Abstract
Background
In the general adult population, lymphopaenia is associated with an increased risk for hospitalisation with infection and infection-related death. The quality of evidence and strength of association between perioperative lymphopaenia across different surgical procedures and mortality/morbidity has not been examined by systematic review or meta-analysis.
Methods
We searched MEDLINE, Embase, Web of Science, Google Scholar, and Cochrane databases from their inception to June 29, 2020 for observational studies reporting lymphocyte count and in-hospital mortality rate in adults. We defined preoperative lymphopaenia as a lymphocyte count 1.0–1.5×109 L−1. Meta-analysis was performed using either fixed or random effects models. Quality was assessed using the Newcastle–Ottawa Scale. The I2 index was used to quantify heterogeneity. The primary outcome was in-hospital mortality rate and mortality rate at 30 days.
Results
Eight studies met the inclusion criteria for meta-analysis, comprising 4811 patients (age range, 46–91 yr; female, 20–79%). These studies examined preoperative lymphocyte count exclusively. Studies were of moderate to high quality overall, ranking >7 using the Newcastle–Ottawa Scale. Preoperative lymphopaenia was associated with a threefold increase in mortality rate (risk ratio [RR]=3.22; 95% confidence interval [CI], 2.19–4.72; P<0.01, I2=0%) and more frequent major postoperative complications (RR=1.33; 95% CI, 1.21–1.45; P<0.01, I2=6%), including cardiovascular morbidity (RR=1.77; 95% CI, 1.45–2.15; P<0.01, I2=0%), infections (RR=1.45; 95% CI, 1.19–1.76; P<0.01, I2=0%), and acute renal dysfunction (RR=2.66; 95% CI, 1.49–4.77; P<0.01, I2=1%).
Conclusion
Preoperative lymphopaenia is associated with death and complications more frequently, independent of the type of surgery.
Prospero registry number
CRD42020190702.
Keywords: complications, death, lymphocyte, lymphopaenia, surgery
Editor's key points.
-
•
The authors systematically reviewed the literature regarding the association between preoperative lymphopaenia and adverse perioperative outcome. Eight studies, including almost 5000 subjects, were analysed; the quality of included studies was high, and heterogeneity was low.
-
•
The authors found a strong and credible association between low preoperative lymphocyte count and major adverse perioperative outcomes, including death, infection, renal dysfunction, and cardiovascular morbidity.
Infections after surgery occur frequently,1 cluster with other complications,2,3 and are associated with lower survival rates even if patients survive to hospital discharge.4,5 In the general population, lymphopaenia is associated with an increased risk of hospitalisation as a result of infection and almost doubled risk of death, after adjusting for potential explanatory factors including blood neutrophil count.6
Lymphopaenia that is evident for several years before infection suggests that an elevated risk for infection is not attributable to either undiagnosed infection or comorbidity.6 Lymphopaenia is a common finding in older individuals who are at most risk of complications after surgery.7 Age-related thymic atrophy and a shift towards myelopoiesis results in a reduction of peripheral lymphocyte numbers.8,9 Relative lymphopaenia is further exacerbated by low-grade chronic inflammation secondary to cancer, cardiovascular disease, and type 2 diabetes.8 Acute viral infections, including coronavirus disease 2019 (Covid-19), are particularly associated with profound lymphopaenia.10 A functional T cell arm of the adaptive immune system is necessary for protection from polymicrobial sepsis and diminishes the inflammatory response to injury.11 Reduced T cell functionality caused by bioenergetic impairment is evident in lymphopaenic patients before elective surgery.3 Lower preoperative lymphocyte counts because of ageing and disease is, therefore, very likely to play an integral role in shaping the immune response to surgery and trauma.12, 13, 14 Further dramatic declines in lymphocyte count from preoperative levels occurs within hours of surgical trauma, with persistent lymphopaenia independently associated with higher mortality in critically ill emergency surgical patients.15,16 Low lymphocyte counts promote lymphopaenia-induced proliferation of antigen-experienced T cells, and lymphocytosis derived inflammation contribute to the development of cardiovascular disease.17 Moreover, a lack of reparative T lymphocyte subsets prevents the resolution of cardiac inflammation and tissue repair.18 Despite multiple lines of biological and clinical enquiry suggesting a link between lymphopaenia and adverse outcomes after surgery, this association has yet to be examined by systematic review and meta-analysis. The objective of this study was, therefore, to systematically examine the relationship between lymphopaenia and mortality, complications after elective surgery, or both.
