Abstract
Study Design:
Prospective randomized clinical trial.
Objectives:
To assess the effectiveness of PEAK Plasmablade (PPB), compared with bipolar sealer and standard electrocautery, in the posterior spinal instrumentation and fusion (PSF) surgery performed for adolescent idiopathic scoliosis (AIS).
Methods:
Ninety-three patients undergoing PSF surgery for AIS were randomized in 2 groups: group-A patients (n = 45) underwent PSF surgery using PPB; group-B patients (n = 48) were treated with bipolar sealer and standard electrocautery. Demographic and surgical data was recorded. All the patients underwent serial blood tests on the day before surgery (T0) and at 24 (T1), 48 (T2), 72 (T3), and 96 (T4) hours postoperatively. Visual analogue scale for pain (VAS) score, the percentage of paracetamol assumption, and the blood transfusion rate were recorded in the time-lapse T1 to T4. Intergroup variability was assessed. Pearson correlation test was performed. A P value <.05 was considered significant.
Results:
In group A, a significantly shorter total operative time (P = .0087), a significantly lower total intraoperative blood loss (TBL) (P = .001), and a higher postoperative hemoglobin (Hb) (P = .01) were recorded. A significant higher mean Hb concentration and mean albumin value was recorded in group A at 24 and 48 hours postoperatively. A significant correlation between TBL and hospital stay was recorded in both groups (group A, P = .00 001; group B, P = .00 006); moreover, in both groups, a significant correlation was observed between TBL and mean VAS at 72 hours postoperatively (group A, P = .0009; group B, P = .0001) and at 96 hours postoperatively (group A, P = .000 044; group B, P = .00 001).
Conclusions:
PPB reduces the intraoperative blood loss in PSF performed for AIS, thus allowing a patient’s faster recovery.
Keywords: adolescent idiopathic scoliosis, blood loss, surgical bleeding, PEAK Plasmablade, posterior spinal instrumentation and fusion, bipolar sealer, standard electrocautery
Introduction
Posterior spinal instrumentation and fusion (PSF) surgery for idiopathic scoliosis may cause severe intraoperative blood loss, ranging from 800 to 1514 mL, 1,2 with a reported maximum of 4500 mL in complex pediatric surgery. 3
Several factors influence the amount of blood loss during PSF surgery; they can be divided into patient-related factors, namely sex, Risser sign, preoperative Cobb angle, preoperative kyphosis magnitude, activated partial thromboplastin time level, fibrinogen level, and menstruation cycle phase–and surgery-related factors, namely number of fused vertebrae, surgical time, stage of surgery, number of osteotomies and surgical approach. 4,5
Severe intraoperative blood loss may lead to different perioperative disadvantages, including poor intraoperative visibility, increased transfusion rates, longer surgical times, higher incidence of perioperative major complications, and longer hospital stay, thus significantly affecting the patients’ quality of life. 5,6
Several blood-sparing techniques are currently used during PSF surgery, to avoid massive intraoperative bleeding, including correct patient positioning, in order to decrease intra-abdominal pressure and the use of controlled hypotension anesthesia; acute pulsed radiofrequency; acute normovolemic hemodilution; local hemostatic agents; intra-operative blood cells salvage; fibrinolytic agents and electrosurgical instruments, such as electrocautery and bipolar sealer. 7 -11
As reported in a recent metanalysis by Lan et al, 10 current evidence suggests that bipolar sealer is superior to standard electrocautery in terms of blood loss, operative time, and transfusion requirement in spine surgery.
The Pulsed-Electron Avalanche Knife (PEAK) PlasmaBlade (PPB) is a new electrosurgical device that produces pulsed plasma–mediated discharges, at the edge of an insulated electrode, to allow precise dissection of soft tissue, with reduced tissue damage and intraoperative bleeding. 12 -15
PPB is currently successfully used to perform tonsillectomy, 13 aesthetic blepharoplasty, 14 and neuromodulation implant revision surgery. 15 None of the previous studies, to the authors’ knowledge, have investigated the role of PBB in major spine surgery.
The current study aims to assess the effectiveness of PPB, compared with bipolar sealer and standard electrocautery, in the PSF surgery performed for adolescent idiopathic scoliosis (AIS).
