Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection has triggered the COVID-19 pandemic. Several factors induce hypoxia in COVID-19. Despite being hypoxic, some SARS-CoV-2-infected individuals do not experience any respiratory distress, a phenomenon termed ‘silent (or happy) hypoxia’. Prolonged undetected hypoxia could be dangerous, sometimes leading to death. A few studies attempted to unravel what causes silent hypoxia, however, the exact mechanisms are still elusive. Here, we aim to understand how SARS-CoV-2 causes silent hypoxia.
Current Opinion in Physiology 2021, 23:100456
This review comes from a themed issue on Microbiome
Edited by Soumita Das, Ellen J Beswick and Irina V Pinchuk
For a complete overview see the Issue
Available online 6th July 2021
https://doi.org/10.1016/j.cophys.2021.06.010
2468-8673/© 2021 Elsevier Ltd. All rights reserved.
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has initiated the current COVID-19 pandemic. COVID-19 symptoms are diverse and extend from mild to severe manifestations of pneumonia, acquired respiratory distress syndrome (ARDS) and multi-organ failure [1]. A prevalent feature associated with COVID-19 is the onset of hypoxemia {low blood oxygen (O2) level}. SARS-CoV-2 replication within the lungs causes an uncontrolled inflammatory response, the ‘cytokine storm’, which impinges on the lung function or perfusion, leading to hypoxemia [2]. This causes a deficiency in tissue oxygenation leading to hypoxia. Compensatory mechanisms like increased ventilation and dyspnea, which are generally initiated in hypoxia, are surprisingly lacking in many COVID-19 patients. This phenomenon is known as ‘silent/happy hypoxia’ or non-dyspneic hypoxemia [3•,4]. Since the patient remains unaware of the condition, undetected hypoxia could be dangerous. Studies indicate that gut dysbiosis (disruption of the gut microbial homeostasis) is an important manifestation in COVID-19 and can hamper respiratory control [5]. This article explores the potential role of gut microbiota-brain communication in causing silent hypoxia in COVID-19.
Hypoxia and hypoxia-sensing
The cause of hypoxia in COVID-19 is multifactorial and includes thrombosis, pulmonary infiltration, viral invasion in pneumocytes, profuse cytokine release and inflammatory responses. Sepsis and pulmonary edema-mediated thickening of the alveolar-capillary barrier, viremia and dysregulated renin-angiotensin-aldosterone system (RAAS) also cause systemic hypoxia in COVID-19 [2,6].
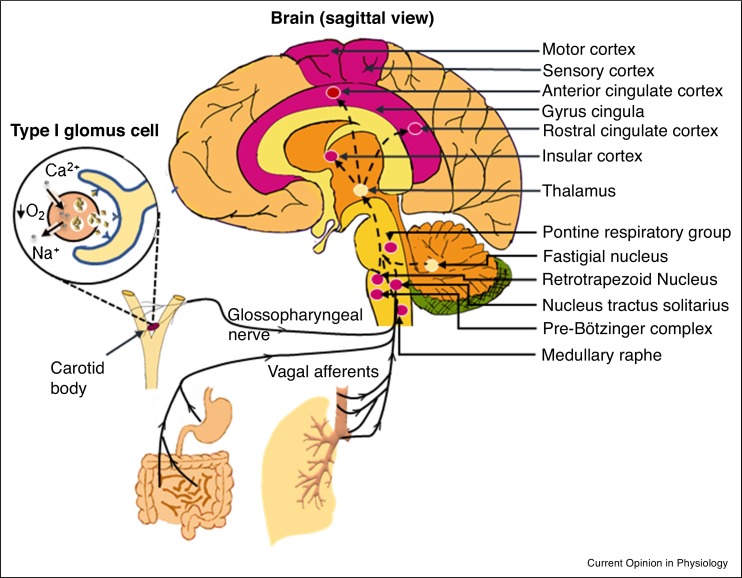
The central chemoreceptors of the respiratory center (RC) (the medulla oblongata and pons in the brainstem) and the peripheral chemoreceptors of the carotid body (CB) sense O2 and carbon dioxide (CO2) in the arterial blood [7,8]. RC is modulated by several metabolites including lactate and are more sensitive in detecting slight increases in CO2-tension (PaCO2) or a drop in pH than PaO2-decrease. CB evokes peripheral chemoreflex and ventilatory activity [9]. Although both RC and CB can detect hypoxia, the CB has the main role in O2 homeostasis. Hypoxia depolarizes glomus cells (type I) in the CB, promoting the release of neurotransmitters that signal the nucleus tractus solitarius (NTS) via a small division of the glossopharyngeal nerve (carotid sinus nerve) [10]. These signals are integrated and relayed to the rostral ventrolateral (‘pressor’) region of the medulla and the hypothalamic paraventricular nucleus that initiate ventilatory output which regulate breathing. Central chemoreceptors communicate (glutaminergic) with the pre-Bötzinger complex (PBC) of the medulla oblongata, the medullary raphe (serotonergic), the fastigial nucleus (glutaminergic) of the cerebellum and the astrocytes of the glial cells [11]. PBC and the retrotrapezoid nucleus/parafacial respiratory group of the brainstem neurons are considered the primary and secondary respiratory rhythm-regulators, respectively. The RC receives signals from these chemoreceptors, the cerebrum and the hypothalamus to determine the rate or depth of respiration as well as the sensation of dyspnea [12]. Figure 1 provides a schematic representation of the major neural components involved in O2-sensing.