Methods
Protocol and registration
We registered the systematic review prospectively with PROSPERO: CRD42020190702. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for this review. Ethical approval was not required for this study.
Study search strategy
We searched Medline, Embase, Web of science, and Cochrane Database for trials reporting perioperative lymphocyte count from inception of each database until June 2020. We used a combination of the following terms (in UK and US English, where applicable) to search the different databases on June 29, 2020: ‘lymphocytopaenia’, ‘lymphocytopenia’, ‘lymphopaenia’, ‘lymphopaenia’, ‘lymphocyte count’, ‘lymphocyte’, ‘surgery’, ‘perioperative’, ‘postoperative’. The electronic search was conducted using the following search strategy: (1) ‘lymphopaenia’ OR ‘lymphocytopenia’ OR ‘lymphocyte count’; (2) ‘surgery’ OR ‘preoperative’ OR ‘perioperative’ OR ‘postoperative’; (3) 1 AND 2 (Supplementary Table S1). The search was completed by two authors (JS and VW), and the results were compared. No search filters or language and publication status restrictions were applied. We extracted records to Endnote (Thomson, Reuters, Philadelphia, PA, USA) to sort and remove duplicates.
Inclusion criteria
Original research articles were considered in this study provided they met the following inclusion criteria: adult patients (age >18 yr) undergoing elective surgical intervention; lymphopaenia count, lymphocyte count, or both reported before surgical intervention; quantitative outcomes of in-hospital mortality, mortality at 30 days, or both.
Exclusion criteria
We excluded non-English articles, review articles, non-research letters, commentaries, animal studies, case reports, and full-text articles with insufficient information.
Study selection
Study selection and data extraction was conducted by two independent researchers (JS and VW). All studies were screened based on title and abstract, followed by full-text review to identify articles meeting our inclusion criteria. The full text of these articles was subsequently reviewed to select papers reporting our primary outcome. References of selected articles and published systematic reviews were also searched to identify any further relevant articles meeting our inclusion criteria. Additionally, authors of relevant papers were contacted for missing information where possible. When there was uncertainty regarding eligibility, a third reviewer was consulted (TJ).
Data collection process and data items
Data were extracted from selected papers by two independent reviewers (JS and VW) to a pre-formatted Excel worksheet (Microsoft, Redmond, WA, USA) containing the following characteristics: first author, year, study type, surgery type, sample size, age, sex, comorbidities, outcome(s) reported, duration of follow-up period, definition of lymphopaenia, timing of blood draw to enumerate lymphocyte count in relation to timing of surgery and mortality (Table 1). Numbers of events were extracted for dichotomous outcomes and means with standard deviation (sd) were extracted for continuous outcomes.
Table 1.
Main characteristics of the studies included in the review. C/V, cardiovascular complications; LOS, length of stay; N/A, not applicable; NR, not reported; NOS, Newcastle–Ottawa Scale; P, prospective; R, retrospective; WCC, white cell count.
| Author | Year | Surgery | Study | NOS | Lymphopaenia | Timing | Age, SD | Female, n (%) | Mortality (n/total) | Infection | C/V | Renal | Los | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ackland and colleagues2 | 2019 | Noncardiac | P | 8 | <1.00×109 L−1 | Preop/day of surgery | 66 (9) | 813 (49.2%) | 39/1654 | ✓ | ✓ | ✓ | 30 days | |
| Pierre-Louis and colleagues22 | 2019 | Vascular | R | 8 | N/A | Within 7 days of surgery | 64 (12) | 83 (20.2%) | 19/410 | 30 days | ||||
| Edwards and colleagues3 | 2015 | Orthopaedic | P | 7 | <1.3; <20% WCC | Preop/day of surgery | 70 (11)∗ | 480 (64.4%) | 1/745 | ✓ | ✓ | ✓ | ✓ | In-hospital |
| Lomivorotov and colleagues23 | 2011 | Cardiac | R | 8 | <1.00 | Within 3 days of surgery | 56 (10) | 498 (36.4%) | 51/1368 | ✓ | ✓ | ✓ | In-hospital | |
| O'Daly and colleagues24 | 2010 | Orthopaedic | R | 9 | <1.5 | Within 2 days of surgery | 81 (10)∗ | 294 (80%) | 14/200; 59/200 | In-hospital 12 months | ||||
| Masuo and colleagues25 | 1998 | Abdominal | R | 8 | N/A | Day of surgery | 83 (4) | NR | 16 589 | In-hospital | ||||
| Conlan21 | 1989 | Orthopaedic | R | 7 | <1.5 | Day of surgery | 79 (NR) | 100 (79.4%) | 62/126 | In-hospital | ||||
| Seltzer and colleagues26 | 1979 | Cardiac/noncardiac | R | 7 | <1.5 | Day of surgery | NR | NR | 2/263 | In-hospital |
Estimated from range of data provided in paper.
