Abstract
Introduction
13C‐breath tests are valuable, noninvasive diagnostic tests that can be widely applied for the assessment of gastroenterological symptoms and diseases. Currently, the potential of these tests is compromised by a lack of standardization regarding performance and interpretation among expert centers.
Methods
This consensus‐based clinical practice guideline defines the clinical indications, performance, and interpretation of 13C‐breath tests in adult and pediatric patients. A balance between scientific evidence and clinical experience was achieved by a Delphi consensus that involved 43 experts from 18 European countries. Consensus on individual statements and recommendations was established if ≥ 80% of reviewers agreed and <10% disagreed.
Results
The guideline gives an overview over general methodology of 13C‐breath testing and provides recommendations for the use of 13C‐breath tests to diagnose Helicobacter pylori infection, measure gastric emptying time, and monitor pancreatic exocrine and liver function in adult and pediatric patients. Other potential applications of 13C‐breath testing are summarized briefly. The recommendations specifically detail when and how individual 13C‐breath tests should be performed including examples for well‐established test protocols, patient preparation, and reporting of test results.
Conclusion
This clinical practice guideline should improve pan‐European harmonization of diagnostic approaches to symptoms and disorders, which are very common in specialist and primary care gastroenterology practice, both in adult and pediatric patients. In addition, this guideline identifies areas of future clinical research involving the use of 13C‐breath tests.
Keywords: breathtest, diagnosis, gastroenterology, gastroparesis, helicobacter pylori, liver cirrhosis, motility, pancreatic exocrine insufficiency, pancreatitis
INTRODUCTION
Breath tests are valuable, noninvasive diagnostic tests that are widely applied for the assessment of gastroenterological symptoms and diseases. 13C‐breath tests provide the opportunity to diagnose Helicobacter pylori (H. pylori) infection, document gastric emptying time, monitor pancreatic exocrine and liver function, and have several additional potential gastroenterological and nongastroenterological applications.
Currently, the potential of breath testing is compromised by a lack of standardization regarding performance and interpretation among expert centers. This is highly relevant because modifications of the volume and/or composition of the test meal, of test performance and of the evaluation of data may markedly influence test results, diagnosis and thus, clinical usefulness of the investigation.
This consensus‐based clinical practice guideline is needed within the gastrointestinal (GI) community to enhance pan‐European harmonization of diagnostic approaches to symptoms and disorders, which are very common in specialist and primary care gastroenterology practice, both in adult and in pediatric patients. The guideline can add significantly to quality of investigation and, thus, the welfare of gastroenterological patients because it will allow a more rational approach to diagnostic evaluation and treatment. The guideline also aims to minimize disparities between health care systems across Europe, to facilitate cooperation between expert groups and the performance of multicenter clinical trials.
METHODS
The structured procedure, which was developed for the creation of this consensus‐based clinical practice guideline, has previously been published. 1 Briefly, this procedure was initiated by three representatives of the contributing societies (heads of guideline, JK, HH, MF) and started with formation of a representative core group of experts nominated from all participating societies and associations. This core group developed statements and recommendations, which were then submitted to reviewers in a three‐stage Delphi voting process. The heads of guideline and the core group members are listed as authors; the reviewers are listed as members of the European 13C‐breath test group.
The following key questions were addressed in the guideline:
What is the role of 13C breath tests in the detection of H. pylori infection, and in the measurement of gastric emptying, pancreatic exocrine and liver function?
What are the general technical requirements and operating procedures for performance of 13C‐breath tests, including preparation, dosage, breath sampling, technical analysis?
What are the reporting requirements?
Are there areas of disagreement and research priorities?
