Abstract
Micropapillary adenocarcinoma has been reported as an aggressive variant of adenocarcinoma in several organs, where it is associated with poor clinical outcome. This study reports the clinicopathological features and outcomes of cervical adenocarcinomas with a micropapillary component (micropapillary cervical adenocarcinomas); this represents the largest reported study of these neoplasms. The study comprised 44 cervical adenocarcinomas of usual (HPV-related)-type (84%), mucinous, not otherwise specified (4.5%), gastric-type (4.5%), endometrioid (4.5%) and adenosquamous carcinoma (2%). The micropapillary component comprised more than 50% of the neoplasm in 34 cases (77%) (group 1), and 10–50% in 10 cases (23%) (group 2). Lymph node metastasis was present in 41 of 44 (93%) cases and typically the nodal tumor retained a prominent micropapillary morphology. Follow-up ranged from 7 to 123 months (mean, 65.9). 17/44 (38.6%) patients had no evidence of disease on follow-up, 6/44 (13.6%) were alive with disease, and 21/44 (47.7%) died of disease. There were no survival differences between group 1 and group 2. On univariate analysis, lymph node metastasis (p:0.0015), lymphovascular space invasion (LVSI)(p:0.002), parametrial involvement (p:0.03) and depth of stromal invasion (p:0.045) were related to tumor recurrence. On multivariate analysis, lymph node metastasis (p:0.001), and extent of LVSI (p: 0.027) were significant independent predictors of tumor recurrence. Our study shows that a micropapillary component in cervical adenocarcinoma may be associated with aggressive behavior and that a micropapillary architecture may occur within a variety of types of cervical adenocarcinoma.
Keywords: Cervix, adenocarcinoma, micropapillary component, follow-up
INTRODUCTION
Histological subtype is one of the parameters which determines the behavior and outcome in cervical adenocarcinoma. Certain adenocarcinoma subtypes have a poor prognosis; for example, gastric-type has a much worse prognosis than usual human papillomavirus (HPV)-related cervical adenocarcinomas. (1) One study revealed that gastric-type adenocarcinomas had a disease-specific survival at 5 years of 42% compared to 91% for usual cervical adenocarcinoma. (2) Such studies imply that histologic subtype affects prognosis and should be taken into consideration when determining treatment modalities.
Micropapillary adenocarcinoma is a variant of adenocarcinoma characterized by small, tightly cohesive groups of neoplastic cells within well-delineated clear spaces resembling vascular channels. (3) Adenocarcinomas with a micropapillary pattern have been described in several organs including, breast, (4) urinary tract, (5) thyroid, (6) lung (7) and ovary. (8) With the exception of ovarian low-grade serous carcinoma, which typically has a micropapillary architecture and often follows a relatively indolent course, micropapillary adenocarcinomas or adenocarcinomas with a micropapillary component are typically aggressive tumors with a marked tendency for vascular invasion and a poor prognosis. (4–7)
To date, only occasional case reports or small series of cervical adenocarcinomas with a micropapillary component have been reported in the literature. (9–13) In some of these cases, the micropapillary component has been admixed with a component of usual-type adenocarcinoma without a micropapillary architecture. In this study, we report the clinicopathological features, including follow-up data, of a series of cervical adenocarcinomas with variable proportions of micropapillary architecture.
MATERIAL AND METHODS
Cases Included in Study
The cases were derived from the institutions to which the authors are affiliated. Only patients who had been treated by radical hysterectomy and pelvic lymphadenectomy (1 patient had radiologically positive pelvic lymph nodes and did not undergo pelvic lymphadenectomy- see results below) and in whom follow-up was available were included in this study. Some of the cases have been previously published in series describing growth patterns in cervical adenocarcinomas. (11) In all cases, the slides were reviewed by pathologists involved in this study, all of whom are specialist gynecological pathologists. A micropapillary architecture was defined as papillary groups of neoplastic cells, sometimes with solid nests or tubules, without fibrovascular cores lying within spaces which do not represent vascular channels, as first described by Siriaunkgul et al. (4)
Cervical adenocarcinomas with a micropapillary architecture were classified into two subgroups depending on the proportion of the micropapillary component: - 1. those with a micropapillary component comprising greater than 50% of the neoplasm and 2. those with a micropapillary component comprising 10% to 50% of the neoplasm. Tumors with a minor component of micropapillary architecture (less than 10%) were excluded from the study.
Pathologic parameters evaluated in each tumor were: - depth of stromal invasion, maximum horizontal dimension, lower uterine segment involvement, parametrial invasion and lymph node metastasis. Lymphovascular space invasion (LVSI) was classified as negative, low (0–4 spaces involved), moderate (5–19 spaces involved), and extensive (≥ 20 spaces involved).
Cervical adenocarcinomas were classified using the International Endocervical Adenocarcinoma Criteria and Classification (IECC) system. (14) Clinical information was recorded, including patient age at diagnosis, surgical procedures, 2014 FIGO stage, (15) and follow-up data (period in months and status).
Immunohistochemistry
Immunohistochemical staining was performed in 40 of the 44 cases with PAX8, cytokeratin7 (CK7), cytokeratin 20 (CK20), carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), estrogen receptor (ER), progesterone receptor (PR), vimentin, CDX2, CD10, Wilm’s tumor 1 (WT1) and p16. A streptavidin-biotin peroxidase complex method was used with appropriate positive and negative controls. An autostainer (Biocare, intelliPATH FLX) was used according to the manufacture’s specifications. The antibodies used and their dilutions are listed in Table 1.
Table 1.