Primary outcome
The primary outcome was mortality rate, which was defined as in-hospital mortality or mortality at 30 days after surgical intervention.
Secondary outcomes
Secondary outcomes were all-cause complications, infection, surgical site infection, pneumonia, thromboembolic events (deep vein thrombosis, pulmonary embolus), acute renal failure, delirium/confusion, and cardiovascular complications. We defined cardiovascular complications as myocardial injury, myocardial ischaemia, myocardial infarction, any arrhythmia, inotropes or vasopressors requirement, and extracorporeal support (ventricular assist devices/extracorporeal membrane oxygenation).
Sensitivity analyses
A priori sensitivity analyses were designed for study-specific thresholds for the definition of lymphopaenia.
Explanatory variable
We used study-specific definitions of lymphopaenia as described by each study.
Risk of bias assessment
The risk of bias and the quality of each included study was evaluated independently using the Newcastle–Ottawa Scale (NOS).19 This scale allows evaluation of non-randomised studies based on three criteria: patient selection, comparability of study groups, and outcome or exposure assessment. Studies with a score <7, a threshold at which studies are considered not of high quality, were excluded from this review.19
Statistical analysis
The meta-analysis was conducted using Review Manager software (RevMan; Computer program; Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark). Dichotomous data were analysed using risk ratio (RR) with 95% confidence intervals (CIs). Estimation of mean (sd) values from median and inter-quartile range was performed for studies in which these data were not presented.20 For continuous variables, we used an inverse variance method to obtain mean difference (MD) and sd. The pre-specified threshold for statistical significance was P<0.05. Between-study heterogeneity was assessed using the I2 statistic test using P<0.1 as the pre-defined threshold for statistical significance. We used random-effects models for pooled analysis regardless of heterogeneity. Sensitivity analysis using the leave-one-out method was performed to identify the potential cause of heterogeneity when indicated. Subgroup analysis was performed for study specific lymphopaenia cut-off points at <1.0×109 and <1.5×109 L−1 and for type of surgical intervention. Potential publication bias was assessed with visual assessment of funnel plots for each meta-analysis outcome.
Results
Study selection
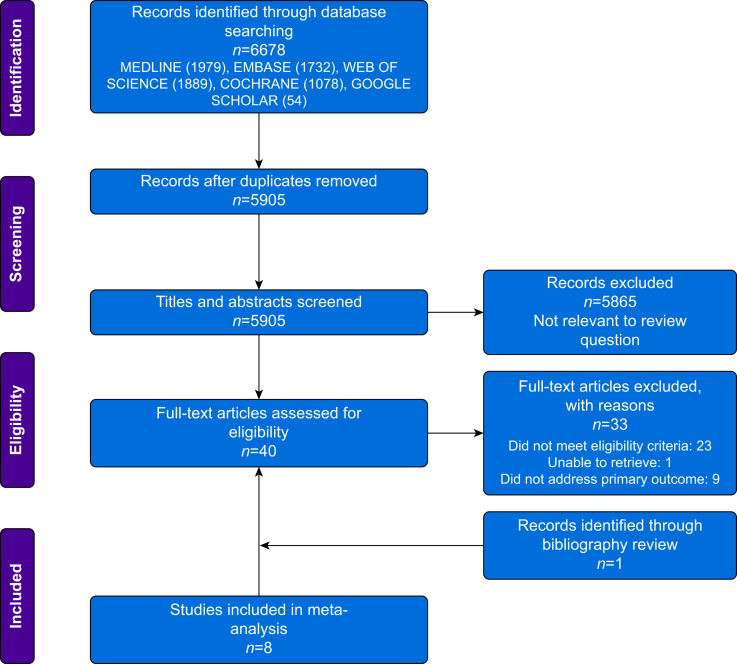
We identified 5905 studies published between 1950 and 2020. After title and abstract screening, we determined that 40 full-text articles may have been eligible, 33 of which were excluded. Hand searching of included articles and published systematic reviews identified one further article meeting our inclusion criteria.21 In total, eight studies published between 1979 and 2019 were included for meta-analysis.2,3,21, 22, 23, 24, 25, 26 The study flow diagram including reasons for exclusion is presented according to PRISMA guidelines (Fig. 1). Several studies reported the neutrophil/lymphocyte ratio, but did not report the absolute lymphocyte count (Supplementary data) or underlying diagnoses that may account for lymphopaenia.
