Abstract
Peripheral neuroblastic tumors are extremely rare in the adult with less just over 20 cases involving adrenal gland described in the literature. We reported herewith the case of a 22-year-old young male who presented with epigastric pain and diarrhea. Imaging studies documented a 3.5cm x 3cm x 4cm solid well-circumscribed right adrenal mass, of heterogeneous structure and with fine calcifications. The lesion turned negative at MIBG scintigraphy. A right robotic-assisted adrenalectomy was performed leading to complete excision of the lesion without complications. Histology was consistent with intermixed stroma-rich ganglioneuroblastoma. A wait-and-see strategy was considered adequate. Two years after diagnosis patient is alive disease-free. Although the definitive diagnosis of a peripheral neuroblastic tumor is obtained after histopathological analysis, CT, and MRI are helpful to further characterize masses and useful in pretreatment risk stratification. Clinicians should be aware of the possibility of GNB development in adult population and its malignant potential.
Keywords: Ganglioneuroblastoma, Neuroblastoma, Adrenal gland, Adult, Computed tomography, Magnetic resonance imaging
Abbreviations: GNB, Ganglioneuroblastoma; PNT, Neuroblastic tumors; NB, Neuroblastoma; GN, Ganglioneuroma; US, Ultrasound; CT, Computed Tomography; MRI, Magnetic Resonance Imaging; INPC, International Neuroblastoma Pathology Classification; INSS, International Neuroblastoma Staging System; INRG, International Neuroblastoma Risk Group; ADC, Apparent Diffusion Coefficient; RT, Radiotherapy
Background
Ganglioneuroblastoma (GNB) is a malignant neoplasm of the autonomic nervous system on the spectrum of peripheral neuroblastic tumors (PNT) [1]. It originates from primitive neuroectodermal cells of the neural crest that migrate during embryonic life, giving rise to the sympathetic ganglia, and the adrenal medulla [2].
These precursor cells may remain undifferentiated (referred to as neuroblasts) or they may mature (to ganglion and Schwann cells) [3,4]. A tumor composed primarily of neuroblasts is a malignant neuroblastoma (NB), 1 composed entirely of mature ganglion cells and other mature tissue a benign ganglioneuroma (GN), while a tumor with both immature, and mature cell types is considered an intermediate-grade GNB [3,4].
GNB usually is a pediatric tumor and is extremely rare in adults[5]. In literature, less than 50 cases of adult patients are reported; the most frequent site is the adrenal gland, other sites are the retroperitoneum, the brain, and the mediastinum. In the adrenal gland less just over 20 cases of adult onset of GNB are described [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20].
The mean age at the diagnosis of adult adrenal GNB was 39 years (range: 20-67 years) and males were predominantly affected (12:6) [7]. Left adrenal gland was more frequently involved, in 1 case bilateral tumors were reported7.
The imaging characteristics of PNT are similar and preoperative diagnosis is difficult. The histologic type cannot be discriminated only with imaging and is based on pathologic analysis findings [21].
We reported the case of a 22-year-old male with an incidental 4 cm adrenal mass turned out to be an intermixed-GNB.
Case report
A 22-year-old male, underwent an abdominal ultrasound (US) for recurrent epigastric pain and diarrhea; the examination revealed a right adrenal mass with heterogeneous hypoechoic appearance with focal hyperechoic areas due to calcifications (Fig. 1). A computed tomography (CT) showed a solid well-circumscribed mass in right adrenal lodge (3.5cm x 3cm x 4cm), with smooth margins, showing heterogeneous density (varying 25-35) and exhibiting fine and punctate calcifications (Fig. 2). Progressive and poor contrast enhancement was observed (Fig. 3A-C). It was in close contact with right renal vessels, neither involvement of lymph nodes nor distant metastases were observed. Magnetic resonance imaging (MRI) confirmed the anatomic relationships of the mass (Fig. 4A-E). MIBG scintigraphy was negative. These findings were compatible, in the first hypothesis, with a PNT.