Antibodies Used in Study Together with Sources and Dilutions.
| Antibody | Clone | Dilution | Source | Vendor |
|---|---|---|---|---|
| PAX 8 | BC12 | 1:200 | Rabbit polyclonal | Biocare |
| CD10 | 56C6 | 1:100 | Mouse monoclonal | Biocare |
| P16 | G175–405 | 1:100 | Mouse monoclonal | Biocare |
| CDX2 | EP25 | 1:100 | Rabbit monoclonal | Biocare |
| WT1 | BC.6F-H2 | 1:100 | Mouse monoclonal | Biocare |
| CK7 | OV-TL 12/30 | 1:100 | Mouse monoclonal | Biocare |
| CK20 | Ks20.8 | 1:100 | Mouse monoclonal | Biocare |
| CEA | N/A | 1:100 | Rabbit polyclonal | Biocare |
| EMA | Mc-5 | 1:150 – 1:250 | Mouse monoclonal | Biocare |
| Vimentin | SP20 | 1:100 | Rabbit monoclonal | Biocare |
| ER | 1D5 | 1:100 | Mouse monoclonal | Biocare |
| PR | SP2 | 1:100 – 1:200 | Rabbit monoclonal | Biocare |
HPV DNA Detection and Typing
HPV genotyping was performed in 40 cases using the Roche linear array testing kit (Roche Molecular Diagnostics) according to the manufacture’s recommendations. This test is designed to identify 37 HPV genotypes, including 14 high-risk types (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68). The hybridization and the color reaction were performed automatically in an AutoLiPA device. The results of hybridization were assessed visually by comparing to the standard grid.
Statistical Analysis
The results were analyzed using SPSS version 21.0 (Statistical Package for the Social Sciences INC., IBM, Armonk, NY, USA). Numerical variables were expressed as the mean ± standard deviation (SD) or median (range) based on distribution pattern, while categorical variables were presented as absolutes values and percentages.
Differences between continuous and categorical variables were assessed by the student’s t-test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables, as well as the Fishe’s exact test or chi-square test, respectively.
The Cox proportional hazards model was used to construct a multivariate model to predict survival. A p value less than 0.05 was considered statistically significant.
RESULTS
Patient age at diagnosis ranged from 32 to 84 years with a mean age of 62.7 years.
Pathological Findings
The tumors ranged in maximum horizontal dimension from 20 to 54mm (mean 42mm). The depth of stromal invasion ranged from 6 to 25mm (mean 15.8mm). Lower uterine segment involvement was present in 12 patients (27%) and parametrial involvement in 14 (32%) patients.
Using the IECC Classification, the adenocarcinomas were classified as usual-type (37; 84%), mucinous, not otherwise specified (NOS) (2; 4.5%), gastric-type (2; 4.5%), endometrioid-type (2; 4.5%) and adenosquamous carcinoma (1; 2%) (Table 2). The micropapillary component comprised more than 50% of the tumor in 34 cases (group 1) and 10 to 50% in 10 cases (group 2). In some cases, the micropapillary component was predominantly present at the invasive edge of the tumor. The micropapillary component consisted of tumor cells arranged in small micropapillary tight nests surrounded by a cleft-like space. The tumor cells generally had atypical nuclei and a moderate amount of cytoplasm and mitotic activity was easily identified. Four cases showed a micropapillary component with more abundant eosinophilic cytoplasm. The gastric-type adenocarcinomas showed well-formed mucinous glands admixed with a micropapillary component.
TABLE 2.
Morphological Subtypes of Adenocarcinoma with a Micropapillary Architecture.
| No of cases | Micropapillary Component | ||
|---|---|---|---|
| 10% - ≤50% | >50% | ||
| Usual-Type | 37 | 3 | 34 |
| Endometrioid | 2 | 2 | 0 |
| Mucinous, NOS | 2 | 2 | 0 |
| Gastric-Type | 2 | 2 | 0 |
| Adenosquamous | 1 | 1 | 0 |
LVSI was seen in all 44 cases (100%). The degree of LVSI was low in 12 patients (27%), moderate in 14 (32%), and extensive in 18 (41%). Pelvic lymphadenectomy was performed in all patients except 1 who had radiologically positive lymph nodes. Forty-one patients (93%) had metastatic disease within lymph nodes: eight patients with 1 positive lymph node, 9 with 2, 12 with 3, 6 with 4, 3 with 5, 2 with 6 and 1 had radiologically positive nodes. Usually the tumor within lymph nodes retained a prominent micropapillary morphology.
Figures 1–5 illustrate representative images of the neoplasms.
Figure 1.
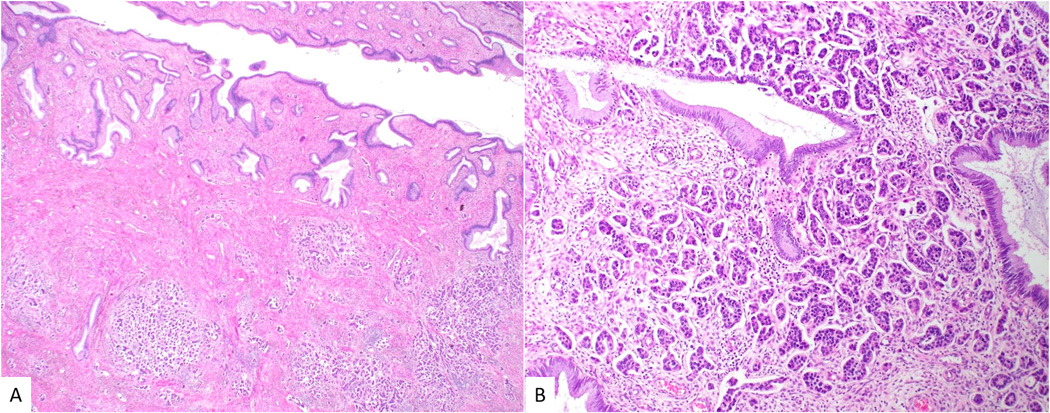
Low-power view of micropapillary adenocarcinoma with multiple tumor nodules beneath normal endocervical glands (A). At higher power, numerous small micropapillary formations are present within clear spaces (B).
Figure 5.