A systematic literature search with the appropriate key words using Medline/Pubmed and the Cochrane database was performed. We limited our search to studies performed in humans, which were published between 01 January 2000 and 25 July 2019. The resulting 446 references were assessed and allocated to the following topics: general methodology, 13C‐urea breath tests (13C‐UBT), 13C‐gastric emptying breath tests (13C‐GEBT), 13C‐pancreatic function breath tests (13C‐PFBT), 13C‐liver function breath tests (13C‐LFBT), and other 13C‐breath tests. Statements and recommendations were developed based on these results, relevant consensus documents including those of participating societies (published after the year 2000), 2 and on pertinent literature known to members of the core group. Statements reflect key aspects and definitions but give no direct instructions on how to act, whereas recommendations advise when and how to perform individual 13C‐breath tests and how to report on breath test results. The wording used to indicate the strength of recommendation is detailed in Table 1. 1
TABLE 1.
Descriptors of grading 1
| Descriptor | Meaning | Wording |
|---|---|---|
| A—Strength high | Evidence or general accord that the procedure or statement is useful or effective. Further research is very unlikely to change our confidence in the estimate of effect | …has to be… |
| …is to be… | ||
| …shall… | ||
| B—Strength moderate | Conflicting evidence or discordant opinions that the procedure or statement is useful or effective. The weight of evidence/opinion is in favor of utility. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate | …should… |
| …can… | ||
| C—Strength low | Conflicting evidence or discordant opinions that the procedure or statement is useful or effective. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate | …could… |
| D—Strength very low | Any estimate of effect is very uncertain | …may… |
Of note, we aimed to develop recommendations on the specific indications for 13C‐breath tests, whereas general indications for H. pylori testing, gastric emptying testing and monitoring of pancreatic exocrine or liver function were cited from current national and international guidelines.
Statements and recommendations were distributed among all reviewers via email for voting and commenting. All reviewers voted on all statements and recommendations according to the 6‐point Likert scale given in Table 2 1 and gave comments in case of disagreement. “Agreement” was established if ≥ 80% of reviewers voted A+/A AND < 10% D+/D.
TABLE 2.
Six‐point Likert‐scale 1
| Point | Description |
|---|---|
| A+ | Agree strongly |
| A | Agree with minor reservation |
| A− | Agree with major reservation |
| D‐ | Disagree with major reservation |
| D | Disagree with minor reservation |
| D+ | Disagree strongly |
Statements and recommendations, which did not receive agreement during the first Delphi round, were modified according to comments as previously described, 1 and all modified statements and recommendations underwent further rounds of the Delphi process. Statements that achieved “agreement” after three Delphi rounds were accepted. Percentages for agreement and disagreement are given for all recommendations and statements. Recommendations additionally include quality of evidence (Q) and strength of recommendation (S). 1
The guideline is organized in a way that information, which will enable the reader to perform clinical tests, is summarized in statements, recommendations and respective tables. Background information detailed in the comments explains the choice of test protocols and further methodological aspects.
GENERAL 13C‐BREATH TEST METHODOLOGY
Since their introduction into clinical practice in the 1970s, 3 stable isotope 13C‐breath tests have gained considerable importance and have been recommended for distinct diagnostic purposes by national and international guidelines and expert consensus papers. 4 , 5 , 6 , 7 This is due to the fact that they are not only reliable, but also noninvasive, relatively simple and safe diagnostic tools. 13C is a stable, nonradioactive carbon isotope with a natural abundance of about 1% of carbon isotopes. The use of non‐radioactive stable‐isotope tracers in biomedical experiments and diagnosis is generally considered ethically acceptable in humans at all ages. Toxicity of 13C has been examined in animals given amounts far in excess of those employed in the clinic. Up to sixty percent enrichment with 13C was achieved over prolonged periods of time without negative effects in adult animals and without signs of teratogenicity or embryotoxicity. 8 , 9 , 10 Thus, the small requirements of 13C as a tracer in most clinical studies, particularly relative to its naturally high abundance, precludes any discernible risk of toxicity. Accordingly, 13C‐enrichment of marker substances does not affect their tolerability. Depending on pharmacological properties of the marker substance itself, most clinically established 13C‐breath tests can also be performed (repeatedly) in young children and pregnant women. This chapter gives important background information on methodological aspects and delineates general recommendations on test performance.