Fig 1.
PRISMA flow diagram showing literature search results. Eight studies were used for the meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Study characteristics
We found that eight eligible studies comprising 4811 patients undergoing noncardiac and cardiac surgery reported only the preoperative lymphocyte count (Table 1).2,3,21, 22, 23, 24, 25, 26 Lymphopaenia was variably defined as a lymphocyte count of 1.00–1.50×109 L−1; 567/4230 subjects had study-specific definitions for preoperative lymphopaenia (Table 1). Two studies reported lymphocyte counts for 581 patients without specifying normal ranges.22,25
Publication bias and study quality
Funnel plot analysis showed symmetrical shapes for all primary and secondary outcomes (Supplementary Figs S1–S4). Studies were of moderate to high quality overall, with ranking >7 using the NOS (Supplementary Table S2).
Primary outcome: lymphopaenia and mortality
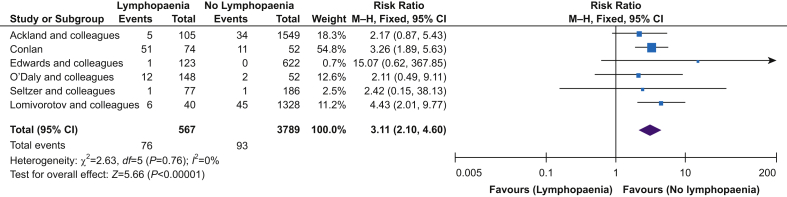
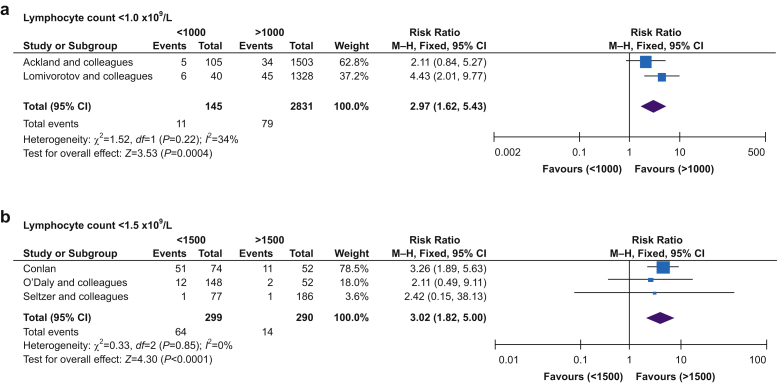
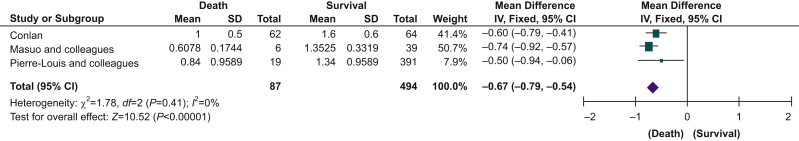
Six studies reported in-hospital mortality, mortality at 30 days, or both. Two studies provided additional unpublished data on request.2,3 Preoperative lymphopaenia was associated with higher mortality (RR=3.22; 95% CI, 2.19–4.72; P<0.001, I2=0%) (Fig. 2). Given the study-specific heterogeneity in defining lymphopaenia, we also performed a subgroup analysis for the degree of lymphopaenia reported, defined as either <1.0×109 or <1.5×109 L−1. For both thresholds, the association of lymphopaenia with higher mortality remained (Fig. 3). Subgroup analysis for surgery type could only be performed for orthopaedic surgery, which showed that lymphopaenia was associated with higher mortality rates (RR=3.21; 95% CI, 1.94–5.33; P<0.001, I2=0%) (Fig. 3). Meta-analysis of three studies showed that patients who died during the follow-up period had a lower preoperative lymphocyte count (MD=–0.67×109 L−1; 95% CI, –0.79 to 0.54; P<0.001, I2=0%) compared with those who survived (Fig. 4).21,22,25 Subgroup analysis for this outcome was not possible because of the small number of studies.
Fig 2.