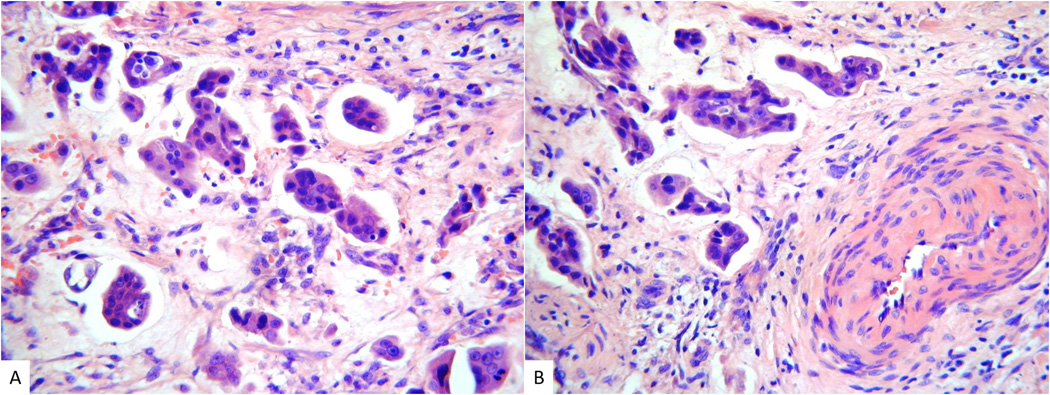
Gastric-type cervical adenocarcinoma with a micropapillary architecture (A and B). Elsewhere there were areas of typical gastric-type adenocarcinoma with well formed mucinous glands.
19 patients (43%) were FIGO stage I, with 2 (4.5%) IA1, 12 (27.2%) IB1 and 5 (11.3%) IB2. 17(38.6%) were stage II and 8 (18%) stage III.
Immunohistochemistry
All adenocarcinomas (40/40,100%) showed diffuse nuclear positivity for PAX8. Thirty-four of 40 cases (85%) were diffusely positive for CK7 and CEA and the others were negative. All adenocarcinomas exhibited positive staining for EMA on the peripheral cell membranes (“inside-out” pattern) (figure 6A).
Figure 6.
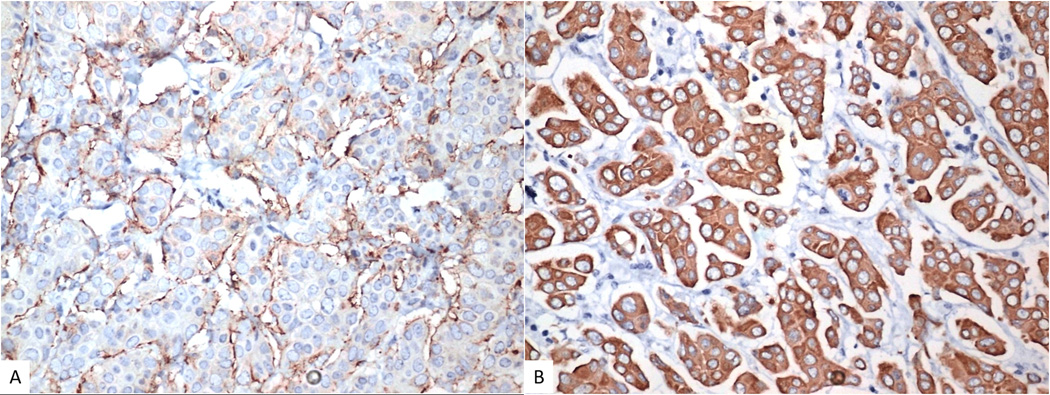
Immunohistochemistry with EMA shows “inside-out” pattern with staining towards the stroma (A). HPV-related micropapillary cervical adenocarcinoma showing diffuse block-type positivity with p16 (B).
p16 exhibited diffuse nuclear and cytoplasmic positivity (block-type staining) in all 33 (100%) usual-type adenocarcinomas (figure 6B) and in the one adenosquamous carcinoma. All the other tumors were p16 negative or exhibited non block-type immunoreactivity.
ER showed focal positive nuclear staining in 2 usual-type adenocarcinomas and was negative in the other cases. PR, vimentin, CK20, CDX2, CD10 and WT1 were negative in all 40 cases.
Human Papilloma Virus (HPV) DNA Detection Rates
HPV DNA was detected in 34 (85%) of the 40 tumors, including all the usual-type adenocarcinomas and the only case of adenosquamous carcinoma. HPV16 and HPV18 accounted for 28 (82%) and 6 (18%) of the HPV positive cases, respectively. All the gastric-type, mucinous NOS and endometrioid adenocarcinomas were HPV negative.
Association Between Lymphovascular Space Invasion (LVSI) and Lymph Node Metastases
To investigate the association between the extent of LVSI in cervical adenocarcinomas with a micropapillary architecture and lymph node metastases, multivariate regression analyses was performed with inclusion of tumors with low, moderate and extensive LVSI. As expected, the degree of LVSI was highly associated with lymph node metastases (p < 0.001)
Clinicopathological Features Associated with Tumour Recurrence/ Metastasis
Tumour recurrence or metastasis was seen in 31 of 44 (70%) patients. The most common sites of recurrence/ metastasis were the lung 11 (35%), retroperitoneum 8 (26%), and liver 7 (23%)
Univariate analysis showed that lymph node metastases (p:0.0015), LVSI (p:0.002), parametrial involvement (p:0.03) and depth of stromal invasion (p:0.045) were significantly associated with tumor recurrence/ metastasis (Table 3).
TABLE 3.
