as an invasive procedure.33 Although cardiac MRI is noninvasive,34,35,10 it also has limitations, including the lack of availability in many hospitals and in the ambulatory setting; in addition, the sensitivity of cardiac MRI to detect edema and vascular permeability decreases over time.35 Therefore, there is a need for new diagnostic methods.
Additional work will be necessary to determine whether hsa-miR-Chr8:96 is suitable for use as a diagnostic test for myocarditis. This miRNA has not yet been evaluated in other cardiac disorders, such as dilated cardiomyopathy, from which myocarditis must be distinguished in the clinical setting. In addition, in our study, we note great variability in the expression of hsa-miR-Chr8:96, which remains unexplained; it is not clear whether this variation reflects the severity of the disease or is attributable to some other factor.
We identified a novel miRNA in mice and humans with myocarditis and found that the human homologue, hsa-miR-Chr8:96, can be used to distinguish patients with myocarditis from those with myocardial infarction and from healthy controls.
Supplementary Material
Acknowledgments
Supported by a grant (PI19/00545, to Dr. Martín) from the Ministry of Science and Innovation through the Carlos III Institute of Health–Fondo de Investigación Sanitaria; by a grant from the Biomedical Research Networking Center on Cardiovascular Diseases (to Drs. Martín, Sánchez-Madrid, and Ibáñez); by grants (S2017/BMD-3671-INFLAMUNE-CM, to Drs. Martín and Sánchez-Madrid; and S2017/BMD-3867-RENIM-CM, to Dr. Ibáñez) from Comunidad de Madrid; by a grant (20152330 31, to Drs. Martín, Sánchez-Madrid, and Alfonso) from Fundació La Marató de TV3; by grants (ERC-2011-AdG 294340-GENTRIS, to Dr. Sánchez-Madrid; and ERC-2018-CoG 819775-MATRIX, to Dr. Ibáñez) from the European Research Council; by grants (SAF2017–82886R, to Dr. Sánchez-Madrid; RETOS2019–107332RB-I00, to Dr. Ibáñez; and SAF2017–90604-REDT-NurCaMeIn and RTI2018–095928-BI00, to Dr. Ricote) from the Ministry of Science and Innovation; by Fondo Europeo de Desarrollo Regional (FEDER); and by a 2016 Leonardo Grant for Researchers and Cultural Creators from the BBVA Foundation to Dr. Martín. The National Center for Cardiovascular Research (CNIC) is supported by the Carlos III Institute of Health, the Ministry of Science and Innovation, the Pro CNIC Foundation, and by a Severo Ochoa Center of Excellence grant (SEV-2015–0505). Mr. Blanco-Domínguez is supported by a grant (FPU16/02780) from the Formación de Profesorado Universitario program of the Spanish Ministry of Education, Culture, and Sports. Ms. Linillos-Pradillo is supported by a fellowship (PEJD2016/BMD-2789) from Fondo de Garantía de Empleo Juvenil de Comunidad de Madrid. Dr. Relaño is supported by a grant (BES2015–072625) from Contratos Predoctorales Severo Ochoa para la Formación de Doctores of the Ministry of Economy and Competitiveness. Dr. Alonso-Herranz is supported by a fellowship from La Caixa–CNIC. Dr. Caforio is supported by Budget Integrato per la Ricerca dei Dipartimenti BIRD-2019 from Università di Padova. Dr. Das is supported by grants (UG3 TR002878 and R35 HL150807) from the National Institutes of Health and the American Heart Association through its Strategically Focused Research Networks.
We thank the patients and other volunteers for their participation in this study; Manuel Gómez for his critical reading of the manuscript; Ana Vanesa Alonso and Lorena Flores for their excellent technical work with the echocardiography acquisition and analysis in the murine models; Ramón F. Maruri for his work analyzing data for the patients from Hospital de la Princesa; Laura Fernández and Belen Díaz from HM Hospitales for patient recruitment; Juan José Lazcano Duque and Elisabet Daniel Palomares for their work with mouse colony management; and the staff members at the Bioinformatics, Genomics, Advanced Imaging, Cellomics, and Comparative Medicine units at the CNIC for their support in this work.
