Abstract
Background
Opioids have been linked to worse oncologic outcomes in surgical patients. Studies in certain cancer types have identified associations between survival and intra-tumoural opioid receptor gene alterations, but no study has investigated whether the tumour genome interacts with opioid exposure to affect survival. We sought to determine whether intraoperative opioid exposure is associated with recurrence-specific survival and overall survival in early-stage lung adenocarcinoma, and whether selected tumour genomics are associated with this relationship. Associations between ketamine and dexmedetomidine and outcomes were also studied.
Methods
Surgical patients (N=740) with pathological stage I–III lung adenocarcinoma and next-generation sequencing data were retrospectively reviewed from a prospectively maintained database.
Results
On multivariable analysis, ketamine administration was protective for recurrence-specific survival (hazard ratio = 0.44, 95% confidence interval 0.24–0.80; P=0.007), compared with no adjunct. Higher intraoperative oral morphine milligram equivalents were significantly associated with worse overall survival (hazard ratio=1.09/10 morphine milligram equivalents, 95% confidence interval 1.02–1.17; P=0.010). Significant interaction effects were found between morphine milligram equivalents and fraction genome altered and morphine milligram equivalents and CDKN2A, such that higher fraction genome altered or CDKN2A alterations were associated with worse overall survival at higher morphine milligram equivalents (P=0.044 and P=0.052, respectively). In contrast, alterations in the Wnt (P=0.029) and Hippo (P=0.040) oncogenic pathways were associated with improved recurrence-specific survival at higher morphine milligram equivalents, compared with unaltered pathways.
Conclusions
Intraoperative opioid exposure is associated with worse overall survival, whereas ketamine exposure is associated with improved recurrence-specific survival in patients with early-stage lung adenocarcinoma. This is the first study to investigate tumour-specific genomic interactions with intraoperative opioid administration to modify survival associations.
Keywords: ketamine, lung adenocarcinoma, next-generation sequencing, oncologic outcomes, opioids, surgery
Editor's key points.
-
•
Increased perioperative administration of opioids has been suggested to be associated with risk of earlier cancer recurrence and death in some but not all cancers, although causality has not been established.
-
•
It is also not known whether or what genomic factors, and whether other anaesthetic medications, might alter these associations.
-
•
This study of outcomes from lung adenocarcinoma surgery found that ketamine might improve recurrence-specific survival, and opioid restriction might improve overall survival after surgery for lung cancer.
-
•
Specific genomic alterations were found to be protective, whereas others were found to be associated with worse cancer-related outcomes.
Opioid misuse in the USA has steadily increased during the past two decades, with nearly half of all opioid-related deaths attributable to prescription opioids.1 Despite the risk of dependence, opioids are a necessary component of the perioperative analgesic regimen. Perioperative care teams nationwide have recently implemented opioid-sparing regimens, or enhanced recovery after surgery protocols, to optimise pain management while limiting unnecessary exposure to opioids.2 These enhanced recovery after surgery protocols have proved to reduce hospital length of stay and postoperative complication rates and have even improved oncologic outcomes in surgical patients.3,4
Research suggests that opioids may augment tumour growth and metastasis, possibly through reduction of natural killer cell activity, T-lymphocyte proliferation, and cytokine secretion.5 Furthermore, although surgery remains the primary and most effective treatment option for most resectable cancers, tumour resection itself can induce systemic dissemination of cancer cells despite optimal surgical technique.6 The surgical environment, which combines the immunosuppressive effects of intraoperatively administered opioids with the surgical stress response, has been hypothesised to magnify the proliferation of minimal residual disease.7,8
Recent literature, however, suggests that the relationship between opioid exposure and oncologic outcomes may vary according to cancer type or subtype. While opioids may support tumour pathogenesis in some solid cancers,9, 10, 11, 12 they may be neutral or even protective in others.13,14 This evolving perspective suggests that overall reduction in the use of opioid analgesia is not the universal endpoint, but rather should be tailored to specific cancer subtypes and, perhaps, ultimately to the specific patient. The real determinant underlying the relationship between opioids and cancer outcomes may be variation in individual tumour genomics.
Preliminary studies in breast, oesophageal, and laryngeal squamous cell cancer have identified associations between intra-tumour opioid receptor gene alterations or expression levels and survival.15, 16, 17 At present, few data are available that address the patient tumour genomic profile as it relates to the effect opioid exposure has on oncologic outcomes. Demonstrating that variation in a genomic factor can alter the opioid-dose-to-outcome relationship is a necessary but unrealised prerequisite for the development of a precision approach to perioperative analgesia in patients with cancer. Precision medicine has become a basic tenet of modern oncology. The treatment for lung adenocarcinoma, for example, includes tumour genomic profiling, through either genomic alterations or programmed death-ligand 1 (PD-L1) expression level, to more effectively tailor therapeutic regimens.18, 19, 20 We imagine a model in which, using the preoperative tumour biopsy, the anaesthesiologist can tailor intraoperative analgesic regimens according to the patient's unique clinical and genomic signature.