Univariate analysis of clinicopathological factors for micropapillary adenocarcinoma recurrence (n:34)
| N (%) | |||
|---|---|---|---|
| Patients With Recurrence (N=26) | Patients Without Recurrence (N=8) | P | |
| Tumor size in mm | |||
| median(min/max) | 44 (22/53) | 37.5 (20/54) | 0.48 |
| Depth of invasion in mm | |||
| mean(SD) | 16.9 (6.9) | 12.4 (4.6) | 0.045 |
| Lower Uterine Segment Involvement | |||
| Yes | 9 (34.6) | 2 (25) | 1.00 |
| No | 17 (65.4) | 6 (75) | |
| Lymphovascular space invasion | |||
| 0 – 4 vessels | 3 (11.5) | 6 (75.0) | 0.002 |
| 5 – 19 vessels | 9 (34.6) | 2 (25.0) | |
| ≥ 20 vessels | 14 (53.8) | 0 | |
| Parametrial involvement | |||
| Yes | 12 (46.2) | 0 | 0.03 |
| No | 14 (53.8) | 8 (100) | |
| Lymph nodes status | |||
| Median (min/max) | 14 (10/19) | 6 (2/19) | 0.039 |
| Patient without metastasis | 0 | 3 (50%) | 0.0015 |
All the single risk factors affecting recurrence rate were further studied using COX proportional hazards regression model. The results revealed that lymph node metastasis (p:0.001), and extent of LVSI (p: 0.027) were significant independent predictors of tumor recurrence/ metastasis.
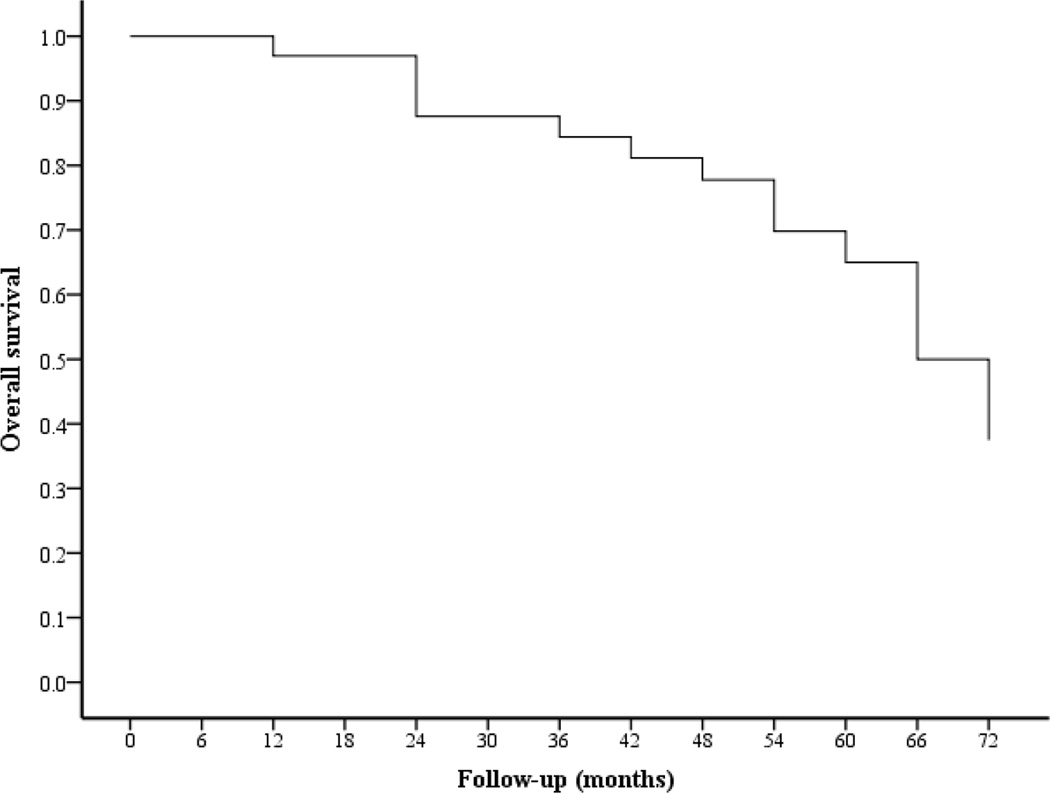
Overall Survival
With follow-up periods that ranged from 7 to 123 months (mean 65.9 months), 5-year overall survival rate was 33% (Figure 7). At last follow-up, 21 patients (47.7%) had died of disease, six patients (13.6%) were alive with disease and 17 (38.6%) were alive with no recurrence of disease. Using Cox regression model, lymph node metastasis, depth of stromal invasion, parametrial involvement and FIGO stage were significant independent predictors for survival (Table 4).
Figure 7.
Disease-specific survival estimates for cervical micropapillary adenocarcinoma
TABLE 4.
