Abstract
The regulation of glucose-stimulated insulin secretion and glucose excursion has a sensory component that operates in a sex-dependent manner.
Objective
Here, we aim to dissect the basis of the sexually dimorphic interaction between sensory neurons and pancreatic β cells and its overall impact on insulin release and glucose homeostasis.
Methods
We used viral retrograde tracing techniques, surgical and chemodenervation models, and primary cell-based co-culture systems to uncover the biology underlying sex differences in sensory modulation of pancreatic β-cell activity.
Results
Retrograde transsynaptic labeling revealed a sex difference in the density of sensory innervation in the pancreas. The number of sensory neurons emanating from the dorsal root and nodose ganglia that project in the pancreas is higher in male than in female mice. Immunostaining and confocal laser scanning microscopy confirmed the higher abundance of peri-islet sensory axonal tracts in the male pancreas. Capsaicin-induced sensory chemodenervation concomitantly enhanced glucose-stimulated insulin secretion and glucose clearance in male mice. These metabolic benefits were blunted when mice were orchidectomized prior to the ablation of sensory nerves. Interestingly, orchidectomy also lowered the density of peri-islet sensory neurons. In female mice, capsaicin treatment did not affect glucose-induced insulin secretion nor glucose excursion and ovariectomy did not modify these outcomes. Interestingly, same- and opposite-sex sensory-islet co-culture paradigms unmasked the existence of potential gonadal hormone-independent mechanisms mediating the male-female difference in sensory modulation of islet β-cell activity.
Conclusion
Taken together, these data suggest that the sex-biased nature of the sensory control of islet β-cell activity is a result of a combination of neurodevelopmental inputs, sex hormone-dependent mechanisms and the potential action of somatic molecules encoded by the sex chromosome complement.
Keywords: Sex difference, Islet β cells, Sensory neurons, Glucose homeostasis
Highlights
-
•
Pancreas-projecting sensory neurons are more abundant in males than in females.
-
•
Male sex hormones regulate the density of peri-islet sensory innervation in adulthood.
-
•
Male sex hormones regulate sensory modulation of β-cell activity and glucose clearance.
-
•
Sex chromosome mechanisms regulate sensory modulation of islet β-cell activity.
1. Introduction
Sex difference in physiological and behavioral responses is a byproduct of combinatory effects of cell-autonomous factors encoded by the sex chromosome complement, the organizational action of testosterone in males, and the activational effect of male and female gonadal hormones after puberty [1]. However, it has been experimentally difficult to uncouple the effects of gonadal hormones from the sex chromosome genes. Sex steroid hormones are largely believed to be the primary signals driving the sexual dimorphisms of non-gonadal tissues [2]. In mammals, males undergo a testosterone surge during fetal and neonatal development and the resulting high levels of testosterone, secreted by the testes, masculinize several regions of the brain and ultimately lead to sexually dimorphic physiology and behavior in adulthood [1,3]. When given to females, during a specific developmental time window, testosterone elicits irreversible lifetime lasting masculinization of the female brain, thus highlighting the powerful effect of sex steroids irrespective of the sex genotype [4,5]. The hypothesis that the action of gonadal hormones outweighs the effects of the sex chromosome complement has been recently challenged with the advent of mouse models allowing the separation of the action of sex hormones from the effect of sex chromosomes [6]. New data have emerged to depict how gonadal hormones interact with sex chromosomes to control biological pathways in a sex-specific manner in health and disease [7]. In the context of pancreatic islet β cells, sex differences are apparent in physiological and diabetic conditions [[8], [9], [10]]. Glucose-stimulated insulin secretion (GSIS) is higher in females than in males; in rodents [9] and humans [11]. The superior GSIS in women was highlighted in clinical studies showing that insulin response to a glucose load, in otherwise healthy individuals, was higher in women than in men despite similar insulin sensitivity in both sexes [12,13]. Endogenous estrogens enhance insulin synthesis and secretion and protect pancreatic β cells from metabolic injuries [14]. Testosterone also increases GSIS in cultured human and mouse pancreatic islets through potentiation of islet-derived GLP-1 action. Mice lacking androgen receptors, specifically in pancreatic β cells, display impaired GSIS and glucose excursion [15]. Consistent with the insulinotropic action of testosterone, developmental and postnatal androgen excess in females results in insulin hypersecretion and hyperinsulinemia and may lead to β-cell dysfunction as observed in women with polycystic ovary syndrome [16,17]. Beyond their impact on islet β-cell function, several studies highlighted the critical roles of sex hormones in β-cell mass [14,[18], [19], [20], [21]]. Interestingly, pancreatic β-cell failure is sexually dimorphic as well; the prevalence of type 1 and type 2 diabetes is higher in males than in females [22,23]. However, the molecular basis of this sex-specific pattern in β-cell function and dysfunction is unknown [24]. Sex hormones are powerful modifiers but might not be the sole players in the development of sexually dimorphic patterns in metabolic health and disease. With the advent of the Four Core Genotypes (FCG) mouse model, it is now possible to separate the effects of sex hormones from those of sex chromosomes [6]. Emerging data suggest that the sex chromosome complement—independently of gonadal hormones—plays a role in adiposity, food intake, insulin resistance and glucose homeostasis [25], although no such effects have been described in β-cell biology. Deconstructing the cellular and molecular underpinnings of the sexual dimorphism in β-cell activity would, therefore, unmask risk and protective factors for diabetes. Importantly, understanding the “sexome” of β-cell biology will unleash novel translational potential that can be leveraged to develop sex- and gender-based therapeutic interventions.
We recently reported that the pancreas-projecting sensory neurons directly regulate islet β-cell function in a sex-dependent manner [10]. Here, we probed the sex-biased nature of this islet-neuron crosstalk to determine the underlying mechanisms of the well-known sexual dimorphism in β-cell function and glucose homeostasis. We employed retrograde tracing approaches and showed that the number of spinal and vagal sensory afferents projecting in the pancreas is lower in female mice as compared to age-matched males. Moreover, the combined use of chemodenervation and gonadectomy models revealed the critical roles of male sex hormones in the abundance of pancreatic peri-islet afferent neurons and the sensory regulation of β-cell activity. Interestingly, we developed sensory neuron-islet co-culture systems and demonstrated that the sex-biased interaction between sensory neurons and islet β cells can be recapitulated in vitro independently of circulating gonadal hormones. Finally, same- and opposite-sex neuron-islet co-cultures unveiled potential sex hormone-independent factors—in pancreatic islets and sensory neurons—which mediate the male-female difference in sensory modulation of insulin secretion. Together, these studies suggest that the sex-specific sensory modulation of β-cell activity likely has neurodevelopmental origins and is influenced by a combination of gonadal-hormone and potential sex-chromosome mechanisms in adulthood.