Lymphopaenia and mortality. Funnel plot analysis showed symmetrical shape. CI, confidence interval; M-H, Mantel–Haenszel.
Fig 3.
Subgroup analysis for mortality based on different cut-offs for lymphopaenia and type of surgery. Funnel plot analysis showed symmetrical shape for all three groups. CI, confidence interval; M-H, Mantel–Haenszel.
Fig 4.
Lymphocyte count and mortality. Funnel plot analysis showed symmetrical shape. CI, confidence interval; sd, standard deviation.
Secondary outcomes
All-cause complications
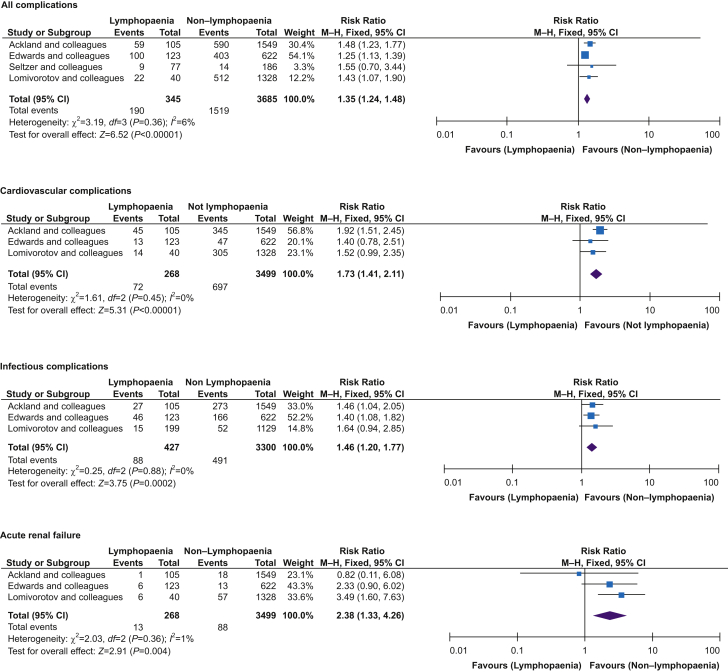
Four studies reported perioperative complications.2,3,23,26 Lymphopaenia was associated with an increased risk of all-cause complications in the perioperative period (RR=1.33; 95% CI, 1.21–1.45; P<0.001; I2=6%) (Fig. 5). For specific secondary outcomes, only cardiovascular, renal, and infectious complications were reported.
Fig 5.
Lymphopaenia and postoperative complications. Funnel plot analysis showed symmetrical shape for all groups. CI, confidence interval; M-H, Mantel–Haenszel.
Cardiovascular complications
Cardiovascular complications were reported in three studies.2,3,23 Lymphopaenia was associated with an increased risk of cardiovascular complications in the perioperative period (RR=1.77; 95% CI, 1.45–2.15; P<0.001, I2=0%) (Fig. 5).
Postoperative infections
Three studies reported infectious complications.2,3,23 Lymphopaenia was associated with an increased risk of infections in the perioperative period (RR=1.45; 95% CI, 1.19–1.76; P=0.001, I2=0%) compared with normal lymphocyte count (Fig. 5).
Acute renal failure
Three studies reported the incidence of acute renal failure requiring renal replacement for which the risk was higher in patients with lymphopaenia (RR=2.66; 95% CI, 1.49–4.77; P=0.001, I2=1%) (Fig. 5).2,3,23
Discussion
Our meta-analysis of eight studies including 4811 patients exclusively detailing lymphocyte count before surgery found that relative lymphopaenia was consistently associated with higher risk of death and more frequent major postoperative complications after surgery. Patients who died during the follow-up period had a lower preoperative lymphocyte count compared with survivors.
Despite the clear biologic rationale for lymphopaenia promoting infectious complications and organ dysfunction fuelled by dysregulation of inflammation, there are surprisingly few studies in the surgical population. Many studies have focused on neutrophil/lymphocyte ratio, but have seldom reported absolute differential leucocyte counts. In the absence of enumerating specific leucocyte subsets, determining whether the roles for the relative presence, or absence, of a particular cell type is impossible. Our data identifying lymphopaenia as a key leucocyte are in accord with findings in a study of the general population in Denmark, which utilised data obtained from 98 344 individuals enrolled in the Copenhagen General Population Study.6 This Danish study, the largest of its type thus far, found a consistent independent association between a low lymphocyte count and increased risk of several infections, adjusted for age, smoking, BMI, alcohol intake, plasma C-reactive protein, blood neutrophil count, recent infection, medication use, and comorbidities (including autoimmune disease, immunodeficiency, and haematologic disease). Although unaccounted for confounding variables cannot be excluded, these epidemiological data suggest that lymphopaenia is not an epiphenomenon merely reflecting inflammatory, metabolic, or neuroendocrine stressors.
