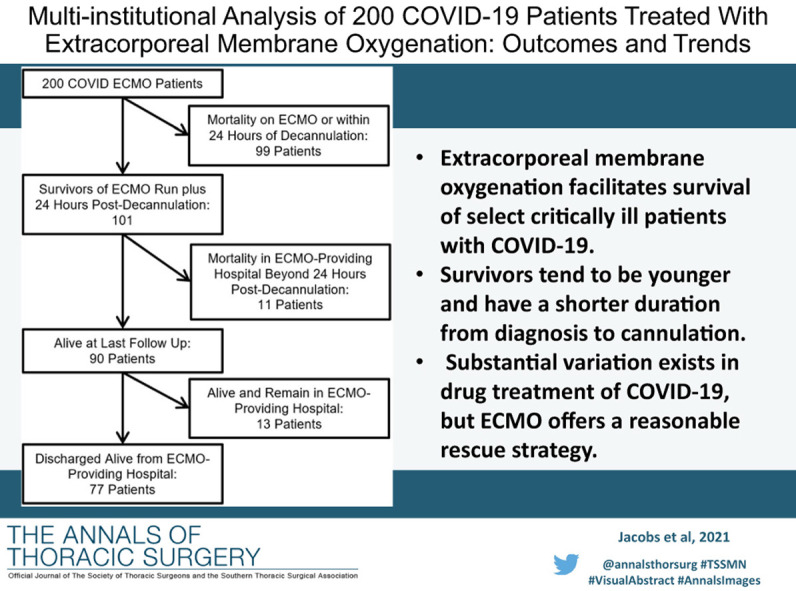
Abstract
Background
The role of extracorporeal membrane oxygenation (ECMO) in the management of patients with COVID-19 continues to evolve. The purpose of this analysis is to review our multi-institutional clinical experience involving 200 consecutive patients at 29 hospitals with confirmed COVID-19 supported with ECMO.
Methods
This analysis includes our first 200 COVID-19 patients with complete data who were supported with and separated from ECMO. These patients were cannulated between March 17 and December 1, 2020. Differences by mortality group were assessed using χ2 tests for categoric variables and Kruskal-Wallis rank sum tests and Welch’s analysis of variance for continuous variables.
Results
Median ECMO time was 15 days (interquartile range, 9 to 28). All 200 patients have separated from ECMO: 90 patients (45%) survived and 110 patients (55%) died. Survival with venovenous ECMO was 87 of 188 patients (46.3%), whereas survival with venoarterial ECMO was 3 of 12 patients (25%). Of 90 survivors, 77 have been discharged from the hospital and 13 remain hospitalized at the ECMO-providing hospital. Survivors had lower median age (47 versus 56 years, P < .001) and shorter median time from diagnosis to ECMO cannulation (8 versus 12 days, P = .003). For the 90 survivors, adjunctive therapies on ECMO included intravenous steroids (64), remdesivir (49), convalescent plasma (43), anti-interleukin-6 receptor blockers (39), prostaglandin (33), and hydroxychloroquine (22).
Conclusions
Extracorporeal membrane oxygenation facilitates survival of select critically ill patients with COVID-19. Survivors tend to be younger and have a shorter duration from diagnosis to cannulation. Substantial variation exists in drug treatment of COVID-19, but ECMO offers a reasonable rescue strategy.
Visual Abstract
Drs Sestokas and Stammers disclose a financial relationship with SpecialtyCare.
The Supplemental Table can be viewed in the online version of this article (10.1016/j.athoracsur.2021.06.026) on http://www.annalsthoracicsurgery.org.
As of January 28, 2021, 100,986,160 patients around the world have been diagnosed with Coronavirus Disease 2019 (COVID-19), with 2,177,611 associated deaths (2.16% mortality worldwide).1 Meanwhile, in the United States, as of January 28, 2021, 25,599,961 patients have been diagnosed with confirmed COVID-19, with 429,214 associated deaths (1.68% mortality in the US).1 Most deaths of patients with COVID-19 are due to severe respiratory failure, with a small group succumbing to combined pulmonary and cardiac failure.2 , 3
We previously published an analysis of our initial 32 COVID-19 patients with severe pulmonary compromise supported with extracorporeal membrane oxygenation (ECMO)4 and concluded that “ECMO may play a useful role in salvaging select critically ill patients with COVID-19. Additional patient experience and associated clinical and laboratory data must be obtained to further define the optimal role of ECMO in patients with COVID-19 and acute respiratory distress syndrome (ARDS). These initial data may provide useful information to help define the best strategies to care for these challenging patients, and may also provide a framework for much-needed future research about the use of ECMO to treat patients with COVID-19.”