Statement 1.1
13 C‐breath tests are used for investigation of a variety of gastrointestinal and liver functions and for diagnosis of Helicobacter pylori infection (100%, 0%).
In addition to the clinically established indications extensively discussed in this consensus report, several other tests have been developed for gastroenterological and other purposes (compare “Other tests”).
Statement 1.2
The general principle requires that the digestive/metabolic process under investigation represents the rate‐limiting step in the sequence of events leading to occurrence of 13 CO 2 in the exhaled air (100%, 0%).
For instance, within the time frame used for testing, the presence of bacterial urease in the stomach determines whether 13C‐urea is metabolized leading to a selective increase in 13CO2‐exhalation in H. pylori positive patients.
Statement 1.3
Most 13 C‐breath tests require sample collection and measurement over several hours. For selected indications (e.g., 13 C‐urea breath test in adults) measurements at two time points can be sufficient (100%, 0%).
Analysis of 13C‐breath tests is frequently based on kinetic data of the 13CO2 exhalation characteristics (e.g., gastric emptying tests) or on quantitative analysis of the whole metabolic process, that is, by analysis of cumulative 13CO2 exhalation (e.g., pancreatic or liver function tests). Accordingly, several breath samples have to be collected at predefined intervals. Depending on the metabolic and whole body distribution pathways of each 13C‐labeled substrate and the process under investigation, breath sampling over several hours may be necessary. For instance, protocols for pancreatic function testing usually require 4 to 9 h of sample collection at 15 or 30 min intervals. 11 , 12 , 13 , 14 On the other hand, 13C‐UBT in adults are usually based on two point measurements. 15 , 16 However, even for H. pylori testing, some experts calculate cumulative 13C‐exhalation over 1 h based on breath samples collected at 15 min intervals. The major advantage is that the patient's anthropometry is taken into account: a small and light person has a much higher measurement of δ over baseline value (DOB) than a very tall and heavy person for the same cumulative percentage of administered dose. In this way, the test can also be applied in children.
Statement 1.4
Isotope‐ratio mass spectrometry is the reference method for measurement of the 13 CO 2 concentration in the exhaled air (100%, 0%).
Statement 1.5
Isotope‐selective nondispersive infrared spectroscopy can be used alternatively (91%, 0%).
At present, mass‐spectrometry is the most accurate and efficient method for measuring carbon isotope ratios in exhaled breath (IRMS), but its application is restricted by the high cost of the equipment and operational complexity. Nondispersive infrared spectrometry (NDIRS) is the most widely used alternative method. Apart from lower costs, the devices are smaller, easier to handle and can be used on site, for example, in outpatient facilities. 17 On the other hand, NDIRS measurements usually require higher sample volumes. Samples are frequently collected in aluminum bags (200–1300 ml) instead of 10 ml glass tubes as used for IRMS, which limits its use in large laboratories to which samples are delivered from distant sites.
Studies comparing the results of IRMS and NDIRS measurements of identical samples have shown comparable results for both methods. Most of these studies have investigated samples from 13C‐UBT. 17 , 18 , 19 , 20 , 21 Data show correlation coefficients of up to 0.999 for both analytical methods. A meta‐analysis assessing the diagnostic accuracy of 13C‐UBT in adult patients with dyspepsia showed no significant difference for studies reporting NDIRS or IRMS results. 22
Similarly, results of 13C‐octanoic acid breath tests (13C‐OABT), which measure gastric emptying, have been compared using IRMS and NDIRS. 23 As expected, precision and repeatability of 13C‐measurements with NDIRS were inferior to IRMS. However, correlation coefficients for 13C‐exhalation and all gastric emptying parameters as computed on the basis of IRMS and NDIRS measurements were >0.98. Mean gastric emptying half time calculated using nonlinear regression (NLR) analysis was almost identical (87 ± 39 min vs. 90 ± 39 min). For the 13C‐methacetin breath test, a dynamic liver function test, molecular correlations spectroscopy, a method similar to NDIRS, showed comparable results to IRMS in Bland‐Altman and correlation analysis. 24 Breath samples were collected continuously via a nasal cannula with one sample analyzed about every 3 min.