Lymphopaenia appears to be dose-dependently associated with adverse outcomes. Our study mirrored the findings of the Copenhagen General Population Study, where the association with excess risk for acquiring infections persisted whether lymphopaenia was defined as lymphocyte count below the 2.5th percentile or, alternatively, two widely implemented cut-offs (1.0×109 and 1.5×109 L−1). Moreover, using repeat measurements of lymphocyte count made over 10 yr in 5181 individuals, the Danish study found that most individuals with lymphopaenia had persistently lower lymphocyte counts. Using a statistical technique that considered both measurement and biological variability overtime (regression dilution bias), longstanding lymphopaenia remained associated with increased risk of infection. Similarly, in a large US cohort study of 31 178 participants enrolled in the National Health and Nutrition Examination Survey, a dose–response relationship was observed between the degree of lymphopaenia and all-cause mortality.17 Lymphocyte counts ≤1.5×109 L−1, present in 20.1% participants, were associated with age- and sex-adjusted excess risk for mortality (hazard ratio=1.3; 95% CI, 1.2–1.4), compared with individuals with an absolute lymphocyte count >1.5×109 L−1. However, the risk of for mortality was even higher (hazard ratio=1.8; 95% CI, 1.6–2.1) in 3.0%, individuals with even more pronounced lymphopaenia (<1.0×109 L−1). We found that relative lymphopaenia was associated with poorer outcomes independent of the type of surgery. Taken together, these studies suggest strongly that lymphopaenia is a pathologic driver for acquiring infections and acute-on-chronic inflammation triggering organ injury, independent of aetiology (comorbidity), age, and chronicity of lower lymphocyte count.
Our study only included papers that documented absolute lymphocyte counts, but we should note that a large number of studies reported total white blood cell count, neutrophil/lymphocyte ratio, or both.27 Many of these papers suggest both measures may serve as a guide to identify the highest risk surgical patients but clearly cannot provide mechanistic clues as to which subset of leucocytes may be most instrumental. Our data support a role for several pathologic mechanisms demonstrating causative role for lymphopaenia in conferring a higher risk of acquiring infections and organ dysfunction. Human and laboratory data show that relative lymphopaenia is linked to decreased T cell activation, proliferation, and lymphopoiesis,28 accompanied by a propensity for increased apoptosis29,30 because of impaired mitochondrial function.3,31,32 Reduced CD4 T-helper cell numbers impair the production of specific antibodies by B lymphocytes and compromise phagocytic capacity.33 Cytotoxic T lymphocytes eliminate malignant cells, the metastases of which requires the formation of neutrophil extracellular traps.34 None of the analysed studies describe the relative contribution of lymphocyte subpopulations such as CD4 and CD8 T cells, B cells, and natural killer cells, to the overall lymphopaenic phenotype and postoperative complications. For example, after surgery, CD8 T cell apoptosis frequency is associated with postoperative infections.35 Persistent lymphopaenia after the onset of sepsis correlates with mortality.36 These longstanding observations in sepsis are now mirrored by patients infected with Covid-19, who are frequently lymphopaenic and have worse outcomes after noncardiac surgery.37,38 Apoptosis-resistant lymphocytes improve survival in experimental sepsis.39 Pre-treatment of septic mice with anti-apoptotic antiretroviral agents improves survival in a lymphocyte-dependent manner, as T cell-deficient (RAG1–/–) mice did not benefit from this treatment.40
Strengths of this analysis include the analysis of a wide range of types of surgery, which suggests these data are generalisable. We published our methodology prospectively via PROSPERO before undertaking this study. As lymphocyte counts appear to be similar across different ethnicities and sexes, the results are likely generalisable.41 A limitation is that various cut-off values for lymphopaenia definition were used in the included studies. This precludes making preliminary recommendations on potentially clinically useful thresholds that may indicate higher risk. Several studies had a small sample size, which is likely to underestimate the association between perioperative lymphopaenia and mortality. Few studies report (secondary) morbidity outcomes. Although funnel plots including <10 studies may not be sufficient to distinguish real asymmetry from chance and to accurately detect publication bias,42,43 studies were of moderate to high quality as adjudged by the NOS.