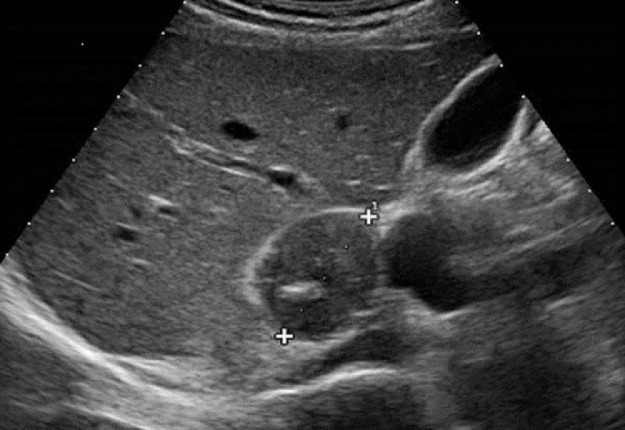
Fig. 1.
Abdominal ultrasound revealed the presence of a right adrenal mass with heterogeneous hypoechoic appearance with focal hyperechoic areas due to calcifications.
Fig. 2.
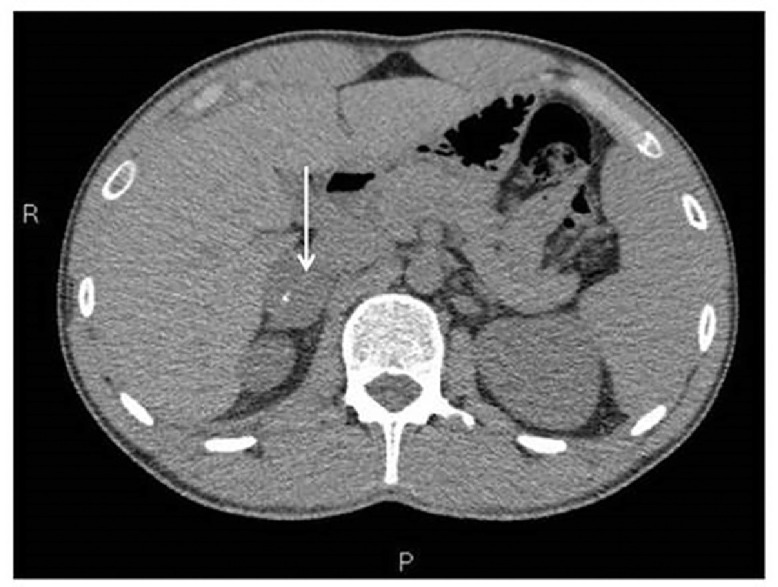
Unenhanced CT confirmed the presence of a solid well-circumscribed mass, with smooth margins, measuring 3,5 × 3 × 4 cm, showing heterogeneous density (varying from 25-30 HU) and exhibiting fine and punctuate calcifications.
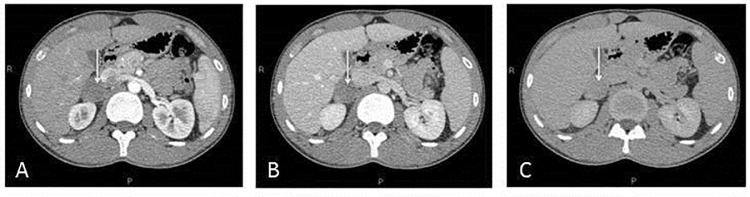
Fig. 3.
(A-C). Contrast-enhanced CT demonstrated progressive and modest enhancement of the lesion (3A arterial phase, 3B venous phase, and 3C late phase). The mass was located between inferior vena cava, the right kidney and the medial margin of right lobe of the liver. The lesion came into close contact with right renal vessels with no signs of infiltration. Lymph nodes involvement or distant metastases were not observed.
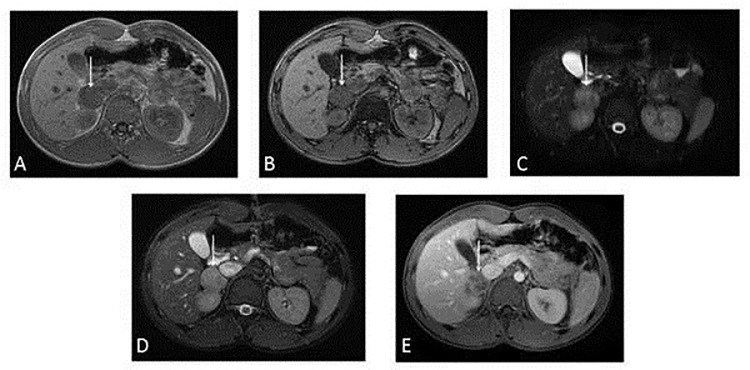
Fig. 4.