Theoretically, these results should be transferable to other 13C‐breath tests since 13CO2 is the ultimate metabolic product, which is analyzed in all of these tests. However, caution may be necessary when applying tests, that only result in small increases in 13C‐exhalation, for example, 13C‐pancreatic function tests, for which highly accurate measuring devices are required. 25
Statement 1.6
The δ‐value (‰) is the measuring parameter and is defined as the 13 CO 2 / 12 CO 2 ratio in a given sample in comparison to the 13CO 2/12 CO 2 ratio in a reference material (97%, 0%).
Statement 1.7
Differences between δ‐values obtained after application of a marker substance and the baseline δ‐value (δ over baseline, DOB) are used for calculation of outcome parameters (97%, 0%).
Conventionally, 13C‐content of breath samples is expressed as δ ‰ PDB units, that is, relative to the international standard, which originally was the calcium carbonate of the fossil Belemnitella of the Pee Dee formation (PDB) in South Carolina, USA. Zero δ ‰ corresponds to 1.112372% 13C atoms within CaCO3. Thus, if a sample of carbon dioxide has a 13C/12C ratio which is less than that of the standard by 5 per mil, it is said to have a δ‐value of −5‰. 26 , 27 DOB values are the differences between δ‐values obtained before (baseline) and after application of the marker substance. They reflect the increase in 13C‐exhalation, which is the basis for calculation of test parameters. For most established 13C‐UBT‐protocols, the DOB value at 30 min is the relevant outcome parameter (compare below). For most other tests, based on DOB values and the assumption of a stable CO2‐production rate of 300 mmol per square meter of body surface per hour, the quantity of 13C appearing in breath per unit time is calculated. These data are usually expressed as percentage of 13C‐dose administered.
Statement 1.8
13 CO 2 concentrations in samples collected using breath tubes for isotope‐ratio mass spectrometry (IRMS) remain stable for at least 4 weeks so that measurement of breath samples can be delayed by this period (84%, 0%).
Statement 1.9
13 CO 2 concentrations in samples collected using aluminum bags for nondispersive infrared spectroscopy (NDIRS) remain stable for at least 72 h so that measurement of breath samples can be delayed by this period (93%, 0%).
For IRMS, stability of samples for a minimum period of 4 weeks has been demonstrated. A brief report even suggests that with 10 ml samples stored in glass tubes at room temperature in the absence of light, 13C‐concentrations are stable for 8 months. 28 For NDIRS there are hardly any published data on sample stability. Mana et al. showed that samples are stable for 72 h. 18 Personal experience of the authors suggests longer stability (1‐2 weeks by additional sealing of the rubber tubes of aluminum bags with gas‐tight tapes).
Statement 1.10
Digestive and metabolic processes can be influenced by several factors including demographic parameters, fasting or fed state, composition and size of a test meal, physical activity, pre‐existing diseases and drug intake (100%, 0%).
13C‐exhalation from marker substances depends not only on the process under investigation (e.g., gastric emptying), but also on absorption of the marker substance and/or its metabolites, further (mostly hepatic) metabolism leading to production of 13CO2, its transport to the lung and pulmonary excretion and distribution to other body compartments leading to a relevant loss of label, for example, into muscles or bone. 29 All of these functions as well as the process under investigation can be influenced by demographic and physiological parameters, concomitant diseases and drug intake. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 A study investigating the influence of clinical parameters on the results of 13C‐OABT in more than 1200 patients has shown that 13C‐exhalation was increased in women and correlated with age. Diabetes mellitus and inflammatory bowel disease were associated with decreased, and bacterial overgrowth and malignant disease with increased 13C‐exhalation. 39
Figure 1 summarizes the general principle of 13C‐breath testing.
FIGURE 1.