Materials and Methods
Patients and Methods
Between January 2014 and February 2018, a total of 93 patients (87 females, 6 males; average age 16.6 years, range 14-19 years), undergoing PSF surgery for thoracolumbar AIS at our institution, were enrolled in this clinical prospective randomized study. The clinical study proposal was approved by the Medical Ethical Committee of the authors’ institution, as per the 1964 Declaration of Helsinki. All patients gave informed consent before enrollment in the study.
Inclusion criteria were: AIS, number of fusion level >6; autotransfusion eligibility. Exclusion criteria were: adult scoliosis; nonidiopathic scoliosis; coagulopathy; thrombocytopenia or thromobcytopathy; previous spinal surgery.
The patients were randomized, in 1:1 fashion, in 2 groups: group-A patients underwent PSF surgery using PPB; group-B patients were treated with bipolar sealer and standard electrocautery. Covariate-adaptive randomization, performed with an online statistical computing web programming, was used to randomize the patients into the 2 groups.
The following demographic and operative data was recorded: age, gender, body mass index (BMI), Lenke curve types, preoperative major Cobb angle, preoperative hemoglobin (Hb), number of instrumented vertebrae, spine surgical exposure time, total surgical time, total intraoperative blood loss (TBL), blood loss during exposure (EBL), autologous blood transfusion rate (BTR). All the data was gathered and blindly analyzed.
TBL was assessed using the method by Kwan et al 6 :
TBL (mL) = (final volume accumulated in the reservoir) − (total volume of anticoagulant citrate dextrose [ACD]) − (total irrigation fluid used intraoperatively) + (total unfiltered blood).
The perfusionist calculated the total volume of ACD used and total unfiltered blood, as the difference between used and dry reservoir. The total irrigation fluid was strictly assessed by the scrub nurse before use. Blood loss from surgical gowns and drapes was not evaluated.
The blood loss during exposure (EBL) was also assessed, at the end of surgical exposure of the posterior bony elements, using the same method.
Surgical Procedure
All the PSF procedures were performed by the same senior spine surgeon (P.A.), assisted by a junior surgeon (P.C. or B.D.); the same anesthesiology team followed all the procedures. Cell salvage autologous blood recovery system was used during all surgical procedures.
Exposure was defined as the time interval between skin incision and the insertion of the first pedicle screw. 6
All the patients underwent PSF surgery using a system of titanium rods and screws (Solera System, Medtronic). Bilateral pedicle screws insertion was performed at each instrumented level; all the pedicle screws were implanted using free-hand technique. 16 Facetectomies were realized at all instrumented vertebrae, to improve the curve correction. Spinal fusion was obtained performing laminar decortication and using autologous bone graft, obtained from facet joints, spinous processes, and decorticated laminae of each instrumented vertebra. An intradermic suture was performed in all the patients. No wound drains were placed in both groups.
Postoperative Evaluation
In the first 48 hours after surgery, all the patients received the same single-use elastomeric pump containing morphine, antiemetics, and nonsteroidal anti-inflammatory drugs (NSAIDs) for postoperative pain management; paracetamol 1000 mg was added as required. From postoperative day 3, until discharge time, pain was treated only with paracetamol 1000 mg given as required.
All the patients underwent serial blood tests, including complete blood count and C-reactive protein (CRP), on the day before surgery (T0) and at 24 (T1), 48 (T2), 72 (T3), and 96 (T4) hours postoperatively.
Visual analogue scale for pain (VAS) score, the percentage of paracetamol assumption, and the BTR were recorded in the time interval T1 to T4. Autologous blood transfusion trigger was set at a Hb level <7.5 g/dL.
PEAK PlasmaBlade Features
PPB is a relatively new electrosurgical device, which uses brief (40 μs), precise pulses of radiofrequency energy to cut and coagulate soft tissues.
This technology received the Food and Drug Administration clearance for general surgery in 2008, with subsequent additional use indications in plastic and reconstruction, ear-nose-throat (ENT), gynecologic/obstetric, orthopedic, arthroscopic, spinal. and neurological procedures. 14
PBB works by inducing an electrical plasma along the edge of a thin (12.5 μm), 99.5% insulated cutting blade. Due to the thermal protection shield technology, it operates at significantly lower temperatures than traditional electrocautery (40to 170 °C, compared with 200 to 350 °C of standard electrocautery). PPB can cut and coagulate using rapid cyclical progression radiofrequency waves lasting from 10 to 100 ms.
