Purpose
The objectives were to evaluate dosing schedules of labetuzumab govitecan, an antibody-drug conjugate targeting carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) for tumor delivery of 7-ethyl-10-hydroxycamptothecin (SN-38), in an expanded phase II trial of patients with relapsed or refractory metastatic colorectal cancer.
Patients and Methods
Eligible patients with at least one prior irinotecan-containing therapy received labetuzumab govitecan once weekly at 8 and 10 mg/kg, or two times per week at 4 and 6 mg/km on weeks 1 and 2 of 3-week repeated cycles. End points were safety, response, pharmacokinetics, and immunogenicity.
Results
Eighty-six patients who had undergone a median of five prior therapies (range, one to 13) were each enrolled into one of the four cohorts. On the basis of Response Evaluation Criteria in Solid Tumors 1.1, 38% of these patients had a tumor as well as plasma carcinoembryonic antigen reduction from baseline after labetuzumab govitecan treatment; one patient achieved a partial response with a sustained response spanning > 2 years, whereas 42 patients had stable disease as the best overall response. Median progression-free survival and overall survival were 3.6 and 6.9 months, respectively. The major toxicities (grade ≥ 3) among all cohorts were neutropenia (16%), leukopenia (11%), anemia (9%), and diarrhea (7%). The antibody-drug conjugate’s mean half-life was 16.5 hours for the four cohorts. Anti-drug/anti-antibody antibodies were not detected. The two once-weekly dose schedules, showing comparable toxicity and efficacy, were chosen for further study.
Conclusion
Monotherapy with labetuzumab govitecan demonstrated a manageable safety profile and therapeutic activity in heavily pretreated patients with metastatic colorectal cancer, all with prior irinotecan therapy. Further studies of labetuzumab govitecan treatment alone or in combination with other therapies in earlier settings are indicated.
INTRODUCTION
In the United States, an estimated 134,490 patients were diagnosed with colorectal cancer (CRC) in 2016, and 49,190 died.1,2 At least 50% of patients with CRC develop metastases, with a 5-year survival rate of 12.5%.2,3 Treatment of metastatic CRC (mCRC) includes chemotherapy; monoclonal antibodies targeting vascular endothelial growth factor or epidermal growth factor receptor; and the recently approved oral therapies regorafenib, trifluridine, and tipiracil.3-6 These agents, given in combination or sequentially, yield survival approaching 3 years7,8; further advances will require new approaches.
An antibody-drug conjugate (ADC) could deliver cytotoxic agents directly to tumors while minimizing systemic toxicity.9-12 Although irinotecan is a potent drug, it has significant GI and hematologic toxicity.13-15 Because the topoisomerase-I inhibitor, 7-ethyl-10-hydroxycamptothecin (SN-38), is the active metabolite of irinotecan,14 we demonstrated that ADCs using SN-38 have promising activity in several solid tumor xenograft models.16-20 Labetuzumab is a slowly internalizing humanized antibody whose clinical safety and antitumor activity as a radioconjugate have been reported.21-23 This agent targets the carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) (CD66e) antigen expressed on many solid cancers,24,25 including > 80% of CRCs.24,25 We developed labetuzumab govitecan (IMMU-130), an ADC that uses a proprietary linker to site-specifically couple SN-38 to labetuzumab.16,26 This results in the delivery of much higher amounts of active SN-38 to tumors than systemic irinotecan, while producing lower serum levels of glucuronidated SN-38 (SN-38G),27 potentially reducing the occurrence of diarrhea.28
The first clinical study of labetuzumab govitecan evaluated doses given every 14 days to patients with mCRC who had been treated previously with an irinotecan-containing regimen.29 Neutropenia was dose-limiting, and 16 mg/kg was the maximum tolerated dose (MTD). Disease stabilization was observed, and one patient receiving 18 treatments experienced a partial response (PR) lasting 4.7 months, including an 87% decrease in plasma carcinoembryonic antigen (CEA).29 SN-38 is most effective in S-phase cells, and because preclinical studies suggested that more frequent dosing might be more efficacious,26 this study was undertaken to evaluate two intensified dose regimens.
PATIENTS AND METHODS
This was a phase I/II, open-label, multicenter trial in heavily pretreated patients with relapsed or refractory mCRC who had received at least one prior irinotecan-containing regimen. The primary objective was to evaluate the safety and tolerability of two schedules, each with two doses. Secondary objectives included assessment of efficacy, pharmacokinetics (PK), and immunogenicity.
Patients ≥ 18 years of age with mCRC with measurable disease but no lesion ≥ 10 cm were enrolled. Requirements included CEA serum levels > 5 ng/mL but < 1,000 ng/mL, Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate laboratory values. Other eligibility criteria included those reported previously for sacituzumab govitecan.30 The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients signed informed consent, and the protocols were approved by responsible investigational review boards.
Labetuzumab govitecan was administered by intravenous infusion either once weekly or twice weekly on weeks 1 and 2 of 3-week cycles and continued until progression, withdrawal of consent, or intolerance. Toxicities were managed by supportive hematopoietic growth-factor therapy, dose delay or reduction as specified in the protocol, and standard supportive care. Safety evaluations occurred weekly, with adverse events (AEs) defined by Medical Dictionary for Regulatory Activities Preferred Term and System Organ Class, version 10, and graded in accordance with the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0. The UGT1A1 genotype was determined at study entry; serum samples were evaluated for human antihuman antibody at baseline and during treatment, and computed tomography (CT) scans (chest, abdomen, pelvis, and other involved areas) and serum CEA levels were assessed at baseline and every 8 weeks until progression. Blood for PK studies was collected before and 30 minutes after each infusion, with additional samples between infusions in select patients.
Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 were used to categorize overall best response as a confirmed complete response or PR, stable disease (SD), or disease progression (PD),31 with progression-free survival (PFS) measured from treatment initiation until PD or death from any cause, and overall survival (OS) from treatment initiation to death from any cause. An enzyme-linked immunosorbent assay (ELISA), similar to the one reported previously,30 measured serum levels of human antibodies against labetuzumab govitecan, and bioanalytical assays measured serum levels of labetuzumab govitecan, total antibody (labetuzumab), unconjugated drug (SN-38Free and SN-38GFree), and total drug (SN-38Total and SN-38GTotal).30,32 PFS and OS were estimated by Kaplan-Meier methods (MedCalc Statistical Software version 16.4.3; MedCalc Software, Ostend, Belgium), PK parameters by noncompartmental methods (PK Solutions 2.0; Summit Research Services, Montrose, CO), and other results by descriptive statistics.