We investigated the association between intraoperative opioid exposure and oncologic outcomes in a cohort of patients treated with surgery for lung adenocarcinoma for whom next-generation sequencing data were available. Our primary hypothesis was that higher intraoperative opioid dose is associated with worse recurrence-specific survival and overall survival. We also examined whether the use of the opioid-sparing analgesic adjuncts ketamine and dexmedetomidine independently improves patient outcomes. We further leveraged patient-specific genomics data to explore the hypothesis that alterations in underlying tumour genomics may affect the opioid exposure–survival relationship and therefore modify the predicted opioid-dose-to-outcome response curve.
Methods
Patients
After institutional review board approval to conduct this study (IRB# 18-391, Memorial Sloan Kettering Cancer Center, approval date September 7, 2018), we retrospectively reviewed a prospectively maintained database to identify patients with primary pathological stage I–III lung adenocarcinoma who underwent complete (R0) resection from 2010 to 2019. Demographic, radiographic, pathologic, genomic, and follow-up patient data were reviewed. Predominant invasive lung adenocarcinoma histologic subtype was designated as either lepidic, acinar, papillary, micropapillary, solid, or unknown. The Elixhauser–van Walraven score, a well-validated comorbidity index, was used to quantify patient comorbid status.21
All patients had consented to next-generation sequencing (MSK-IMPACT) with samples obtained from their primary tumour.22 The CONSORT diagram shows exclusion criteria for the patient cohort (Supplementary Fig. S1). Metachronous and synchronous tumours were excluded, with metachronous tumours differentiated from recurrent tumours in accordance with the Martini and Melamed criteria, and confirmation of clonal relatedness was performed using next-generation sequencing data, as previously described by our group.23,24
MSK-IMPACT sequencing
Tumour genomic profiling was performed on all lung adenocarcinoma samples using the MSK-IMPACT platform, as previously described.22 Genomic factors of interest—including tumour mutational burden, fraction genome altered, and all genes altered at ≥5% frequency in the cohort—were selected for further analysis. Also included were the 10 canonical oncologic signalling pathways (cell cycle, Hippo, Myc, Notch, Nrf2, Pi3K, RTK/RAS, TGFβ, p53, and Wnt).25 A total of 121 genes were identified a priori in the 10 oncogenic signalling pathways (Supplementary Table S1). A pathway was considered altered in a tumour if at least one gene within the corresponding pathway template was altered. For analysis of co-occurrence and mutual exclusivity, we assessed all genes known to be drivers in lung adenocarcinoma.26 Mutual exclusivity and co-occurrence alterations in genes and oncogenic signalling pathways were assessed using Fisher's exact test, and P-values were adjusted to correct for multiple comparisons using the false discovery rate correction. Further details can be found in the Supplementary Methods.
Intraoperative analgesic agents
Doses of fentanyl, hydromorphone, and morphine administered intraoperatively were extracted from electronic anaesthesia records, converted to oral morphine milligram equivalents, and summed to give the total intraoperative dose, where 10 morphine milligram equivalents is equivalent to fentanyl 50 μg i.v. (a standard intraoperative bolus dose). Intraoperative administration of ketamine or dexmedetomidine was also identified and quantified (Supplementary Fig. S2). Specific analysis measures are found in the Supplementary Methods.
Statistical analysis
The primary objective of the study was to quantify the association between intraoperative opioid dose and oncologic outcomes. The primary outcome was recurrence-specific survival. Time to event was determined from the time of surgical resection to the time of first recurrence; this was otherwise censored at the time of last follow-up. Recurrence-specific survival, time to recurrence, was chosen in place of the alternative recurrence-free survival in order to determine whether opioids and the adjuncts were associated with disease progression in patients with stage I–III lung adenocarcinoma. The secondary outcome was overall survival, which was defined as the time to death from any cause.
To estimate the association between intraoperative opioid dose or use of adjuncts (ketamine and dexmedetomidine) and oncologic outcomes, we used univariable and multivariable Cox proportional hazard regressions to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), treating intraoperative morphine milligram equivalents as a continuous variable and administration of adjuncts as a categorical variable. For each endpoint (recurrence-specific survival and overall survival), variables with P<0.1 were included in the multivariable model, and some clinically relevant variables were retained. In the case of recurrence-specific survival analysis, patients who died without recurrence were considered to be censored using a cause-specific hazard model rather than a subdistribution hazard model; in this aetiological framework, the cause-specific hazard is more appropriate as it directly quantifies the hazard among subjects who are actually at risk of developing the event of interest, whereas in the subdistribution hazard model, individuals who experienced the competing event remain in the risk set.27 Each model was designed for the overall survival and recurrence-specific survival outcomes and was adjusted for relevant clinicopathological features. An additional multivariable model was constructed for each genomic factor of interest, for each outcome, by adding to the multivariable model a term for presence of factor alteration and a term for interaction between the factor and opioid dose.