As it happens in traditional electrosurgical devices, PPB has 2 different modalities, that is, CUT and COAG, used to respectively cut and coagulate soft tissue. Each modality has a 10-point intensity; in the current study, both CUT and COAG modalities were set at 6 in all PSF surgeries.
Randomization and Blinding
Randomization was performed by a statistician, independent of the outcome assessment, using a statistical program, to ensure an equal group distribution. All the data was analyzed in a blind manner by an external statistician who was unaware of the randomization process.
Statistical Analysis
Statistical analysis was performed using STATA/MP 14 for Windows (Stata Corp LP). The Kolmogorov-Smirnov test was performed to check the normality of the data distribution. Intragroup variability was assessed using paired t test, whereas unpaired t test and chi-square test were used to compare the 2 groups. Pearson correlation test was finally performed. The data is presented in terms of mean value and standard deviation (SD); a P value <.05 was considered significant.
Results
A total of 93 patients (87 females and 6 males; average age 16.6 years; range 14-19 years) were recruited for the current study.
The main data of the study is summarized in Table 1: 45 patients were recruited in group A, whereas 48 patients were recruited in group B. In group A, a significantly shorter total operative time (P = .0087), a significantly lower intraoperative TBL (P = .001), and a higher postoperative Hb value (P = .01), compared with group B, were recorded (Table 1). Consequently, a significantly lower BTR (P = .0001) and mean hospital length of stay (P = .0011) were observed in group A (Table 1).
Table 1.
Main Data of the Study.
| Group A | Group B | P | |
|---|---|---|---|
| Patients, n | 45 | 48 | — |
| Age, years | |||
| Mean ±SD | 16.2 ± 2.54 | 17 ± 2.36 | .35 |
| Range | 14-19 | 15-19 | — |
| Gender, n (%) | |||
| Male | 3 (6.67) | 3 (6.25) | .59 |
| Female | 42 (93.33) | 45 (93.74) | .52 |
| Body mass index, kg/m2 | |||
| Mean ± SD | 18.4 ± 2.55 | 18.1 ± 2.47 | .55 |
| Lenke classification, n (%) | |||
| Lenke 1 | 12 (26.67%) | 11 (22.92%) | .21 |
| Lenke 2 | 7 (15.56) | 8 (16.67) | .38 |
| Lenke 3 | 6 (13.33) | 7 (14.58) | .33 |
| Lenke 4 | 5 (11.11) | 6 (12.5) | .28 |
| Lenke 5 | 8 (17.78) | 9 (18.75) | .33 |
| Lenke 6 | 7 (15.56) | 7 (14.58) | .31 |
| Preoperative major Cobb angle, deg | |||
| Mean ± SD | 67.5 ± 13.5 | 65.6 ± 11.8 | .28 |
| Preoperative hemoglobin, g/dL | |||
| Mean ± SD | 12.85 ± 1.49 | 13.05 ± 1.28 | .63 |
| Total operative time, min | |||
| Mean ± SD | 373.4 ± 34.5 | 446.0 ± 38.6 | .0087 |
| Instrumented vertebrae | |||
| Mean ± SD | 11.3 ± 2.72 | 11.5 ± 2.85 | .66 |
| Intraoperative total blood loss, mL | |||
| Mean ± SD | 344.62 ± 55.24 | 576.35 ± 89.56 | .0001* |
| Intraoperative total blood loss per kilogram of body weight, mL/kg | |||
| Mean ± SD | 4.45 ± 0.526 | 4.877 ± 0.743 | .645 |
| Volume of reinfused blood, mL | |||
| Mean ± SD | 184.56 ± 32.44 | 325.3 ± 52.336 | .003* |
| Postoperative hemoglobin, g/dL | |||
| Mean ± SD | 10.4 ± 0.62 | 9.43 ± 1.18 | .01* |
| Blood transfusion rate, n of patients (%) | 3/45 (6.67) | 9/48 (18.75) | .0001* |
| Hospital stay, days | |||
| Mean ± SD | 6.57 ± 1.59 | 11.18 ± 3.66 | .0011* |
| Mean arterial pressure prior to incision, mm Hg | |||
| Mean ± SD | 71.43 ± 3.56 | 74.55 ± 4.22 | .232 |
* Significant P value (unpaired t test). **Significant P value (chi-square test).