Figure 1.
Components of the neuronal system involved in O2 sensing. The sagittal view of the brain showing components of the neuronal system involved in sensing O2 level. Vagal afferents and afferent neurons of the glossopharyngeal nerve from the peripheral chemoreceptors reach the medulla and the hypothalamus. Retrotrapezoid nucleus of the medulla contains the central chemoreceptors which is connected to the pre-Bötzinger complex in the medulla oblongata and the cerebellum. Afferent connections from these regions to the thalamus relay the signal to the corticolimbic network that ultimately control ventilatory responses. Areas which are possibly damaged in COVID-19 are colored in magenta. Inset: Decrease in partial pressure of O2 in the blood causes depolarization of the type I glomus cells of the CB and release neurotransmitters.
Respiratory-responses hugely vary among individuals and are further complicated by respiratory virus infections. SARS-CoV-2 reaches the central nervous system (CNS) by various routes. As discussed later, the neuroinvasive potential of SARS-CoV-2 might directly impair hypoxia-response by targeting the chemosensors [13, 14, 15]. In addition, SARS-CoV-2 can disturb the intricately balanced gut-brain axis [16] to ultimately impact the functioning of the RC.
Gut dysbiosis in COVID-19
The symbiotic relationship of gut microbes with the host regulates metabolic pathways, immune and neuroendocrine crosstalk [17]. Gut microbes can interact with the brain via the vagus nerve and produce many neuroactive substances such as metabolites, endocrine modulators and neurotransmitters.
The Bacteroidetes (Bacteroides, Alistipes, Prevotella) and Firmicutes (Eubacteria, Clostridium, Faecalibacterium, Roseburia) are the most dominant phyla in the human gut, followed by the Actinobacteria, Proteobacteria, Fusobacteria and Verrucomicrobia [18•]. The loss of microbial diversity in COVID-19 correlates with increased inflammation [19••]. Firmicutes (Ruminococcus torques and Ruminococcus gnavus) and Bacteroidetes (Bacteroides dorei) get enriched while other Firmicutes (such as Eubacterium rectale, Faecalibacterium prausnitzii) and Actinobacteria (such as Bifidobacterium adolescentis, Bifidobacterium bifidum) are depleted in COVID-19 [20••]. The GI tract and the respiratory epithelia express angiotensin-converting enzyme 2 (ACE2) which acts as the binding receptor of SARS-CoV-2 and is involved in the maintenance of the gut microbiota [21]. Interestingly, Bacteroides downregulate ACE2 in the rodent gut and are depleted in COVID-19 patients [21]. An impaired ratio of Bacteroidetes to Firmicutes is reflective of the disease severity. Shotgun metagenomics of patients’ fecal samples exhibit depletion of commensals and an upsurge in the population of opportunistic pathogens [20••]. Opportunistic pathogens Clostridium ramosum, C. hathewayi, Coprobacillus sp., Streptococcus sp. and Actinomyces sp. increase with the disease severity [20••,22]. The symbionts F. prausnitzii, Ruminococcus obeum, E. rectale, Dorea formicigenerans, Lachnospiraceae bacterium and Alistipesonderdonkii are depleted in COVID-19. Since systematic efforts to understand the contribution of SARS-CoV-2-mediated gut dysbiosis towards silent hypoxia have never been made, here we summarize the mechanisms that might be involved.