Several recently published analyses describe cohorts of COVID-19 patients supported with ECMO.4, 5, 6, 7, 8 Early data from Wuhan, China, reported an alarmingly high rate of mortality of 83% (5 of 6) among COVID-19 patients supported with ECMO.5 , 6 More recent data, however, reveal improved survival of COVID-19 ECMO patients.4 , 7 , 8 Both recent individual institutional reports,7 as well as recent reports from multi-institutional registries.8 present detailed analyses with promising results. Our previous report from our multi-institutional database4 corroborates these findings from individual institutions7 and multi-institutional registries,8 and in addition, provides more granular, detailed information than a large-scale registry and more generalizable information than can be garnered from analysis of a single institution. Clearly, the role of ECMO in the management of severely ill patients with COVID-19 continues to evolve. The purposes of this manuscript are (1) to review our multi-institutional clinical experience based on 200 consecutive patients with confirmed COVID-19 with severe pulmonary compromise who were supported with and separated from ECMO at 29 hospitals; and (2) to document outcomes and trends in management over time.
Material and Methods
A prospective, multi-institutional cohort study was conducted of all patients with confirmed COVID-19 who were supported with ECMO at 29 different hospitals. Supplemental Table 1 documents the regional distribution of these 200 patients at 29 hospitals in 18 states in the United States. A multi-institutional database was created and utilized to assess these patients. This database is prospectively maintained on all patients and has been used for data collection and analysis. The database used is a component of the SpecialtyCare Operative Procedural Registry (SCOPE [https://specialtycareus.com/]). (SpecialtyCare is a US provider of Allied Health services, and the SCOPE registry contains data from more than 1 million perfusion procedures at more than 300 hospitals in more than 40 states. This manuscript describes the experience with ECMO to support a subset of these patients with COVID-19.) Data captured included patient characteristics, pre-COVID-19 risk factors and comorbidities, confirmation of COVID-19 diagnosis, features of ECMO support, specific medications utilized to treat COVID-19, and short-term outcomes through hospital discharge.
This analysis includes our first 200 patients with complete data who had confirmed COVID-19 and were supported with ECMO, starting with our first COVID-19 patient who was placed on ECMO on March 17, 2020, and ending with a patient placed on ECMO on December 1, 2020. These 200 patients include 188 patients supported with venovenous ECMO and 12 patients supported with venoarterial ECMO. The initial cohort included our first 206 patients who had confirmed COVID-19 and were supported with and decannulated from ECMO; 6 patients (1 survivor and 5 nonsurvivors) were excluded from this analysis because of incomplete data. Inclusion in the analysis required complete data in the following fields: ECMO start date; ECMO end date; outcome (alive or dead); no more than one missing pre-COVID comorbidities (asthma, cancer, chronic renal failure, diabetes mellitus, heart disease, hypertension, obesity); and no more than one missing adjunctive therapeutic interventions (antiviral medications, antimalarial medications, convalescent plasma, interleukin-6 blockers, prostaglandin, steroids).
Criteria for placement on ECMO were determined by the individual patient care teams at each of the contributing 29 hospitals; all patients who were supported with ECMO had the diagnosis of COVID-19 with severe respiratory failure deemed to be refractory to conventional management. The decision to initiate ECMO, the mode of therapy (ie, venovenous, venoarterial, and so forth), and the cannulation strategy were each determined by the individual ECMO teams, in keeping with their respective individual institutional protocols and guidelines. Tables 1 and 2 provide P/F ratio, which is defined as the arterial partial pressure of oxygen (Pao 2) of the patient divided by the fraction of inspired oxygen (FIO2, expressed as a decimal) that the patient is receiving.
Table 1.