This meta-analysis shows that preoperative lymphopaenia is associated with excess postoperative mortality and a higher incidence of postoperative complications including cardiac, infection, and renal failure. Given the consistent findings for lower lymphocyte count and outcomes across surgery types and independent of comorbidity, these data suggest a plausible causative role for lymphopaenia in determining surgical outcomes. Aside from a role for lymphopaenia as a biomarker, these data suggest that emerging immunoadjuvant therapy that target defects in adaptive immunity may play a role in patients undergoing major surgery.44
Authors' contributions
VW, JS, and GLA had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: GLA, VW, JS
Acquisition, analysis, or interpretation of data: all authors
Drafting of the manuscript: VW, JS, TFJ, AGA, GLA
Critical revision of the manuscript for important intellectual content: all authors
Obtained funding: GLA, SMH
Administrative, technical, or material support: GLA, SMH
Supervision: GLA, SMH
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
VW: Rod Flower WHRI medical student scholarship; JS: British Journal of Anaesthesia non-clinical PhD research training fellowship; TJ: Barts Charity Clinical Research Training Fellowship; GLA: British Oxygen Company research chair grant in anaesthesia from the Royal College of Anaesthetists, National Institute for Health Research Advanced Fellowship (NIHR300097).
Handling editor: Jonathan Hardman
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.02.023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Barnes J., Hunter J., Harris S. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: infection and sepsis. Br J Anaesth. 2019;122:500–508. doi: 10.1016/j.bja.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackland G.L., Abbott T.E.F., Cain D. Preoperative systemic inflammation and perioperative myocardial injury: prospective observational multicentre cohort study of patients undergoing non-cardiac surgery. Br J Anaesth. 2019;122:180–187. doi: 10.1016/j.bja.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards M.R., Sultan P., del Arroyo A.G. Metabolic dysfunction in lymphocytes promotes postoperative morbidity. Clin Sci (Lond) 2015;129:423–437. doi: 10.1042/CS20150024. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien W.J., Gupta K., Itani K.M.F. Association of postoperative Infection with risk of long-term infection and mortality. JAMA Surg. 2019;155:1–8. doi: 10.1001/jamasurg.2019.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moonesinghe S.R., Harris S., Mythen M.G. Survival after postoperative morbidity: a longitudinal observational cohort study. Br J Anaesth. 2014;113:977–984. doi: 10.1093/bja/aeu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warny M., Helby J., Nordestgaard B.G., Birgens H., Bojesen S.E. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brass D., Mckay P., Scott F. Rational testing: investigating an incidental finding of lymphopenia. BMJ. 2014;348:g1721. doi: 10.1136/bmj.g1721. [DOI] [PubMed] [Google Scholar]
- 8.Akbar A.N., Henson S.M., Lanna A. Senescence of T Lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37:866–876. doi: 10.1016/j.it.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Macaulay R., Akbar A.N., Henson S.M. The role of the T cell in age-related inflammation. Age. 2013;35:563–572. doi: 10.1007/s11357-012-9381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou H., Zhang B., Huang H. Using IL-2R/lymphocytes for predicting the clinical progression of patients with COVID-19. Clin Exp Immunol. 2020;201:76–84. doi: 10.1111/cei.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelley O., Murphy T., Paterson H., Mannick J.A., Lederer J.A. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20:123–129. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 12.Christou N.V., Rode H., Larsen D., Loose L., Broadhead M., Meakins J.L. The walk-in anergic patient. How best to assess the risk of sepsis following elective surgery. Ann Surg. 1984;199:438–444. doi: 10.1097/00000658-198404000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nohr C.W., Latter D.A., Meakins J.L., Christou N.V. In vivo and in vitro humoral immunity in surgical patients. Ann Surg. 1984;200:373–380. doi: 10.1097/00000658-198409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lord J.M., Midwinter M.J., Chen Y.F. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384:1455–1465. doi: 10.1016/S0140-6736(14)60687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiarelli M., Achilli P., Tagliabue F. Perioperative lymphocytopenia predicts mortality and severe complications after intestinal surgery. Ann Transl. 2019;7:311. doi: 10.21037/atm.2019.06.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vulliamy P.E., Perkins Z.B., Brohi K. Persistent lymphopenia is an independent predictor of mortality in critically ill emergency general surgical patients. Eur J Trauma Emerg Surg. 2016;42:755–760. doi: 10.1007/s00068-015-0585-x. [DOI] [PubMed] [Google Scholar]