2. Methods
2.1. Animals
All mice studied were on the C57BL/6J background (stock #000664, The Jackson Laboratory). Mice were housed in pathogen-free facilities and maintained on a 12-hour light/dark cycle in the Animal Care Facility at the Child Health Institute of New Jersey. All studies and protocols were approved by the Rutgers University Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines. Blood glucose was monitored using an automated glucose monitor (Bayer).
2.2. Retrograde viral tracing
All tracing studies were performed in five-week-old male and female wild-type C57BL/6J mice. Pseudorabies virus (PRV) was prepared as previously described [26]. Ten microliters of PRV-Bartha (109 plaque-forming units [pfu]/mL) encoding green fluorescent protein (PRV-152 containing CMV-GFP) [27] and red fluorescent protein (PRV-614 containing CMV-RFP) [28] were injected, in laparotomy settings, in the pancreas and liver, respectively. Two-microliter aliquots of PRV-152 were injected in different parts of the pancreas, including the splenic, gastric, and duodenal regions. For liver injections, 2-μL aliquots of PRV-614 were injected in different regions/lobes. Mice were sacrificed five days after infection. Dorsal root and nodose ganglia were collected, fixed in Z-Fix (Cat# NC9937162, Fisher Scientific) for 2 h, incubated in 30% sucrose for 16 h, and then embedded in optimal cutting temperature (OCT) compound. Five-micrometer sections were cut and mounted for imaging of endogenous GFP and RFP fluorescence.
2.3. Pancreas immunostaining and morphometric analysis
Mice were sacrificed with CO2 and intracardially perfused with 25 mL of 0.1 M phosphate-buffered saline solution (PBS), followed by 25 mL Zamboni's fixative (4% formaldehyde/12.5% picric acid solution in 0.1 M PBS) at 4 °C over a 2-min period. The pancreas was dissected and fixed at 4 °C for 24 h in the perfusion fixative and then cryoprotected for 24 h in 30% sucrose in 0.1 M PBS. The pancreas was embedded in TissueTek® (Sakura, Torrance, CA, USA) and cut on a cryostat at a thickness of 30 μm. For staining, tissue sections were incubated for 30 min at room temperature (RT) in a blocking solution of 10% goat serum in PBS with 0.3% Triton X-100 and then incubated overnight at RT in the blocking solution containing the primary antibodies rabbit anti-CGRP (Sigma, C8198, 1:3000) and mouse anti-insulin (Sigma, I2018, 1:2000). Tissue sections were thereafter incubated with secondary goat anti-rabbit IgG-Alexa 488 (Jackson Immunoresearch, 111-545-003, 1:200) and goat anti-mouse cy3 (APExBIO, 1:200) for 2 h at RT before sections were mounted for confocal imaging. Quantification of CGRP axonal tracts surrounding pancreatic islets was performed based on image pixels using ImageJ software. The CGRP staining around islets was quantified within ~50-μm radial distance and was normalized to the total CGRP signal within the imaged field.
2.4. Gonadectomy
For orchidectomy experiments, 10-week-old male mice were deeply anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and received an analgesic (Buprenorphine 0.05 mg/kg BW) by subcutaneous injection. Using a sterile scalpel, a single incision (0.5 cm) on the ventral side of the scrotum was made. The testicular fat pad was gently pulled through the incision using blunt forceps and the cremaster muscle was cut. After exposing the testicular content, the cauda epididymis, the caput epididymis, and the vas deferens along with testicular blood vessels were exposed while holding the testicular sac with sterile tooth forceps. To prevent bleeding after testis removal, a single ligature around the blood vessels was performed. The cauda epididymis and the caput epididymis were severed and the testis was gently removed by severing blood vessels. The remaining content was placed back in the testicular sac. For sham operations, the testis was placed back in the scrotum. The skin was sutured with non-absorbable sutures using interrupted suture technique and double knots. Similar steps above were repeated to ablate the other testis. For ovariectomy experiments, 10-week-old female mice were anesthetized as indicated above. After the onset of anesthesia, mice were shaved bilaterally over the lumbar spine. Using small scissors, a single midline incision (0.5 cm), penetrating the skin, was made in the lower back, directly under the rib cage. Subcutaneous connective tissue was gently freed from the underlying muscle layer on each side using blunt forceps. Ovaries were located and a small incision (less than 1 cm) was made on each side to gain access to the peritoneal cavity. The ovarian fat pad was retracted with blunt forceps to expose the oviduct. For sham operations, the ovaries were placed back in the peritoneal cavity. For ovariectomy, a single ligature was performed 0.5 cm far from the ovary to prevent bleeding following removal of the ovary. The gonad was removed by gentle severing of the oviduct. The uterus and the remaining part of the oviduct were placed back in the abdominal cavity and the muscle layer was sutured using absorbable sutures. The skin incision was closed with non-absorbable sutures. The steps described above were repeated to sever the other ovary.
2.5. Islet-DRG co-culture
DRG neurons were prepared as previously described [29]. Briefly, thoracic DRG were harvested from 3-week-old mice and dissociated in collagenase (5 mg/mL) and dispase (1 mg/mL) at 37 °C for 70 min, and then cultured in 12-well plates coated with Poly-d-lysine (100 μg/mL) and laminin (10 μg/mL). DRG neurons were plated at a density of 5 × 104 cells/well in Neurobasal media containing B27 (200 mM), NGF (50 ng/mL), GDNF (2 ng/mL) and AraC (10 μM) and were allowed to grow alone before acute co-culture with pancreatic islets. Islets were isolated as previously described [[30], [31], [32]]. After 3 days of axonal outgrowth, DRG neurons were washed in HEPES and co-cultured using transwell inserts (Corning, cat#3477) with 50 size-matched 8-week-old primary islets in HEPES for GSIS studies. Islets cultured alone or with DRG neurons were incubated in HEPES solution containing 5.5 mM glucose for 60 min and then 16.5 mM glucose for 60 min, successively. Supernatants were collected for insulin ELISA assays (Crystal Chem, cat#90080) to determine basal and glucose-stimulated insulin release. Insulin secreted was normalized to total insulin content.
2.6. Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Data were analyzed using Student's ‘t’ test or one-way ANOVA with Tukey's multiple-comparisons tests. A “p” value of less than 0.05 was considered statistically significant.