(A-E). Magnetic resonance imaging (MRI) confirmed the anatomic relationships of the mass as seen on CT. On T1-weighted images the lesion was isointense (4A) without drop of signal in T1 weighted opposition phase sequence (4B). In fat-suppressed fast spin-echo T2-weighted sequence (4C) and in FIESTA sequence (4D) the lesion appeared mildly and heterogeneous hyperintense. After contrast medium injection it is characterized by a progressive, heterogeneous contrast enhancement (Figure 4E).
The patient was then addressed to the Endocrine Unit for biochemical evaluation of the mass. The complete physical examination was negative. Blood pressure and heart rate were normal. Results of complete blood count, plasma levels of electrolytes, tests of coagulation, kidney, and liver function and urinalysis were normal. Testing for adrenal gland function were all normal: in particular, urinary excretion of catecholamines and their metabolites, including homovanillic acid and vanillylmandelic acid were normal.
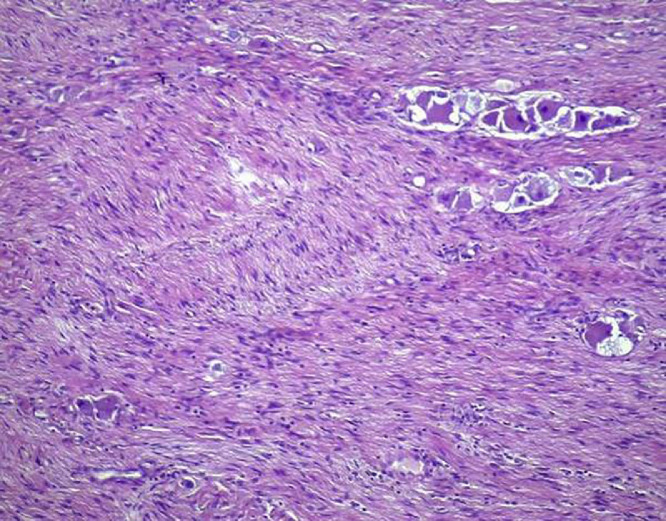
Patient underwent lateral transperitoneal robotic right adrenalectomy using Xi Da Vinci surgical system. Operative time and estimated blood loss were 100 minutes and 50 mL, respectively. No intraoperative complications occurred and post-operative course was uneventful. The surgical sample was composed of the adrenal gland with a whitish nodular mass of 4.2 cm in its greater diameter, with yellowish streaks on cut surface (Fig. 5). Microscopically the tumor shows well-defined microscopic nests of neuroblastic cells (10% of the tumor volume) in a background of naked neuropil, which are intermixed in an expanding Schwannian stroma constituting 60% of the tumor volume (Fig. 6). The mitosis-karyorrhexis index (MKI) was <2%. By FISH, N-MYC status was unamplified. These findings were consistent with intermixed stroma-rich ganglioneuroblastoma according to Shimada et al., arising from the adrenal gland [4]. Chemotherapy was not proposed based on the favorable histology. No recurrence was observed during a 24-months’ follow-up. Written informed consent for the publication of this case was obtained from the patient.
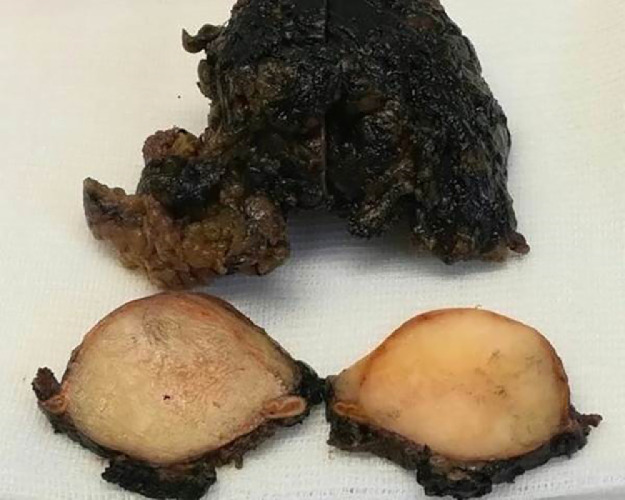
Fig. 5.