Gut dysbiosis disrupts hypoxia-sensing in SARS-CoV-2 infection
SARS-CoV-2 directly infects enterocytes by binding with ACE2 and causes gut dysbiosis [21,23]. Like many other viruses, SARS-CoV-2 disrupts the intestinal barrier function, causes hematological dissemination of gut microbes and initiates systemic inflammation [23]. High levels of proinflammatory cytokines, interferon γ (IFN-γ), tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) are found in the blood of COVID-19 patients [24]. These cytokines travel via the systemic circulation and alter the blood–brain barrier (BBB) permeability [16]. Systemic inflammation increases the level of circulating reactive O2 species (ROS) that may further affect the brainstem and the cerebrum [25,26]. The brain has a limited antioxidant capacity and, therefore, is known to be prone to oxidative stress [27]. Oxidative stress causes neuroinflammation and mitochondrial DNA damage in the NTS [28]. Studies involving germ-free mice also indicate that gut dysbiosis compromises the BBB integrity, consequently allowing the transmission of proinflammatory cytokines to the brain causing neuroinflammation [29]. α-synuclein is generated in the gut due to SARS-CoV-2-mediated cytokine storm, bacterial endotoxins {mainly, lipopolysaccharide (LPS)} and is subsequently transported to the brain by the vagus nerve causing neuronal damage [30]. LPS may also reach the brain, cause neuroinflammation and BBB disruption [31]. Another major mechanism behind SARS-CoV-2 entry into the brain is the reverse axonal transport from the peripheral nerves [32].
Neurons or glial cells, which express ACE2, get infected by the virus [4]. Studies on neurotropic flaviviruses indicate that astrocytes, by virtue of performing aerobic glycolysis, might provide the ideal replicative environment for SARS-CoV-2 [13]. The CNS damage can be triggered by neurotropic or neuroimmune effects of SARS-CoV-2 on the brainstem [33••]. The PBC-infection might directly hamper hypoxia-sensing [34]. Ventilatory responses and dyspnea are tightly regulated by PaCO2. Prevailing hypotheses explaining the COVID-19-associated silent hypoxia are associated with existing hypocapnia (low PaCO2 in the blood) that prevents brainstem-involvement [35]. During SARS-CoV-2 infection-induced hypoxia, the brain raises the metabolic rate and produces lactate but the cerebral blood flow, which is well-maintained, carries away the excess CO2 generated during the process [36]. This hypocapnic hypoxia may hamper the function of central chemoreceptors and cause dyspnea. A study involving a small group of COVID-19 patients show that PaCO2 lower than 39 mm Hg blunts the CNS-response to hypoxia [37]. In contrast, CB detects changes in PaO2 in the arterial blood but it cannot sense O2-saturation. In pyrexia, prevalent in COVID-19 patients, the O2-dissociation curve shifts to the right (i.e. causes hemoglobin-desaturation) rendering CB-chemoreceptors unstimulated and contributes to silent hypoxia. Poor respiratory control and BBB integrity in the elderly and diabetic COVID-19 patients may explain the prevalence of silent hypoxia in these populations.
The vagus nerve forms a major neural route connecting the gut to the brain and has innervations in the respiratory tract and the NTS [38]. As dysbiosis modulates the vagal tone, it can perturb the input signaling to the NTS [38,39], thereby affecting respiration. Damage to the lung vagal receptors and respiratory muscle mechanoreceptors further explains the absence of dyspnea in COVID-19 [40]. Microbe-released metabolites alter immune-inflammatory responses in the CNS [41]. As inflammatory mediators cause CNS neurodegeneration [16,42], gut dysbiosis-induced neuroinflammation damages the RC and might be a potential mechanism behind silent hypoxia [16,41]. These studies highlight gut-dysbiosis as a critical deregulator of neuronal function.
Gut microbiota-derived circulating metabolites blunt hypoxia-sensing in COVID-19
The gut microbiota generates several neurotropic metabolites, neurotransmitters, peptides and gaseous substances, many of which show altered levels in COVID-19 (Table 1 ). Fermentation of undigested starch and dietary fibers by the colonic bacteria generates short chain fatty acids (SCFA) such as butyrate, propionate and acetate as the major metabolites [43•]. Bacteroidetes mainly produce acetate and propionate, but butyrate is largely produced by Firmicutes which modulate rate/depth of breathing [44,45]. SCFA-producing commensal Firmicutes, for example, Roseburia, Eubacterium and F. prausnitzii are depleted in COVID-19 [20••]. Butyrate and propionate regulate serotonin, dopamine, adrenaline or noradrenaline which alter the brain-neurochemistry [46]. SCFA, especially butyrate, maintain the intestinal tight junctions, BBB integrity, show neuroprotective effects [47] and even are capable of ACE2 downregulation in the colonic organoids of rats [48]. Murine RC and CB are responsive to SCFA by the mediation of Olfr78, a Gs-coupled receptor involved in mild-moderate hypoxia-sensing [49]. All the evidence implicate that SARS-CoV-2-mediated depletion of SCFA can impair hypoxia-sensing.