3. Results
3.1. Sex difference in the density of pancreas-projecting sensory neurons
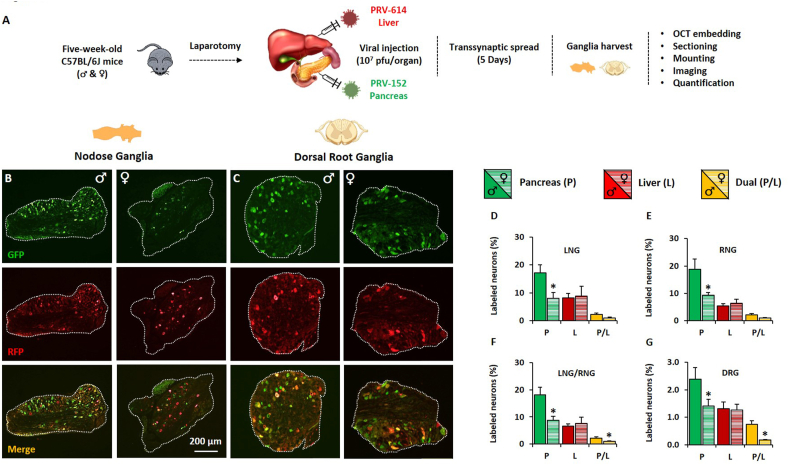
Bartha is an attenuated vaccine strain of the PRV that can cross synapses and spread retrogradely from postsynaptic to presynaptic neurons. Owing to its transsynaptic transport properties, Bartha-PRV allows for tracing and quantification of central and peripheral neuronal projections to infected target organs [33]. Based on our recent report suggesting a role for sensory neurons in the sex-specific regulation of pancreatic β-cell function [10], we used fluorescent PRV Bartha recombinants to determine whether the pancreas sensory innervation density is different in male and female mice. Briefly, 10 μL of PRV-152 (109 pfu/mL) encoding green fluorescent protein (GFP) was injected in the pancreas and 10 μL of PRV-614 (109 pfu/mL) encoding red fluorescent protein (RFP) in the liver of 5-week-old C57BL/6J male and female mice. Co-tracing of axonal tracts from the liver was performed to contrast the abundance of sensory neurons in the pancreas with that of a distinct but anatomically close organ. Five days post-injection, dorsal root (DRG) and nodose ganglia (NG) were harvested and mounted for imaging and quantification (Figure 1A). We collected the DRG located at the lower cervical C7 through the upper lumbar L2 (17 pairs) and harvested both the right and left NG. Manual counting of labeled and non-labeled ganglion cells was carried out to determine the percentage of DRG and NG neurons projecting directly in the pancreas (GFP-labeled), in the liver (RFP-labeled), or dually in both tissues (GFP/RFP-labeled). The percentage of labeled (GFP, RFP, GFP/RFP) cells is calculated as the ratio of labeled cells over the total number of cells identified based on background fluorescence; an average of 1000 cells was counted per ganglion in both males and females. Our data analysis revealed that the percentage of vagal sensory neurons innervating the pancreas (P) is halved in females when compared to age-matched males (Figure 1B). This was observed in both left (LNG) and right (RNG) nodose ganglia (Figure 1D,E). The proportional number of vagal afferent projections to the pancreas, within the same sex, is similar between LNG (Figure 1D) and RNG (Figure 1E). The percentage of vagal afferents projecting in the liver (L) is similar in males and females (Figure 1D–F). Interestingly, we found that some neurons bifurcate to project in both the pancreas and liver (Figure 1B). The proportion of these dually projecting (P/L) vagal neurons is decreased in female LNG (Figure 1D) and RNG (Figure 1E). This decrease was statistically significant when LNG and RNG were combined (LNG/RNG) for analysis (Figure 1F). Similar to the sex difference encountered in the NG innervation pattern, the percentage of pancreas-projecting DRG neurons is 40% lower in females when compared to age-matched males. No sex difference was observed in the percentage of DRG neurons projecting in the liver. Remarkably, the proportion of GFP/RFP double-positive neurons was four-fold higher in males as compared to females (Figure 1C,G). Together, retrograde transsynaptic tracing studies revealed a major sex difference in the density of pancreas-projecting sympathetic and parasympathetic sensory neurons, which suggests a neurodevelopmental feature contributing to the sexual dimorphism in the neuromodulation of islet β-cell activity.
Figure 1.
Sex difference in the density of pancreas-projecting sensory neurons Retrogradely labeled sensory neurons projecting in the pancreas and liver. Five-week-old male and female C57BL/6J mice were injected with 10 μL of PRV-152 (109 pfu/mL) in the pancreas and with 10 μL PRV-614 (109 pfu/mL) in the liver. Five days after injection, nodose and dorsal root ganglia were harvested for imaging and morphometric analysis. A. Schematic of the experimental design. B. Representative images of nodose ganglia derived from male (left panel) and female (right panel) mice displaying GFP, RFP and merged signals. C. Representative images of dorsal root ganglia derived from male (left panel) and female (right panel) mice displaying GFP, RFP and merged signals. D. Quantification of the percentage of GFP+, RFP+ and GFP+RFP+ double-positive cells in left nodose ganglia (LNG). E. Quantification of the percentage of GFP+, RFP+ and GFP+RFP+ double-positive cells in right nodose ganglia (RNG). F. Quantification of the percentage of GFP+, RFP+ and GFP+RFP+ double-positive cells in LNG and RNG. G. Quantification of the percentage of GFP+, RFP+ and GFP+RFP+ double-positive cells in dorsal root ganglia. Data represent mean ± SEM. Student's t-test: ∗p < 0.05 (n = 7–8).
3.2. Male sex hormones regulate the density of peri-islet sensory innervation in adulthood
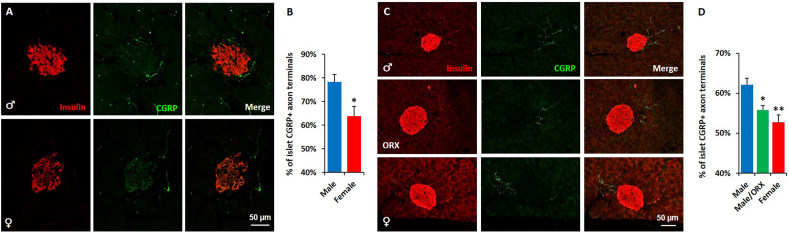
To validate the enhanced density of islet-projecting afferent neurons in the male pancreas, we employed immunostaining and confocal microscopy techniques. Pancreatic tissues harvested from 6-week-old male and female C57BL/6J mice were co-stained with specific antibodies against the sensory and endocrine markers calcitonin-gene related protein (CGRP) and insulin, respectively. The fluorescence intensity of CGRP+ terminal endings within ~50-μm radial distance from islets was determined using ImageJ. The percentage of peri-islet CGRP+ sensory axon terminals was normalized to the total CGRP signal captured within the imaged field. This quantification method allows for selective quantification of peri-islet innervation by discarding signals from varicosities in acinar tissue. Consistent with the conclusions drawn from retrograde viral tracing studies, our morphometric studies revealed that the percentage of peri-islet CGRP+ sensory terminals is increased in male pancreases when compared to those derived from females (Figure 2A,B). To determine whether this enhanced peri-islet sensory innervation in males is secondary to the action of gonadal hormones, we compared the abundance of peri-islet CGRP+ sensory neurons among control males, orchidectomized males and females. As observed in six-week-old mice, peri-islet sensory innervation was more abundant in six-month-old males than in age-matched females; although the overall density of afferents innervating islets is slightly lower in the aging mice (Figure 2C,D). Interestingly, male mice that underwent gonadectomy had lower peri-islet CGRP+ sensory axonal endings when compared to control sham-operated mice. The percentage of peri-islet sensory terminals in gonadectomized male mice was comparable to that observed in age-matched female mice (Figure 2C,D). Together, these studies demonstrate a sex difference in the density of peri-islet sensory innervation. This feature was observed at a young age and was maintained via the action of male gonadal hormones throughout adulthood.