Table 2 shows the exposure stage details. Exposure surgical time (P = .001) and exposure blood loss (EBL; P = .0001) were significantly lower in group A, compared with group B. It should be noted that the instrumented vertebrae number (Table 2) does not affect this datum.
Table 2.
Exposure Stage Details: Comparison Between the Two Groups (Unpaired t Test).
| Group A (Mean ± SD) | Group B (Mean ± SD) | P | |
|---|---|---|---|
| Mean arterial pressure during exposure, mm Hg | 73.65 ± 5.67 | 78.45 ± 4.55 | .097 |
| Exposure surgical time, min | 99.29 ± 19.17 | 160.1 ± 31.29 | .001* |
| Exposure surgical time per instrumented vertebrae, min | 8.73 ± 1.95 | 12.8 ± 3.03 | .0004* |
| Exposure stage blood loss, mL | 38.57 ± 27.48 | 91.03 ± 26.02 | .0001* |
| Exposure stage blood loss per instrumented vertebrae, mL | 3.415 ± 2.66 | 7.34 ± 1.6 | .00 457* |
| Exposure stage blood loss per instrumented vertebrae per minute, mL/min | 0.034 ± 0.027 | 0.625 ± 0.373 | .0006* |
* Significant P value.
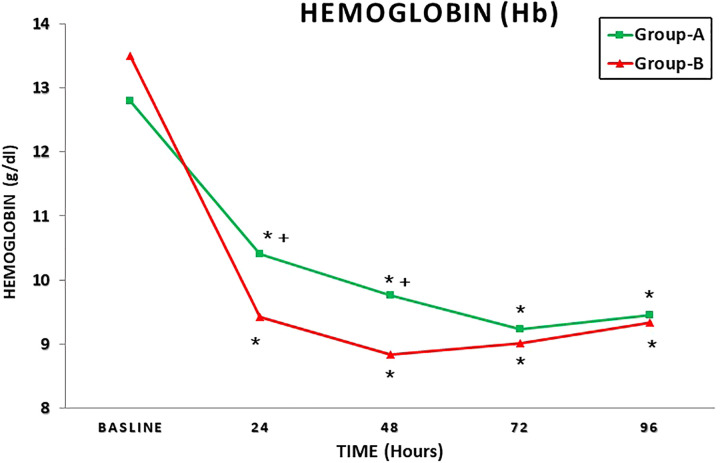
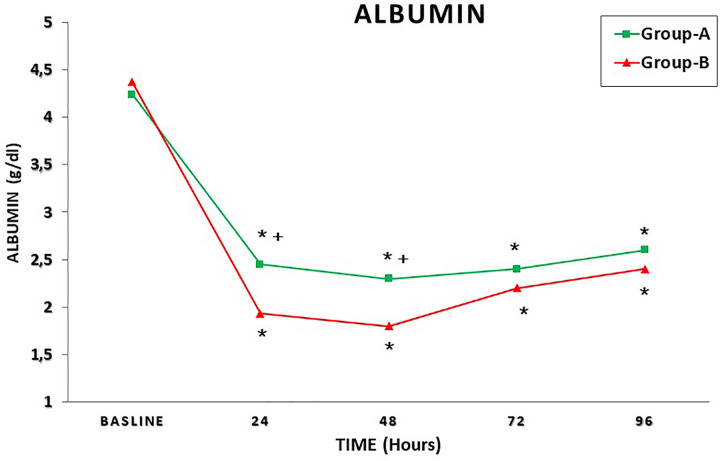
Table 3 summarizes the postoperative blood tests results. A significant higher mean Hb concentration and mean albumin value was recorded in group A at 24 and 48 hours postoperatively (Table 3).
Table 3.