Appendix
The authors’ full names and academic degrees are as follows: Rafael Blanco-Domínguez, M.Sc., Raquel Sánchez-Díaz, Ph.D., Hortensia de la Fuente, M.D., Ph.D., Luis J. Jiménez-Borreguero, M.D., Adela Matesanz-Marín, Ph.D., Marta Relaño, Ph.D., Rosa Jiménez-Alejandre, M.Sc., Beatriz Linillos-Pradillo, M.Sc., Katerina Tsilingiri, Ph.D., María L. Martín-Mariscal, M.D., Laura Alonso-Herranz, Ph.D., Guillermo Moreno, M.Sc., Roberto Martín-Asenjo, M.D., Marcos M. García-Guimaraes, M.D., Katelyn A. Bruno, Ph.D., Esteban Dauden, M.D., Ph.D., Isidoro González-Álvaro, M.D., Ph.D., Luisa M. Villar-Guimerans, M.D., Ph.D., Amaia Martínez-León, M.D., Ane M. Salvador-Garicano, Ph.D., Sam A. Michelhaugh, B.A., Nasrien E. Ibrahim, M.D., James L. Januzzi, M.D., Jan Kottwitz, M.D., Sabino Iliceto, M.D., Mario Plebani, M.D., Cristina Basso, M.D., Ph.D., Anna Baritussio, M.D., Ph.D., Mara Seguso, M.Sc., Renzo Marcolongo, M.D., Mercedes Ricote, Ph.D., DeLisa Fairweather, Ph.D., Héctor Bueno, M.D., Ph.D., Leticia Fernández-Friera, M.D., Ph.D., Fernando Alfonso, M.D., Ph.D., Alida L.P. Caforio, M.D., Ph.D., Domingo A. Pascual-Figal, M.D., Ph.D., Bettina Heidecker, M.D., Ph.D., Thomas F. Lüscher, M.D., Ph.D., Saumya Das, M.D., Ph.D., Valentín Fuster, M.D., Ph.D., Borja Ibáñez, M.D., Ph.D., Francisco Sánchez-Madrid, Ph.D., and Pilar Martín, Ph.D.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
R. Blanco-Domínguez, the Vascular Pathophysiology Area
R. Sánchez-Díaz, the Vascular Pathophysiology Area CIBER de Enfermedades Cardiovasculares.
H. de la Fuente, Centro Nacional de Investigaciones Cardiovasculares (CNIC), the Department of Immunology CIBER de Enfermedades Cardiovasculares.
L.J. Jiménez-Borreguero, Centro Nacional de Investigaciones Cardiovasculares (CNIC), the Department of Cardiology CIBER de Enfermedades Cardiovasculares.
A. Matesanz-Marín, the Vascular Pathophysiology Area
M. Relaño, the Vascular Pathophysiology Area
R. Jiménez-Alejandre, the Vascular Pathophysiology Area
B. Linillos-Pradillo, the Vascular Pathophysiology Area
K. Tsilingiri, the Vascular Pathophysiology Area
M.L. Martín-Mariscal, Instituto de Investigación Sanitaria, Hospital Universitario de la Princesa, Fundación Jiménez Díaz
L. Alonso-Herranz, the Myocardial Pathophysiology Area
G. Moreno, the Cardiology Department, Hospital Universitario 12 de Octubre, and Instituto de Investigación Sanitaria Hospital 12 de Octubre
R. Martín-Asenjo, the Cardiology Department, Hospital Universitario 12 de Octubre, and Instituto de Investigación Sanitaria Hospital 12 de Octubre
M.M. García-Guimaraes, Centro Nacional de Investigaciones Cardiovasculares (CNIC), the Department of Cardiology
K.A. Bruno, the Department of Cardiovascular Medicine, Mayo Clinic, Jacksonville, FL
E. Dauden, Centro Nacional de Investigaciones Cardiovasculares (CNIC), the Department of Dermatology
I. González-Álvaro, Centro Nacional de Investigaciones Cardiovasculares (CNIC), the Department of Rheumatology
L.M. Villar-Guimerans, the Department of Immunology, Hospital Ramón y Cajal
A. Martínez-León, Madrid, Hospital Universitario Central de Asturias, Oviedo
A.M. Salvador-Garicano, the Cardiovascular Division and Corrigan Minehan Heart Center, Massachusetts General Hospital Harvard Medical School, Boston.
S.A. Michelhaugh, the Cardiovascular Division and Corrigan Minehan Heart Center, Massachusetts General Hospital Harvard Medical School, Boston.