Table 1.
Altered gut microbiota leads to the dysregulation of neurotropic metabolites in COVID-19 patients altering neuronal responses
| Bacterial phylum/genus (and status in COVID-19) | Microbial metabolite/neurotransmitter | Impacts of the microbial metabolites/neurotransmitters | References |
|---|---|---|---|
| E. rectale, F. prausnitzii (decreased) | Butyrate | Neuroprotective, anti-inflammatory, antioxidant | [20••,44,47] |
| Roseburia sp., Akkermansia muciniphilia, Ruminococcus (decreased) | Propionate | Neuroprotective, anti-inflammatory, antioxidant | [20••,44,47] |
| Bifidobacterium sp. (decreased) | Acetate | Neuroprotective, anti-inflammatory | [5,20••,47] |
| B. dorei, B. ovatus, B. caccae, B. vulgatus (increased) | γ-aminobutyric acid (GABA) | Neuroinhibitor | [19••,59,60] |
| Enterococcus sp., Clostridium sp. (increased) | Dopamine | Neuroinhibitor, blunts ventilation under normocapnic hypoxia | [9,20••,57,61, 62, 63] |
| Corynebacterium sp., Brevibacterium sp., Ruminococcus sp. (decreased) | Glutamate | Neurostimulator | [20••,59,64] |
Inflammatory bowel diseases (IBD), which include ulcerative colitis (UC) and Crohn’s disease (CD), show striking-similarities with COVID-19 in their pathophysiological mechanisms. IBD are associated with immune dysregulation, damaged intestinal barrier and gut dysbiosis [50]. Eventually, inflammatory processes spread extraintestinally and affect other organs including the respiratory organs and the brain. IBD patients display “pathological hypoxia” frequently but some patients remain nondyspneic [51] and asymptomatic unless assessed by lung function test [52]. The gut microbiome of IBD patients, has fewer SCFA producers such as Roseburia and F. prausnitzii accompanied by depletion of beneficial Faecalibacterium sp., Ruminococcus and increased Clostridium sp. abundance [50]. As in COVID-19, SCFA, specifically butyrate, is consistently low in the gut of individuals with IBD. Interestingly, ACE2 receptors are induced in IBD [53] and possibly correlates with the SCFA downregulation. These reports signify the need for future studies to unravel the relationship of gut metabolites with respiratory controls dependent and independent of SARS-CoV-2 infection.
The molecular mechanism of hypoxia-sensing is still elusive; however, the role of hypoxia-adaptive hypoxia-inducible factor-1 (HIF-1) and HIF-2 are well-known. Hypoxia stabilizes the α-subunit of HIF. HIF-1α deficiency and HIF-2α accumulation contributes towards a blunted hypoxic response by the CB [54]. Moreover, direct invasion by SARS-CoV-2 induces inflammatory responses in the CB [15]. In contrast to SARS-CoV-2, other viruses attacking the respiratory system such as the influenza virus and respiratory syncytial virus, which do not have any association with silent hypoxia, increase SCFA or valerate [55]. SCFA increase HIF-1α stability in enterocytes which contributes in improving the intestinal barrier function [56]. It will be interesting to know whether SCFA downregulation in COVID-19 contributes towards HIF-1α downregulation in CB and blunting of hypoxia-response.
Gut microbiota produces various neuromodulators [43•,57]. Among the neuronal compounds detected in the rat glomus cells are NO, enkephalins, neurotensins, neuropeptide Y, substance P, dopamine, GABA, vasoactive intestinal peptide and tyrosine hydroxylase [58]. The major catecholamine functional in the CB is dopamine which exerts inhibitory signals to both hypoxia-sensing and ventilatory efforts [9]. Pathogenic Clostridium sp., positively correlated with COVID-19 in elderly people, can synthesize dopamine and thus, possibly impairs hypoxia-sensing. The chemoreceptors present at the cardiorespiratory center of the NTS, the medulla oblongata and the cerebellum are glutaminergic and inhibited by GABA [59]. Enrichment of GABA synthesizing Bacteroides population in COVID-19 might inhibit these neurons impacting O2-sensing [20••,60].