Postoperative Blood Results: Comparison Between Groups.
| Group A | Group B | P | |
|---|---|---|---|
| Baseline (T0) | |||
| Hemoglobin, g/dL | 12.85 ± 1.49 | 13.05 ± 1.28 | .63 |
| Albumin, g/dL | 4.24 ± 1.05 | 4.37 ± 0.77 | .47 |
| C-reactive protein, mg/dL | 0.9 ± 0.37 | 0.83 ± 0.42 | .81 |
| 24 hours postoperatively (T1) | |||
| Hemoglobin, g/dL | 10.4 ± 0.61 | 9.43 ± 1.18 | .012* |
| Albumin, g/dL | 2.45 ± 0.76 | 1.93 ± 0.95 | .01* |
| C-reactive protein, mg/dL | 73.25 ± 9.75 | 76.79 ± 11.77 | .10 535 |
| 48 hours postoperatively (T2) | |||
| Hemoglobin, g/dL | 9.76 ± 1.356 | 8.83 ± 0.92 | .01* |
| Albumin, g/dL | 2.3 ± 0.66 | 1.8 ± 1.01 | .007* |
| C-reactive protein, mg/dL | 111.45 ± 49.9 | 113.24 ± 52.88 | .22 |
| 72 hours postoperatively (T3) | |||
| Hemoglobin, g/dL | 9.23 ± 1.54 | 9.02 ± 0.78 | .15 |
| Albumin, g/dL | 2.38 ± 0.9 | 2.2 ± 0.88 | .13 |
| C-reactive protein, mg/dL | 128.24 ± 51.59 | 130.44 ± 56.65 | .342 |
| 96 hours postoperatively (T4) | |||
| Hemoglobin, g/dL | 9.459 ± 0.76 | 9.33 ± 0.66 | .21 |
| Albumin, g/dL | 2.57 ± 0.83 | 2.42 ± 0.93 | .38 |
| C-reactive protein, mg/dL | 139.55 ± 64.33 | 142.67 ± 72.4 | .143 |
* Significant P value.
Table 4 shows the Pearson correlation test between TBL, hospital length of stay, and mean VAS, recorded in the time interval T1 to T4. A significant correlation between TBL and the hospital length of stay was recorded in both groups (group A, P = .00 001; group B, P = .00 006); moreover, in both groups, a significant correlation was observed between TBL and mean VAS at 72 hours postoperatively (group A, P = .0009; group B, P = .0001) and 96 hours postoperatively (group A, P = .000 044; group B, P = .00 001).
Table 4.
Pearson Correlation Test Between Total Intraoperative Blood Loss and Hospital Stay, Visual Analogue Scale (VAS) at 24, 48, 72, and 96 Hours Postoperatively.
| Group A | Group B | |||
|---|---|---|---|---|
| R | P | R | P | |
| Hospital stay | 0.6152 | .00 001* | 0.5442 | .00 006* |
| VAS | ||||
| At 24 hours postoperatively | 0.2213 | .114 | 0.163 | .285 |
| At 48 hours postoperatively | 0.2473 | .105 | 0.1956 | .1827 |
| At 72 hours postoperatively | 0.5478 | .0009* | 0.68 | .00 001* |
| At 96 hours postoperatively | 0.5696 | .000 044* | 0.5931 | .00 001* |
* Significant P value.