N.E. Ibrahim, the Cardiovascular Division and Corrigan Minehan Heart Center, Massachusetts General Hospital Harvard Medical School, Boston.
J.L. Januzzi, the Cardiovascular Division and Corrigan Minehan Heart Center, Massachusetts General Hospital Harvard Medical School, Boston.
J. Kottwitz, Kanntonsspital St. Gallen Klinik für Anesthesiologie und Intensivmedizin, St. Gallen, Switzerland
S. Iliceto, University of Padua, Padua, Italy, Cardiology
M. Plebani, University of Padua, Padua, Italy, the Department of Cardiac, Thoracic, Vascular Sciences and Public Health, the Department of Laboratory Medicine
C. Basso, University of Padua, Padua, Italy, the Cardiovascular Pathology Unit
A. Baritussio, University of Padua, Padua, Italy, Cardiology
M. Seguso, University of Padua, Padua, Italy, the Department of Cardiac, Thoracic, Vascular Sciences and Public Health, the Department of Laboratory Medicine
R. Marcolongo, University of Padua, Padua, Italy, the Department of Medicine, Hematology and Clinical Immunology
M. Ricote, the Myocardial Pathophysiology Area
D.L. Fairweather, the Department of Cardiovascular Medicine, Mayo Clinic, Jacksonville, FL
H. Bueno, the Myocardial Pathophysiology Area the Cardiology Department, Hospital Universitario 12 de Octubre, and Instituto de Investigación Sanitaria Hospital 12 de Octubre.
L. Fernández-Friera, the Myocardial Pathophysiology Area HM Hospitales–Centro Integral de Enfermedades Cardiovasculares.
F. Alfonso, Centro Nacional de Investigaciones Cardiovasculares (CNIC), the Department of Cardiology CIBER de Enfermedades Cardiovasculares.
A.L.P. Caforio, University of Padua, Padua, Italy, Cardiology
D.A. Pascual-Figal, the Vascular Pathophysiology Area CIBER de Enfermedades Cardiovasculares; the Cardiology Department, Hospital Universitario Virgen de la Arrixaca, Murcia.
B. Heidecker, Charite Universitätsmedizin Berlin, Campus Benjamin Franklin, Berlin
T.F. Lüscher, University of Padua, Padua, Italy, the Department of Medicine, Hematology and Clinical Immunology
S. Das, the Cardiovascular Division and Corrigan Minehan Heart Center, Massachusetts General Hospital Harvard Medical School, Boston.
V. Fuster, the Vascular Pathophysiology Area University of Padua, Padua, Italy, the Department of Medicine, Hematology and Clinical Immunology.
B. Ibáñez, the Myocardial Pathophysiology Area Instituto de Investigación Sanitaria, Hospital Universitario de la Princesa, Fundación Jiménez Díaz; CIBER de Enfermedades Cardiovasculares.
F. Sánchez-Madrid, the Vascular Pathophysiology Area Centro Nacional de Investigaciones Cardiovasculares (CNIC), the Department of Immunology; CIBER de Enfermedades Cardiovasculares.