Overview of 200 Patients With COVID-19 Supported by Extracorporeal Membrane Oxygenation
| COVID Patient Variables | Overall |
|---|---|
| (n = 200) | |
| Nonsurvivors | 110 (55) |
| Survivors | 90 (45) |
| Diagnosis to intubation, d, mean (SD) | 7.45 (6.82) |
| Diagnosis to intubation, d | 6.50 (2-12) |
| Intubation to cannulation, d, mean (SD) | 4.81 (4.72) |
| Intubation to cannulation, d | 4 (1-6) |
| Diagnosis to cannulation, d, mean (SD) | 11.1 (8.22) |
| Diagnosis to cannulation, d | 10 (5-16) |
| ECMO, d, mean (SD) | 20.3 (16.1) |
| ECMO, d | 15 (9-28) |
| ECMO, h, mean (SD) | 475 (386) |
| ECMO, h | 339 (200-670) |
| Age, y, mean (SD) | 49.8 (12.1) |
| Age, y | 51 (40-59) |
| Sex | |
| Female | 62 (31) |
| Male | 138 (69) |
| Asthma | |
| No | 167 (83.5) |
| Yes | 33 (16.5) |
| Cancer | |
| No | 194 (97) |
| Yes | 6 (3) |
| Chronic renal failure | |
| No | 187 (94) |
| Yes | 12 (6.0) |
| Diabetes mellitus | |
| No | 124 (62) |
| Yes | 76 (38) |
| Heart disease | |
| No | 178 (89) |
| Yes | 22 (11) |
| Hypertension | |
| No | 106 (53) |
| Yes | 94 (47) |
| Obesity | |
| No | 72 (36) |
| Yes | 128 (64) |
| One or more comorbid conditions | |
| No | 32 (16) |
| Yes | 168 (84) |
| Prone position before ECMO | |
| No | 73 (36.7) |
| Yes | 126 (63.3) |
| P/F ratioa before ECMO, mean (SD) | 69.5 (27) |
| Tracheostomy performed | |
| No | 130 (65) |
| Yes | 70 (35) |
| Number of circuit changes | 0 (0-1) |
| One of more circuit changes | |
| No | 130 (67.4) |
| Yes | 63 (32.6) |
| CVVH or CRRT used | |
| No | 135 (68.9) |
| Yes | 61 (31.1) |
| ECMO type | |
| Venoarterial | 12 (6) |
| Venovenous | 188 (94) |
| Anticoagulation type | |
| Argatroban | 11 (5.5) |
| Bivalirudin | 28 (14.1) |
| Heparin | 160 (80.4) |
| Antiviral medication | |
| No | 91 (45.5) |
| Yes | 109 (54.5) |
| Convalescent plasma | |
| No | 90 (47.6) |
| Yes | 99 (52.4) |
| Hydroxychloroquine | |
| No | 154 (77) |
| Yes | 46 (23) |
| Interleukin-6 blocker | |
| No | 122 (61.6) |
| Yes | 76 (38.4) |
| Prostaglandin | |
| No | 116 (58.3) |
| Yes | 83 (41.7) |
| Steroids | |
| No | 56 (28) |
| Yes | 144 (72) |
Values are median (interquartile range) or n (%) unless otherwise indicated.
CRRT, continuous renal replacement therapy; CVVH, continuous venovenous hemofiltration; ECMO, extracorporeal membrane oxygenation.
The P/F ratio is the arterial partial pressure of oxygen (Pao2) divided by the fraction of inspired oxygen (Fio2) expressed as a decimal.
Table 2.
Comparison of the 110 Survivors to 90 Nonsurvivors
| COVID Patient Variables | Nonsurvivors |
Survivors |
P Value |
|---|---|---|---|
| (n = 110) | (n = 90) | ||
| Diagnosis to intubation, d, mean (SD) | 9.18 (7.42) | 5.21 (5.24) | .001 |
| Diagnosis to intubation, d | 9.00 (2.25-13.8) | 3.50 (1-9.25) | .005 |
| Intubation to cannulation, d, mean (SD) | 5.30 (5.30) | 4.18 (3.80) | .196 |
| Intubation to cannulation, d | 4 (1-8) | 3 (1-5) | .314 |
| Diagnosis to cannulation, d, mean (SD) | 12.8 (8.96) | 9.10 (6.76) | .001 |
| Diagnosis to cannulation, d | 12 (6-17) | 8 (4-14) | .003 |
| ECMO, d, mean (SD) | 21 (15.9) | 19.3 (16.4) | .472 |
| ECMO, d | 18 (9.25-28) | 12.5 (8-27.2) | .25 |
| ECMO, h, mean (SD) | 493 (381) | 454 (393) | .488 |
| ECMO, h | 412 (217-668) | 292 (192-648) | .251 |
| Age, y, mean (SD) | 52.5 (11.8) | 46.4 (11.7) | <.001 |
| Age, y | 56 (45.2-61) | 47 (36-56.8) | <.001 |
| Sex | |||