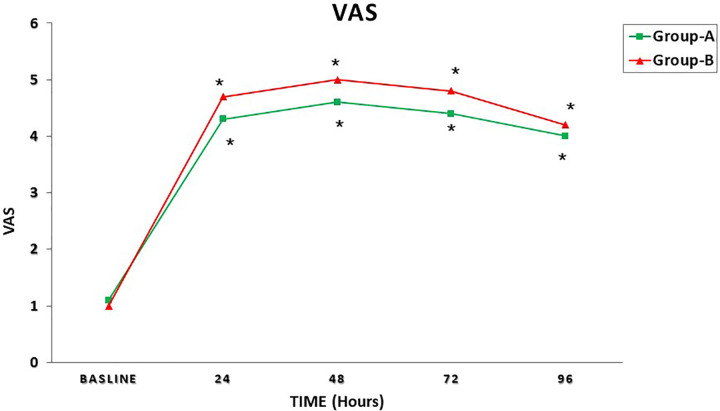
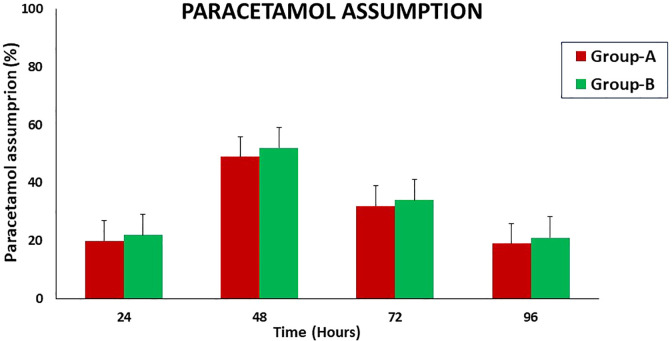
Figure 1 shows the mean Hb concentration in the time interval T0 to T4 in both groups; Figure 2 represents the mean serum albumin concentration in the time interval T0 to T4 in both groups; Figure 3 summarizes the mean VAS in the time interval T0 to T4; and Figure 4 shows the paracetamol assumption in the time interval T1 to T4 in both groups.
Figure 1.
Mean hemoglobin concentration in the time interval T0 to T4 in both groups. + P < .05; P values refer to a comparison between groups (unpaired t test); *P < .05; P values refer to the difference between postoperative and baseline (paired t test).
Figure 2.
Mean albumin concentration in the time interval T0-T4 in both groups. + P < .05; P values refer to a comparison between groups (unpaired t test); *P < .05; P values refer to the difference between postoperative and baseline (paired t test).
Figure 3.
Mean visual analogue scale for pain (VAS) score in the time interval T0 to T4 in both groups. + P < .05; P values refer to a comparison between groups (unpaired t test); *P < .05; P values refer to the difference between postoperative and baseline (paired t test).
Figure 4.
Paracetamol assumption in both groups in the time interval T1 to T4 in both groups.
Discussion
PSF, performed in patients with idiopathic scoliosis, is often associated with excessive blood loss due to the long median incision, extensive soft tissue dissection, and surgical time. 1,2
An excessive perioperative blood loss could harm the patient’s health, since it may cause a higher blood transfusion rate and a consequently higher risk of infection, thus leading to longer recovery time and higher health and social costs. 1 -4
Therefore, the spinal surgical community is making a great effort to reduce the intraoperative blood loss in major spine surgery. 7,8,17,18
Standard electrocautery is widely used in PSF, but it develops a significant quantity of smoke in operating room and it could interfere with pacemakers. 19
Bipolar sealer is a surgical instrument that uses radiofrequency energy to coagulate, in association with saline irrigation, the soft tissues at lower temperatures (ie, <100 °C) than standard electrocautery. 10 The introduction of bipolar sealer in spine surgery has helped with a significant reduction in blood loss, in both idiopathic scoliosis and degenerative spinal diseases treatment 10 ; however, it is not able to cut the skin and soft tissues, therefore it must be used in association with classic scalpel and electrocautery.
PPB is a new electrosurgical device that can cut with the precision of the traditional scalpel and perform, at the same time, an accurate hemostasis, thus minimizing intraoperative and postoperative bleeding, tissue damage and scar formation. 12 -15 It is a Food and Drug Administration–approved device that can be used in several surgical procedures. 12 -15
The current study has evaluated the role of PPB, compared with bipolar sealer and standard electrocautery, in the PSF surgery performed for AIS. For this purpose, 93 patients (87 females, 6 males; average age 16.6 years, range 14-19 years) undergoing PSF for AIS were randomized into 2 groups, treated with PPB (group A) and bipolar sealer in association with standard electrocautery (group B).
The exclusive use of PPB in the exposure stage, in this study, has significantly reduced the exposure surgical time (P = .001) and the exposure stage blood loss (P = .0001), thus confirming that PPB is effective in reducing intraoperative bleeding. Our data, in detail, shows that in patients treated with PPB there is a halving of the exposure stage blood loss per instrumented vertebra (P = .00 457).
The use of PPB also caused, in this study, a significant reduction of the total operative time (P = .0087) and intraoperative TBL (P = .0001); a significantly higher postoperative mean Hb concentration (P = .01) was observed in group-A patients, compared with group-B patients.