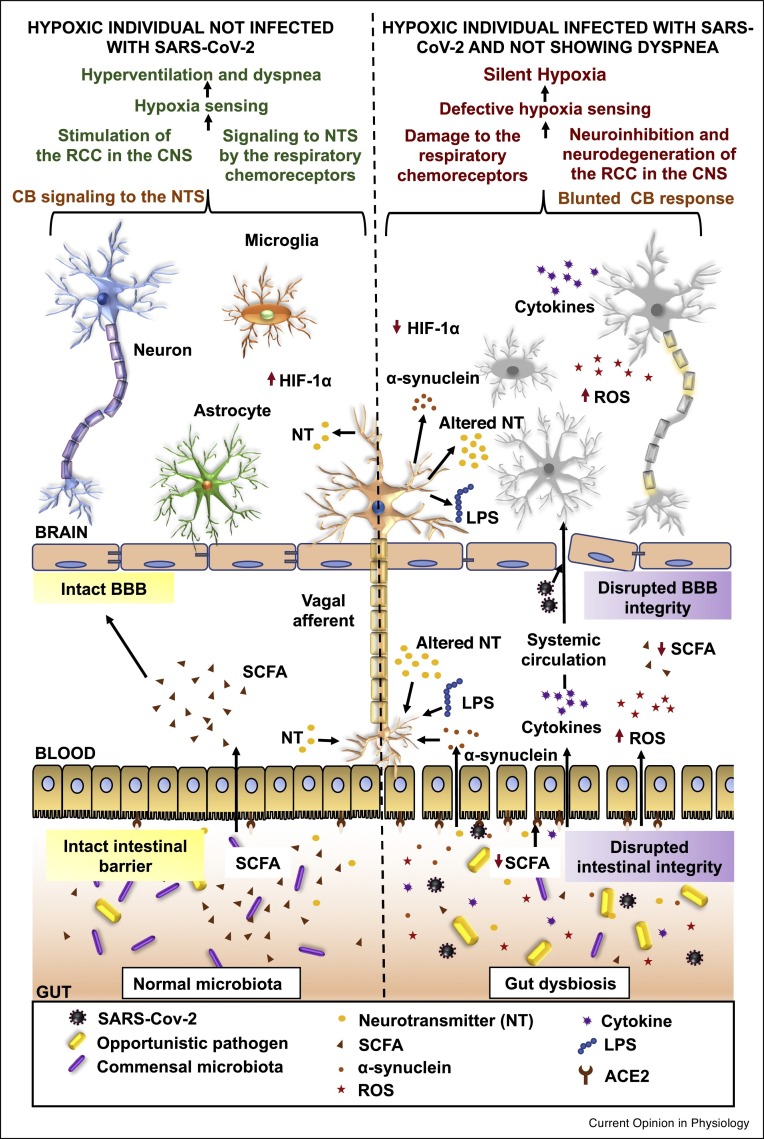
In summary, we theorize that SARS-CoV-2 modulates gut microbes which fine-tune gut-derived metabolites, potentially altering hypoxia-sensing (Figure 2 ).
Figure 2.
Summary figure comparing the gut-brain communication during hypoxia in the uninfected and SARS-CoV-2-infected non-dyspneic hypoxic individuals. The gut microbiota is involved in maintaining the intestinal barrier, the BBB integrity as well as overall homeostasis in the host. In COVID-19, SARS-CoV-2-mediated altered inflammatory and metabolic responses damage the intestinal barrier and the BBB. As a result, in the infected individuals, viral particles, increased inflammatory mediators, ROS, neurotropic gut microbial metabolites and depleted SCFA can cause damage to the central and peripheral neurons involved in hypoxia-sensing.
Conclusions and future remarks
COVID-19-research is still in its nascent stage. The problem associated with silent hypoxia in COVID-19 is the lack of dyspnea which also deters the opportunity to study the gut microbiota-brain axis during this stage. Increased testing can help in identifying infected individuals even if they do not show any respiratory distress and bring them under medical surveillance. Early detection of circulating metabolites in asymptomatic individuals would help in the prediction of silent hypoxia. The focus should be on exploring the reversal of gut dysbiosis in COVID-19 through microbiota-modification therapy (food, prebiotic/probiotic and fecal material transplant) [65] which look promising in reversing gut dysbiosis in several diseases.