Figure 2.
Male sex hormones regulate the density of peri-islet sensory innervation in adulthood. The pancreas from six-week-old male and female C57BL/6J mice were co-stained with insulin (red) and CGRP (green) antibodies. A. Representative confocal images of pancreatic islets from male (upper) and female (lower) mice. B. Quantification of the peri-islet CGRP+ axon terminals in A. The pancreas from six-month-old control male, orchidectomized male and control female C57BL/6J mice were co-stained as describe above. Data represent mean ± SEM. Student's t-test: ∗p ≤ 0.05 (n = 5). C. Representative confocal images of pancreatic islets from male (to panel), orchidectomized male (middle panel) and female (bottom panel) mice. D. Quantification of the peri-islet CGRP+ axon terminals in C. Data represent mean ± SEM. One-way ANOVA with Tukey's multiple-comparisons test: ∗p < 0.01 and ∗∗p < 0.01 (n = 6) vs male group.
3.3. Male sex hormones influence sensory neuromodulation of β-cell activity and glucose clearance
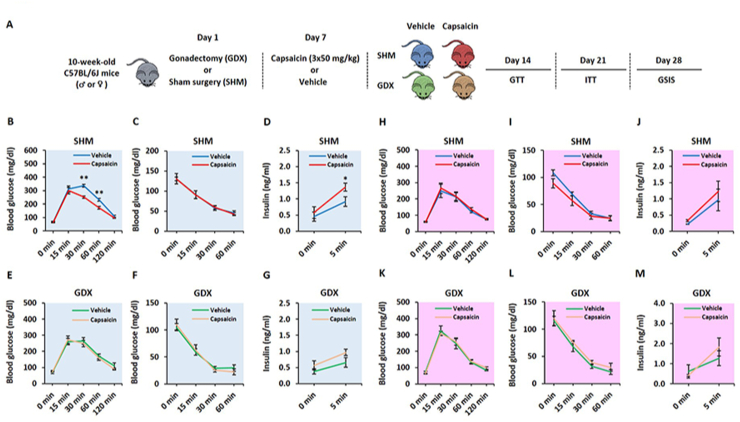
To investigate the role of sex hormones in sensory modulation of post-absorptive glucose levels and GSIS, we used the capsaicin-induced chemodenervation paradigm and gonadectomized (GNX) mouse models. Briefly, 10-week-old male and female mice were subjected to bilateral orchidectomy and ovariectomy, respectively. Sham surgeries were performed for control mice (SHM). Seven days after surgery, vehicle or capsaicin (50 mg/kg) was subcutaneously injected in SHM and GNX mice on three consecutive days into the scruff of the neck. Glucose (GTT) and insulin (ITT) tolerance tests, and GSIS assays were conducted over three weeks after chemodenervation (Figure 3A). Consistent with our recent studies [10], ablation of sensory neurons in male mice improved insulin secretion and glucose clearance without altering insulin sensitivity (Figure 3B–D). No significant differences in glucose-induced insulin release, glucose excursion and insulin sensitivity were observed in sensory denervated age-matched female mice (Figure 3H–J). Interestingly, male mice that underwent orchidectomy before capsaicin treatment did not display improvement in GSIS and glucose tolerance after sensory denervation (Figure 3E,G). Similar to SHM mice, insulin sensitivity was unaffected in capsaicin-versus vehicle-treated GNX male mice (Figure 3F). Finally, ovariectomized female capsaicin- and vehicle-treated mice displayed similar GSIS, glucose clearance, and insulin sensitivity (Figures 3K-M). Collectively, these data suggest that gonadal hormones play an integral role in the sensory neuromodulation of β-cell activity in male but not female mice.
Figure 3.
Male sex hormones influence sensory neuromodulation of β-cell activity and glucose clearance. Ten-week-old male and female C57BL/6J mice were subjected to gonadectomy (or sham surgery) and then subcutaneously injected with vehicle or capsaicin (50 mg/kg) on three consecutive days into the scruff of the neck. Seven days after sensory chemodenervation, metabolic phenotyping was performed. A. Schematic of the experimental design. B. GTT, C. ITT and D. GSIS in sham male mice. E. GTT, F. ITT and G. GSIS in orchidectomized male mice. H. GTT, I. ITT and J. GSIS in sham female mice. K. GTT, L. ITT and M. GSIS in ovariectomized female mice. Data represent mean ± SEM. ∗p < 0.05, ∗∗p < 0.01 (n = 6–7 per group).