- 17.Zidar D.A., Al-Kindi S.G., Liu Y. Association of lymphopenia with risk of mortality among adults in the US general population. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alessio F.R., Kurzhagen J.T., Rabb H. Reparative T lymphocytes in organ injury. J Clin Invest. 2019;129:2608–2618. doi: 10.1172/JCI124614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlan D.P. Value of lymphocyte counts as a prognostic index of survival following femoral neck fractures. Injury. 1989;20:352–354. doi: 10.1016/0020-1383(89)90012-0. [DOI] [PubMed] [Google Scholar]
- 22.Pierre-Louis W.S., Bath J., Mikkilineni S. Neutrophil to lymphocyte ratio as a predictor of outcomes after amputation. Ann Vasc Surg. 2019;54:84–91. doi: 10.1016/j.avsg.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Lomivorotov V.V., Efremov S.M., Boboshko V.A. Preoperative total lymphocyte count in peripheral blood as a predictor of poor outcome in adult cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25:975–980. doi: 10.1053/j.jvca.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 24.O’Daly B.J., Walsh J.C., Quinlan J.F. Serum albumin and total lymphocyte count as predictors of outcome in hip fractures. Clin Nutr. 2010;29:89–93. doi: 10.1016/j.clnu.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Masuo K., Kumagai K., Tanaka T. ‘Physiological’ age as an outcome predictor for abdominal surgery in elderly patients. Surg Today. 1998;28:997–1000. doi: 10.1007/BF02483951. [DOI] [PubMed] [Google Scholar]
- 26.Seltzer M.H., Bastidas J.A., Cooper D.M., Engler P., Slocum B., Fletcher H.S. Instant nutritional assessment. JPEN J Parenter Enteral Nutr. 1979;3:157–159. doi: 10.1177/014860717900300309. [DOI] [PubMed] [Google Scholar]
- 27.Dolan R.D., Lim J., McSorley S.T., Horgan P.G., McMillan D.C. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep. 2017;7:16717. doi: 10.1038/s41598-017-16955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norddahl G.L., Pronk C.J., Wahlestedt M. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell. 2011;8:499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Tumpey T.M., Lu X., Morken T., Zaki S.R., Katz J.M. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol. 2000;74:6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols J.E., Niles J.A., Roberts N.J. Human lymphocyte apoptosis after exposure to influenza A virus. J Virol. 2001;75:5921–5929. doi: 10.1128/JVI.73.13.5921-5929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sena L.A., Li S., Jairaman A. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandarpurkar M., Wilson-Fritch L., Corvera S. Ian4 is required for mitochondrial integrity and T cell survival. Proc Natl Acad Sci U S A. 2003;100:10382–10387. doi: 10.1073/pnas.1832170100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furst D.E. Serum immunoglobulins and risk of infection: how low can you go? Semin Arthritis Rheum. 2009;39:18–29. doi: 10.1016/j.semarthrit.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Cools-Lartigue J., Spicer J., McDonald B. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delogu G., Moretti S., Antonucci A. Apoptosis and surgical trauma. Arch Surg. 2000;135:1141–1147. doi: 10.1001/archsurg.135.10.1141. [DOI] [PubMed] [Google Scholar]
- 36.Drewry A.M., Samra N., Skrupky L.P., Fuller B.M., Compton S.M., Hotchkiss R.S. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42:383–391. doi: 10.1097/SHK.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng H., Hébert H.L., Chatziperi A. Perioperative management of patients with suspected or confirmed COVID-19: review and recommendations for perioperative management from a retrospective cohort study. Br J Anaesth. 2020;125:895–911. doi: 10.1016/j.bja.2020.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang K.C., Unsinger J., Davis C.G. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. Faseb J. 2007;21:708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 40.Weaver J.G.R., Rouse M.S., Steckelberg J.M., Badley A.D. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. Faseb J. 2004;18:1185–1191. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- 41.Bain B.J. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49:664–666. doi: 10.1136/jcp.49.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Debray T.P.A., Moons K.G.M., Riley R.D. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: a comparison of new and existing tests. Res Synth Methods. 2018;9:41–50. doi: 10.1002/jrsm.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau J., Ioannidis J.P.A., Terrin N., Schmid C.H., Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francois B., Jeannet R., Daix T. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.