The 5-year disease-specific survival of patients with micropapillary adenocarcinoma related to several clinicopathological variables
| N (%) | |||
|---|---|---|---|
| Dead (N=17) | Alive + (N=17) | P | |
| Age in years [mean (SD)] | 65.2 (8) | 60.3 (13) | 0.196 |
| Tumor size (mm) | |||
| median(min/max) | 46 (35/53) | 40 (20/54) | 0.007 |
| Depth of invasion (mm) | |||
| Mean (SD) | 18.1 (7.1) | 13.7 (5.6) | 0.057 |
| Lower Uterine Segment Involvement | |||
| Yes | 6 (35.3) | 5 (29.4) | 1.00 |
| No | 11 (64.7) | 12 (70.6) | |
| Lymphovascular space invasion | |||
| 0 – 4 vessels | 0 | 9 (52.9) | <0.001 |
| 5 – 19 vessels | 3 (17.6) | 8 (47.1) | |
| ≥ 20 vessels | 14 (82.4) | 0 | |
| Parametrial involvement | |||
| Yes | 9 (52.9) | 3 (17.6) | 0.071 |
| No | 8 (47.1) | 14 (82.4) | |
| Lymph node status | |||
| Median (min/max) | 14 (10/19) | 10 (2/19) | 0.231 |
| Patient without metastasis | 0 | 3 (50%) | 0.0015 |
| Median (min/max) | 33% (14/75) | 14% (6/31) | <0.0001 |
| FIGO Stage | |||
| I | 4 (23.5) | 10 (58.8) | 0.0001 |
| II | 5 (29.4) | 7 (41.2) | |
| III | 8 (47.1) | 0 | |
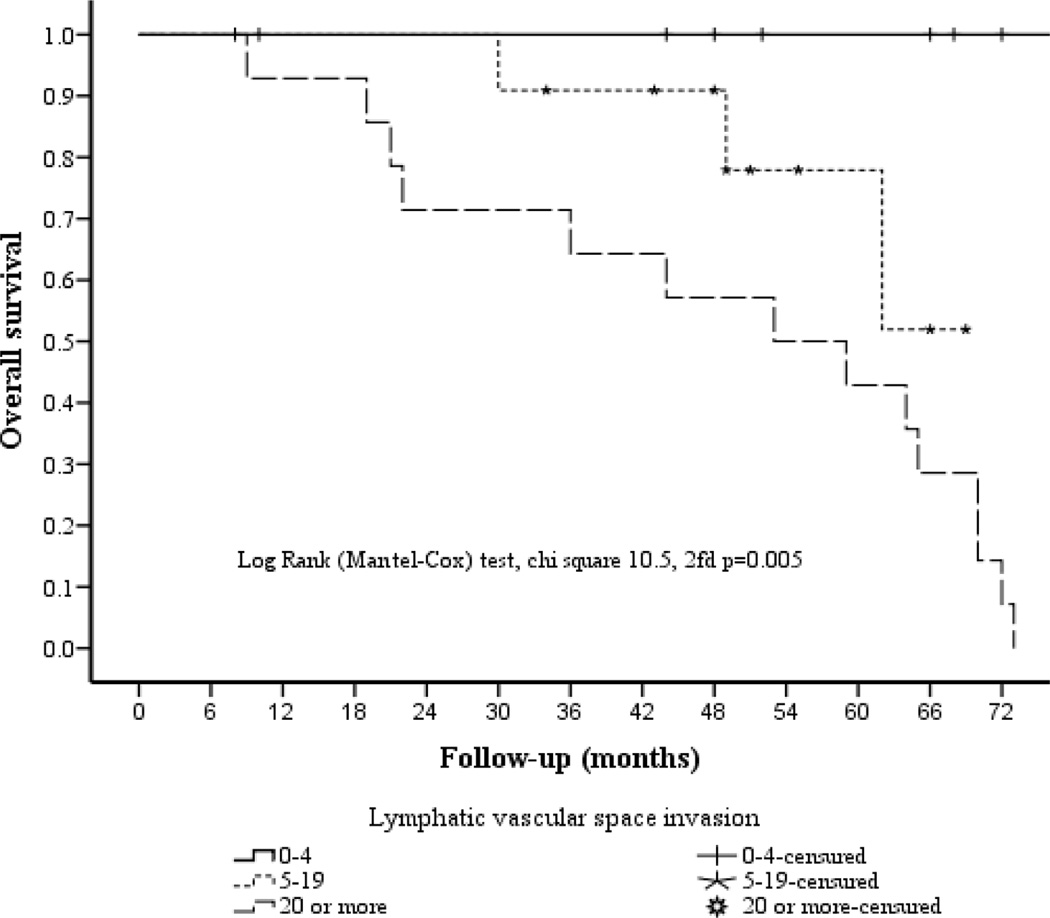
Relationship Between Survival and Degree of Lymphovascular Space Invasion (LVSI).
The association between the degree of LVSI and survival is depicted in Figure 8.
Figure 8.
Overall 5-year survival according to degree of lymphovascular space invasion
When correlated with the number of lymphovascular spaces involved by tumor (low, moderate, extensive), there was a significant difference in survival of100%, 51.9%, and 0%, respectively.
Impact of Amount of Micropapillary Component on Prognosis
A comparison between the two groups of patients with different proportions of micropapillary component, group 1 and group 2, did not show any statistically significant differences, with respect to any of the clinicopathological parameters evaluated.
DISCUSSION
Adenocarcinomas exhibiting a micropapillary growth pattern (micropapillary adenocarcinomas) were first reported in the breast where these neoplasms were shown to have a propensity for frequent LVSI and an aggressive clinical course. (4). The same morphology has been recognized in adenocarcinomas in several other organs, including the urinary tract (5), thyroid, (6) lung (7) and ovary. (8) In all these organs, the morphological features are of tight papillary groups of neoplastic cells, sometimes with solid nests or tubules, and without fibrovascular cores lying within empty spaces which do not represent vascular channels. They display an “inside-out” arrangement, with the luminal aspect of the cell present on the outer surface of the cluster. Using electron microscopy, Luna-Moré et al, (16) found that tumor cells of micropapillary adenocarcinomas have microvilli on their cell membranes, lining the outer surfaces of the cell clusters. This peculiar arrangement of cells within the clusters with their apical surfaces polarized to the outside supports the microscopic observation of the “inside-out” growth pattern. EMA staining highlights this “inside-out” pattern.
In this study, we report the clinicopathological features of a series of 44 cervical adenocarcinomas with a micropapillary architecture. We classified these into two groups according to the proportion of micropapillary component but there were no significant differences in the various clinicopathological features or behavior between the two groups. We have seen other cervical adenocarcinomas with a micropapillary component comprising less than 10% of the neoplasm but these were excluded from our study. Most of the previously reported cervical adenocarcinomas with a micropapillary architecture were of usual HPV-related type when the morphological subtype was specified (see below). However, in this report, we also describe other morphological subtypes of cervical adenocarcinoma, such as gastric-type, with a micropapillary architecture.