3.4. Sex chromosome complement contributes to sex differences in sensory-islet crosstalk
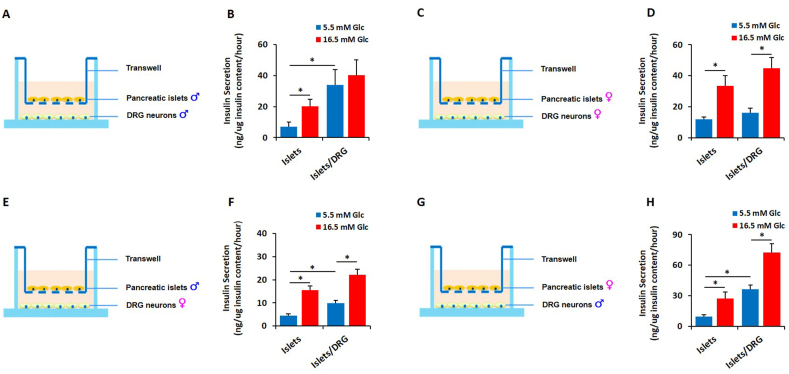
We reason that if the male-female difference observed in the sensory control of insulin release [10] is due—partially—to intrinsic properties encoded by the sex chromosome complement, co-culture systems using components derived from same- or opposite-sex mice should reveal sexually dimorphic functions. To test this hypothesis, we developed an acute primary co-culture model comprising pancreatic islets and DRG sensory neurons. Using transwell-insert systems, we conducted GSIS studies on primary male and female mouse islets cultured alone or in the presence of sensory neurons derived from same- or opposite-sex mice. Because islets and sensory neurons required distinct primary culture conditions, they were cultured separately after isolation and then co-cultured in HEPES media for the insulin secretion assays. All GSIS studies were performed in serum-free HEPES media. Islet-neuron co-cultures were incubated in 5.5 mM glucose-containing HEPES media for 60 min (basal secretion) and then in 16.5 mM glucose for an additional 60 min (stimulated secretion). Basal and stimulated insulin release was normalized to total insulin content. Consistent with the loss-of-function in vivo data [10], male islets co-cultured with male DRG neurons exhibited blunted GSIS when compared to male islets cultured alone. Interestingly, basal insulin secretion is higher in male islets co-cultured with same-sex DRG neurons (Figure 4A,B). Conversely, female islets cultured with female DRG neurons exhibited normal responses; both basal and stimulated insulin secretions were identical to the ones observed when islets were cultured alone (Figure 4C,D). As expected [9,11,12,24,34], female islets exhibit a qualitatively superior GSIS when compared to age-matched male islets (Figure 4B,D). To determine whether this sex difference in sensory-islet intercommunication lies within islets or sensory neurons, we conducted opposite-sex co-culture experiments where islets from one sex were co-cultured with DRG neurons from the other sex. In these experimental settings, male islets displayed a moderate increase in basal insulin secretion when co-cultured with female DRG neurons (Figure 4E,F); this increase was less pronounced than the one observed in male–male co-culture (Figure 4A,B). However, when the same co-culture was challenged with high glucose concentrations, the GSIS was not affected (Figure 4E,F). As opposed to male DRG neurons (Figure 4A,B), sensory neurons harvested from female mice did not have a suppressive effect on GSIS in male pancreatic islets (Figure 4E,F). Finally, DRG neurons derived from male mice strongly enhanced basal insulin secretion in female islets (Figure 4G,H). In contrast to male pancreatic islets (Figure 4A,B), GSIS was not affected when female islets were co-incubated with male sensory neurons (Figure 4G,H). Together, these co-culture paradigms suggest the potential existence of somatic intrinsic (in β cells) and extrinsic (in DRG neurons) factors that regulate GSIS differently in males and females.
Figure 4.
Cell-autonomous factor(s) regulate the sexually dimorphic sensory-islet crosstalk. DRG neurons were harvested from three-week-old C57BL/6J mice, seeded at the density of 5 × 104 cells/well and co-cultured with fifty size-matched islets derived from eight-week-old C57BL/6J mice. GSIS was conducted as described in the “Methods” section. A-B. Schematic of the experimental design and quantification of GSIS for male islets co-cultured with male DRG neurons. C-D. Schematic of the experimental design and quantification of GSIS for female islets co-cultured with female DRG neurons. E-F. Schematic of the experimental design and quantification of GSIS for male islets co-cultured with female DRG neurons. G-H. Schematic of the experimental design and quantification of GSIS for female islets co-cultured with male DRG neurons. Data represent mean ± SEM. ∗p < 0.05 (n = 5–7 per group).
4. Discussion
Males and females display phenotypic differences in the regulation of energy balance [1,23], including the control of food intake [35,36], energy expenditure [37,38], body weight gain [22,39], body composition [40,41], insulin secretion [9,12,24,34], and insulin action [42,43]. Some of these sex differences are driven by neuronal networks/conduits and implicate gonadal hormones [35,44] and sex chromosome effects [45]. We recently demonstrated that the pancreatic β-cell function has a sensory component that operates in a sexually dimorphic manner [10]. Herein we report the biological underpinnings of this male-female dichotomy. First, retrograde viral labeling revealed a higher number of pancreas-projecting DRG and NG neurons in post-weaned males than females. Second, gonadectomy studies uncovered a major role for male sex hormones in the density of peri-islet afferents and the sensory modulation of insulin secretion and glucose excursion. Finally, same- and opposite-sex co-culture paradigms unmasked the existence of potential sex chromosome complement-encoded molecules that regulate β-cell activity, distinctively, in males and females.
Our studies reported an increase in the abundance of sensory neurons in the male versus female pancreas, which highlights a previously unknown sex-biased structural aspect of the neuronal regulation of the pancreas physiology. Along with the reported inhibitory role of sensory neurons in insulin secretion [10,46], this observation supports further the physiological role of pancreas-projecting afferent neurons in the negative-feedback control of insulin secretion and provides new biological insight into the generally observed higher GSIS in females [24]. Intriguingly, some sensory neurons bifurcate to innervate both the liver and pancreas. This “convergence/divergence” pattern allows the same neuron to receive/emit information from/to multiple viscera. Such neuronal networks might facilitate organ–organ crosstalk independently of circulating biomolecules and central inputs as recently reported for the regulation of adaptive islet β-cell regeneration by liver neuronal mechanisms [47,48]. Why are males subject to a stronger sensory-regulated GSIS than females? A possible explanation may lie within a biological need to adjusting the strict dependence of the brain on glucose as the main source of energy [49] with the size difference between male and female brains [50]. Males have larger brains than females and, therefore, likely require built-in mechanisms to favor routing higher amounts of glucose to the brain for its optimal functioning. The excessive sensory wiring of the male pancreas may exert strengthened negative feedback that impairs glucose-induced insulin release. This physiological deficiency in GSIS reduces insulin-dependent glucose uptake in peripheral metabolic tissues and accelerates insulin-independent glucose transport to the brain. This sparing effect is consistent with the recently reported idea that physiologically deficient GSIS plays a critical role in the brain distribution of dietary glucose [51]. From the clinical perspective, it is conceivable that the extensive innervation of the pancreas predisposes men to common pancreatic disorders—diabetes and pancreatic cancer are more prevalent in men than in women [22,23,52,53]. Alterations in the activity of the pancreas-innervating neurons have been associated with the development of type 1 diabetes [[54], [55], [56]]. Moreover, the density of pancreas-projecting DRG neurons is increased in the tumor microenvironment [57] where they support tumor growth [58]. In accordance with their tumor growth-enhancing capabilities, experimental ablation of DRG sensory neurons slows the initiation and progression of pancreatic ductal carcinoma [59].
A major finding revealed by our studies is that the sensory control of islet β-cell function is sensitive to male gonadal sex hormones. This suggests a physiological role for gonadal-sensory-islet crosstalk in the regulation of insulin secretion and post-absorptive glucose levels in males. The lack of male sex hormones dampens the insulinotropic action of sensory chemodenervation and indicates antagonistic action of sex hormones in the sensory modulation of islet β cells. Several theories can be postulated as to how testosterone may interact with capsaicin-sensitive pancreatic sensory neurons. First, testosterone interacts directly with the transient receptor potential melastatin 8 (TRPM8) [60,61], which is known to exhibit reciprocal interactions with TRPV1; the capsaicin receptor [62]. These TRPV1/TRPM8 interactions could occur on the same neurons or involve two independent TRPM8 and TRPV1 pancreatic neuronal subsets. Second, it is also possible that testosterone acts on TRPM8-expressing sensory afferents that innervate non-pancreatic tissues. In agreement with this scenario, genetic ablation of the trpm8 gene results in decreased GSIS secondary to enhanced insulin clearance; an effect that is associated with the loss of the activity of TRPM8-expressing neurons innervating the hepatic portal vein [63]. Additional studies are warranted to elucidate the physiological interactions between testosterone and TRPV1-expressing sensory afferents and their roles in the regulation of islet β-cell function and glucose clearance. Third, testosterone may modulate the number of peripheral neurons in adulthood [64,65] as occurring in the brain during neonatal testosterone surge [3]. Ablation of male gonads would lower the density of sensory neurons in the male pancreas, which would abolish their suppressive effect on glucose-induced insulin release. The absence of an extra beneficial capsaicin effect in gonadectomized males might be a consequence of an already altered sensory innervation within the pancreas after testosterone depletion. The notion that hormones regulate adult organ innervation is not limited to testosterone. Recent studies reported a role for leptin signaling in the wiring of the pancreas and adipose tissue [66,67].