On the basis of initial clinical findings, where a single dose of 16 mg/kg given every 3 weeks was tolerated safely,29 6 mg/kg was selected as a starting dose for twice weekly dosing, and 8 mg/kg for once weekly dosing. Phase I dose escalation used a 3 + 3 design to determine the MTD for each dosing schedule. The MTD was defined as the highest dose level for which zero or one of six patients encountered dose-limiting toxicity (DLT) during cycle 1, with DLT defined as grade 4 neutropenia for ≥ 5 days; grade ≥ 3 thrombocytopenia or anemia for ≥ 5 days; grade ≥ 3 nausea, vomiting, or diarrhea for > 48 hours; or any other grade ≥ 3 nonhematologic toxicity, including infusion reactions after premedication with antihistamines, H2 blockers, and steroids. Treatment started at 6 mg/kg in the twice-weekly regimen, with adjustment to increase to 9 mg/kg or decrease to 4 mg/kg if necessary, and 8 mg/kg in the once-weekly regimen, with contingencies to study 10, 12, 14, and 16 mg/kg doses or a lower level of 6 mg/kg. For both dosing schedules, enrollment was expanded in phase II, targeting up to 25 patients in two dose levels at or below the MTD to estimate whether this single-agent treatment provides an objective response rate of ≥ 15% in this population. This estimate was derived from the results of the prior phase I trial.29
RESULTS
Patient Disposition and Treatment
Ninety-one patients were enrolled between February 2013 and January 2016. In the twice-weekly cohort, doses of 6 and 9 mg/kg were given, with the first evidence of toxicity occurring at 9 mg/kg, where two of three patients could not complete cycle 1 without dose reduction because of dose-limiting neutropenia. The 6 mg/kg dose was subsequently declared the MTD after zero of six DLTs occurred during cycle 1, and 4 mg/kg was also selected subsequently for expanded bi-weekly enrollment in phase II because of the intention to have repeated therapy cycles. In the once-weekly cohort, patients in phase I had zero of three DLTs at 8 mg/kg and one of six DLTs (grade 4 neutropenia > 7 days) at 10 mg/kg. One patient also received 12 mg/kg without DLT, but this higher dose was not pursued. Thus, the 8 and 10 mg/kg once-weekly doses were selected for enrollment in phase II.
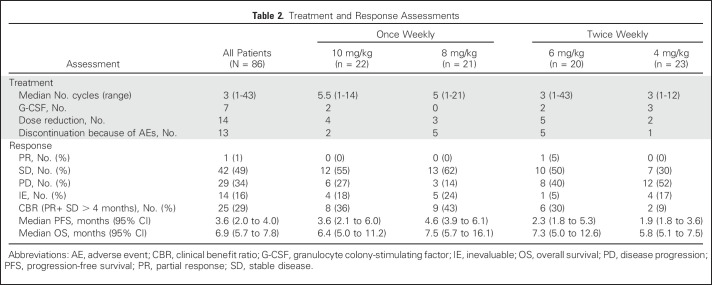
In total, 86 patients were enrolled in the four expanded cohorts that are the focus of this report; they received labetuzumab govitecan either twice weekly at a dose level of 4 mg/kg (n = 23) or 6 mg/kg (n = 20), or once weekly at a dose level of 8 mg/kg (n = 21) or 10 mg/kg (n = 22). Patient demographics and baseline characteristics are listed in Table 1. All 86 patients have now discontinued treatment after a median of three treatment cycles (range, one to 43). Seven patients received hematopoietic cytokine support for neutropenia (five only once; two repeatedly), but only after experiencing a grade 3 event. Neutropenia was also the primary indication for a 25% dose reduction, which occurred once in 14 patients; additional reductions were infrequent. Most patients were treated until radiologic PD, but 13 withdrew from the study because of AEs (neutropenia, small bowel obstruction, nausea and vomiting, constipation, hyperbilirubinemia, or elevated liver function test [one each] or clinical deterioration [two patients], including patients with new brain or osteolytic lesions). No treatment-related deaths occurred. Treatment metrics are listed for all 86 patients and each dose cohort in Table 2.
Table 1.
Demographics and Baseline Characteristics (N = 86)
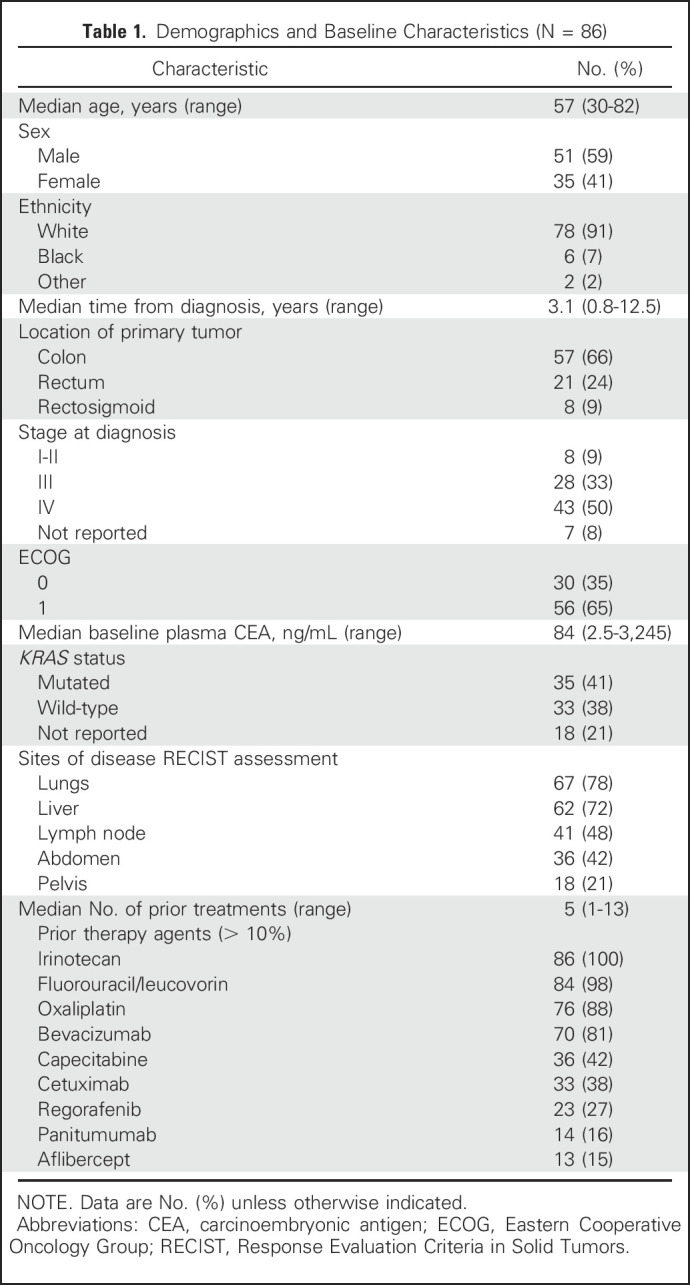
Table 2.