It is important to be aware that cervical adenocarcinomas may exhibit a micropapillary pattern to avoid diagnostic confusion and misdiagnosis as other neoplasms where a micropapillary architecture is better known. The differential diagnoses will not be discussed in detail but other tumors which may be considered include serous carcinomas (low-grade and high-grade) from elsewhere in the female genital tract, mesonephric carcinoma and metastasis from extragenital micropapillary adenocarcinoma. It is now clear that primary serous carcinomas of the cervix probably do not exist and most cases which are reported as such represent metastasis from elsewhere within the female genital tract or usual HPV-related cervical adenocarcinomas with a papillary architecture and marked nuclear atypia; (14) it is probable that some of the previously reported cases of primary cervical serous carcinoma represent the micropapillary adenocarcinomas which we describe. In distinguishing between a micropapillary cervical adenocarcinoma and an extragenital metastasis, the demonstration of a component of usual HPV-related cervical adenocarcinoma may be of value. Immunohistochemistry will also assist, the panel of markers chosen depending on the exact differential diagnosis under consideration. For example, if a breast or urothelial micropapillary adenocarcinoma is considered, GATA3 will usually be positive while PAX8 will typically be negative. The latter marker will be positive in most cervical micropapillary adenocarcinomas; it was positive in all 40 cases in our study where immunohistochemistry was undertaken.
All 40 of our cases were negative with WT1, helping to exclude an adnexal low-grade or high-grade serous carcinoma. All HPV-related tumors showed diffuse and strong positivity for p16. High-grade adnexal serous carcinomas and uterine serous carcinomas will often also exhibit this pattern of p16 immunoreactivity. However, almost all adnexal high-grade serous carcinomas and uterine serous carcinomas exhibit mutation-type staining with p53 while this pattern of immunoreactivity is extremely uncommon in HPV-related carcinomas (17) The lack of staining for CD10 and vimentin in all cases and diffuse block-type staining with p16 in most does not support a diagnosis of mesonephric carcinoma. (18)
Cervical adenocarcinomas can be classified into two main groups, namely those related to high-risk HPV and those unrelated to high-risk HPV. The former, which represent the vast majority, are referred to as cervical adenocarcinomas of usual type (19,20). HPV-independent adenocarcinomas are much more uncommon and include gastric-type, (21,22) mesonephric, (23) clear cell and endometrioid adenocarcinomas. In this series, HPV was detected in 34 out of 40 tumors (85%) where molecular tests were undertaken. HPV 16 was identified in 28 cases and HPV 18 in six. All of these 34 cases exhibited diffuse block-type immunoreactivity with p16. This illustrates that most micropapillary cervical adenocarcinomas are HPV-related neoplasms, although as discussed a small percentage of our cases comprised non-HPV-related cervical adenocarcinomas. In a review of the literature, we identified only 4 cases of micropapillary cervical adenocarcinoma where HPV-testing was performed and all were high-risk HPV positive. (12,13) In one study, all 3 cases tested were HPV 18 positive (12) and in another case in situ hybridization using a probe which detects high-risk HPVs types 16, 18, 31, 33 and 51 was positive. (13)
Recently, Stewart et al (12) reported 8 cervical carcinomas (adenocarcinomas and adenosquamous carcinomas) with a micropapillary architecture; up until now, this represents the largest reported series of cervical carcinomas with a micropapillary architecture. Three of their six patients with > 12 months follow-up died of metastatic disease and another was alive with distant metastasis. There have been four other reported cases of cervical micropapillary adenocarcinomas. (9–13,24) Three of the four reported patients presented with stage IV disease; two of these patients died 1 month and 6 months after diagnosis while the third was disease-free at 48 months. The four patient presented with pelvic and para-aortic lymph node metastasis. In the current study, twenty-one (47.7%) of the 44 patients with follow-up died of disease and 31 (70.4%) developed recurrent disease. These studies illustrate that cervical adenocarcinomas with a micropapillary architecture exhibit extremely aggressive behavior similar to adenocarcinomas with a micropapillary architecture in most other organs.
LVSI has long been identified as an important prognostic indicator in many tumor types. It has been reported as an independent predictor of patient outcome in cervical carcinoma. (25,26) In several organs, adenocarcinomas with a micropapillary architecture have a particular propensity for LVSI. In the present study, LVSI was present in all our micropapillary adenocarcinomas and the extent of LVSI was found to be an independent predictor of lymph node metastases and survival. In our series of micropapillary cervical adenocarcinomas, 43% were FIGO stage I, 38.6% stage II and 18% stage III. This again highlights the propensity for aggressive behavior in cervical adenocarcinomas with a micropapillary architecture.
Conclusions
This is the largest published series of cervical micropapillary adenocarcinomas. We show that the micropapillary component could be a significant prognostic factor even in the early stage. While most of these represent HPV-associated neoplasms, a small percentage are of the various non-HPV-related types.
Figure 2.