We believe the surgical time was significantly reduced in group A, since the lower blood loss and the lower smoke production in the exposure phase, allowed a better view of the surgical field. Bleeding was easily and quickly stopped using the PPB, therefore we had better and faster control of the operating field.
Due to the reduced intraoperative blood loss, a significantly lower BTR was recorded in group-A patients (P = .0001). Moreover, a significantly higher mean Hb value and serum albumin value was recorded in patients treated with PPB at 24 and 48 hours postoperatively (Table 3).
We also hypothesized that the use of PPB could reduce the intraoperative inflammatory stress, in PSF performed for AIS; however, no significant changes of CRP between groups was observed postoperatively in the current study (Table 3).
We also thought that PPB, reducing the intraoperative blood loss and operating at significantly lower temperatures than standard electrocautery, could reduce the postoperative pain, but neither significant mean VAS differences nor a significant different paracetamol assumption percentage between groups were observed postoperatively in the current study (Figures1 and 2).
It should be noted, however, that in both groups a significant positive correlation between TBL and mean VAS at 72 and 96 hours postoperatively was recorded. This data may be explained considering that in the first 48 hours after surgery, all the patients received the same single-use elastomeric pump containing morphine, antiemetics, and NSAIDs, therefore the VAS recorded in this time interval was less affected by the intraoperative blood loss, compared with the VAS recorded at T2 and T3.
Finally, a significant positive correlation between intraoperative TBL and hospital stay was observed in both groups. This datum confirms that, as reported in the literature, the intraoperative blood loss has an important impact on the patient’s recovery, thus the reduction of intraoperative bleeding should be an important aim to be reached during major spine surgery.
It important to remark that PPB is not endowed with significantly higher costs, compared with standard electrocautery 20 ; thus, based on the findings reported on the current study, the use of PPB seems to be cost-effective in PSF performed for AIS.
This study has some limitations. The intraoperative blood loss has been estimated at best, but the blood on the surgical sheet, on the surgeon’s gloves, and the surgical instruments was not assessed. Moreover, this study analyzes in-depth the blood loss during the exposure phase, without taking into the factors that could cause hemorrhage in the other stages of PSF surgery.
Conclusions
The use of PPB in PSF surgery performed for AIS, in the current prospective randomized clinical trial, contributed to reduce the intraoperative blood loss, mainly in the exposure stage, and the surgical operating time. This data had a positive impact on the patients’ recovery and on the health and social costs, since a significantly shorter hospital stay was recorded in patients treated with PPB.
Consequently, the routine use of PPB in major spinal surgery could have several advantages both for the surgeon and the patient.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Davide Bizzoca  https://orcid.org/0000-0002-7516-2333
https://orcid.org/0000-0002-7516-2333
References
- 1. Szpalski M, Gunzburg R, Sztern B. An overview of blood-sparing techniques used in spine surgery during the perioperative period. Eur Spine J. 2004;13(suppl 1):S18–S27. doi:10.1007/s00586-004-0752-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu SS. Blood loss in adult spinal surgery. Eur Spine J. 2004;13(suppl 1):S3–S5. doi:10.1007/s00586-004-0753-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shapiro F, Sethna N. Blood loss in pediatric spine surgery. Eur Spine J. 2004;13(suppl 1):S6–S17. doi:10.1007/s00586-004-0760-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu X, Xiao H, Wang R, Huang Y. Prediction of massive blood loss in scoliosis surgery from preoperative variables. Spine (Phila Pa 1976). 2013;38:350–355. [DOI] [PubMed] [Google Scholar]