Treatment and Response Assessments
Safety
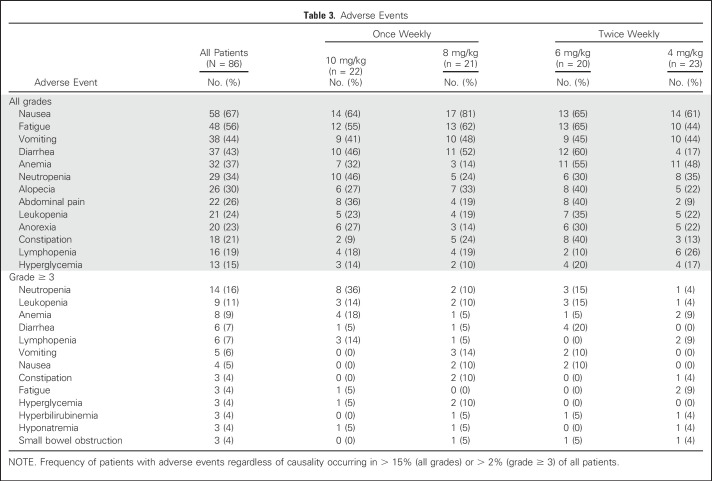
The most frequent AEs were nausea, fatigue, vomiting, and diarrhea. The most frequent grade ≥ 3 events were neutropenia (16%), leukopenia (11%), anemia (9%), and diarrhea (7%; Table 3). Overall, toxicity rates seem to be similar among the four cohorts, and although grade ≥ 3 neutropenia appears more frequent in the 10 mg/kg once-weekly group and grade ≥ 3 diarrhea in the 6 mg/kg twice-weekly group, the small numbers in each cohort limit definitive conclusions. UGT1A1 status was determined in 54 patients as 1*1* (n = 27), 1*28* (n = 23) or 28*28* (n = 4), with grade ≥ 3 neutropenia occurring in two of 27 (7%), three of 23 (13%), and one of four (25%) patients, respectively, and febrile neutropenia and diarrhea occurring in one patient in each of the 1*1* and 1*28* cohorts, but not reported in the 28*28* patients.
Table 3.
Adverse Events
Antitumor Activity
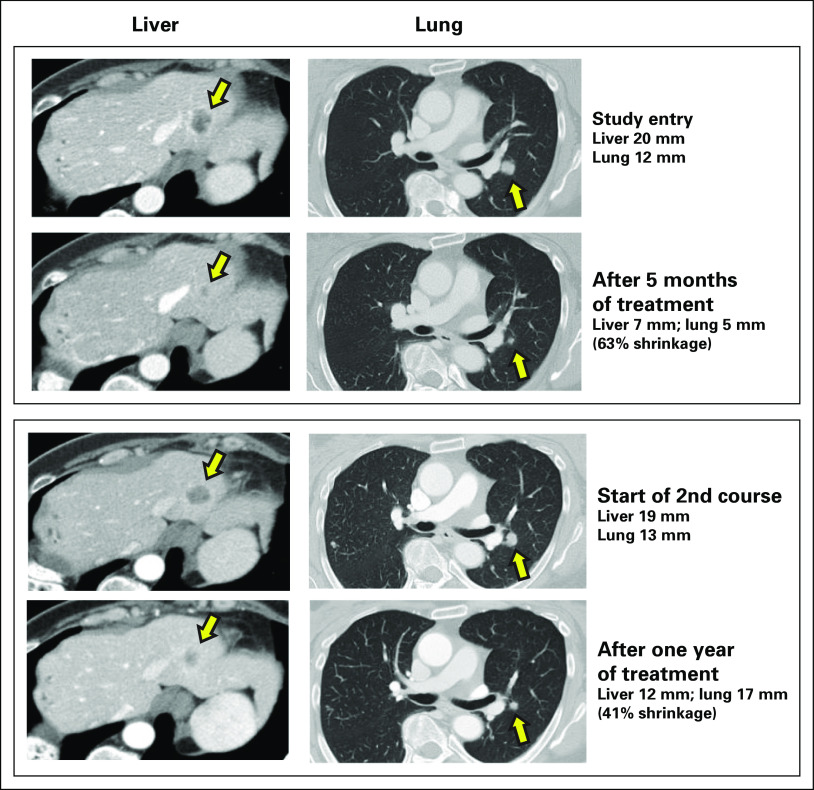
Of the 86 patients, 14 did not have any postbaseline radiologic assessments for tumor response. This included one patient who withdrew after cycle 1 because of hyperbilirubinemia and underwent imaging that was unchanged from baseline but too early to qualify as SD by RECIST1.1; one patient who had unevaluable images after a collapsed lung obscured target pulmonary lesions; and 12 other patients who withdrew during cycles 1 or 2 before any postbaseline imaging because of disease-related complications. Of the other 72 patients with CT-assessable responses, 38% (27 of 72) had a reduction from baseline of the sum of diameters of their target lesions after treatment. One patient, who had received only fluorouracil, leucovorin, and irinotecan plus bevacizumab for metastatic disease and remained progression free until approximately 1 year later, achieved a confirmed PR by RECIST1.1. Otherwise, 42 patients had a best response of SD, and 29 had PD at first postbaseline assessment. The single PR lasted for 13 months, with an 88% reduction of lung and liver lesions and an 88.6% reduction in plasma CEA (from 21.0 to 2.4 ng/mL). After the patient took a 2-month drug holiday, a comparable response to the regrown liver and lung metastases occurred after treatment resumed, spanning a period of 2.7 years in total (Fig 1). In addition to radiologic tumor reduction from baseline in 38% of patients undergoing sequential CT scans, 38% of 66 patients with elevated CEA titers also exhibited a reduction in their postbaseline serum CEA levels.
Fig 1.