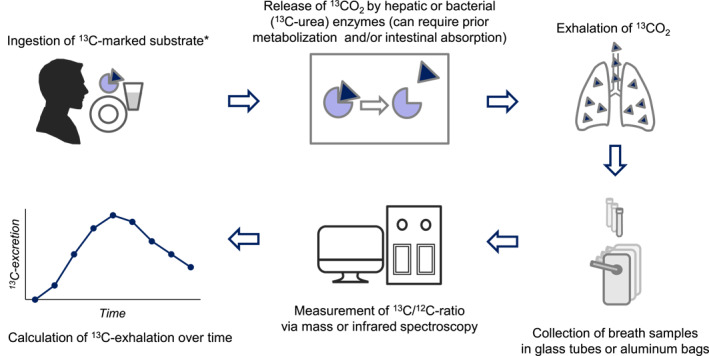
General principle of 13C‐breath testing. The 13C‐marked substrate is ingested, frequently with a specific test meal or test solution. Hepatic or bacterial enzymes release 13CO2, this requires prior intraluminal metabolization and/or intestinal absorption for most tests. 13CO2 is absorbed into the blood stream, transported to the lung, and exhaled. Breath samples are collected in glass tubes (mass spectroscopy) or aluminum bags (infrared spectroscopy) for measurement of 13C/12C‐ratio. Delta over basal values are used for calculation of 13C‐excretion. * for specific liver function tests the 13C‐marked substrate is applied intravenously
What are the general prerequisites for performance of 13C‐breath tests for clinical or research purposes?
Recommendation 1.1
13 C‐breath tests performed for clinical reasons have to adhere strictly to standardized study protocols adequately validated in a representative patient population (100%, 0%; Q:C S:A).
Recommendation 1.2
For research projects, test parameters such as composition of the test meal or duration of breath sampling can be varied to evaluate the impact of the variation on test results (89%, 3%; Q:D S:B).
As discussed above, 13C‐breath tests are indirect tests depending on several digestive and metabolic processes, which ultimately lead to exhalation of 13C‐enriched breath. All intermediary steps as well as the process under investigation can be influenced by demographic and physiological parameters, concomitant diseases and drug intake. Moreover, alterations of marker substance, dose, or other components of the test meal/solution can markedly influence test results. Accordingly, it is of pivotal importance that tests are validated in representative patient populations and that clinicians adhere to strictly standardized study protocols. Research projects are required to better delineate the impact of variations on test results in order to optimize test procedures.
Which dietary restrictions need to be observed before 13C‐breath testing?
Recommendation 1.3
13 C‐rich food, ingested before the test (e.g., corn, pineapple, broccoli, sugarcane) can increase the baseline δ‐value and thereby compromise the measurements. Accordingly, they should be avoided at least 48 h before 13 C‐breath testing (85%, 0%; Q:C S:B).
Isotopic fractionation—change in isotopic ratios between materials, due to the different rates at which various isotopes undergo chemical reactions—is a well‐established phenomenon. 27 Carbon isotopes are strongly fractionated during photosynthesis, when plants metabolize carbon dioxide. Three types of photosynthesis occur in the plant world, commonly referred to as the C3, C4, and CAM pathways. 27 While most plants traditionally consumed in European diets perform C3 photosynthesis, leading to comparably lower 13C‐content, other plants such as corn, pineapple, broccoli, and sugarcane are C4 plants with relatively higher 13C‐abundance. Their consumption prior to a 13C‐breath test increases basal 13C‐exhalation, and further metabolization may influence 13C‐exhalation over time. Avoidance for 48 h before breath testing is deemed satisfactory by most experts, while some recommend 72 h.
Which drugs and medical interventions need to be avoided before and during 13C‐breath testing?
Recommendation 1.4
Drugs with potential influence on test results should be avoided before the test, unless essential long‐term medication is concerned or the effect of the drug on the digestive/metabolic process is to be determined (92% 0%; Q:D S:B).
Recommendation 1.5
Dialysis solutions and glucose infusions mostly contain glucose that originates from hydrolysis of maize starch naturally enriched in 13 C and should therefore be avoided during 13 C‐breath testing (94%, 0%; Q:D S:B).