To better illustrate the impact of incremental increases in intraoperative morphine milligram equivalents on outcomes, predicted 5-yr overall survival and recurrence-specific survival estimates, corresponding to a range of morphine milligram equivalents, were generated on the basis of the final multivariable analyses for a representative patient. Survival endpoints were measured from the time of surgery, and patients were censored at the time of last follow-up. Overall survival and recurrence-specific survival were estimated using the Kaplan–Meier approach, by morphine milligram equivalents and type of adjunct, and were compared using log-rank tests. Further details on statistical analysis, representative patient construction, and calculation of predicted opioid-dose-to-outcome response curves are found in the Supplementary Methods.
The linearity assumptions for all continuous variables (e.g. morphine milligram equivalents) were assessed using restricted cubic splines. All statistical tests were two-sided, with P<0.05 indicating statistical significance. R 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses.
Results
Patient characteristics
In total, 740 patients were included in the study. The majority were women (n=489 [66%]), and most were former or current smokers (n=543 [73%]). The median age at surgery was 68 yr (inter-quartile range [IQR], 61–73 yr); the median Elixhauser–van Walraven comorbidity score was 12 (IQR, 4–15) (Fig. 1, Supplementary Table S2).
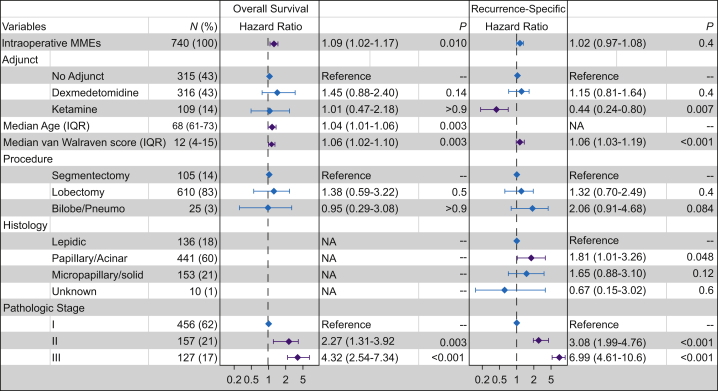
Fig 1.
Multivariable analysis for all intraoperative analgesics and clinicopathologic factors significantly associated with overall survival and recurrence-specific survival on univariable analysis. Purple error bars indicate significant difference compared with the reference group for each variable. Bilobe, bilobectomy; IQR, inter-quartile; MME, morphine milligram equivalents; NA, not applicable; Pneumo, pneumonectomy.
Clinicopathologic and analgesic variables
The median number of intraoperative morphine milligram equivalents for the overall cohort was 42 (IQR, 30–60). Patients who received dexmedetomidine had significantly lower intraoperative morphine milligram equivalents (median, 40 [IQR, 30–57]), compared with patients who received no adjunct (median, 50 [IQR, 30–61]) (Supplementary Table S2). All patients had pathologically diagnosed lung adenocarcinoma and were differentiated into groups on the basis of histologic subtype: lepidic (n=136 [18%]), acinar or papillary (n=441 [60%]), micropapillary or solid (n=153 [21%]), and unknown (n=10 [1.4%]). Four hundred and fifty-six patients (62%) had pathologic stage I disease, 157 (21%) had stage II disease, and 127 (17%) had stage III disease (Fig. 1). Thirteen percent of the cohort (n=96) received induction chemotherapy, and most patients underwent lobectomy (n=610 [82%]). Almost one-third of patients (n=227 [31%]) received adjuvant therapy, 31 (4.2%) received combination chemoradiation, 153 (21%) received chemotherapy alone, and 29 (3.9%) received radiation therapy (Supplementary Table S2). Median follow-up was 2.74 yr (IQR, 1.76–3.82 yr).
Association between intraoperative analgesics and outcomes
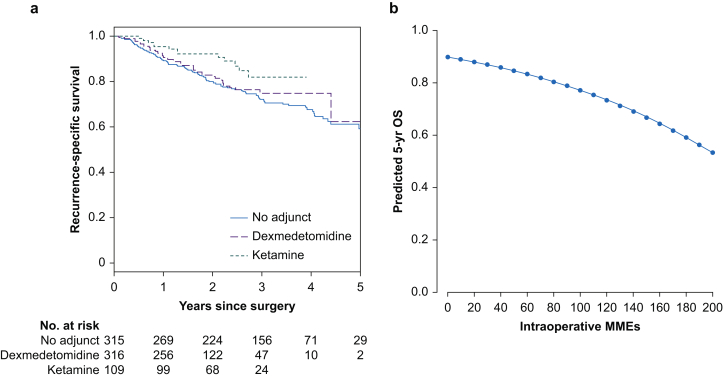
Five-year recurrence-specific survival was 61.8% (95% CI, 54.7–69.7%), and 5-yr overall survival was 74.4% (95% CI, 68.6–80.6%). There were 95 deaths and 160 locoregional or distant recurrence events during the study period (2010–9). The linearity assumptions for intraoperative morphine milligram equivalents were not violated for either recurrence-specific survival (P=0.32) or overall survival (P=0.35); hence, this primary exposure factor was treated as a continuous variable in the analyses. Intraoperative opioid dose was not significantly associated with recurrence-specific survival (Fig. 1). Ketamine administration was significantly associated with improved recurrence-specific survival, compared with no adjunct therapy, on both univariable (HR, 0.51 [95% CI, 0.28–0.91]; P=0.023) and multivariable (HR, 0.44 [95% CI, 0.24–0.80]; P=0.007) analysis (Fig 1, Fig 2a, Supplementary Table S3). Higher intraoperative morphine milligram equivalents were associated with worse overall survival on both univariable (HR, 1.09/10 morphine milligram equivalents [95% CI, 1.02–1.16]; P=0.011) and multivariable (HR, 1.09/10 morphine milligram equivalents [95% CI, 1.02–1.17]; P=0.010) analysis. Of note, ketamine administration was not associated with significant differences in overall survival. Dexmedetomidine administration was not associated with either outcome (Fig 1, Fig 2b, Supplementary Table S3).