This 51-year-old woman was initially diagnosed with rectal adenocarcinoma, stage IIIb. After primary surgery, she received infusional fluorouracil, leucovorin, and oxaliplatin, capecitabine, and local radiation. Approximately 1.5 years later, she underwent a right hepatectomy for liver recurrence, followed by 12 cycles of fluorouracil, leucovorin, and irinotecan with bevacizumab. Her disease progressed approximately 1 year later and she entered the study with a plasma carcinoembryonic antigen (CEA) level of 21 ng/mL and multiple pulmonary and hepatic metastases, including two target lesions for Response Evaluation Criteria in Solid Tumors 1.1 response assessment: a 12-mm left perihilar lesion and a 20-mm lesion at the hepatic dome. She responded to treatment with 6 mg/kg twice-weekly labetuzumab govitecan with a 25% reduction of target lesions at first postbaseline assessment and with a partial response with a 46% reduction at 3 months, which was confirmed with a 63% reduction on subsequent assessment. After 13 months of treatment, there had been a 88% reduction of target lesions, including complete disappearance of the liver lesion, and the plasma CEA was reduced to 2.4 ng/mL. Because of the demands of the twice-weekly regimen over this period, she took a 3-month drug holiday, after which her disease returned with a plasma CEA level of 37.6 ng/mL, a 13-mm perihilar lesion, and a 19-mm hepatic dome lesion. She then resumed treatment, but at 8 mg/kg once-weekly, and again responded, with onset of a partial response at 4 months (41% reduction). One year after resuming treatment, her plasma CEA was 5.3 ng/mL and she continued to maintain 41% shrinkage, eventually progressing 6 months later. During the entire course of her treatment, which spanned 2.7 years and consisted of > 40 treatment cycles, no antilabetuzumab or anti–SN-38 antibodies were detected.
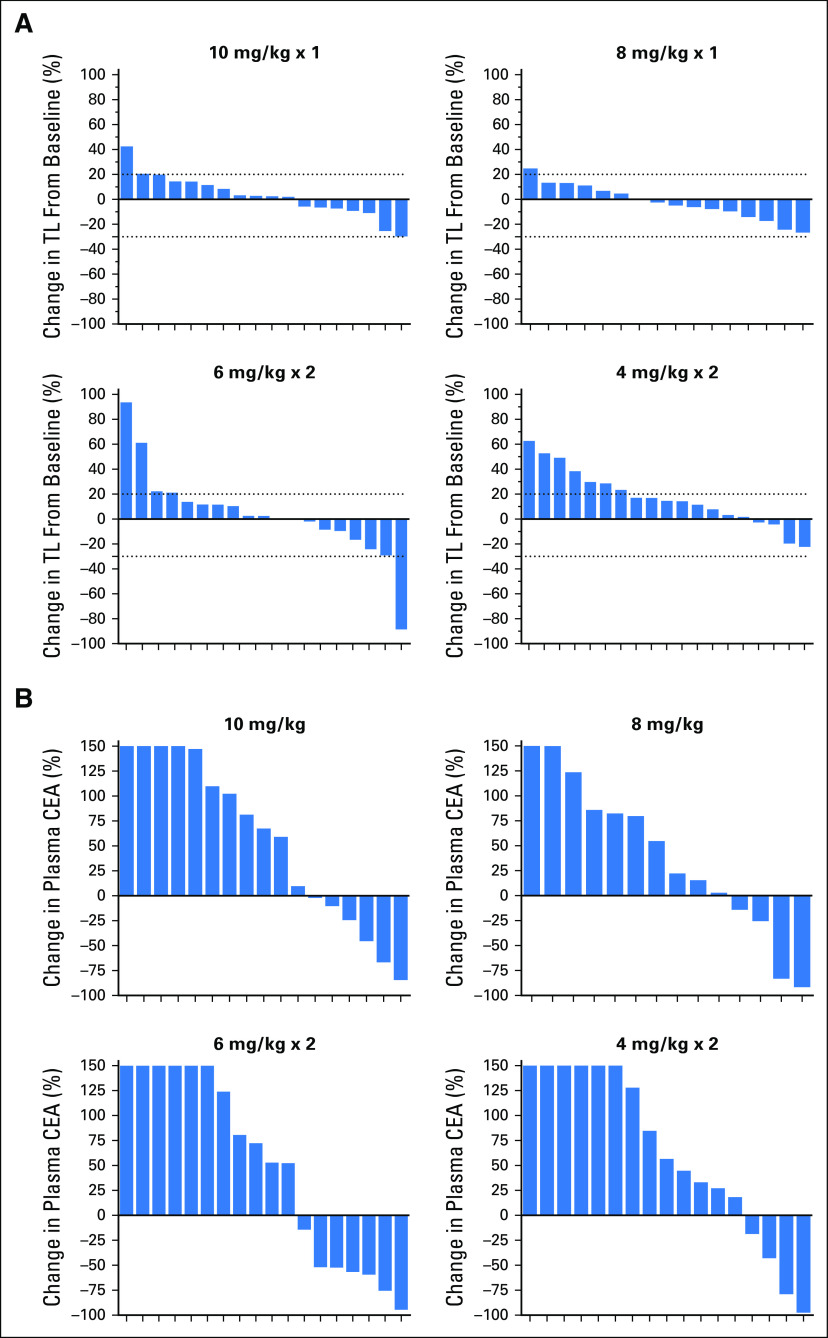
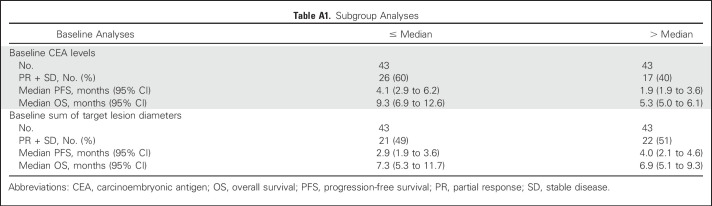
Because 24 patients had SD lasting at least 4 months, the clinical benefit rate (PR + SD ≥ 4 months) was 29% (25 of 86). The median PFS for all 86 patients was 3.6 months (95% CI, 2.0 months to 4.0 months), with 16% (14 of 86) remaining progression free for at least 6 months, including three patients who maintained this status for at least 1 year. The median OS was 6.9 months (95% CI, 5.7 months to 7.8 months), with 24% (21 of 86) surviving for at least 1 year, including three patients who survived at least 2 years (one for 3 years). Prior treatment in these 24 patients included bevacizumab, fluorouracil, irinotecan and oxaliplatin-containing chemotherapies, and regorafenib. In the regorafenib subset (n = 23), the median PFS and OS were 3.9 and 6.7 months, respectively. Additional exploratory analyses found that plasma CEA levels predicted better PFS and OS, but no substantial association was seen between tumor size (as measured by the sum of baseline target lesion diameters) or baseline KRAS mutation status and PFS or OS (Appendix Tables A1 and A2, online only). Waterfall plots of tumor and CEA plasma level reduction (38% each) provide evidence of treatment activity in all four dose groups (Fig 2). Response metrics for each group are listed in Table 2, with Kaplan-Meier PFS and OS graphs for each dose group presented in Figure 3.
Fig 2.