| Female | 29 (26.4) | 33 (36.7) | .157 |
| Male | 81 (73.6) | 57 (63.3) | |
| Asthma | |||
| No | 91 (82.7) | 76 (84.4) | .893 |
| Yes | 19 (17.3) | 14 (15.6) | |
| Cancer | |||
| No | 106 (96.4) | 88 (97.8) | .692 |
| Yes | 4 (3.6) | 2 (2.2) | |
| Chronic renal failure | |||
| No | 102 (93.6) | 85 (94.4) | 1 |
| Yes | 7 (6.4) | 5 (5.6) | |
| Diabetes mellitus | |||
| No | 64 (58.2) | 60 (66.7) | .279 |
| Yes | 46 (41.8) | 30 (33.3) | |
| Heart disease | |||
| No | 96 (87.3) | 82 (91.1) | .525 |
| Yes | 14 (12.7) | 8 (8.9) | |
| Hypertension | |||
| No | 59 (53.6) | 47 (52.2) | .955 |
| Yes | 51 (46.4) | 43 (47.8) | |
| Obesity | |||
| No | 37 (33.6) | 35 (38.9) | .534 |
| Yes | 73 (66.4) | 55 (61.1) | |
| One or more comorbid conditions | |||
| No | 15 (13.6) | 17 (18.9) | .416 |
| Yes | 95 (86.4) | 73 (81.1) | |
| Prone position before ECMO | |||
| No | 38 (34.9) | 35 (38.9) | .661 |
| Yes | 71 (65.1) | 55 (61.1) | |
| P/F ratioa pre-ECMO, mean (SD) | 73.1 (31.9) | 64.9 (18.1) | .08 |
| Tracheostomy performed | |||
| No | 76 (69.1) | 54 (60) | .233 |
| Yes | 34 (30.9) | 36 (40) | |
| Number of circuit changes | 0 (0-1) | 0 (0-1) | .914 |
| One or more circuit changes | |||
| No | 71 (67.6) | 59 (67) | 1 |
| Yes | 34 (32.4) | 29 (33) | |
| CVVH or CRRT used | |||
| No | 72 (67.3) | 63 (70.8) | .71 |
| Yes | 35 (32.7) | 26 (29.2) | |
| ECMO type | |||
| Venoarterial | 9 (8.2) | 3 (3.3) | .255 |
| Venovenous | 101 (91.8) | 87 (96.7) | |
| Anticoagulation type | |||
| Argatroban | 6 (5.5) | 5 (5.6) | .961 |
| Bivalirudin | 16 (14.7) | 12 (13.3) | |
| Heparin | 87 (79.8) | 73 (81.1) | |
| Antiviral medication | |||
| No | 50 (45.5) | 41 (45.6) | 1 |
| Yes | 60 (54.5) | 49 (54.4) | |
| Convalescent plasma | |||
| No | 47 (45.6) | 43 (50) | .651 |
| Yes | 56 (54.4) | 43 (50) | |
| Hydroxychloroquine | |||
| No | 86 (78.2) | 68 (75.6) | .787 |
| Yes | 24 (21.8) | 22 (24.4) | |
| Interleukin-6 blocker | |||
| No | 71 (65.7) | 51 (56.7) | .246 |
| Yes | 37 (34.3) | 39 (43.3) | |
| Prostaglandin | |||
| No | 59 (54.1) | 57 (63.3) | .243 |
| Yes | 50 (45.9) | 33 (36.7) | |
| Steroids | |||
| No | 30 (27.3) | 26 (28.9) | .924 |
| Yes | 80 (72.7) | 64 (71.1) |
Values are median (interquartile range) or n (%) unless otherwise indicated.
CRRT, continuous renal replacement therapy; CVVH, continuous venovenous hemofiltration; ECMO, extracorporeal membrane oxygenation.
The P/F ratio is the arterial partial pressure of oxygen (Pao2) divided by the fraction of inspired oxygen (Fio2) expressed as a decimal.
Descriptive analysis of the entire cohort was performed using mean and standard deviation or median and interquartile range (IQR), as appropriate. The primary outcome of interest was mortality during the index hospitalization. Potential differences in categoric variables by mortality group were assessed using χ2 tests, and potential differences in continuous variables by mortality group were assessed using Kruskal-Wallis rank sum tests and Welch’s analysis of variance, as appropriate.
Institutional Review Board approval and waiver of the need for consent were obtained. The human subjects research protocol for this study was reviewed and approved by an independent Institutional Review Board. Institutional Ethics Review Board approval was obtained for the use of data from the SCOPE database (Protocol #012017, ADVARRA Center for IRB Intelligence, Columbia, Maryland).