Fig 2.
Association between analgesic adjunct use and recurrence and between intraoperative opioid dose and survival in patients with lung adenocarcinoma. (a) Five-year Kaplan–Meier curves for recurrence-specific survival for patients with lung adenocarcinoma who received ketamine, dexmedetomidine, or no adjunct intraoperatively. (b) Five-year predicted curve for overall survival (OS) with increasing intraoperative morphine milligram equivalents (MMEs).
Interaction of gene alterations with opioid dose
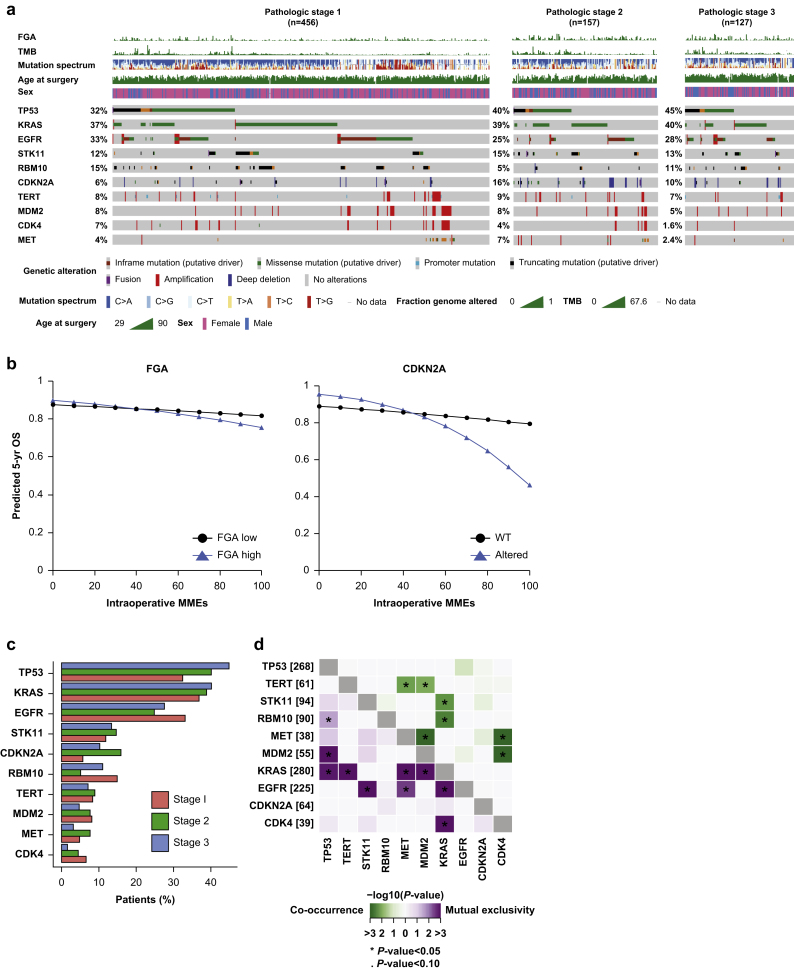
We performed an exploratory analysis to assess any modifying effect of tumour-specific genomic alterations, delineated by stage in the OncoPrint, on the opioid-survival relationship (Fig. 3a). Higher tumour mutational burden (P=0.026) and fraction genome altered (P=0.044), treated as continuous variables, were associated with worse overall survival with increasing opioid dose, although the actual size of the tumour mutational burden effect estimate was small (Fig. 3a, Supplementary Table S4). When fraction genome altered was broken down into 25th and 75th percentiles for our predicted model curves, higher fraction genome altered had a profound interaction with the opioid-overall survival relationship (Fig. 2b). CDKN2A alteration (8.6% alteration rate [n=64]) was associated with worse overall survival with increasing opioid dose, compared with wild-type CDKN2A (P=0.052) (Fig. 3b). CDKN2A alteration rates were significantly higher in pathologic stage II (16%) and stage III (10%) tumours, compared with stage I tumours (6%; P=0.002) (Fig. 3c). More than half of the total driver alterations (33 of 61) were homozygous deletions (Fig. 3a).
Fig 3.