Author contribution
Akshita, Soumyadeep and Asima prepared the original draft; reviewing and editing were done by Alok, Pratyush, Pragyesh, Debashish, Supriya, Indrajit, Arup and Asima; artworks were done by Soumyadeep, Pratyush and Alok. The entire work was planned and supervised by Asima. All authors approved this version of the manuscript to be published.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Akshita, Alok, Debashish, Indrajit obtained fellowships from DAE, India. Soumyadeep and Pratyush’s fellowships were supported by DST, India. Supriya’s fellowship was supported by CSIR, India. Infect-eRA/DBT grant (BT/In/Infect-eRA/33/BS/2016-17) to Arup and institutional funding from NISER-Bhubaneswar, DAE to Asima are thankfully acknowledged.
References
- 1.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santamarina M.G., Boisier D., Contreras R., Baque M., Volpacchio M., Beddings I. COVID-19: a hypothesis regarding the ventilation-perfusion mismatch. Crit Care. 2020;24:395. doi: 10.1186/s13054-020-03125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Brouqui P., Amrane S., Million M., Cortaredona S., Parola P., Lagier J.C., et al. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int J Infect Dis. 2021;102:233–238. doi: 10.1016/j.ijid.2020.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study from France involved a large number of COVID-19 patients and observed that most of the admitted COVID-19 patients didn’t face any difficulty in breathing although they had pneumonia. Some patients showed hypocapnic hypoxia but were still nondyspneic. Patients with asymptomatic hypoxia were associated with very poor prognosis.
- 4.Nouri-Vaskeh M., Sharifi A., Khalili N., Zand R. Dyspneic and non-dyspneic (silent) hypoxemia in COVID-19: possible neurological mechanism. Clin Neurol Neurosurg. 2020;198 doi: 10.1016/j.clineuro.2020.106217. 106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor K.M., Lucking E.F., Cryan J.F., O’Halloran K.D. Bugs, breathing and blood pressure: microbiota-gut-brain axis signalling in cardiorespiratory control in health and disease. J Physiol. 2020;598:4159–4179. doi: 10.1113/JP280279. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann J., Mori V., Bates J.H.T., Suki B. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun. 2020;11 doi: 10.1038/s41467-020-18672-6. 4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gourine A.V. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J Physiol. 2005;568:715–724. doi: 10.1113/jphysiol.2005.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prabhakar N.R., Peng Y.J. Peripheral chemoreceptors in health and disease. J Appl Physiol (1985) 2004;96:359–366. doi: 10.1152/japplphysiol.00809.2003. [DOI] [PubMed] [Google Scholar]
- 9.Zera T., Moraes D.J.A., da Silva M.P., Fisher J.P., Paton J.F.R. The logic of carotid body connectivity to the brain. Physiology (Bethesda) 2019;34:264–282. doi: 10.1152/physiol.00057.2018. [DOI] [PubMed] [Google Scholar]
- 10.Ruyle B.C., Klutho P.J., Baines C.P., Heesch C.M., Hasser E.M. Hypoxia activates a neuropeptidergic pathway from the paraventricular nucleus of the hypothalamus to the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol. 2018;315:R1167–R1182. doi: 10.1152/ajpregu.00244.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummins E.P., Strowitzki M.J., Taylor C.T. Mechanisms and consequences of oxygen and carbon dioxide sensing in mammals. Physiol Rev. 2020;100:463–488. doi: 10.1152/physrev.00003.2019. [DOI] [PubMed] [Google Scholar]
- 12.Fukushi I., Pokorski M., Okada Y. Mechanisms underlying the sensation of dyspnea. Respir Investig. 2021;59:66–80. doi: 10.1016/j.resinv.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Tavcar P., Potokar M., Kolenc M., Korva M., Avsic-Zupanc T., Zorec R., et al. Neurotropic viruses, astrocytes, and COVID-19. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.662578. 662578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villadiego J., Ramírez-Lorca R., Cala F., Labandeira-García J.L., Esteban M., Toledo-Aral J.J., et al. Is carotid body infection responsible for silent hypoxemia in COVID-19 patients? Function. 2020;2 doi: 10.1093/function/zqaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porzionato A., Emmi A., Stocco E., Barbon S., Boscolo-Berto R., Macchi V., et al. The potential role of the carotid body in COVID-19. Am J Physiol Lung Cell Mol Physiol. 2020;319:L620–L626. doi: 10.1152/ajplung.00309.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serra D., Almeida L.M., Dinis T.C.P. The impact of chronic intestinal inflammation on brain disorders: the microbiota-gut-brain axis. Mol Neurobiol. 2019;56:6941–6951. doi: 10.1007/s12035-019-1572-8. [DOI] [PubMed] [Google Scholar]
- 17.Kho Z.Y., Lal S.K. The human gut microbiome - a potential controller of wellness and disease. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01835. 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7 doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]; The review summarizes the impact of gut microbiota in shaping the health of humans over their entire course of lives. Gut microbiota-mediated effects are not restricted to their site of residence. Ramifications of gut dysbiosis has been associated with several neurological conditions and disorders.