Drugs can influence test results by altering GI transit, absorption or (postabsorptive) metabolism of the 13C‐labeled substrate. Accordingly, reliable performance of 13C‐breath tests may require avoidance of specific drugs as discussed for the individual tests below. However, if long‐term treatment is mandatory, drug avoidance is not always reasonable because it confounds the normal clinical situation of the patient. Moreover, 13C‐breath tests can be used for monitoring drug effects. For instance, the 13C‐mixed triglyceride breath test (13C‐MTGBT) has been used to monitor improvement of lipid absorption with enzyme replacement therapy in pancreatic exocrine insufficiency (PEI). 11
Dialysis solutions and glucose infusions mostly contain glucose that originates from hydrolysis of maize starch, so that they are naturally enriched in 13C. Accordingly, they may confound test results and should also be avoided. 37
Is physical activity allowed during 13C‐breath testing?
Recommendation 1.6
Physical activity alters gastrointestinal transit of orally administered substrates and markedly increases CO 2 production. Therefore, physical activity has to be avoided during 13 C‐breath testing (100%, 0%; Q:C S:A).
Even moderate physical activity such as walking roughly doubles energy expenditure compared with sedentary subjects and has corresponding effects on endogenous CO2 production. 41 , 42 In addition, exercise leads to a shift toward oxidation of nonlipid components. This increases 13C‐exhalation because the lipid molecules in the body contain substantially lower concentrations of 13C than the nonlipid molecules, due to fractionation processes during lipid synthesis. 35 Moreover, 13C‐breath tests using orally applied marker substances depend on GI transit which is accelerated by moderate exercise, while strenuous exercise has opposite effects. 43 Accordingly, it has been shown that physical activity during 13C‐OABT markedly alters 13CO2‐exhalation in healthy volunteers as well as respective normal values. 36
In summary, physical activity has profound and complex effects on 13C‐exhalation and breath test results. For standardization purposes under clinical conditions, patients must be asked to strictly avoid physical activity during tests.
Which concomitant diseases may influence 13C‐breath test results?
Recommendation 1.7
Disturbances of gastrointestinal motor and secretory function, hepatic and pulmonary function can generally affect the time course and/or amount of 13 C‐exhalation. This has to be taken into account for performance and interpretation of 13 C‐breath tests (100%, 0%; Q:B S:A).
Concomitant diseases affecting digestive and metabolic processes which ultimately lead to exhalation of 13C‐enriched breath may confound breath test results. In particular, major disturbances of GI transit and absorption, hepatic and lung function have to be considered. However, the influence of concomitant diseases on many 13C‐breath tests appears to be small:
Even in patients with nonalcoholic steatohepatitis or liver cirrhosis (∼50% Child–Pugh score C), 13C‐octanoic acid metabolism was found to be normal 44 , 45 , 46 and the 13C‐OABT correlated well with scintigraphy in patients who were critically ill. 47
Disturbances of 13CO2‐exhalation in patients with lung diseases are only expected in patients with very severe disease and reduced CO2‐diffusion capacity. Data on potential impairment of 13C‐breath tests in such cases are not available in the literature.
Since digestion of dietary lipids by pancreatic lipase cannot occur before the meal has entered the duodenum, results of the 13C‐MTGT are influenced by and correlate with gastric emptying parameters. 14 This predominantly applies to early postprandial 13C‐exhalation rates (1–3 h), which have insufficient specificity for detection of PEI. However, this problem can be overcome by prolongation of the breath sampling period so that gastric emptying is of minor importance for 13C‐exhalation.