The surgical sample sent for pathologic examination was composed of the adrenal gland with a whitish nodular mass of 4.2 cm in its greater diameter, with yellowish streaks on cut surface.
Fig. 6.
The histopathological examination (hematoxylin and eosin stain, 10x) showed well-defined microscopic nests of neuroblastic cells (10% of the tumor volume) in a background of naked neuropil, which are intermixed in an expanding Schwannian stroma constituting 60% of the tumor volume.
The mitosis-karyorrhexis index (MKI) was <2%. By FISH, N-MYC status was unamplified.
Discussion
The International Neuroblastoma Pathology Classification (INPC) established in 1999 and revised in 2003, redefined the histologic features of NT and proposed 4 tumor categories: NB, GNB-intermixed, GN, and GNB-nodular [22], [23], [24].
The 4 categories are divided in 2 distinct prognostic groups: Favorable Histology, including GNB-intermixed and GN, and Unfavorable Histology including NB and GNB-nodular [25].
GNB is divided into 2 types. The first type is nodular-GNB characterized by a grossly visible neuroblastic nodular component (stroma-poor) which is usually hemorrhagic and/or necrotic coexisting with an intermixed-GNB (stroma-rich) or GN (stroma-dominant) component. The other type is intermixed-GNB which is composed of intermixing of neuroblastic cells and ganglion cells. By definition, more than 50% of tumor tissue shows a GN appearance [1]. According to Decarolis et al. GNB-intermixed behaves similarly to GN and can be considered to be at the benign end of the PNT spectrum [22].
Two systems, the International Neuroblastoma Staging System (INSS) and the International Neuroblastoma Risk Group (INRG) staging system are widely recognized to predict the prognosis of patients with neuroblastoma [1].
The maturation sequences of the tumors are promoted by so-called cross-talk between neuroblasts and Schwann cells comparable to the embryologically well-defined relationship in neural crest development toward ganglion structure of the autonomic nervous system1.
It is important to consider biological factors, in particular amplification of N-MYC (which is amplified in 20%-30% of neuroblastomas) and deletion of the short arm of chromosome 1 that are related to poor prognosis [4].
GNB is a pediatric tumor and adult onset is extremely rare. In literature, less than 50 cases of adult onset are reported. The most frequent site is the adrenal gland; other localizations are the retroperitoneum, the brain and the mediastinum. To the best of our knowledge, in the adrenal gland 22 cases are reported, including our patient, and only 2 cases were consistent with intermixed-GNB at histology (Table 1).
Most of patients with adult onset of adrenal GNB (14/22) were male, from 20 to 73 years old (mean age 36,8 years). Because typically the lesions grew silently, at time of diagnosis big masses were found (mean size 9,8 cm).
Eight patients showed distant metastasis or local recurrence; metastatic sites were bone or bone marrow in 4 cases, lymph node in 2 cases and liver in 2 cases. Most of the patients were treated only with surgery, showing no recurrence during follow up (mean follow-up duration 18.6 months’) and 2 received also radio- and chemotherapy. Bone metastases were detected after 2.5 years in a patient who refused radio- and chemotherapy after surgical removal [6]. One patient died 3 months’ after diagnosis due to heart failure [20].
Clinical presentation of PNT is not specific and often represented by pain, abdominal swelling or other symptoms due to compression. Some cases, such as the 1 reported, may be found incidentally [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20].
Laboratory tests include a blood and urine measurement of catecholamines and their metabolites, since NB are often responsible for an abnormality in the production, secretion or catabolism of catecholamines [5].
The preoperative diagnosis of PNT remains challenging because radiological features of NB, GNB, and GN are similar and the definitive discrimination of the histologic type is based on pathologic analysis findings only [26].