- 19••.Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors have studied the gut microbiota of COVID-19 patients and correlate with disease severity. Cytokine levels of patients have been assessed and correlated with the immunomodulatory effects of the microbiota. The authors infer that depletion in certain populations of the gut microbiota correlates with more aggressive inflammatory responses in COVID-19.
- 20••.Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors have analyzed the microbiome from the fecal matter of COVID-19 patients. The analysis reveals a correlation between disease severity and gut microbiota composition within patients. Certain members from Bacteroides sp. show a negative correlation with SARS-CoV-2 load in patients. Moreover, cured patients exhibit altered gut microbiota in comparison to healthy persons.
- 21.Viana S.D., Nunes S., Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities — role of gut microbiota dysbiosis. Ageing Res Rev. 2020;62 doi: 10.1016/j.arr.2020.101123. 101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X., Liao B., Cheng L., Peng X., Xu X., Li Y., et al. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. 2020;104:7777–7785. doi: 10.1007/s00253-020-10814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57–69. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumitrescu L., Popescu-Olaru I., Cozma L., Tulba D., Hinescu M.E., Ceafalan L.C., et al. Oxidative stress and the microbiota-gut-brain axis. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/2406594. 2406594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ke Y., Li D., Zhao M., Liu C., Liu J., Zeng A., et al. Gut flora-dependent metabolite trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med. 2018;116:88–100. doi: 10.1016/j.freeradbiomed.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Nuzzo D., Picone P. Potential neurological effects of severe COVID-19 infection. Neurosci Res. 2020;158:1–5. doi: 10.1016/j.neures.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu M.H., Chen I.C., Lee C.H., Wu C.W., Lee Y.C., Kung Y.C., et al. Anti-neuroinflammation ameliorates systemic inflammation-induced mitochondrial DNA impairment in the nucleus of the solitary tract and cardiovascular reflex dysfunction. J Neuroinflammation. 2019;16:224. doi: 10.1186/s12974-019-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Toth M., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009759. 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Follmer C. Viral infection-induced gut dysbiosis, neuroinflammation, and alpha-synuclein aggregation: updates and perspectives on COVID-19 and neurodegenerative disorders. ACS Chem Neurosci. 2020;11:4012–4016. doi: 10.1021/acschemneuro.0c00671. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J., Bi W., Xiao S., Lan X., Cheng X., Zhang J., et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9 doi: 10.1038/s41598-019-42286-8. 5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 33••.Groiss S.J., Balloff C., Elben S., Brandenburger T., Muttel T., Kindgen-Milles D., et al. Prolonged neuropsychological deficits, central nervous system involvement, and brain stem affection after COVID-19-A case series. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.574004. 574004. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors infer that the brainstem function is impaired in COVID-19 and patients experience impediments in cognitive abilities.
- 34.Gandhi S., Srivastava A.K., Ray U., Tripathi P.P. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients? ACS Chem Neurosci. 2020;11:1379–1381. doi: 10.1021/acschemneuro.0c00217. [DOI] [PubMed] [Google Scholar]
- 35.Barreto-Filho J.A., Seabra-Garcez J.D., Garcez F.B., Moreira T.S., Drager L.F. Non-dyspnogenic acute hypoxemic respiratory failure in COVID-19 pneumonia. J Appl Physiol (1985) 2021;130:892–897. doi: 10.1152/japplphysiol.00522.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machado B.H., Paton J.F.R. Relevance of carotid bodies in COVID-19: a hypothetical viewpoint. Auton Neurosci. 2021;233 doi: 10.1016/j.autneu.2021.102810. 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fulling C., Dinan T.G., Cryan J.F. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101:998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 39.McVey Neufeld K.A., Bienenstock J., Bharwani A., Champagne-Jorgensen K., Mao Y., West C., et al. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci Rep. 2019;9 doi: 10.1038/s41598-019-50807-8. 14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baig A.M. Computing the effects of SARS-CoV-2 on respiration regulatory mechanisms in COVID-19. ACS Chem Neurosci. 2020;11:2416–2421. doi: 10.1021/acschemneuro.0c00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu S., Jiang Y., Xu K., Cui M., Ye W., Zhao G., et al. The progress of gut microbiome research related to brain disorders. J Neuroinflammation. 2020;17:25. doi: 10.1186/s12974-020-1705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Caspani G., Swann J. Small talk: microbial metabolites involved in the signaling from microbiota to brain. Curr Opin Pharmacol. 2019;48:99–106. doi: 10.1016/j.coph.2019.08.001. [DOI] [PubMed] [Google Scholar]; The authors have reviewed how gut microbiota can influence the crosstalk between the gut and the CNS. Gut microbes can release as well as modify small molecules such as SCFA and neurotransmitters. These molecules are suggested to be directly or indirectly involved in relaying signals to the brain.