Beyond the action of circulating sex steroid hormones, we revealed that sensory modulation of basal and glucose-induced insulin release might be regulated by cell-autonomous factors encoded by sex chromosomes (XX or XY) and/or epistatic interactions of the latter with the autosomes. We demonstrated that female islets exhibit superior GSIS when compared to their male counterparts in vitro. This finding is consistent with data from other studies [9,12,24,34] and highlights further the possible existence of islet sex-specific molecules that regulate insulin release differently in males and females [68]. Interestingly, male but not female islets exhibited higher basal insulin secretion but blunted GSIS when co-cultured with same-sex sensory neurons. These observations allude to the commonly encountered impairment of islet β-cell function in insulin resistance—high basal insulin secretion but modest/poor GSIS [69,70]—and suggest a plausible link between increased islet innervation in insulin resistance settings [71,72] and the maladaptive β-cell function in such metabolically demanding conditions [73,74]. The capacity of sensory neurons to blunt GSIS was observed only in males. The basal and glucose-induced insulin secretions are similar between female islets cultured alone and those seeded with same-sex sensory neurons. The ability of female islets to release insulin in response to elevated glucose levels was maintained even when forcibly co-cultured with male sensory neurons. Along with the structural data suggesting increased density of sensory neurons in the male pancreas and the further increased islet innervation in metabolically challenged conditions [71,72], our studies highlight a possible sexually dimorphic role for islet-projecting sensory neurons in the development of obesity-associated type 2 diabetes [22,23]. A major caveat, however, in our studies is that our experiments do not determine the proportional contribution of sex-hormone dependent versus potentially sex-chromosome dependent events in the sensory-mediated regulation of β-cell activity. It is important to mention that while our in vitro studies (Figure 4) are suggestive of sex chromosome effects, they do not exclude the potential epigenetic action of neonatal testosterone that would give rise to phenotypic differences between sexes in adulthood [75,76]. Equally important, we do not rule out potential de novo synthesis of steroids in neurons nor in islet β cells [14,77,78] as contributors to the seemingly in vitro hormone-independent effects. Future studies that combine the use of the FCG paradigm [6] along with sensory secretome/epigenome will be useful to deconstruct such biological insights.
In summary, we demonstrated that the sex-biased sensory modulation of islet β-cell activity results from potential neurodevelopmental origins, sex hormone-dependent mechanisms and possibly the action of cell-autonomous factors encoded by the sex chromosome complement. The identification of these sex-specific islet- and sensory-derived molecules mediating sensory modulation of pancreatic β cells will be useful for the currently ongoing efforts gearing toward developing novel β-cell-centered preventive and/or therapeutic measures to counter diabetes.
Acknowledgments
We thank Dr. Arnold Rabson for critically reading the manuscript and for helpful discussion. This research was supported by the NIDDK-supported Human Islet Research Network (HIRN, RRID:SCR_014393; https://hirnetwork.org; UC4 DK104162; to A.E.) and the R01 DK122167 (to A.E.). W.C. was supported by the K01 DK120740. The Child Health Institute of New Jersey was supported by the Robert Wood Johnson Foundation (grant #74260). E.A.E was supported by the Center for Neuroanatomy with Neurotropic Viruses NIH 2P40OD010996-16.
Conflict of interest
None.
References
- 1.Mauvais-Jarvis F., Arnold A.P., Reue K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metabolism. 2017;25(6):1216–1230. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jost A. Recherches sur la différenciation sexuelle de l'embryon de lapin. Archives d'Anatomie Microscopique et de Morphologie Experimentale. 1947;36:271–315. [Google Scholar]
- 3.Corbier P., Edwards D.A., Roffi J. The neonatal testosterone surge: a comparative study. Archives Internationales de Physiologie de Biochimie et de Biophysique. 1992;100(2):127–131. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- 4.Arnold A.P., Gorski R.A. Gonadal steroid induction of structural sex differences in the central nervous system. Annual Review of Neuroscience. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 5.Phoenix C.H., Goy R.W., Gerall A.A., Young W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female Guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 6.De Vries G.J., Rissman E.F., Simerly R.B., Yang L.Y., Scordalakes E.M., Auger C.J. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. Journal of Neuroscience. 2002;22(20):9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold A.P., Cassis L.A., Eghbali M., Reue K., Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(5):746–756. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efrat S. Sexual dimorphism of pancreatic beta-cell degeneration in transgenic mice expressing an insulin-ras hybrid gene. Endocrinology. 1991;128(2):897–901. doi: 10.1210/endo-128-2-897. [DOI] [PubMed] [Google Scholar]
- 9.Li T., Jiao W., Li W., Li H. Sex effect on insulin secretion and mitochondrial function in pancreatic beta cells of elderly Wistar rats. Endocrine Research. 2016;41(3):167–179. doi: 10.3109/07435800.2015.1124437. [DOI] [PubMed] [Google Scholar]
- 10.Bou Karam J., Cai W., Mohamed R., Huang T., Meng L., Homan E.P. TRPV1 neurons regulate beta-cell function in a sex-dependent manner. Mol Metab. 2018;18:60–67. doi: 10.1016/j.molmet.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu A., Dube S., Basu R. Men are from mars, women are from venus: sex differences in insulin action and secretion. Advances in Experimental Medicine & Biology. 2017;1043:53–64. doi: 10.1007/978-3-319-70178-3_4. [DOI] [PubMed] [Google Scholar]
- 12.Basu R., Dalla Man C., Campioni M., Basu A., Klee G., Toffolo G. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55(7):2001–2014. doi: 10.2337/db05-1692. [DOI] [PubMed] [Google Scholar]
- 13.Horie I., Abiru N., Eto M., Sako A., Akeshima J., Nakao T. Sex differences in insulin and glucagon responses for glucose homeostasis in young healthy Japanese adults. J Diabetes Investig. 2018;9(6):1283–1287. doi: 10.1111/jdi.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauvais-Jarvis F. Role of sex steroids in beta cell function, growth, and survival. Trends in Endocrinology and Metabolism. 2016;27(12):844–855. doi: 10.1016/j.tem.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro G., Xu W., Jacobson D.A., Wicksteed B., Allard C., Zhang G. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metabolism. 2016;23(5):837–851. doi: 10.1016/j.cmet.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarro G., Allard C., Morford J.J., Xu W., Liu S., Molinas A.J. Androgen excess in pancreatic beta cells and neurons predisposes female mice to type 2 diabetes. JCI Insight. 2018;3(12) doi: 10.1172/jci.insight.98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Meara N.M., Blackman J.D., Ehrmann D.A., Barnes R.B., Jaspan J.B., Rosenfield R.L. Defects in beta-cell function in functional ovarian hyperandrogenism. The Journal of Cinical Endocrinology and Metabolism. 1993;76(5):1241–1247. doi: 10.1210/jcem.76.5.8496316. [DOI] [PubMed] [Google Scholar]