Results
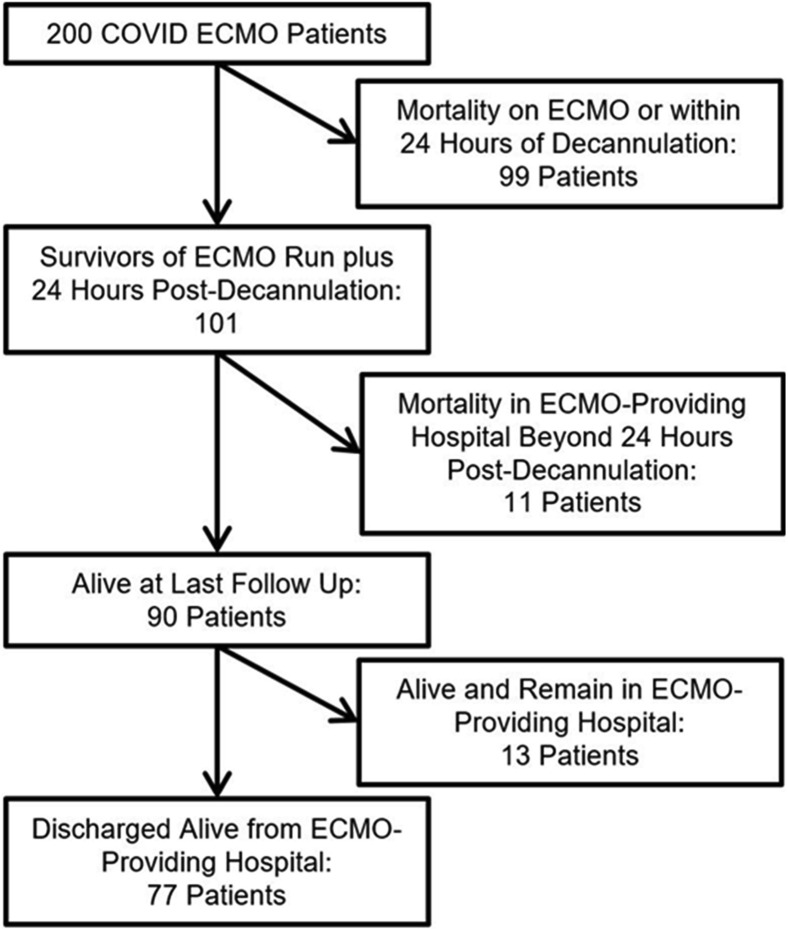
Two hundred consecutive patients with COVID-19 were supported with ECMO at 29 different hospitals. All 200 patients have since been separated from ECMO: 90 patients survived (45%) and 110 patients died (55%). Of the 90 survivors, 77 patients have been discharged from the hospital to date. Table 1 provides detailed data about all 200 patients with COVID-19 treated with ECMO. Of note, of 200 patients, 128 (64%) were obese, 94 (47%) had hypertension, 76 (38%) had diabetes, 33 (16.5%) had asthma, 22 (11%) had heart disease, 12 (6%) had chronic renal failure, and 6 (3%) had cancer. The median time on ECMO was 15 days (IQR, 9 to 28).
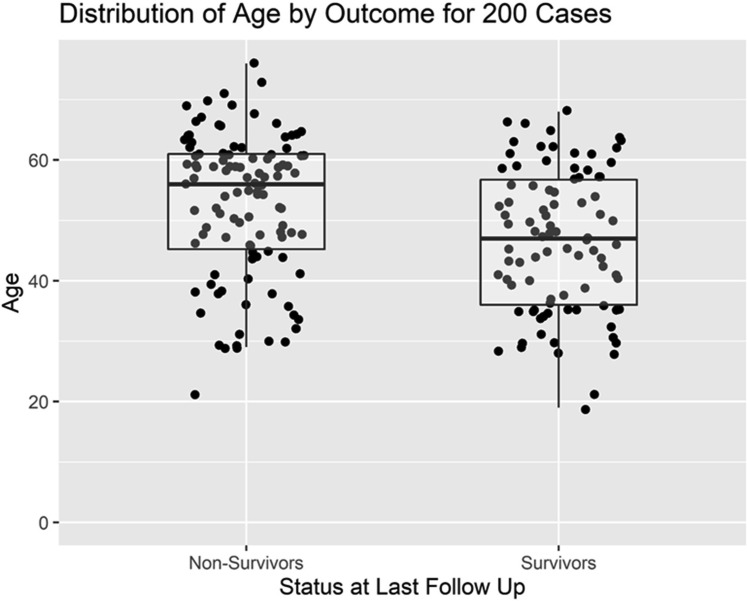
Table 2 provides detailed data comparing the characteristics of 90 survivors with 110 nonsurvivors. Survivors were generally younger, with a lower median age (47 versus 56 years, P < .001). Survivors also had a shorter median interval from the diagnosis of COVID-19 to cannulation for ECMO (8 versus 12 days, P = .003). Although duration on ECMO was shorter among survivors than nonsurvivors, this trend was not statistically significant: median time on ECMO for survivors was 12.5 days (IQR, 8 to 27), and median time on ECMO for nonsurvivors was 18 days (IQR, 9 to 28).