- 5. Meert KL, Kannan S, Mooney JF. Predictors of red cell transfusion in children and adolescents undergoing spinal fusion surgery. Spine (Phila Pa 1976). 2002;27:2137–2142. [DOI] [PubMed] [Google Scholar]
- 6. Kwan MK, Chiu CK, Chan CYW. Single vs two attending senior surgeons: assessment of intra-operative blood loss at different surgical stages of posterior spinal fusion surgery in Lenke 1 and 2 adolescent idiopathic scoliosis. Eur Spine J. 2017;26:155–161. doi:10.1007/s00586-016-4803-y [DOI] [PubMed] [Google Scholar]
- 7. Yuan QM, Zhao ZH, Xu BS. Efficacy and safety of tranexamic acid in reducing blood loss in scoliosis surgery: a systematic review and meta-analysis. Eur Spine J. 2017;26:131–139. doi:10.1007/s00586-016-4899-0 [DOI] [PubMed] [Google Scholar]
- 8. Szpalski M, Gunzburg R, Aebi M, Weiskopf R. Research and evidence about blood sparing in spine surgery. Eur Spine J. 2004;13(suppl 1):S1–S2. doi:10.1007/s00586-004-0751-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Florentino-Pineda I, Blakemore L, Thompson GH, Poe-Kochert C, Adler P, Tripi P. The effect of epsilon-aminocaproic acid on perioperative blood loss in patients with idiopathic scoliosis undergoing posterior spinal fusion: a preliminary prospective study. Spine (Phila Pa 1976). 2001;26:1147–1151. [DOI] [PubMed] [Google Scholar]
- 10. Lan T, Hu S, Yang XJ, et al. The efficacy of bipolar sealer on blood loss in spine surgery: a meta-analysis. Eur Spine J. 2017;26:1796–1802. doi:10.1007/s00586-017-5045-3 [DOI] [PubMed] [Google Scholar]
- 11. Mankin KP, Moore CA, Miller LE, Block JE. Hemostasis with a bipolar sealer during surgical correction of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2012;25:259–263. doi:10.1097/BSD.0b013e3182334ec5 [DOI] [PubMed] [Google Scholar]
- 12. Loh SA, Carlson GA, Chang EI, Huang E, Palanker D, Gurtner GC. Comparative healing of surgical incisions created by the PEAK PlasmaBlade, conventional electrosurgery, and a scalpel. Plastic Reconstr Surg. 2009;124:1849–1859. [DOI] [PubMed] [Google Scholar]
- 13. Yilmazer R, Yazici ZM, Balta M, Erdim I, Erdur O, Kayhan FT. PlasmaBlade vs. cold dissection tonsillectomy: a prospective, randomized, double-blind, controlled study in adults. Ear Nose Throat J. 2017;96:250–256. [DOI] [PubMed] [Google Scholar]
- 14. Punthakee X, Keller GS, Vose JG, Stout W. New technologies in aesthetic blepharoplasty and brow-lift surgery. Facial Plast Surg. 2010;26:260–265. [DOI] [PubMed] [Google Scholar]
- 15. Ughratdar I, Kawsar KA, Mitchell JR, Selway R, Ashkan K. Use of a Pulsed Radiofrequency Energy Device (PEAK Plasmablade) in neuromodulation implant revisions. World Neurosurg. 2018;112:31–36. [DOI] [PubMed] [Google Scholar]
- 16. Piazzolla A, Montemurro V, Bizzoca D, Parato C, Carlucci S, Moretti B. Accuracy of plain radiographs to identify malpositioned free hand pedicle screw in the deformed spine. J Neurosurg Sci. 2019;63:372–378. doi:10.23736/S0390-5616.16.03670-5J [DOI] [PubMed] [Google Scholar]
- 17. Butler JS, Burke JP, Dolan RT, et al. Risk analysis of blood transfusion requirements in emergency and elective spinal surgery. Eur Spine J. 2011;20:753–758. doi:10.1007/s00586-010-1500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sebastián C, Romero R, Olalla E, Ferrer C, García-Vallejo JJ, Muñoz M. Postoperative blood salvage and reinfusion in spinal surgery: blood quality, effectiveness and impact on patient blood parameters. Eur Spine J. 2000;9:458–465. doi:10.1007/s005860000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nercessian OA, Wu H, Nazarian D, Mahmud F. Intraoperative pacemaker dysfunction caused by the use of electrocautery during a total hip arthroplasty. J Arthroplasty. 1998;13:599–602. [DOI] [PubMed] [Google Scholar]
- 20. Kypta A, Blessberger H, Kammler J, et al. Economic assessment of traditional surgical intervention versus use of a new innovative radiofrequency based surgical system in device replacements. PLoS One. 2018;13:e0192587. doi:10.1371/journal.pone.0192587 [DOI] [PMC free article] [PubMed] [Google Scholar]