US is usually the first imaging examination to identify the presence of an adrenal mass because of its wide availability and noninvasiveness [26]. At US, GNB is described as heterogeneously echogenic: there may be anechoic areas within the tumor corresponding to hemorrhage or necrosis, while calcifications are common and appears as focal echogenic areas or diffuse increased echogenicity (from fine calcifications); distal acoustic shadowing may or may not be present. Grey-scale and Doppler US allows accurate localization of the lesion and can help to define the relationship with adjacent organs and vessels [21].
However, due to its high operator dependency and low accuracy in local staging, US is typically followed by CT and MRI imaging to further evaluate the extent of the disease and to assist in staging [26].
CT is the most commonly used imaging modality for assessment of PNT, because it reveals extent of tumor, organ of origin, regional invasion, vascular encasement, lymph nodes involvement, and calcifications [27].
The reported CT findings in GNB vary ranging from a predominantly solid mass to a predominantly cystic mass with a few thin strands of solid tissue [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20],27]. Usually, the disease appears as a homogeneous or slightly heterogeneous mass that tends to surround major blood vessels without compression or occlusion and is poorly enhanced by contrast medium. Calcifications, typically fine and punctate, are seen in approximately 42%-60% of GNB [5].
Compared with CT, MRI imaging offers the advantages of higher contrast resolution in soft tissue and the lack of ionizing radiation. GNB is typically heterogeneous with relatively low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. No absolute change in signal intensity is observed on chemical shift imaging. After intravenous contrast medium administration, it shows early enhancement, in contrast to GN. NB and GNB are more aggressive than GN, and they usually have an irregular contour, sometimes with invasion to adjacent organs and vessels [26,27].
Gahr et al. suggested that diffusion-weighted imaging is effective for differentiating NB from the more benign GN and GNB, which tend to have an apparent diffusion coefficient (ADC) value of no less than 1.1 × 10−3 mm2/s, whereas NB has markedly lower ADC value [28].
Distant metastases must be assessed by using Iodine 123-MIBG scintigraphy and the examination has to be performed before tumor excision. For MIBG-non–avid PNT other options such as FDG-PET and/or CT should be considered [29].
Although the definitive diagnosis of a PNT is obtained after histopathological analysis, CT and MRI are helpful to further characterize masses and useful in pretreatment risk stratification based on clinical criteria and image-defined risk factors [26].
Treatment guidelines were derived from pediatric experience. The therapeutic strategy includes surgery, radiotherapy and chemotherapy [5].
If the tumor is considered radically resectable, surgery represents the treatment of choice. Because of the high possibility of infiltration of regional lymph nodes, local lymph node dissection is recommended [5].
If the tumor is unresectable, a diagnostic biopsy should be performed and treatment options are radiotherapy or cytoreductive chemotherapy. Chemotherapy is the treatment of choice in metastatic disease.
Recurrence of the disease occurs mostly in the first 2 years after surgery. The patient should be examined every 3 months’ in the first 2 years, then every 6 months’. Complete blood count, urinary catecholamine analysis, and imaging of the site of primary tumor should be performed at every examination [30].
Nowadays prognosis of GBN of the adults remains uncertain because of long-term data poorness.
Conclusion
GNB is an intermediate-grade tumor of the autonomic nervous system on the spectrum of PNT. It is usually a pediatric disease with fewer than 50 cases previously reported in adults and just over 20 in the adrenal gland.
Although the definitive diagnosis of PNT is obtained after histopathological analysis, CT and MRI are helpful to further characterize masses and useful in pretreatment risk stratification based on clinical criteria and image-defined risk factors.
Clinicians should be aware of the possibility of GNB development in adult population and its malignant potential.
Ethical considerations
Written informed consent was obtained from the patient. We confirm that this work is original and has not been published elsewhere nor it is currently under consideration for publication elsewhere.
Funding information
No research funding was obtained.
Data availability
The data that support the findings of this study are available from the corresponding author, (LV), upon reasonable request.
Disclaimer
The views and opinions expressed in this research article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
Authors’ contributions
LV and MF contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript; IB contributed to the revision of the manuscript; LP, MF, and GFO contributed to the acquisition and interpretation of the data and to revision of the manuscript and SS contributed to the revision and final edit of the manuscript.