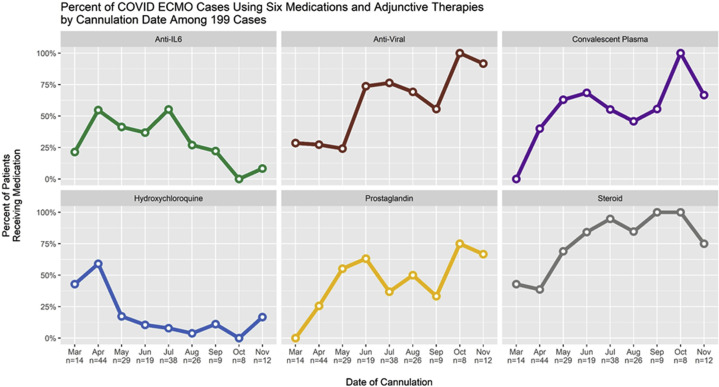
For the 90 surviving patients, adjunctive therapies received while on ECMO were intravenous steroids (64 of 90), antiviral medications (remdesivir [49 of 90]), convalescent plasma (43 of 90), anti-interleukin-6 receptor monoclonal antibodies (tocilizumab or sarilumab [39 of 90]), prostaglandin (33 of 90), and hydroxychloroquine (22 of 90).
This analysis includes all patients with COVID-19 supported with ECMO at the 29 hospitals participating in this study during the period of this analysis. None of these 200 patients was placed on ECMO during cardiopulmonary resuscitation. Extracorporeal cardiopulmonary resuscitation was not utilized for COVID-19 patients at these 29 hospitals. Of 90 survivors, 87 (97%) were supported only with venovenous ECMO. Furthermore, only 3 of 12 patients (25%) supported with venoarterial ECMO survived. Of the 110 patients who died, documented causes of death were respiratory failure (63), multisystem organ failure including acute kidney injury (12), disseminated intravascular coagulation (8), sepsis (7), cardiac arrest (6), cerebral bleeding while on ECMO (5), central nervous system injury (2), air embolism (1), pulmonary embolism (1), pneumothorax (1), and unknown (4).
Figure 1 is a Consolidated Standards of Reporting Trials (CONSORT) flow diagram that depicts the distribution of all 200 patients by category of outcome. Of 90 survivors, 77 have been discharged from the hospital and 13 remain hospitalized at the ECMO-providing hospital. Figure 2 depicts the distribution of the age of the patients, comparing the survivors with the nonsurvivors. Figure 3 depicts the distribution of hours on ECMO, comparing the survivors with the nonsurvivors. Figure 4 depicts the monthly trends over time in the utilization of adjunctive therapies in patients with COVID-19 while supported with ECMO during the 9 months of this analysis.
Figure 1.
Distribution of all 200 patients by category of outcome. (ECMO, extracorporeal membrane oxygenation.)
Figure 2.
Distribution of age of patients, comparing survivors with nonsurvivors.
Figure 3.
Distribution of hours on extracorporeal membrane oxygenation (ECMO), comparing survivors with nonsurvivors.
Figure 4.
Monthly trends over time in the utilization of six adjunctive therapies in patients with COVID-19 while supported with extracorporeal membrane oxygenation (ECMO) during the 9 months of analysis: anti-interleukin-6–receptor monoclonal antibodies (tocilizumab or sarilumab [green line]); antiviral medications (remdesivir [brown line]); convalescent plasma (purple line); hydroxychloroquine (blue line); Flolan (prostaglandin [yellow line]); and intravenous steroids (gray line).
Comment
Our multi-institutional analysis of 200 consecutive COVID-19 patients who were supported with ECMO and subsequently decannulated provides clear evidence that ECMO facilitates salvage and survival of select critically ill patients with COVID-19. Survivors had lower median age (47 versus 56 years, P < .001) and shorter median interval from diagnosis to ECMO cannulation (8 versus 12 days, P = .003). Survival with venovenous ECMO was 87 of 188 patients (46.3%), whereas survival with venoarterial ECMO was 3 of 12 patients (25%). Substantial variation exists in the use of adjunctive drugs and therapies in the treatment of COVID-19, but these findings support the selective use of venovenous ECMO as a reasonable rescue strategy.
Clinical guidelines for the management of patients with COVID-19 have been released by the World Health Organization9 and the Centers for Disease Control and Prevention of the United States.10 The Extracorporeal Life Support Organization11 and the American Society for Artificial Internal Organs12 have also both published guidelines regarding the role of ECMO in treating patients with COVID-19. Nevertheless, the role of ECMO in the management of these challenging patients remains unclear.