- 44.Markowiak-Kopec P., Slizewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12 doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connor K.M., Lucking E.F., Golubeva A.V., Strain C.R., Fouhy F., Cenit M.C., et al. Manipulation of gut microbiota blunts the ventilatory response to hypercapnia in adult rats. EBioMedicine. 2019;44:618–638. doi: 10.1016/j.ebiom.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oleskin A.V., Shenderov B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb Ecol Health Dis. 2016;27 doi: 10.3402/mehd.v27.30971. 30971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J., Richards E.M., Handberg E.M., Pepine C.J., Raizada M.K. Butyrate regulates COVID-19-relevant genes in gut epithelial organoids from normotensive rats. Hypertension. 2021;77:e13–e16. doi: 10.1161/HYPERTENSIONAHA.120.16647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres-Torrelo H., Ortega-Saenz P., Macias D., Omura M., Zhou T., Matsunami H., et al. The role of Olfr78 in the breathing circuit of mice. Nature. 2018;561:E33–E40. doi: 10.1038/s41586-018-0545-9. [DOI] [PubMed] [Google Scholar]
- 50.Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basseri B., Enayati P., Marchevsky A., Papadakis K.A. Pulmonary manifestations of inflammatory bowel disease: case presentations and review. J Crohns Colitis. 2010;4:390–397. doi: 10.1016/j.crohns.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 52.D’Andrea N., Vigliarolo R., Sanguinetti C.M. Respiratory involvement in inflammatory bowel diseases. Multidiscip Respir Med. 2010;5:173–182. doi: 10.1186/2049-6958-5-3-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neurath M.F. COVID-19 and immunomodulation in IBD. Gut. 2020;69:1335–1342. doi: 10.1136/gutjnl-2020-321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prabhakar N.R., Semenza G.L. Regulation of carotid body oxygen sensing by hypoxia-inducible factors. Pflugers Arch. 2016;468:71–75. doi: 10.1007/s00424-015-1719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Groves H.T., Higham S.L., Moffatt M.F., Cox M.J., Tregoning J.S. Respiratory viral infection alters the gut microbiota by inducing inappetence. mBio. 2020;11 doi: 10.1128/mBio.03236-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J., et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopetuso L.R., Scaldaferri F., Petito V., Gasbarrini A. Commensal clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5:23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonkowski S. Vasoactive intestinal polypeptide in the carotid body-a history of forty years of research. A mini review. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Travagli R.A. In: Encyclopedia of Neuroscience. Binder M.D., Hirokawa N., Windhorst U., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2009. Nucleus tractus solitarii; pp. 2908–2911. [Google Scholar]
- 60.Strandwitz P., Kim K.H., Terekhova D., Liu J.K., Sharma A., Levering J., et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4:396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tao W., Zhang G., Wang X., Guo M., Zeng W., Xu Z., et al. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med Microecol. 2020;5 doi: 10.1016/j.medmic.2020.100023. 100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonazzetti C., Morena V., Giacomelli A., Oreni L., Casalini G., Galimberti L.R., et al. Unexpectedly high frequency of enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: an observational study. Crit Care Med. 2021;49:e31–e40. doi: 10.1097/ccm.0000000000004748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leonard E.M., Salman S., Nurse C.A. Sensory processing and integration at the carotid body tripartite synapse: neurotransmitter functions and effects of chronic hypoxia. Front Physiol. 2018;9 doi: 10.3389/fphys.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Thiel I.A.M., Botschuijver S., de Jonge W.J., Seppen J. Painful interactions: microbial compounds and visceral pain. Biochim Biophys Acta Mol Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2019.165534. 165534. [DOI] [PubMed] [Google Scholar]
- 65.Wilcox M.H., McGovern B.H., Hecht G.A. The efficacy and safety of fecal microbiota transplant for recurrent clostridium difficile infection: current understanding and gap analysis. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa114. ofaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]