Vice versa, 13CO2‐excretion following administration of 13C‐octanoic acid was similar in patients with and without PEI, including those with severe exocrine insufficiency characterized by overt steatorrhea. 39 , 48 , 49
13C‐UREA BREATH TEST
H. pylori is a common bacterial pathogen responsible for substantial GI morbidity worldwide. In addition to causing inflammatory gastroduodenal alterations, H. pylori is the major risk factor for gastric cancer development and is associated with various other, partly non‐GI diseases. Table 3 summarizes important indications for H. pylori testing in adults and children recommended by current European guidelines. 4 , 5
TABLE 3.
| Grade of recommendation | ||
|---|---|---|
| Adults | High | Suspicion/evidence of peptic ulcer disease, atrophic gastritis, gastric adenocarcinoma, MALT (mucosa‐associated lymphoid tissue) lymphoma |
| Test‐and‐treat strategy for uninvestigated dyspepsia | ||
| Exclusion of H. pylori gastritis before reliable diagnosis of functional dyspepsia | ||
| Moderate | Aspirin and NSAIDs users with a history of peptic ulcer | |
| Low | Unexplained iron deficiency anemia, idiopathic thrombocytopenic purpura, vitamin B12 deficiency | |
| Children | High | Suspicion/evidence of peptic ulcer disease |
| Monitoring of outcome of eradication therapy | ||
| Low | Chronic immune thrombocytopenic purpura | |
There are differences in the approach to H. pylori infection between adults and children. Thus, the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) explicitly recommends against a test and treat strategy in children, as well as against H. pylori testing as part of the initial investigation in children with iron deficiency or as part of the investigations of causes of short stature. Moreover, these guidelines recommend against H. pylori testing in children with functional abdominal pain disorders. 5
For the diagnosis of H. pylori infection, histology (±culture) from biopsy samples is regarded as the reference standard. 50 , 51 Several other tests are available, including endoscopic biopsy followed by rapid urease testing and noninvasive methods like serology, fecal antigen tests and the 13C‐UBT. The principle of the 13C‐UBT relies upon the capacity of H. pylori, when present in the stomach, to hydrolyze orally administered 13C‐urea to produce 13CO2, which diffuses into the blood, is transported to the lungs and exhaled, so that it can be detected in breath samples. 13C‐urea is innocuous and can be safely administered repeatedly, including in children and pregnant women.
According to the Maastricht V/Florence Consensus report, 13C‐UBT is regarded as the best approach to the diagnosis of H. pylori infection in the context of a “test‐and‐treat strategy” in adults because of its high sensitivity and specificity, and excellent performance. 4 This is confirmed by a recent Cochrane review showing superior diagnostic accuracy compared with other non‐invasive tests. 50
Stool antigen tests may be less acceptable in some societies but also have a high sensitivity and specificity, provided a monoclonal antibody‐based ELISA is used. 4 Some serology tests have high sensitivity and specificity, but these tests may perform differently in different geographic locations according to the antigenic composition of the circulating strains. Thus, the Maastricht V/Florence Consensus recommends that only locally validated tests should be used. 4 Moreover, due to the slow decrease of serum antibody levels, they are inadequate for assessment of H. pylori eradication after treatment.
In clinical practice when there is an indication for endoscopy, and there is no contraindication for biopsy, the rapid urease test (RUT) is recommended as a first‐line diagnostic test. In the case of a positive test, it allows immediate treatment. 4
When should 13C‐UBT be utilized?
Recommendation 2.1
The 13 C‐urea breath test is to be considered as a noninvasive alternative for all indications for Helicobacter pylori testing if endoscopy is not required or if biopsies are contraindicated (92%, 0%; Q:A S:A).
Recommendation 2.2
The 13 C‐urea breath test is a preferred option for confirmation of Helicobacter pylori eradication in adults and children. It has to be performed at least 4 weeks after completion of therapy (89%, 0%; Q:A S:A).
Meta‐analyses confirm that 13C‐UBT achieves ≥95% sensitivity and specificity in both adults and children. 50 , 52 Accordingly, it is a highly accurate test and generally appropriate for all indications for H. pylori testing. However, in clinical practice, when there is an indication for endoscopy, and there is no contraindication for biopsy, the RUT is recommended as a first‐line diagnostic test. 4 Some national guidelines suggest two positive tests for reliable diagnosis, except in cases with a very high a priori likelihood of H. pylori infection, for example, duodenal ulcer, 53 and 13C‐UBT can be readily performed. Moreover, 13C‐UBT is regarded as the best option for confirmation of H. pylori eradication, 4 , 50 with stool antigen tests being an alternative. Testing has to be delayed for at least 4 weeks after the end of therapy, otherwise it may lead to false negative results (compare patient preparation).