Waterfall plots for the four dose groups treated with labetuzumab govitecan once weekly at 8 or 10 mg/kg or twice weekly at 4 or 6 mg/kg. (A) Percent change from baseline of the sum of target lesion (TL) diameters at time of best response for patients with computed tomography–assessable responses. (B) Percent change in plasma carcinoembryonic antigen (CEA) levels from baseline to time of best response for patients with one or more postbaseline CEA values.
Fig 3.
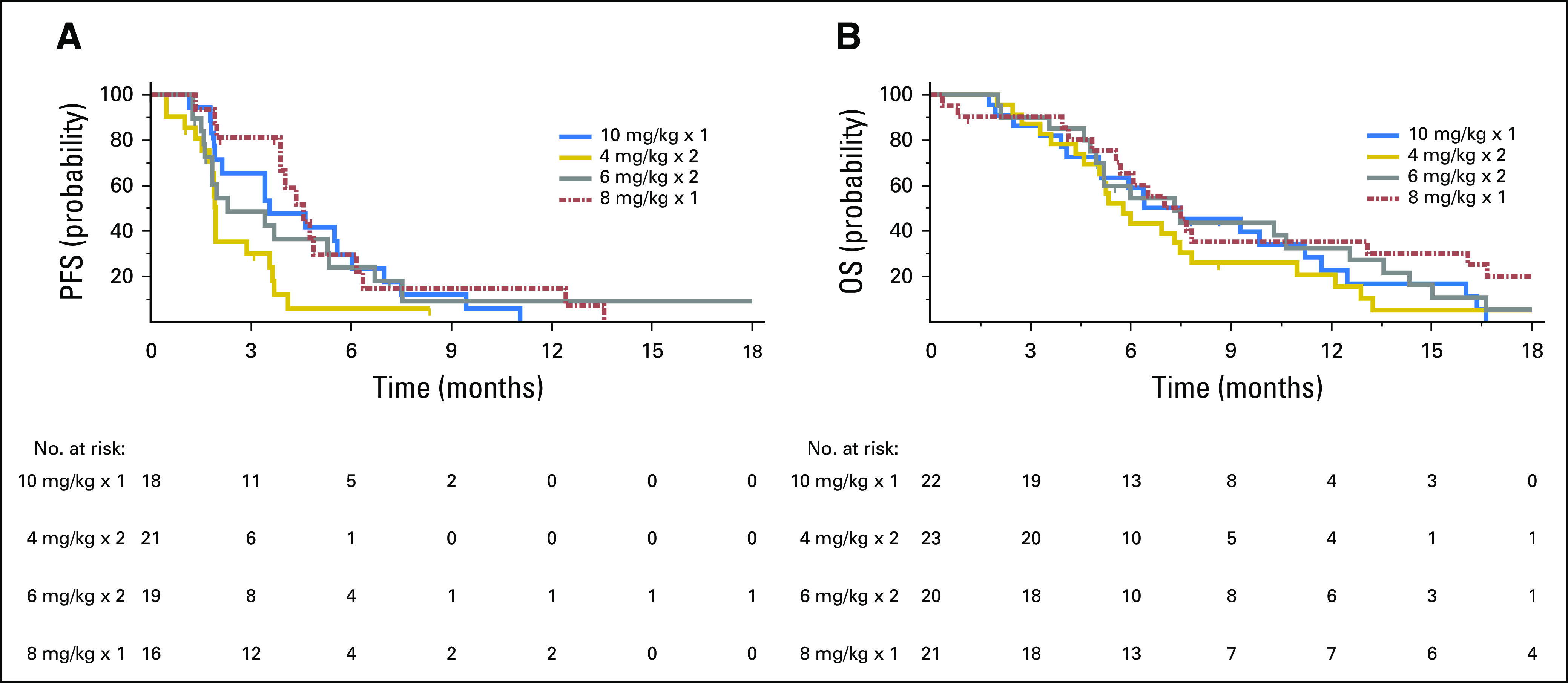
(A) Progression-free survival (PFS) and (B) overall survival (OS) in patients with refractory metastatic colorectal cancer treated with labetuzumab govitecan once weekly at 8 or 10 mg/kg or twice weekly at 4 or 6 mg/kg. Of the 86 patients, 72 continued on-study until progressive disease was documented radiologically at a tumor response assessment, whereas the other 21 patients discontinued study participation before radiologic confirmation of progression and were censored for PFS at the time of their most recent radiologic evaluation. Similarly, 78 of the 86 patients were observed until death, whereas eight patents were lost to follow-up and were censored for OS at last study evaluation.
PK and Immunogenicity
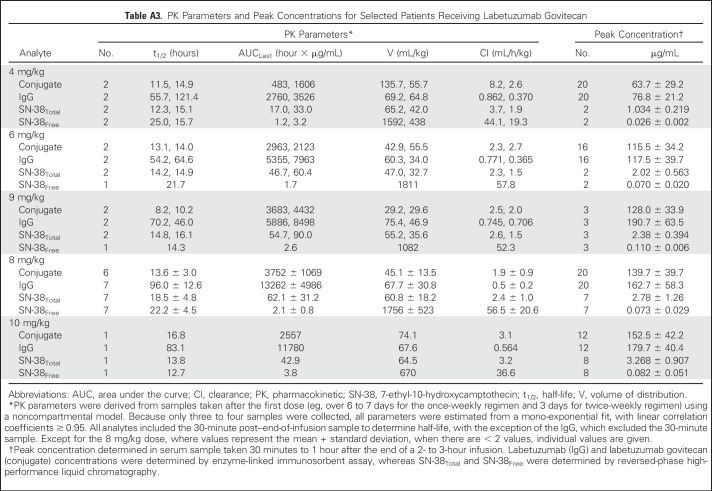
Fourteen patients provided PK profiles for the four primary analyses (Appendix Table A3, online only). Peak drug levels generally increased with dose, with no appreciable differences in clearance between doses. Labetuzumab govitecan was cleared from serum more rapidly than was the IgG. Enzyme-linked immunosorbent assay and high-performance liquid chromatography data monitoring of SN-38Total provided similar estimates of the conjugate’s mean half-life (16.5 ± 4.0 hours by high-performance liquid chromatography analysis), whereas the IgG’s half-life was more variable (84.5 ± 23.6 hours). In patients treated bi-weekly, residual IgG in the serum at the time of the second dose increased peak IgG levels by approximately 42% compared with the first dose, whereas in patients who treated once-weekly, peak levels increased by approximately 20%. Little to no residual ADC or its components was detected 3 days after a dose. In samples assayed for SN-38, SN-38Free never exceeded 5% of SN-38Total, indicating that most SN-38 was bound to IgG. Furthermore, SN-38GFree could be detected in the 30-minute and day-1 serum samples from only nine of 14 patients and was always less than (nonglucuronidated) SN-38Free (eg, 19.9% ± 5.8% and 35.8% ± 12.6%, respectively).