P. Martín, the Vascular Pathophysiology Area CIBER de Enfermedades Cardiovasculares.
References
- 1.Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol 2016;68: 2348–64. [DOI] [PubMed] [Google Scholar]
- 2.Cooper LT Jr. Myocarditis. N Engl J Med 2009;360:1526–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 2009; 30:1995–2002. [DOI] [PubMed] [Google Scholar]
- 4.Caforio AL, Marcolongo R, Basso C, Iliceto S. Clinical presentation and diagnosis of myocarditis. Heart 2015;101:1332–44. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342:1077–84. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018; 72:2231–64. [DOI] [PubMed] [Google Scholar]
- 7.Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- 8.Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139(18):e891–e908. [DOI] [PubMed] [Google Scholar]
- 9.Gannon MP, Schaub E, Grines CL, Saba SG. State of the art: evaluation and prognostication of myocarditis using cardiac MRI. J Magn Reson Imaging 2019; 49(7):e122–e131. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–76. [DOI] [PubMed] [Google Scholar]
- 11.Caforio ALP, Cheng C, Perazzolo Marra M, et al. How to improve therapy in myocarditis: role of cardiovascular magnetic resonance and of endomyocardial biopsy. Eur Heart J Suppl 2019;21:Suppl B:B19–B22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol 2008;99:95–114. [DOI] [PubMed] [Google Scholar]
- 13.Kovalcsik E, Antunes RF, Baruah P, Kaski JC, Dumitriu IE. Proteasome-mediated reduction in proapoptotic molecule Bim renders CD4+CD28null T cells resistant to apoptosis in acute coronary syndrome. Circulation 2015;131:709–20. [DOI] [PubMed] [Google Scholar]
- 14.Rangachari M, Mauermann N, Marty RR, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med 2006;203:2009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers JM, Cooper LT, Kem DC, et al. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 2016;1(9):e85851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan J, Cao AL, Yu M, et al. Th17 cells facilitate the humoral immune response in patients with acute viral myocarditis. J Clin Immunol 2010;30:226–34. [DOI] [PubMed] [Google Scholar]
- 17.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol 2015;12:135–42. [DOI] [PubMed] [Google Scholar]
- 18.Cruz-Adalia A, Jiménez-Borreguero LJ, Ramírez-Huesca M, et al. CD69 limits the severity of cardiomyopathy after autoimmune myocarditis. Circulation 2010; 122:1396–404. [DOI] [PubMed] [Google Scholar]
- 19.Myers JM, Fairweather D, Huber SA, Cunningham MW. Autoimmune myocarditis, valvulitis, and cardiomyopathy. Curr Protoc Immunol 2013;15:Unit 15.14.1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patriki D, Gresser E, Manka R, Emmert MY, Lüscher TF, Heidecker B. Approximation of the incidence of myocarditis by systematic screening with cardiac magnetic resonance imaging. JACC Heart Fail 2018;6:573–9. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler G, Ntounia-Fousara S, Granda B, Rathjen T, Dalmay T. Identification of new central nervous system specific mouse microRNAs. FEBS Lett 2006;580: 2195–200. [DOI] [PubMed] [Google Scholar]
- 22.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 2014;15:509–24. [DOI] [PubMed] [Google Scholar]
- 23.Rao DS, O’Connell RM, Chaudhuri AA, Garcia-Flores Y, Geiger TL, Baltimore D. MicroRNA-34a perturbs B lymphocyte development by repressing the forkhead box transcription factor Foxp1. Immunity 2010;33:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halushka PV, Goodwin AJ, Halushka MK. Opportunities for microRNAs in the crowded field of cardiovascular biomarkers. Annu Rev Pathol 2019;14:211–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakahama T, Hanieh H, Nguyen NT, et al. Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17-producing T-helper cell differentiation. Proc Natl Acad Sci U S A 2013;110:11964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corsten MF, Papageorgiou A, Verhesen W, et al. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res 2012;111:415–25. [DOI] [PubMed] [Google Scholar]
- 27.Xu HF, Ding YJ, Zhang ZX, et al. Micro-RNA-21 regulation of the progression of viral myocarditis to dilated cardiomyopathy. Mol Med Rep 2014;10:161–8. [DOI] [PubMed] [Google Scholar]
- 28.Aleshcheva G, Pietsch H, Escher F, Schultheiss HP. MicroRNA profiling as a novel diagnostic tool for identification of patients with inflammatory and/or virally induced cardiomyopathies. ESC Heart Fail 2021;8:408–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Xing Q, Zhou X, et al. Circulating miRNA-21 is a promising biomarker for heart failure. Mol Med Rep 2017;16: 7766–74. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Li B, Qin F, Bai F, Sun C, Liu Q. Expression of serum microRNA-155 and its clinical importance in patients with heart failure after myocardial infarction. J Int Med Res 2019;47:6294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon T, Taleb S, Danchin N, et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur Heart J 2013;34:570–7. [DOI] [PubMed] [Google Scholar]
- 32.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34: 2636–48. [DOI] [PubMed] [Google Scholar]
- 33.Sato N Call for action to establish standard diagnostic and therapeutic approaches for myocarditis. Int J Cardiol 2019;284:61–2. [DOI] [PubMed] [Google Scholar]
- 34.Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic accuracy of cardiovascular magnetic resonance in acute myocarditis: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2018; 11:1583–90. [DOI] [PubMed] [Google Scholar]
- 35.Lurz P, Luecke C, Eitel I, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-trial. J Am Coll Cardiol 2016;67:1800–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