Association between intraoperative opioid dose and genomic alterations in patients with lung adenocarcinoma. (a) OncoPrint of alteration frequencies of all genes altered ≥5% for the overall study cohort. Patients are subdivided by pathologic tumour stage. (b) Five-year predicted overall survival (OS) for patients with high and low fraction genome altered and altered and wild-type (WT) CDKN2A with increasing intraoperative morphine milligram equivalents (MMEs). (c) Comparative bar graphs representing the alteration rate for each genomic factor by stage. (d) Co-occurrence and mutual exclusivity between genes across all tumours. FGA, fraction genome altered; TMB, tumour mutational burden.
Interaction of canonical pathway alterations with opioid dose
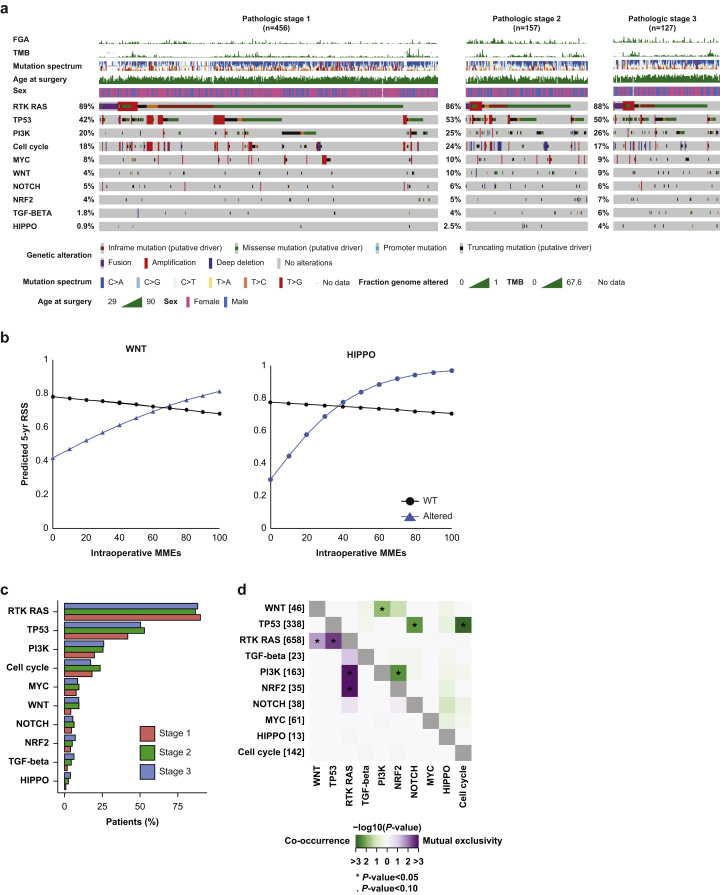
We also investigated alterations in the 10 canonical oncogenic pathways and determined the sum total of pathways altered (Fig. 4a). Alterations in the Wnt pathway (6.2% alteration rate [n=46]) were associated with improved 5-yr recurrence-specific survival with increasing opioid exposure (P=0.029), compared with no alterations in the pathway. A similar result was found for the Hippo pathway (1.8% alteration rate [n=13]; P=0.040) (Fig. 4b). Of note, rates of Wnt and Hippo pathway alterations increased with higher pathologic stage, but were not significantly different (P=0.114 and P=0.306, respectively) (Fig. 4c).
Fig 4.
Association between intraoperative morphine milligram equivalents (MMEs) and oncogenic pathway alterations in patients with lung adenocarcinoma. (A) OncoPrint of alteration frequencies of 10 canonical oncogenic pathways for the overall study cohort. Patients are subdivided by pathologic tumour stage. (B) Five-year predicted recurrence-specific survival (RSS) for patients with altered and unaltered Wnt and Hippo pathways. (C) Comparative bar graphs representing the alteration rate for each oncologic signalling pathway by stage. (D) Co-occurrence and mutual exclusivity between oncogenic pathways across all tumours. FGA, fraction genome altered; TMB, tumour mutational burden; WT, wild-type.
Co-occurrence and mutual exclusivity of genes and pathways
We assessed co-occurrences and mutual exclusivity between altered genes and pathways (Fig 3, Fig 4d). The Wnt pathway was found to significantly co-occur with the Pi3K pathway (P=0.020), although the interaction of the Pi3K pathway with opioid dose was not significantly associated with either overall survival or recurrence-specific survival (P=0.127 and P=0.119, respectively) (Supplementary Table S4).