Kon and colleagues7 reported a retrospective analysis of all patients with COVID-19 admitted to New York University Langone Health Manhattan campus from March 10, 2020, to April 24, 2020, who were evaluated for ECMO support. Among 321 patients intubated for COVID-19, 77 (24%) were evaluated for ECMO support, and 27 (8.4%) were supported with venovenous ECMO. No patients were supported with venoarterial ECMO. At the time of publication of their manuscript, survival was 96.3%, with only 1 death to date in more than 350 days of total ECMO support. Thirteen patients (48.1%) remained on ECMO support, and 13 patients (48.1%) were successfully decannulated. Of the 13 decannulated patients, 7 (25.9%) were discharged from the hospital and 6 (22.2%) remained in hospital, with 4 on room air. The researchers concluded, “The early outcomes presented here suggest that the judicious use of ECMO support in severe COVID-19 may be clinically beneficial.”7
In contrast, the use of venoarterial ECMO in patients with COVID-19 has been associated with poor survival. Indeed, in patients with COVID-19, if the extent of end organ damage necessitates venoarterial ECMO, then the prognosis is poor in comparison with patients having isolated respiratory dysfunction requiring only venovenous ECMO. Furthermore, if the disease is so severe that the patient has a cardiac arrest refractory to cardiopulmonary resuscitation without ECMO, the patient is unlikely to survive and the use of venoarterial ECMO is likely to be futile.
Barbaro and colleagues8 reported a cohort study of 1035 patients aged 16 years or more with confirmed COVID-19 who had ECMO support initiated between January 16 and May 1, 2020, at 213 hospitals in 36 countries, using data from the Extracorporeal Life Support Organization registry. At the time of publication, of these 1035 patients, 67 (6%) remained hospitalized, 311 (30%) were discharged home or to an acute rehabilitation center, 101 (10%) were discharged to a long-term acute care center or unspecified location, 176 (17%) were discharged to another hospital, and 380 (37%) died. The estimated cumulative incidence of inhospital mortality 90 days after the initiation of ECMO was 37.4% (95% confidence interval, 34.4% to 40.4%). Mortality was 39% (380 of 968) in patients with a final disposition of death or hospital discharge. In the subset of patients receiving venovenous ECMO and characterized as having acute respiratory distress syndrome, estimated inhospital mortality 90 days after the initiation of ECMO was 38% (95% confidence interval, 34.6% to 41.5%). Extracorporeal membrane oxygenation for circulatory support was independently associated with higher inhospital mortality (hazard ratio 1.89; 95% confidence interval, 1.20 to 2.97).
Shih and colleagues13 recently reported an analysis of 37 patients with severe COVID-19 acute respiratory distress syndrome who “were initiated on venovenous ECMO support at one of four ECMO referral hospitals within a large health care system. Initiation of ECMO occurred on median day 11.5 following admission, and, of the successfully decannulated patients, median time on ECMO was 17 days. Survival to discharge from ECMO center has occurred in 21/37 patients (56.8%).” These findings are also consistent with our analysis. Recently, successful transition from the initial intent of bridge to recovery to subsequent bridge to lung transplantation has been described in a small number of patients with COVID-19.14
Value of This Analysis
Our study adds to the body of knowledge and the literature by providing more granular multi-institutional data about our cohort of 200 patients with COVID-19 supported with ECMO at 29 hospitals. As previously described, several published analyses have studied the outcomes of ECMO in patients with COVID-19, and these outcomes have been quite heterogenous.4, 5, 6, 7, 8 Our analysis of the SpecialtyCare SCOPE registry adds another dataset of multi-institutional data to the growing body of literature about the use of ECMO in patients with COVID-19 and demonstrates that support with ECMO facilitates salvage and survival of select critically ill patients with COVID-19.
In our analysis, survival of patients supported with only venovenous ECMO was 46.3% (87 of 188). Survival of patients requiring venoarterial ECMO was poor (3 of 12; 25%). Our finding of higher survival with venovenous ECMO in comparison with venoarterial ECMO in patients with COVID-19 is consistent with the published literature, but is not statistically significant (P = .255). It is likely that if the extent of end organ damage necessitates venoarterial ECMO in patients with COVID-19, then the prognosis is poor in comparison with patients having isolated respiratory dysfunction requiring only venovenous ECMO. Our study also reveals that, not surprisingly, survivors were younger than nonsurvivors (median age 47 for survivors versus 56 years for nonsurvivors, P < .001). This finding is consistent with the study from Barbaro and colleagues,8 in which patients more than 40 years of age had an increasing risk of mortality compared with patients aged 16 to 39 years. Our study also reveals that survivors had a shorter median interval from the diagnosis of COVID-19 to cannulation for ECMO (8 versus 12 days, P = .003). This finding supports earlier consideration for use of ECMO in patients with COVID-19 and severe respiratory failure.
Finally, our study also documents that substantial variation exists in the use of adjunctive therapies in the treatment of COVID-19. The use of these various adjunctive medications and treatments has changed over time as more information has been obtained regarding the role and potential success of these medications.