- 18.Le May C., Chu K., Hu M., Ortega C.S., Simpson E.R., Korach K.S. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiano J.P., Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nature Reviews Endocrinology. 2012;8(6):342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 20.Choi S.B., Jang J.S., Park S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology. 2005;146(11):4786–4794. doi: 10.1210/en.2004-1653. [DOI] [PubMed] [Google Scholar]
- 21.Sorenson R.L., Brelje T.C., Roth C. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology. 1993;133(5):2227–2234. doi: 10.1210/endo.133.5.8404674. [DOI] [PubMed] [Google Scholar]
- 22.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biology of Sex Differences. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiology & Behavior. 2018;187:20–23. doi: 10.1016/j.physbeh.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gannon M., Kulkarni R.N., Tse H.M., Mauvais-Jarvis F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol Metab. 2018;15:82–91. doi: 10.1016/j.molmet.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Link J.C., Chen X., Arnold A.P., Reue K. Metabolic impact of sex chromosomes. Adipocyte. 2013;2(2):74–79. doi: 10.4161/adip.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Card J.P., Enquist L.W. Transneuronal circuit analysis with pseudorabies viruses. Curr Protoc Neurosci. 2014;68:1 5 1–39. doi: 10.1002/0471142301.ns0105s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demmin G.L., Clase A.C., Randall J.A., Enquist L.W., Banfield B.W. Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection. Journal of Virology. 2001;75(22):10856–10869. doi: 10.1128/JVI.75.22.10856-10869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banfield B.W., Kaufman J.D., Randall J.A., Pickard G.E. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. Journal of Virology. 2003;77(18):10106–10112. doi: 10.1128/JVI.77.18.10106-10112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huebner E.A., Budel S., Jiang Z., Omura T., Ho T.S., Barrett L. Diltiazem promotes regenerative axon growth. Molecular Neurobiology. 2019;56(6):3948–3957. doi: 10.1007/s12035-018-1349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Ouaamari A., Dirice E., Gedeon N., Hu J., Zhou J.Y., Shirakawa J. SerpinB1 promotes pancreatic beta cell proliferation. Cell Metabolism. 2016;23(1):194–205. doi: 10.1016/j.cmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Ouaamari A., Kawamori D., Dirice E., Liew C.W., Shadrach J.L., Hu J. Liver-derived systemic factors drive beta cell hyperplasia in insulin-resistant states. Cell Reports. 2013;3(2):401–410. doi: 10.1016/j.celrep.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Ouaamari A., Zhou J.Y., Liew C.W., Shirakawa J., Dirice E., Gedeon N. Compensatory islet response to insulin resistance revealed by quantitative proteomics. Journal of Proteome Research. 2015;14(8):3111–3122. doi: 10.1021/acs.jproteome.5b00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanley S., Pinto S., Segal J., Perez C.A., Viale A., DeFalco J. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(15):7024–7029. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kautzky-Willer A., Brazzale A.R., Moro E., Vrbikova J., Bendlova B., Sbrignadello S. Influence of increasing BMI on insulin sensitivity and secretion in normotolerant men and women of a wide age span. Obesity. 2012;20(10):1966–1973. doi: 10.1038/oby.2011.384. [DOI] [PubMed] [Google Scholar]
- 35.Nohara K., Zhang Y., Waraich R.S., Laque A., Tiano J.P., Tong J. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology. 2011;152(4):1661–1669. doi: 10.1210/en.2010-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Wang L., Loh D.H., Colwell C.S., Tache Y., Reue K. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Hormones and Behavior. 2015;75:55–63. doi: 10.1016/j.yhbeh.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferraro R., Lillioja S., Fontvieille A.M., Rising R., Bogardus C., Ravussin E. Lower sedentary metabolic rate in women compared with men. Journal of Clinical Investigation. 1992;90(3):780–784. doi: 10.1172/JCI115951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke L.K., Doslikova B., D’Agostino G., Greenwald-Yarnell M., Georgescu T., Chianese R. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol Metab. 2016;5(3):245–252. doi: 10.1016/j.molmet.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi H., Clegg D.J. Sex differences in the regulation of body weight. Physiology & Behavior. 2009;97(2):199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bredella M.A. Sex differences in body composition. Advances in Experimental Medicine & Biology. 2017;1043:9–27. doi: 10.1007/978-3-319-70178-3_2. [DOI] [PubMed] [Google Scholar]
- 41.Schorr M., Dichtel L.E., Gerweck A.V., Valera R.D., Torriani M., Miller K.K. Sex differences in body composition and association with cardiometabolic risk. Biology of Sex Differences. 2018;9(1):28. doi: 10.1186/s13293-018-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerre-Millo M., Leturque A., Girard J., Lavau M. Increased insulin sensitivity and responsiveness of glucose metabolism in adipocytes from female versus male rats. Journal of Clinical Investigation. 1985;76(1):109–116. doi: 10.1172/JCI111932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macotela Y., Boucher J., Tran T.T., Kahn C.R. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58(4):803–812. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morford J.J., Wu S., Mauvais-Jarvis F. The impact of androgen actions in neurons on metabolic health and disease. Molecular and Cellular Endocrinology. 2018;465:92–102. doi: 10.1016/j.mce.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X., McClusky R., Itoh Y., Reue K., Arnold A.P. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology. 2013;154(3):1092–1104. doi: 10.1210/en.2012-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karlsson S., Scheurink A.J., Steffens A.B., Ahren B. Involvement of capsaicin-sensitive nerves in regulation of insulin secretion and glucose tolerance in conscious mice. American Journal of Physiology. 1994;267(4 Pt 2):R1071–R1077. doi: 10.1152/ajpregu.1994.267.4.R1071. [DOI] [PubMed] [Google Scholar]