Discussion
Preclinical evidence suggests that the interaction between exogenous opioids and the tumour immune environment may underlie pathogenic changes in tumour biology.6,28 However, the results of recent clinical studies suggest that this interaction does not always translate to a more aggressive pathology for all cancer types.29 For example, whereas greater opioid exposure was associated with shortened progression-free survival and overall survival in advanced-stage prostate cancer and renal cell carcinoma,12,30 opioids were found to be protective against recurrence in squamous oesophageal cancer.14 With regard to lung adenocarcinoma, in the present study, we found that increasing intraoperative opioid dose was associated with worse overall survival, which is broadly consistent with findings from recent studies in non-small cell lung cancer more generally.9,11
Differences in underlying tumour genomics may conceivably account for differences in the opioid–oncologic–outcome relationship. Recent studies found that the A118G polymorphism in the mu-opioid receptor (MOR) was associated with increased cancer-specific mortality in breast cancer and increased overall cancer development in oesophageal squamous cell cancer.15,17 In the present study, we attempted to uncover specific variations in tumour genomics of the primary tumour that interact with the association between intraoperative opioid dose and oncologic outcomes in lung adenocarcinoma. We found that alterations in specific genes and pathways can modify the predicted opioid-dose-to-outcome response curve, in both magnitude and directionality (i.e. from antisurvival to prosurvival). Note, that these alterations may not be reflected in the tumour recurrence genomic profile. The present study is, to our knowledge, the first analysis to show that increasing fraction genome altered is associated with worse overall survival with increasing intraoperative opioid dose. Nonetheless, fraction genome altered has been well described in association with increased lung adenocarcinoma pathogenicity, including subtype invasiveness and selective response to immunotherapy.31,32 Similarly, CDNK2A alterations, most frequently homozygous deletions, are common in smoking-associated non-small cell lung cancer, but the present study is the first time that alteration of this gene and increased opioid exposure have been shown to be correlated with decreased survival.33 The Hippo signalling pathway is a highly conserved pathway that plays a major role in cell polarity, cell–cell adhesion, and contact inhibition.34 Of note, Hippo pathway alteration has been shown to be associated with worse 2-yr disease-free survival in lung adenocarcinoma,25 which is consistent with our results, which predict shorter recurrence-specific survival with Hippo alteration at lower morphine milligram equivalents. However, as opioid dose increased, Hippo pathway alteration was associated with decreased tumour recurrence—patients with alterations in this pathway had improved recurrence-specific survival with increasing dose, whereas patients without alteration had worse recurrence-specific survival.
The Wnt pathway plays a primary role in cellular differentiation in early-stage lung cancer. A critical downstream effector of this regulatory pathway is B-catenin.35 In the present study, Wnt pathway alteration in conjunction with increasing opioid dose was associated with lower rates of tumour recurrence. Of interest, the Wnt pathway significantly co-occurred with the Pi3K pathway in this cohort. Although, at present, no study has implicated the direct effects of opioids on the Wnt pathway, Wnt activates the Pi3K pathway via B-catenin, and it has been suggested that the Pi3K pathway mediates the role of opioids on tumorigenesis.36 For example, Lennon and colleagues37 found that MOR upregulation promotes lung cancer progression via downstream signalling involving Akt and mTOR, members of the Pi3K pathway.
MOR signalling may similarly connect to other canonical oncogenic pathways—in particular, Wnt and Hippo—although these connections remain unknown at present. The expression of opioid target receptors in lung cancer would seem to be a necessary condition for opioid effects on tumour pathology, and this is supported by data showing associations between MOR expression and oncologic outcomes.38 We would suggest, however, that the expression and variation of MOR on tumour cells is not in itself sufficient to explain the effects of opioids, insofar as we have found that alterations of other genes and pathways interact with opioid exposure to affect the predicted opioid-dose-to-outcome response curve.
Although opioids are the primary focus of the present work, we also explored adjunct analgesic agents used intraoperatively. There is a growing trend in favour of ketamine and dexmedetomidine use, with a concomitant decrease in opioid use, for intraoperative analgesia.39 Recent evidence suggests that ketamine may be protective against recurrence. Intraoperative ketamine exposure was associated with improved recurrence-free survival in a cohort of 2775 surgical patients with localised kidney cancer.12 Although the mechanism of action underlying these favourable outcomes is yet to be fully elucidated, ketamine antagonism at the N-methyl-D-aspartate (NMDA) receptor is likely crucial, as NMDA receptor signalling has been demonstrated to facilitate breast cancer metastasis in a mouse model.40 The divide between a worse overall survival-opioid-dose result and an improved recurrence-specific survival-ketamine result supports the theory of different underlying mechanisms for these drugs.
To our knowledge, this is the first study to explore the interactions of patient-specific tumour genomic alterations on the relationship between intraoperative opioid analgesia and oncologic outcomes. The strengths of the study include its large cohort size and detailed clinicopathological and genomic annotations. Limitations include the retrospective nature and the lack of detailed data on postoperative opioid exposure. Additionally, the tumour genomics analysis was exploratory and thus not driven by specific gene and pathway hypotheses, although this may also be a strength—uncovering previously unknown interactions between opioids and specific genomic factors. Also, although conversion to a common morphine milligram equivalents measure is consistent with clinical studies in general, it is possible that different opioids may have varying oncologic significance. To this point, fentanyl accounted for the overwhelming majority of opioids administered (Supplementary Fig. S2b).
While precision medicine in oncology, and lung adenocarcinoma in particular, is already a reality, to date there exists no comparable progress in onco-anaesthesia, for either non-small cell lung cancer or other solid cancers, which would enable tailoring of perioperative analgesic techniques on the basis of tumour genomics. We believe that the present work offers an incremental development in precision perioperative anaesthesia to generate hypotheses regarding the impact of common analgesics on oncologic outcomes for individual patients. The specific genomic factors uncovered here, and their predicted interactions with intraoperative opioid dose to modify outcomes, constitute actionable evidence that could be expanded in future studies evaluating these mechanistic pathways.