Future Directions
Much remains to be learned about the role of ECMO in these patients. From our analysis, no specific demographic, clinical, or laboratory data, to date, is predictive of outcome with ECMO in patients with COVID-19, with the exception of younger age. Survivors tend to be younger and have a shorter duration from diagnosis to cannulation. Meanwhile, the role of multiple medications in the treatment of COVID-19 remains unclear: none of the adjunct therapies appeared to be associated with survival.
It is known that COVID-19 patients have faced challenges with thrombosis, and one third of the patients in this series required at least one circuit change. In the more recent era of our series bivalirudin has been used more commonly; however, the impact of the use of bivalirudin versus heparin needs additional investigation.
Several factors provide evidence that COVID-19 is different than other causes of respiratory failure, such as the flu; (1) no cause of respiratory failure has ever generated such a large utilization of ECMO in the history of medicine; (2) no cause of respiratory failure has ever generated this level of concern about the risks to health care providers caring for patients supported with ECMO; and (3) no cause of respiratory failure has ever placed this level of stress and this amount of resource consumption on the health care system.
Nevertheless, we believe that many of the lessons that have been learned by caring for COVID-19 patients supported with ECMO will likely be applicable to a variety of other etiologies of respiratory failure, now and in the future, as exemplified by the following lessons: (1) Earlier initiation of ECMO for patients with COVID-19 and respiratory failure appears to be associated with better outcomes, and this finding is likely true for other forms of respiratory failure as well. (2) Prolonged venovenous ECMO runs allow for the recovery of the native lungs in some patients with COVID-19 and facilitate bridge to lung transplantation in others. The use of such prolonged venovenous ECMO runs to support adults with respiratory failure is likely to become more common secondary to these valuable lessons learned during the COVID-19 pandemic.
Study Limitations
This analysis is based on the available data in our database. Potential limitations include patient selection bias, institutional bias, confounding bias, and potential underpowering of the analysis. Additional follow-up is required on all surviving patients. Further patient accrual will enhance continued analysis of outcomes. We plan to continue gathering data to provide additional insight into guideposts for patient selection and predictors of outcomes. It is our hope that by sharing our experience, other centers and patients may benefit.
Conclusion
Our experience and analysis of 200 consecutive patients at 29 hospitals reveal that ECMO facilitates salvage and survival of select critically ill patients with COVID-19. Survivors tend to be younger. Survival of patients supported with only venovenous ECMO is 46.3% in our cohort. Survivors had a shorter median interval from the diagnosis of COVID-19 to cannulation for ECMO, supporting earlier consideration for use of ECMO in patients with COVID-19 and severe respiratory failure. Substantial variation exists in drug treatment of COVID-19, but ECMO offers a reasonable rescue strategy. Additional gathering and analysis of data will inform appropriate selection of patients and provide guidance as to best use of ECMO in terms of timing, implementation, duration of support, and best criteria for discontinuation. Expansion of studies such as the current analysis presented here will provide a means to further define the role of ECMO in the management of severely compromised patients with COVID-19 and will serve to refine the optimal use of ECMO in these patients, with the goal of continuing to enhance survival.
Supplementary Data
References
- 1.Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) https://coronavirus.jhu.edu/map.html Available at:
- 2.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2019;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 3.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs J.P., Stammers A.H., St Louis J., et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: experience with 32 patients. ASAIO J. 2020;66:722–730. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry B.M. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. doi: 10.1016/S2213-2600(20)30119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kon Z.N., Smith D.E., Chang S.H., et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg. 2021;111:537–543. doi: 10.1016/j.athoracsur.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbaro R.P., MacLaren G., Boonstra P.S., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance, 13 March 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf Available at:
- 10.Centers for Disease Control and Prevention Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html Available at:
- 11.Bartlett R.H., Ogino M.T., Brodie D., et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopal K., Keller S., Akhanti B., et al. Advanced pulmonary and cardiac support of COVID-19 patients: emerging recommendations from ASAIO—a “living working document. ASAIO J. 2020;66:588–598. doi: 10.1097/MAT.0000000000001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih E., DiMaio J.M., Squiers J.J., et al. Venovenous extracorporeal membrane oxygenation for patients with refractory coronavirus disease 2019 (COVID-19): multicenter experience of referral hospitals in a large health care system. J Thorac Cardiovasc Surg. 2022;163:1071–1079.e3. doi: 10.1016/j.jtcvs.2020.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs J.P., Falasa M., Machuca T.N. Commentary: extracorporeal membrane oxygenation for patients with refractory coronavirus disease 2019 (COVID-19): what do we know and what do we need to learn? J Thorac Cardiovasc Surg. 2022;163:1080–1082. doi: 10.1016/j.jtcvs.2020.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.