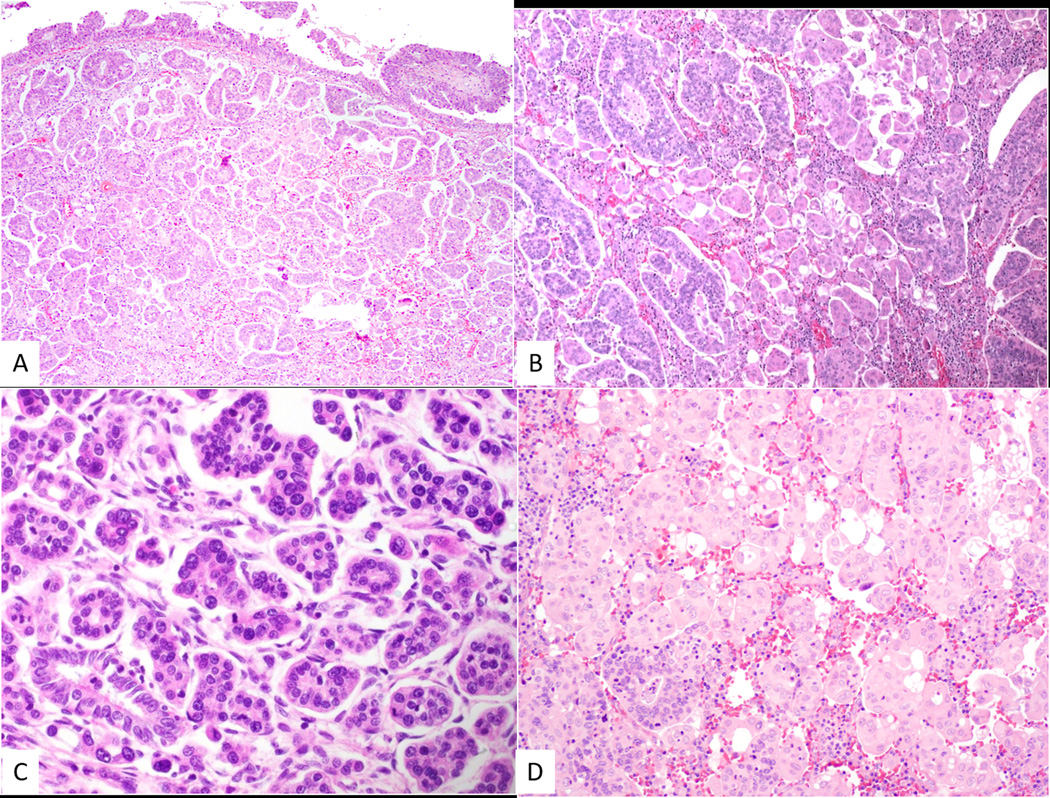
Transition from usual-type cervical adenocarcinoma (top of photomicrograph) to micropapillary adenocarcinoma (A). Tumor with admixture of usual-type adenocarcinoma and micropapillary adenocarcinoma (B). Micropapillary adenocarcinoma with micropapillary aggregates of atypical cells exhibiting mitotic activity surrounded by clear spaces (C). Case of micropapillary adenocarcinoma where the tumor cells have abundant eosinophilic cytoplasm (D).
Figure 3.
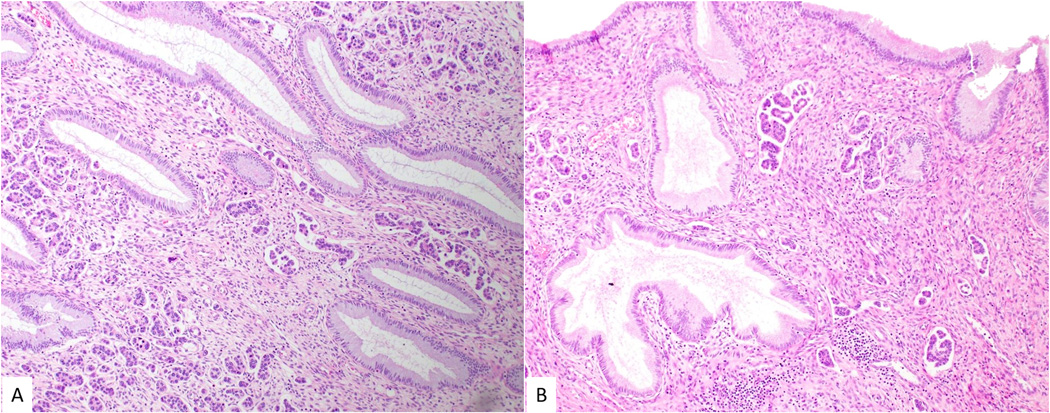
Micropapillary cervical adenocarcinoma exhibiting prominent lymphovascular space invasion, as well as groups of tumor cells surrounded by clear spaces (A and B).
Figure 4.
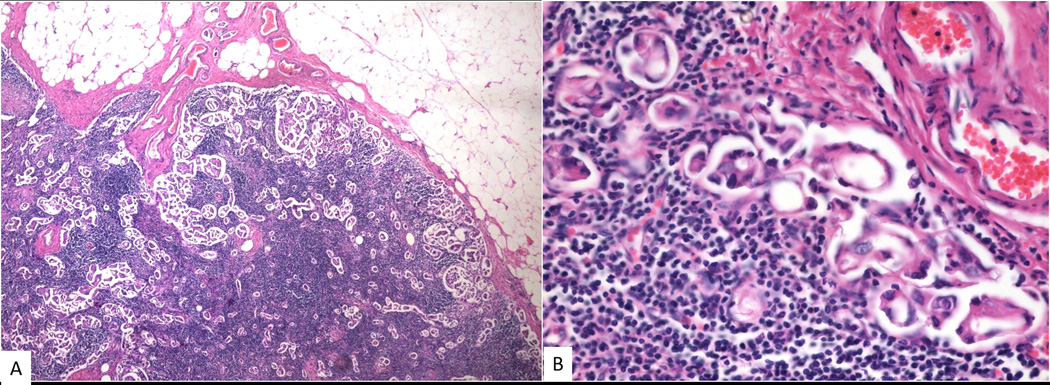
Lymph node metastasis. The tumor within the lymph node retains the prominent micropapillary architecture with micropapillary formations surrounded by clear spaces (A and B).
Contributor Information
Isabel Alvarado-Cabrero, Chief of the Pathology Department Hospital de Oncología, CMN, SXXI Instituto Mexicano del Seguro Social Mexico.
W. Glenn McCluggage, Belfast Health and Social Care Trust, Belfast, United Kingdom.
Rafael Estevez-Castro, Hospital de Especialidades, CMN, SXXI Instituto Mexicano del Seguro Social Mexico.
Delia Pérez-Montiel, Instituto Nacional de Cancerología México.
Simona Stolnicu, University of Medicine and Pharmacy, Tirgu Mures, Romania.
Raji Ganesan, Birmingham Women’s and Children’s NHS foundation Trust, United Kingdom.
Josefa Vella, Birmingham Women’s and Children’s NHS Foundation Trust, United Kingdom.
Rosario Castro, Instituto de Patología y Citología Dominican Republic.
Javier Canedo-Matute, Laboratorio de Patología y Citología (LAPACI), Colombia.
Jessica Gomez-Cifuentes, Fundacion Universitaria de Ciencias de la Salud Hospital San José, Colombia.