Authors' contributions
Devised the project idea and proposal outline: JSM, DRJ, GWF
Performed the data acquisition and executed the project proposal: JGC with the supervision and assistance of JSM, DRJ, GWF
Performed and verified the data analysis and designed the survival figures: KST
Drafted the manuscript and interpreted results: JGC
Discussed the results and contributed to the final manuscript: all authors.
Acknowledgements
JSM and HVG acknowledge John Chodera of the Sloan Kettering Institute for his support and insightful discussions. JSM also acknowledges Sahrena London for helpful conversations.
Handling editor: Michael Avidan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.03.030.
Contributor Information
David R. Jones, Email: jonesd2@mskcc.org.
Joshua S. Mincer, Email: mincerj@mskcc.org.
Declarations of interest
PJMcC's spouse has an equity interest in Johnson & Johnson. PSA has received research funding from ATARA Biotherapeutics and Acea Biosciences, has served on the Scientific Advisory Board or as consultant to ATARA Biotherapeutics, Bayer, Carisma Therapeutics, Imugene, and Takeda Therapeutics, and has patents, royalties, and intellectual property on mesothelin-targeted CARs and other T-cell therapies, method for detection of cancer cells using virus, and pending patent applications on T-cell therapies. GR has financial relationships with Scanlan. JMI is a consultant for Genentech and has an equity interest in LumaCyte LLC. MJB serves as a consultant for AstraZeneca. GWF is on the speaker's bureau and serves as a consultant for Edwards Life Sciences. DRJ serves as a consultant for Merck and AstraZeneca. All other authors have no potential conflicts to disclose.
Funding
National Cancer Institute (R01CA217169, R01CA240472 to DRJ, R01CA236615 to PSA, T32CA009501 to JGC, P30 CA008748 to Memorial Sloan Kettering Cancer Center), Hamilton Family Foundation (to DRJ), and US Department of Defense (LC160212 to PSA). Marie-Josée and Henry R. Kravis Center for Molecular Oncology.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rudd R.A., Seth P., David F., Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 2.Batchelor T.J.P., Rasburn N.J., Abdelnour-Berchtold E. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS(R)) Society and the European Society of Thoracic Surgeons (ESTS) Eur J Cardiothorac Surg. 2019;55:91–115. doi: 10.1093/ejcts/ezy301. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson A., Lowe M.C., Parker J., Lewis S.R., Alderson P., Smith A.F. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101:172–188. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson U.O., Oppelstrup H., Thorell A., Nygren J., Ljungqvist O. Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery: a retrospective cohort study. World J Surg. 2016;40:1741–1747. doi: 10.1007/s00268-016-3460-y. [DOI] [PubMed] [Google Scholar]
- 5.Cata J.P., Gottumukkala V., Sessler D.I. How regional analgesia might reduce postoperative cancer recurrence. Eur J Pain Suppl. 2011;5:345–355. [Google Scholar]
- 6.Bar-Yosef S., Melamed R., Page G.G., Shakhar G., Shakhar K., Ben-Eliyahu S. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. J Am Soc Anesthesiol. 2001;94:1066–1073. doi: 10.1097/00000542-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Looney M., Doran P., Buggy D.J. Effect of anesthetic technique on serum vascular endothelial growth factor C and transforming growth factor β in women undergoing anesthesia and surgery for breast cancer. J Am Soc Anesthesiol. 2010;113:1118–1125. doi: 10.1097/ALN.0b013e3181f79a69. [DOI] [PubMed] [Google Scholar]
- 8.Retsky M., Demicheli R., Hrushesky W.J. Does surgery induce angiogenesis in breast cancer? Indirect evidence from relapse pattern and mammography paradox. Int J Surg. 2005;3:179–187. doi: 10.1016/j.ijsu.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Maher D.P., Wong W., White P.F. Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: a retrospective analysis. Br J Anaesth. 2014;113(Suppl 1):i88–94. doi: 10.1093/bja/aeu192. [DOI] [PubMed] [Google Scholar]
- 10.Cata J.P., Zafereo M., Villarreal J. Intraoperative opioids use for laryngeal squamous cell carcinoma surgery and recurrence: a retrospective study. J Clin Anaesth. 2015;27:672–679. doi: 10.1016/j.jclinane.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Cata J.P., Keerty V., Keerty D. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Canc Med. 2014;3:900–908. doi: 10.1002/cam4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silagy A.W., Hannum M.L., Mano R. Impact of intraoperative opioid and adjunct analgesic use on renal cell carcinoma recurrence: role for onco-anaesthesia. Br J Anaesth. 2020;125:e402–e404. doi: 10.1016/j.bja.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montagna G., Gupta H.V., Hannum M. Intraoperative opioids are associated with improved recurrence-free survival in triple-negative breast cancer. Br J Anaesth. 2021;126:367–376. doi: 10.1016/j.bja.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du K.N., Feng L., Newhouse A. Effects of intraoperative opioid use on recurrence-free and overall survival in patients with esophageal adenocarcinoma and squamous cell carcinoma. Anesth Analg. 2018;127:210–216. doi: 10.1213/ANE.0000000000003428. [DOI] [PubMed] [Google Scholar]