- 47.Imai J., Katagiri H., Yamada T., Ishigaki Y., Suzuki T., Kudo H. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science. 2008;322(5905):1250–1254. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto J., Imai J., Izumi T., Takahashi H., Kawana Y., Takahashi K. Neuronal signals regulate obesity induced beta-cell proliferation by FoxM1 dependent mechanism. Nature Communications. 2017;8(1):1930. doi: 10.1038/s41467-017-01869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mergenthaler P., Lindauer U., Dienel G.A., Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends in Neurosciences. 2013;36(10):587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu L.R., Fernandes D.J., Szulc-Lerch K.U., Dazai J., Nieman B.J., Turnbull D.H. Mouse MRI shows brain areas relatively larger in males emerge before those larger in females. Nature Communications. 2018;9(1):2615. doi: 10.1038/s41467-018-04921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kume S., Kondo M., Maeda S., Nishio Y., Yanagimachi T., Fujita Y. Hypothalamic AMP-activated protein kinase regulates biphasic insulin secretion from pancreatic beta cells during fasting and in type 2 diabetes. EBioMedicine. 2016;13:168–180. doi: 10.1016/j.ebiom.2016.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arslan A.A., Helzlsouer K.J., Kooperberg C., Shu X.O., Steplowski E., Bueno-de-Mesquita H.B. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Archives of Internal Medicine. 2010;170(9):791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen G.M., de Andrade M., Goggins M., Hruban R.H., Bondy M., Korczak J.F. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiology Biomarkers & Prevention. 2006;15(4):704–710. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 54.Guyot M., Simon T., Ceppo F., Panzolini C., Guyon A., Lavergne J. Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nature Biotechnology. 2019;37(12):1446–1451. doi: 10.1038/s41587-019-0295-8. [DOI] [PubMed] [Google Scholar]
- 55.Razavi R., Chan Y., Afifiyan F.N., Liu X.J., Wan X., Yantha J. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127(6):1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 56.Winer S., Tsui H., Lau A., Song A., Li X., Cheung R.K. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nature Medicine. 2003;9(2):198–205. doi: 10.1038/nm818. [DOI] [PubMed] [Google Scholar]
- 57.Ceyhan G.O., Demir I.E., Altintas B., Rauch U., Thiel G., Muller M.W. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochemical and Biophysical Research Communications. 2008;374(3):442–447. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 58.Sinha S., Fu Y.Y., Grimont A., Ketcham M., Lafaro K., Saglimbeni J.A. PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Research. 2017;77(8):1868–1879. doi: 10.1158/0008-5472.CAN-16-0899-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saloman J.L., Albers K.M., Li D., Hartman D.J., Crawford H.C., Muha E.A. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(11):3078–3083. doi: 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asuthkar S., Elustondo P.A., Demirkhanyan L., Sun X., Baskaran P., Velpula K.K. The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions. Journal of Biological Chemistry. 2015;290(5):2659–2669. doi: 10.1074/jbc.M114.610824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohandass A., Krishnan V., Gribkova E.D., Asuthkar S., Baskaran P., Nersesyan Y. TRPM8 as the rapid testosterone signaling receptor: implications in the regulation of dimorphic sexual and social behaviors. The FASEB Journal. 2020 doi: 10.1096/fj.202000794R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takaishi M., Uchida K., Suzuki Y., Matsui H., Shimada T., Fujita F. Reciprocal effects of capsaicin and menthol on thermosensation through regulated activities of TRPV1 and TRPM8. The Journal of Physiological Sciences. 2016;66(2):143–155. doi: 10.1007/s12576-015-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCoy D.D., Zhou L., Nguyen A.K., Watts A.G., Donovan C.M., McKemy D.D. Enhanced insulin clearance in mice lacking TRPM8 channels. American Journal of Physiology. Endocrinology and Metabolism. 2013;305(1):E78–E88. doi: 10.1152/ajpendo.00542.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rasika S., Nottebohm F., Alvarez-Buylla A. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(17):7854–7858. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spritzer M.D., Roy E.A. Testosterone and adult neurogenesis. Biomolecules. 2020;10(2) doi: 10.3390/biom10020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Croizier S., Prevot V., Bouret S.G. Leptin controls parasympathetic wiring of the pancreas during embryonic life. Cell Reports. 2016;15(1):36–44. doi: 10.1016/j.celrep.2016.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang P., Loh K.H., Wu M., Morgan D.A., Schneeberger M., Yu X. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature. 2020;583(7818):839–844. doi: 10.1038/s41586-020-2527-y. [DOI] [PubMed] [Google Scholar]
- 68.Hall E., Volkov P., Dayeh T., Esguerra J.L., Salo S., Eliasson L. Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome Biology. 2014;15(12):522. doi: 10.1186/s13059-014-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 70.Kahn S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46(1):3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 71.Dai C., Brissova M., Reinert R.B., Nyman L., Liu E.H., Thompson C. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes. 2013;62(12):4144–4153. doi: 10.2337/db12-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giannulis I., Mondini E., Cinti F., Frontini A., Murano I., Barazzoni R. Increased density of inhibitory noradrenergic parenchymal nerve fibers in hypertrophic islets of Langerhans of obese mice. Nutrition, Metabolism, and Cardiovascular Diseases. 2014;24(4):384–392. doi: 10.1016/j.numecd.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hull R.L., Kodama K., Utzschneider K.M., Carr D.B., Prigeon R.L., Kahn S.E. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia. 2005;48(7):1350–1358. doi: 10.1007/s00125-005-1772-9. [DOI] [PubMed] [Google Scholar]
- 74.Kaiyala K.J., Prigeon R.L., Kahn S.E., Woods S.C., Porte D., Jr., Schwartz M.W. Reduced beta-cell function contributes to impaired glucose tolerance in dogs made obese by high-fat feeding. American Journal of Physiology. 1999;277(4):E659–E667. doi: 10.1152/ajpendo.1999.277.4.E659. [DOI] [PubMed] [Google Scholar]
- 75.Baum M.J. New evidence that an epigenetic mechanism mediates testosterone-dependent brain masculinization. Endocrinology. 2009;150(9):3980–3982. doi: 10.1210/en.2009-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baum M.J. Evidence that a sex difference in neonatal DNA methylation organizes two distinct phenotypic characteristics of neurons in the murine forebrain. Endocrinology. 2017;158(6):1569–1571. doi: 10.1210/en.2017-00364. [DOI] [PubMed] [Google Scholar]
- 77.Baulieu E.E., Robel P. Neurosteroids: a new brain function? The Journal of Steroid Biochemistry and Molecular Biology. 1990;37(3):395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 78.Ogishima T., Mitani F., Suematsu M. Cytochrome P-450(17alpha) in beta-cells of rat pancreas and its local steroidogenesis. The Journal of Steroid Biochemistry and Molecular Biology. 2008;111(1–2):80–86. doi: 10.1016/j.jsbmb.2008.04.008. [DOI] [PubMed] [Google Scholar]