All authors read and approved the final version of the manuscript.
Ethical considerations
Written informed consent for the publication of this case was obtained from the patient.
The data that support the findings of this study are available from the corresponding author, (LV), upon reasonable request.
Footnotes
Acknowledgments: No acknowledgements are present.
Competing Interests: The authors declare that they have no financial or personal relationship that may have inappropriately influenced them in writing this article.
References
- 1.King-yin Lam A. Update on adrenal tumours in 2017 world health organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28:213–227. doi: 10.1007/s12022-017-9484-5. [DOI] [PubMed] [Google Scholar]
- 2.Lonergan GJ, Schwab CM, Suarez ES. Neuroblastoma, ganglioneuroblastoma and ganglioneuroma: radiologic-pathologic correlation. Radiographics. 2002;22(4):911–934. doi: 10.1148/radiographics.22.4.g02jl15911. [DOI] [PubMed] [Google Scholar]
- 3.Joshi VV. Peripheral neuroblastic tumors: pathologic classification based on recommendations of international neuroblastoma pathology committee (modification of Shimada classification) Pediatr Dev Pathol. 2000;3:184–199. doi: 10.1007/s100240050024. [DOI] [PubMed] [Google Scholar]
- 4.Shimada H, Ambros IM, Dehner LP, Hata JI, Joshi VV, Roald B. The international neuroblastoma pathology classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 5.Bolzacchini E, Martinelli B, Pinotti G. Adult onset of ganglioneuroblastoma of the adrenal gland: case report and review of the literature. Surgical Case Rep. 2015 11;1:79. doi: 10.1186/s40792-015-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizuno S, Iida T, Fujita S. Adult-onset adrenal ganglioneuroblastoma – bone metastasis two years after surgery: report of a case. Surg Today. 2010;40(5):482–486. doi: 10.1007/s00595-008-4084-0. [DOI] [PubMed] [Google Scholar]
- 7.Benedini S, Grassi G, Aresta C, Tufano A, Carmignani LF, Rubino B. Adrenal ganglioneuroblastoma in adults: a case report and review of the literature. Case Rep Endocrinol. 2017;2017 doi: 10.1155/2017/5796236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorrentino S, Gigliotti AR, Sementa AR. Neuroblastoma in the adult: the italian experience with 21 patients. J Pediatr Hematol Oncol. 2014;36:e499–e505. doi: 10.1097/MPH.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 9.Slapa RZ, Jakubowski W, Kasperlik-Zaluska AA, Szopinski K, Debski R, Samsel M. Adrenal ganglioneuroblastoma in pregnant woman: diagnosis with three-dimensional ultrasound. Eur Radiol. 2002;12:S121–S126. doi: 10.1007/s00330-002-1457-4. [DOI] [PubMed] [Google Scholar]
- 10.Heidari Z, Ali Kaykhaei M, Jahantigh M, Sheikhi V. Adrenal ganglioneuroblastoma in an adult: a rare case report. Int J Endocrinol Metab. 2018;16(1):e63055. doi: 10.5812/ijem.63055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding X, Hou Y, Ma X, Zhang H, Wang C, Wang Y. Adult adrenal ganglioneuroblastoma: A rare case report. Can Urol Assoc J. 2015;9(1-2):e75–e77. doi: 10.5489/cuaj.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qui W, Li T, Sun XD, Lv GY. Onset of adrenal ganglioneuroblastoma in an adult after delivery. Ann Surg Treat Res;89(4):220-223. [DOI] [PMC free article] [PubMed]
- 13.Kumata H, Nishimura R, Nakanishi C, Inoue C, Tezuka Y, Endo H. Surgical strategy for an adult patient with a catecholamine-producing ganglioneuroblastoma and a cerebral aneurysm: a case report. Surg Case Rep. 2018;4:119. doi: 10.1186/s40792-018-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koike K, Iihara M, Kanbe M, Omi Y, Aiba M, Obara T. Adult-type ganglioneuroblastoma in the adrenal gland treated by a laparoscopic resection: report of case. Surg Today. 2003;33:785–790. doi: 10.1007/s00595-003-2565-8. [DOI] [PubMed] [Google Scholar]