- 15.Bortsov A.V., Millikan R.C., Belfer I., Boortz-Marx R.L., Arora H., McLean S.A. μ-Opioid receptor gene A118G polymorphism predicts survival in patients with breast cancer. Anesthesiology. 2012;116:896–902. doi: 10.1097/ALN.0b013e31824b96a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H., Sun M., Zhou D. Increased mu-opioid receptor expression is associated with reduced disease-free and overall survival in laryngeal squamous cell carcinoma. Br J Anaesth. 2020;125:722–729. doi: 10.1016/j.bja.2020.07.051. [DOI] [PubMed] [Google Scholar]
- 17.Wang S., Li Y., Liu X.-D., Zhao C.-X., Yang K.-Q. Polymorphism of A118G in μ-opioid receptor gene is associated with risk of esophageal squamous cell carcinoma in a Chinese population. Int J Clin Oncol. 2013;18:666–669. doi: 10.1007/s10147-012-0441-5. [DOI] [PubMed] [Google Scholar]
- 18.Antonia S.J., Villegas A., Daniel D. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 19.Herbst R.S., Baas P., Kim D.W. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 20.Mok T.S., Wu Y.L., Ahn M.J. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Walraven C., Austin P.C., Jennings A., Quan H., Forster A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 22.Cheng D.T., Mitchell T.N., Zehir A. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martini N., Melamed M.R. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–612. [PubMed] [Google Scholar]
- 24.Chang J.C., Alex D., Bott M. Comprehensive next-generation sequencing unambiguously distinguishes separate primary lung carcinomas from intrapulmonary metastases: comparison with standard histopathologic approach. Clin Canc Res. 2019;25:7113–7125. doi: 10.1158/1078-0432.CCR-19-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J., Sanchez-Vega F., Caso R. Analysis of tumor genomic pathway alterations using broad-panel next-generation sequencing in surgically resected lung adenocarcinoma. Clin Canc Res. 2019;25:7475–7484. doi: 10.1158/1078-0432.CCR-19-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakravarty D., Gao J., Phillips S.M. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00011. PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuster N.A., Hoogendijk E.O., Kok A.A., Twisk J.W., Heymans M.W. Ignoring competing events in the analysis of survival data may lead to biased results: a non-mathematical illustration of competing risk analysis. J Clin Epidemiol. 2020;122:42–48. doi: 10.1016/j.jclinepi.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Tai Y.-H., Wu H.-L., Chang W.-K., Tsou M.-Y., Chen H.-H., Chang K.-Y. Intraoperative fentanyl consumption does not impact cancer recurrence or overall survival after curative colorectal cancer resection. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-11460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaram-Davila J., Davis M., Reddy A. Opioids and cancer mortality. Curr Treat Opt Oncol. 2020;21:1–13. doi: 10.1007/s11864-020-0713-7. [DOI] [PubMed] [Google Scholar]
- 30.Zylla D., Gourley B.L., Vang D. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119:4103–4110. doi: 10.1002/cncr.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizvi H., Sanchez-Vega F., La K. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caso R., Sanchez-Vega F., Tan K.S. The underlying tumor genomics of predominant histologic subtypes in lung adenocarcinoma. J Thorac Oncol. 2020;15:1844–1856. doi: 10.1016/j.jtho.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam K.W., Zhang W., Soh J. CDKN2A/p16 inactivation mechanisms and their relationship to smoke exposure and molecular features in non–small-cell lung cancer. J Thorac Oncol. 2013;8:1378–1388. doi: 10.1097/JTO.0b013e3182a46c0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhanasekaran S.M., Balbin O.A., Chen G. Transcriptome meta-analysis of lung cancer reveals recurrent aberrations in NRG1 and Hippo pathway genes. Nat Commun. 2014;5:5893. doi: 10.1038/ncomms6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Hu T., Li C. Convergence between Wnt-β-catenin and EGFR signaling in cancer. Mol Canc. 2010;9:1–7. doi: 10.1186/1476-4598-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lennon F.E., Mirzapoiazova T., Mambetsariev B., Salgia R., Moss J., Singleton P.A. Overexpression of the μ-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology. 2012;116:857–867. doi: 10.1097/ALN.0b013e31824babe2. [DOI] [PubMed] [Google Scholar]
- 38.Singleton P.A., Moss J., Karp D.D., Atkins J.T., Janku F. The mu opioid receptor: a new target for cancer therapy? Cancer. 2015;121:2681–2688. doi: 10.1002/cncr.29460. [DOI] [PubMed] [Google Scholar]
- 39.Gao M., Rejaei D., Liu H. Ketamine use in current clinical practice. Acta Pharmacologica Sinica. 2016;37:865–872. doi: 10.1038/aps.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng Q., Michael I.P., Zhang P. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573:526–531. doi: 10.1038/s41586-019-1576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.