Vilma M Rivas-Lemus, Hospital San Juan de Dios de Santa Ana El Salvador.
Kay J. Park, Department of Pathology, Memorial Sloan Kettering Cancer Center, USA.
Robert A. Soslow, Department of Pathology, Memorial Sloan Kettering Cancer Center, USA.
Esther Oliva, Department of Pathology, Massachusetts General Hospital, USA.
Raquel Valencia-Cedillo, Hospital de Oncología, CMN, SXXI Instituto Mexicano del Seguro Social, Mexico.
References
- 1.Pirog EC. Cervical Adenocarcinoma: Diagnosis of Human Papillomavirus-Positive and Human Papillomavirus-Negative Tumors. Arch Pathol Lab Med. 2017;141;1653–1667. [DOI] [PubMed] [Google Scholar]
- 2.Karamurzin YS, Kiyokawa T, Parkash V, et al. Gastric-Type endocervical adenocarcinoma: An aggressive tumor with unusual metastatic patterns and poor prognosis. Am J Surg Pathol 2015;39:1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen AC, Paulino AC, Schwartz MR, et al. Population based comparison of prognostic factors in invasive micropapillary and invasive ductal carcinoma of the breast. Br J Cancer 2014;111:619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol 1993;6:660–662. [PubMed] [Google Scholar]
- 5.Amin MB, Ro JY, el-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urnary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol 1994;18:1224–1232. [DOI] [PubMed] [Google Scholar]
- 6.Ieni A, Barresi V, Cardia R, et al. The micropapillary/hobnail variant of papillary thyroid carcinoma: A review series described in the literature compared to series from one southern Italy pathology institution. Rev Endocr Metab Disord 2016;17:521–527. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Modern Pathology 2008; 21:992–1001. [DOI] [PubMed] [Google Scholar]
- 8.Bell DA. Low-grade serous tumors of the ovary. Int J Gynecol Pathol 2014;33 :348–356. [DOI] [PubMed] [Google Scholar]
- 9.Kajiyama A, Ishii E, Nakagawa M, et al. Adenocarcinoma of uterine cervix with micropapillary pattern. A case report. J. Japan. Soc. Clin. Cytol 2013;3:231–236. [Google Scholar]
- 10.Toyoda S, Kita T, Sugiura A, et al. Cervical adenocarcinoma with stromal micropapillary pattern. Diagn Cytopathol 2016;44:133–136. [DOI] [PubMed] [Google Scholar]
- 11.Alvarado-Cabrero I, Roma AA, Park KJ, Rutgers JKL, Silva EG. Factors predicting pelvic lymph node metastasis, relapse, and disease outcome in pattern C endocervical adenocarcinomas. Int J Gynecol Pathol 2017;36:476–485. [DOI] [PubMed] [Google Scholar]
- 12.Stewart CJR, Koay MHE, Leslie C, Acott N, Leung YC. Cervical carcinomas with a micropapillary component: a clinicopathological study of eight cases. Histopathology 2018; 72: 626–633. [DOI] [PubMed] [Google Scholar]
- 13.Munakata S, Hosoi A, Yamamoto T. Invasive micropapillary carcinoma of the uterine cervix: case report of a rare entity. Int J Gynecol Pathol 2018; 37: 368–371. [DOI] [PubMed] [Google Scholar]
- 14.Stolnicu S, Barsan I, Hoang L, et al. Criteria and Classification for Invasive Adenocarcinomas of the Endocervix. Am J Surg Pathol 2018;42:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet 2014; 125: 97–98. [DOI] [PubMed] [Google Scholar]
- 16.Luna-Moré S, González B, Acevedo C, Rodrigo I, Luna C. Invasive micropapillary carcinoma of the breast: a new special type of invasive mammary carcinoma. Pathol Res Pract. 1994;190:668–674. [DOI] [PubMed] [Google Scholar]
- 17.Stolnicu S, Barsan I, Hoang L, et al. Diagnostic Algorithmic proposal based on comprehensive immunohistochemical evaluation of 297 invasive endocervical adenocarcinomas. Am J Surg Pathol 2018; 42: 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AN HJ, Kim Is. Prevalence of human papilloma virus DNA in various histological subtypes of cervical adenocarcinoma: a population-based study. Mod Pathol 2005;18:528–534. [DOI] [PubMed] [Google Scholar]
- 19.Ronnett BM. Endocervical adenocarcinoma: selected diagnostic challenges. Mod Pathol 2016; 29: S12–28. [DOI] [PubMed] [Google Scholar]
- 20.McCluggage WG. Recent developments in non-HPV related adenocarcinomas of the lower female genital tract and their precursors. Adv Anat Pathol 2016; 23:58–69. [DOI] [PubMed] [Google Scholar]
- 21.Kusanagi Y, Kojima A, Mikami Y, et al. Absence of high-risk human papilloma virus detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol 2010; 177:2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talia KL, McCluggage WG. The developing spectrum of gastric-type cervical glandular lesions. Pathology 2018; 50:122–133. [DOI] [PubMed] [Google Scholar]
- 23.Kenny SL, McBride HA, Jamison J, McCluggage GW. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-β. Am J Surg Pathol 2012; 36:799–807. [DOI] [PubMed] [Google Scholar]
- 24.Azria E, Dufeu M, Fernández P, Walker F, Luton D. Cervical adenocarcinoma presenting as a cardiac tamponade in a 57-year-old woman: a case report. J. Med. Case Rep 2011;5: 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mabuchi Y, Yahata T, Kobayashi A, et al. Clinicopathologic factors of cervical adenocarcinomas stages IB to IIB. Int J Gynecol Cancer 2015; 25: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 26.Zaganelli FL, Carvalho FM, Almeida BG, et al. Intratural lymphatic vessel density and clinicopathologic features of patients with early-stage cervical cancer after radical hysterectomy. Int J Gynecol Cancer 2010; 20: 1225–1231. [DOI] [PubMed] [Google Scholar]