- 15.Hisoshige K, Sonoda S, Fujita M, Takasugi M, Kuroiwa A, Inatomi H. Primary adrenal ganglioneuroblastoma in an adult. Intern Med. 1995;34:1168–1173. doi: 10.2169/internalmedicine.34.1168. [DOI] [PubMed] [Google Scholar]
- 16.Rousseau P, Bernard A, Favre JP, Arnould L, Cheynel N, Manuelian M. Ganglioneuroblastoma in the adult. Presse Med. 1998;27(33):1677–1679. [PubMed] [Google Scholar]
- 17.Mizuno S, Iida T, Fujita S. Adult-onset adrenal ganglioneuroblastoma – bone metastasis two tears after surgery: report of a case. Surg Today. 2010;40:482–486. doi: 10.1007/s00595-008-4084-0. [DOI] [PubMed] [Google Scholar]
- 18.Gunlusoy B, Arslan M, Selek E, Sural S, Ayder AR. A case report: Adrenal ganglioneuroblastoma in a 59-year old man. Int Urol Nephrol. 2004;36:481–483. doi: 10.1007/s11255-004-0851-z. [DOI] [PubMed] [Google Scholar]
- 19.Lonie J, Boles R, Boldery J. Adrenal ganglioneuroblastoma in an adult. ANZ J Surg. 2019;89:129–130. doi: 10.1111/ans.14157. [DOI] [PubMed] [Google Scholar]
- 20.Koizumi T, Kanbayashi T, Ichiyoshi T, Nakamura M, Moriyama S. Ganglioneuroblastoma with disseminated bone marrow infiltration in an adult. Int Med J. 1992;31:1322–1324. doi: 10.2169/internalmedicine.31.1322. [DOI] [PubMed] [Google Scholar]
- 21.Swift CC, Eklund MJ, Kraveka JM, Alazraki AL. Updates in diagnosis, management, and treatment of neuroblastoma. Radiographics. 2018;38:566–580. doi: 10.1148/rg.2018170132. [DOI] [PubMed] [Google Scholar]
- 22.Decarolis B, Simon T, Krug B. Treatment and outocome of Ganglioneuroma and Ganglioneuroblastoma intermixed. BMC Cancer. 2016;16:542. doi: 10.1186/s12885-016-2513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castleberry RP, Pritchard J, Ambros P, Brodeur GM, Castel V, Cohn SL. The International Neuroblastoma Risk Groups (INRG): a preliminary report. Eur J Cancer. 1997;33(12):2112–2116. doi: 10.1016/s0959-8049(97)00202-5. [DOI] [PubMed] [Google Scholar]
- 24.Brodeur GM, Pritchard J, Berthold F, Castel V, Castelberry RP, De Barnerdi B. Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. J Clin Oncol. 1993;11(8):1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 25.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force Report. J Clin Oncol. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brisse HJ, McCarville MB, Granata C. Guidelines for Imaging and Staging of Neuroblastic Tumors: Consensus Report from the international neuroblastoma Risk Group Project. Radiology. 2011;261(1):243–257. doi: 10.1148/radiol.11101352. [DOI] [PubMed] [Google Scholar]
- 27.Guo YK, Yang ZG, Li Y, Deng YP, Ma ES, Min PQ. Uncommon adrenal masses: CT and MRI features with histopathological correlation. Eur J Radiol. 2007;67:359–370. doi: 10.1016/j.ejrad.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Gahr N, Darge K, Hahn G, Kreher BW, von Buiren M, Uhl M. Diffusion-weighted MRI for differentiation of neuroblastoma and ganglioneuroblastoma/ganglioneuroma. Eur J Radiol 79(3):443-6. [DOI] [PubMed]
- 29.Matthay KK, Shulkin B, Ladenstein R, Michon J, Giammarile F, Lewington V. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: a report of the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer. 2010;102(9):1319–1326. doi: 10.1038/sj.bjc.6605621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy JM, La Quaglia MP. Advances in the surgical treatment of neuroblastoma: a review. Eur Pediatr Surg. 2014;24(6):450–456. doi: 10.1055/s-0034-1396421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, (LV), upon reasonable request.