Antibody responses against labetuzumab and SN-38 were evaluated in 460 samples (including baseline) from 84 patients (66 patients had more than one post-treatment sample; 26 had more than five). All samples were negative (below assay sensitivity) for antibodies to labetuzumab or SN-38.
DISCUSSION
Labetuzumab govitecan is an ADC that incorporates a moderately toxic drug, SN-38, with an antibody against CEACAM5, the same CEA measured in plasma and having a high expression in many solid cancers, particularly CRC.33,34 This phase I/II study enrolled patients with progressive disease who had received prior therapy with an irinotecan-containing regimen; one half of these patients had completed five prior lines of therapy. Waterfall plots of tumor reduction and plasma CEA reductions (both in 38% of patients) provided objective evidence of treatment activity in all four dose groups. One patient achieved a PR by RECIST1.1 and achieved another PR after a second course of therapy following a treatment break, with the entire treatment spanning 2.7 years.
This ADC was well tolerated, with a manageable toxicity profile. Generally, in this advanced and refractory population, labetuzumab govitecan provided encouraging clinical activity in the form of SD. Median PFS was 4.6 and 3.6 months and median OS was 7.5 and 6.4 months for the preferred doses of 8 or 10 mg/kg, respectively, given once weekly. These initial results suggest that additional study of this agent in combination with other therapies would be appropriate, especially because preclinical studies indicated that its combination with bevacizumab is effective.26
Although this trial evaluated labetuzumab govitecan in patients who had undergone a median of five prior therapies, it is interesting to speculate on its potential in a third-line setting, where regorafenib currently is recommended on the basis of the CORRECT trial reporting a median PFS and median OS of 1.9 and 6.4 months, respectively, in 505 patients treated in at least third line (97% of patients).35 In this study, patients given labetuzumab govitecan after regorafenib had a median PFS of 4.0 months and an OS of 6.7 months. This compares well to the median PFS and median OS of 1.6 and 2.1 months, respectively, reported in a retrospective analysis of standard chemotherapy given after regorafenib, mostly with fourth-line therapy.4
Irinotecan dose-limiting toxicities (neutropenia and diarrhea) are related to SN-38 exposure.28,36 Conversion of irinotecan to SN-38 is an inefficient process; only a small fraction of total irinotecan is converted to SN-38. In fact, studies have shown that approximately 40% to 60% of the lactone ring of irinotecan/SN-38 is converted to a carboxylate form, which greatly reduces potency.14 In contrast, the linker used with labetuzumab govitecan binds to the 20th position of SN-38’s lactone ring, a process that has been shown to stabilize the lactone ring, protecting the ADC’s potency.37 SN-38 is inactivated via glucuronidation by UGT1A1-metabolizing enzymes,38 but the SN-38 in this ADC is protected from glucuronidation.32 The incidence of diarrhea in patients given labetuzumab govitecan compares favorably with the incidence in those given irinotecan monotherapy: 83% of patients receiving irinotecan had late diarrhea (31% were grades 3 or 4),39 compared with 46% of those receiving labetuzumab govitecan at 10 mg/kg once weekly (only one grade ≥ 3 [5%] reported in that cohort). At all dose levels, severe diarrhea was found at a much lower level (only 7% experienced grade ≥ 3). In addition, 36% of patients given labetuzumab govitecan once weekly at 10 mg/kg reported grade ≥ 3 neutropenia during treatment, but none had neutropenic fever. The patients required minimal dose reductions or administration of granulocyte colony-stimulating factor (G-CSF) because of neutropenia. Although the occurrence of neutropenia is somewhat lower than that reported for irinotecan,39 patients with homozygous UGT1A1 *28/*28 genes have a higher risk of severe myelosuppression.40 In this study, subgroup analysis showed that only one of four patients with a homozygous UGT1A1 *28/*28 genotype experienced grade ≥ 3 neutropenia.
A conjugate localizing within the tumor can release SN-38 after internalization of the ADC, but any conjugate held within the tumor microenvironment can also be expected to release SN-38 over time. The ability to enhance selective accretion of SN-38 in the tumor via the antibody-binding moiety is a definite advantage over polyethylene glycol (PEG)-modified SN-38 or irinotecan, which rely solely on sustaining these agents in the blood.41,42
The PK profile of labetuzumab govitecan differs from that of irinotecan. The PK analysis of labetuzumab govitecan in this study depicted a more rapid clearance (shorter half-life) of the intact conjugate than did the antibody (labetuzumab). Thus, labetuzumab govitecan clearance reflects both the elimination of intact ADC from the circulation and the loss of SN-38 from the antibody, which has been estimated in vitro to have a half-life of approximately 1 day in serum.26 At any sampling time over the first 3 days, nearly all the SN-38 in the serum is bound to the conjugate, with < 2% of the SN-38Total in the serum being in its free form. The SN-38Total half-life is the same as that reported for irinotecan.39,43 We hypothesize that labetuzumab govitecan allows for lower plasma SN-38 concentrations while maintaining higher tumor concentrations, thereby increasing the benefit:risk ratio of the therapy. This is supported by nonclinical studies.32
Exploratory analysis revealed no association between KRAS mutation status and tumor response. Plasma CEA levels also indicated no relationship to ADC or antibody clearance. However, there is preliminary evidence of lower plasma CEA levels being prognostic of a better response (Appendix Tables A1 and A2).
The fact that activity was seen in this patient population who had relapsed after receiving an irinotecan-containing regimen previously suggests that SN-38 delivered by this ADC should be evaluated in patients who are clearly resistant to irinotecan.
In conclusion, there was no loss of activity or increased safety concern in the once-weekly compared with the twice-weekly regimen, with the advantage of convenience. The differences between the 8 and 10 mg/kg once-weekly groups were small; therefore, additional studies need to define the optimal dose, either 8 or 10 mg/kg once weekly. Importantly, monotherapy with labetuzumab govitecan has manageable toxicity, less than irinotecan, particularly with regard to diarrhea. Additional clinical studies, especially those in which labetuzumab govitecan is combined with other agents (eg, replacing irinotecan FOLFOXIRI), are warranted.
ACKNOWLEDGMENT
We thank Francois Wilhelm for his contributions to early analyses of the data, and Heather Horne and Pius Maliakal for data analyses. Marion Hartley provided editorial assistance. We thank the participating patients and their families, as well as the clinical support staffs at the participating institutions.
Appendix
Table A1.
Subgroup Analyses
Table A2.
KRAS Status
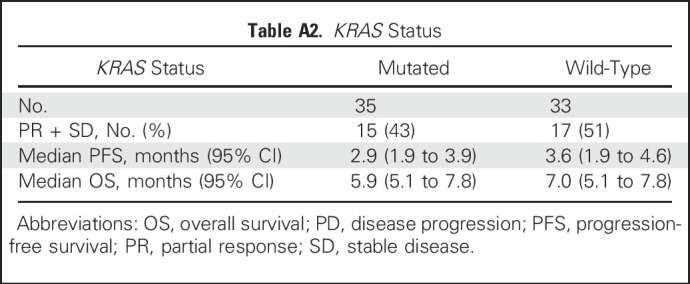
Table A3.
PK Parameters and Peak Concentrations for Selected Patients Receiving Labetuzumab Govitecan
Footnotes
Supported in part by Immunomedics.
Presented in part at the 2015 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015, and at the 2016 Annual Meeting of the American Association for Cancer Research, New Orleans, LA, April 16-20, 2016.
Clinical trial information: NCT01605318.
AUTHOR CONTRIBUTIONS
Conception and design: Efrat Dotan, Steven J. Cohen, William A. Wegener, Robert M. Sharkey, David M. Goldenberg, Jordan D. Berlin
Administrative support: William A. Wegener, Robert M. Sharkey, David M. Goldenberg
Provision of study materials or patients: Efrat Dotan, Steven J. Cohen, Alexander N. Starodub, Christopher H. Lieu, Wells A. Messersmith, Pamela S. Simpson, Michael J. Guarino, John L. Marshall, Richard M. Goldberg, J. Randolph Hecht, Serengulam V. Govindan, Jordan D. Berlin
Collection and assembly of data: Efrat Dotan, Steven J. Cohen, Alexander N. Starodub, Christopher H. Lieu, Wells A. Messersmith, Pamela S. Simpson, Michael J. Guarino, John L. Marshall, Richard M. Goldberg, J. Randolph Hecht, William A. Wegener, Robert M. Sharkey, Serengulam V. Govindan, Jordan D. Berlin
Data analysis and interpretation: Efrat Dotan, Steven J. Cohen, Christopher H. Lieu, Wells A. Messersmith, William A. Wegener, Robert M. Sharkey, David M. Goldenberg
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I/II Trial of Labetuzumab Govitecan (Anti-CEACAM5/SN-38 Antibody-Drug Conjugate) in Patients With Refractory or Relapsing Metastatic Colorectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Efrat Dotan
Research Funding: Pfizer (Inst), OncoMed Pharmaceuticals (Inst), Bayer AG (Inst), Biocompatibles (Inst)
Steven J. Cohen
Consulting or Advisory Role: Taiho Pharmaceutical, Bayer AG, Genentech, Amgen
Alexander N. Starodub
No relationship to disclose
Christopher H. Lieu
Consulting or Advisory Role: Merrimack, Merck
Other Relationship: Immune Design
Wells A. Messersmith
Consulting or Advisory Role: Immunomedics, Gilead Sciences
Research Funding: Pfizer, Roche/Genentech, Millennium Pharmaceuticals, OncoMed Pharmaceuticals, Immunomedics, Alexo Therapeutics
Pamela S. Simpson
No relationship to disclose
Michael J. Guarino
No relationship to disclose
John L. Marshall
Honoraria: Genentech/Roche, Amgen, Bayer AG/Onyx Pharmaceuticals, Taiho Pharmaceutical, Caris Life Sciences, Celgene
Consulting or Advisory Role: Genentech/Roche, Amgen, Bayer/Onyx, Taiho Pharmaceutical, Caris Life Sciences, Celgene
Speakers' Bureau: Genentech/Roche, Amgen, Bayer AG/Onyx Pharmaceuticals, Celgene
Research Funding: Bayer AG/Onyx Pharmaceuticals (Inst), Genentech/Roche (Inst), Pfizer (Inst), Amgen (Inst)
Richard M. Goldberg
Honoraria: Merck, Merck KGaA, Merck Serono, Taiho Pharmaceutical, Novartis
Research Funding: Sanofi (Inst), Bayer AG (Inst), Immunomedics (Inst), Merck (Inst), Bristol-Myers Squibb (Inst)
J. Randolph Hecht
No relationship to disclose
William A. Wegener
Employment: Immunomedics
Stock or Other Ownership: Immunomedics
Robert M. Sharkey
Employment: Immunomedics
Stock or Other Ownership: Immunomedics
Consulting or Advisory Role: Immunomedics
Serengulam V. Govindan
Employment: Immunomedics
Stock or Other Ownership: Immunomedics
Patents, Royalties, Other Intellectual Property: Immunomedics
David M. Goldenberg
Employment: Immunomedics
Leadership: Immunomedics
Stock or Other Ownership: Immunomedics, Immunomedics (I)
Patents, Royalties, Other Intellectual Property: Immunomedics
Jordan D. Berlin
Consulting or Advisory Role: Celgene, Symphogen, Genentech/Roche, Vertex, Pharmacyclics, Aduro Biotech, Cornerstone Pharmaceuticals, ARMO BioSciences, Five Prime Therapeutics, Opsona Therapeutics, Pierre Fabre, Exelixis, ERYTECH Pharma
Research Funding: Genentech/Roche (Inst), OncoMed Pharmaceuticals (Inst), Novartis (Inst), Immunomedics (Inst), Abbvie (Inst), Gilead Sciences (Inst), Merrimack (Inst), Taiho Pharmaceutical (Inst), Five Prime Therapeutics (Inst), Loxo (Inst), Vertex (Inst), Symphogen (Inst), Incyte (Inst), Pharmacyclics (Inst)
Travel, Accommodations, Expenses: Genentech/Roche, Celgene, Vertex
Other Relationship: Momenta Pharmaceuticals, Symphogen, AstraZeneca
REFERENCES
- 1. doi: 10.3322/caac.21395. Siegel RL, Miller KD, Fedewa SA, et al: Colorectal cancer statistics, 2017. CA Cancer J Clin 67:177-193, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A: Cancer Statistics, 2017. CA Cancer J Clin 67:7-30, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Nordlinger B, et al. : Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:iii1-iii9, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Bertocchi P, Aroldi F, Prochilo T, et al. : Chemotherapy rechallenge after regorafenib treatment in metastatic colorectal cancer: Still hope after the last hope? J Chemother 29:102-105, 2017 [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.2174/1568009617666170209095143. Nappi A, Berretta M, Romano C, et al: Metastatic colorectal cancer: Role of target therapies and future perspectives. Curr Cancer Drug Targets . [epub ahead of print on February 8, 2017] [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal RD, Leon E, Hansen HJ, et al. : Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer 7:2, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakih MG: Metastatic colorectal cancer: Current state and future directions. J Clin Oncol 33:1809-1824, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Recondo G, Jr, Díaz-Cantón E, de la Vega M, et al. : Advances and new perspectives in the treatment of metastatic colon cancer. World J Gastrointest Oncol 6:211-224, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casi G, Neri D: Antibody-drug conjugates and small molecule-drug conjugates: Opportunities and challenges for the development of selective anticancer cytotoxic agents. J Med Chem 58:8751-8761, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Esteva FJ, Miller KD, Teicher BA: What can we learn about antibody-drug conjugates from the T-DM1 experience? Am Soc Clin Oncol Educ Book e117-e125, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Peters C, Brown S: Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep 35:e00225, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck A, Goetsch L, Dumontet C, et al. : Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov 16:315-337, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Carbonero R, Supko JG: Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin Cancer Res 8:641-661, 2002 [PubMed] [Google Scholar]
- 14.Mathijssen RH, van Alphen RJ, Verweij J, et al. : Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 7:2182-2194, 2001 [PubMed] [Google Scholar]
- 15.Stein A, Voigt W, Jordan K: Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol 2:51-63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govindan SV, Cardillo TM, Moon SJ, et al. : CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates. Clin Cancer Res 15:6052-6061, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardillo TM, Govindan SV, Sharkey RM, et al. : Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: Preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res 17:3157-3169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govindan SV, Cardillo TM, Sharkey RM, et al. : Milatuzumab-SN-38 conjugates for the treatment of CD74+ cancers. Mol Cancer Ther 12:968-978, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Cardillo TM, Govindan SV, Sharkey RM, et al. : Sacituzumab govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: Characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem 26:919-931, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg DM, Cardillo TM, Govindan SV, et al. : Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 6:22496-22512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajjar G, Sharkey RM, Burton J, et al. : Phase I radioimmunotherapy trial with iodine-131--labeled humanized MN-14 anti-carcinoembryonic antigen monoclonal antibody in patients with metastatic gastrointestinal and colorectal cancer. Clin Colorectal Cancer 2:31-42, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Sahlmann CO, Homayounfar K, Niessner M, et al. : Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study. Cancer 123:638-649, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Sharkey RM, Juweid M, Shevitz J, et al. : Evaluation of a complementarity-determining region-grafted (humanized) anti-carcinoembryonic antigen monoclonal antibody in preclinical and clinical studies. Cancer Res 55(23, Suppl)5935s-5945s, 1995 [PubMed] [Google Scholar]
- 24.Gold P, Goldenberg NA: The carcinoembryonic antigen (CEA): Past, present, and future. McGill J Med 3:46-66, 1997 [Google Scholar]
- 25.Gold P, Shuster J, Freedman SO: Carcinoembryonic antigen (CEA) in clinical medicine: Historical perspectives, pitfalls and projections. Cancer 42:1399-1405, 1978 [DOI] [PubMed] [Google Scholar]
- 26.Govindan SV, Cardillo TM, Rossi EA, et al. : Improving the therapeutic index in cancer therapy by using antibody-drug conjugates designed with a moderately cytotoxic drug. Mol Pharm 12:1836-1847, 2015 [DOI] [PubMed] [Google Scholar]
- 27. Cardillo TM, Sharkey RM, Govindan SV, et al: Superior SN-38 pharmacodynamic and tumor-accretion profıles of labetuzumab govitecan (IMMU-130) versus irinotecan in experimental human colonic cancer models. Proc Am Assoc Cancer Res 77:1042 , 2017 (abstr 4081) [Google Scholar]
- 28.Xie R, Mathijssen RH, Sparreboom A, et al. : Clinical pharmacokinetics of irinotecan and its metabolites in relation with diarrhea. Clin Pharmacol Ther 72:265-275, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Segal NH, Dotan E, Berlin JD, et al. : Abstract CT211: IMMU-130, an SN-38 antibody-drug conjugate (ADC) targeting CEACAM5, is therapeutically active in metastatic colorectal cancer (mCRC): Initial clinical results of two Phase I studies. Cancer Res 74:CT211, 2014 [Google Scholar]
- 30.Starodub AN, Ocean AJ, Shah MA, et al. : First-in-human trial of a novel anti-Trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res 21:3870-3878, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Sharkey RM, McBride WJ, Cardillo TM, et al. : Enhanced delivery of SN-38 to human tumor xenografts with an anti-Trop-2-SN-38 antibody conjugate (sacituzumab govitecan). Clin Cancer Res 21:5131-5138, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Beauchemin N, Draber P, Dveksler G, et al. : Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res 252:243-249, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Hammarström S: The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 9:67-81, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Grothey A, Van Cutsem E, Sobrero A, et al. : Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:303-312, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Hirose K, Kozu C, Yamashita K, et al. : Correlation between plasma concentration ratios of SN-38 glucuronide and SN-38 and neutropenia induction in patients with colorectal cancer and wild-type UGT1A1 gene. Oncol Lett 3:694-698, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao H, Lee C, Sai P, et al. : 20-O-acylcamptothecin derivatives: evidence for lactone stabilization. J Org Chem 65:4601-4606, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Hanioka N, Ozawa S, Jinno H, et al. : Human liver UDP-glucuronosyltransferase isoforms involved in the glucuronidation of 7-ethyl-10-hydroxycamptothecin. Xenobiotica 31:687-699, 2001 [DOI] [PubMed] [Google Scholar]
- 39. http://labeling.pfizer.com/ShowLabeling.aspx?id=533 Pfizer: Camptosar - irinotecan hydrochloride injection, solution. Highlights of prescribing information.
- 40.Hoskins JM, Goldberg RM, Qu P, et al. : UGT1A1*28 genotype and irinotecan-induced neutropenia: Dose matters. J Natl Cancer Inst 99:1290-1295, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Garrett CR, Bekaii-Saab TS, Ryan T, et al. : Randomized phase 2 study of pegylated SN-38 (EZN-2208) or irinotecan plus cetuximab in patients with advanced colorectal cancer. Cancer 119:4223-4230, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Perez EA, Awada A, O’Shaughnessy J, et al. : Etirinotecan pegol (NKTR-102) versus treatment of physician’s choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 16:1556-1568, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Xie R, Mathijssen RH, Sparreboom A, et al. : Clinical pharmacokinetics of irinotecan and its metabolites: A population analysis. J Clin Oncol 20:3293-3301, 2002 [DOI] [PubMed] [Google Scholar]