How should 13C‐UBT be performed?
Recommendation 2.3
If commercially available kits are used for 13 C‐urea breath test, manufacturers' instructions regarding preparation of test solution and test performance have to be followed (97%, 0%; Q:B S:A).
Recommendation 2.4
If the test is prepared on site, investigators have to adhere to well established test protocols (for examples compare Table 4 ) (97, 0%, 0%; Q:B S:A).
TABLE 4.
Established test protocol for 13C‐UBT in adults and children
| Reference | Test solution | Breath sampling | Cut off | Validity | Remarks | |
|---|---|---|---|---|---|---|
| Leodolter 1999 15 | Adults | 75 mg 13 C‐urea dissolved in 200 ml 0.1 mol/L citric acid solution (∼4 g/200 ml water) with two tablets artificial sweetener | Before and 30 min after ingestion | ≥4‰ |
|
Equal performance as test with citric acid solution 10 min before marker ingestion; |
| With 200 ml orange juice instead of citric acid lower SENS (88%) with equally high SPEC (100%) 54 ; with semiliquid meals longer test duration required 55 | ||||||
| Elitsur et al. 2009 56 | Children | 75 mg 13 C‐urea and 2 g citric acid dissolved in 4 ounces (∼120 ml) potable water | Baseline and 15 min after ingestion | ≥2.4‰ | SENS 97.9% | Test performed best in children aged >6 years; in ages 2–5 calculation of urea hydrolysis rate can lead to higher SENS and SPEC compared with DOB values |
| SPEC 96.1% | ||||||
| PPV 90.4% | ||||||
| NPV 99.2% | ||||||
Abbreviations: ACC, accuracy; DOB, δ over baseline; NPV, negative predictive value; PPV, positive predictive value; SENS, sensitivity; SPEC, specificity; UBT, urea breath test.
As discussed above, alterations of marker substance, dose or other components of the test meal/solution can markedly influence test results. Hence, it is of pivotal importance that tests are validated in representative patient populations and that clinicians adhere to strictly standardized study protocols. Since the original description of the 13C‐UBT by Graham et al., 57 several changes of the test protocol have been proposed affecting the dose of 13C‐urea, type of test meal/solution, time of breath collection, cut‐off values, and measuring device. 58 Logan et al. first introduced a modified version with only one breath sample to be analyzed, however this was pooled from several samples collected over 30 min at 5 min intervals. 59 Currently, most studies report DOB values of a single sample collected 30 min after application of 13C‐urea with a threshold of >4‰ for diagnosis of H. pylori infection. At this threshold the summary sensitivity (95% confidence interval [CI]) and specificity (95% CI) from 10 studies (958 participants) were 0.95 (95% CI: 0.79–0.99) and 0.95 (95% CI: 0.87–0.98). 50 A minority of studies used sampling periods of 10 or 20 min and thresholds between DOB>3‰ and DOB>6‰. 50 13C‐urea was usually applied with a citric acid solution; however, orange juice 54 or semiliquid meals have also been used. 55 Examples for well‐established test protocols are given in Table 4.
How should patients prepare for the test?
Recommendation 2.5
Ideally, adult patients should have fasted overnight. If this is not feasible, a fasting period of 4 to 6 h is sufficient (97%, 0%; Q:C S:B).
Recommendation 2.6
Children of all ages should have fasted for 4 h (100%, 0%; Q:B S:B).
Theoretically, food in the stomach may dilute the marker substance and thereby impair the contact between the infected mucosa and the substrate leading to lower 13CO2 production and decreased sensitivity of the test. Although this aspect is still controversial, some studies suggest that fasting before the 13C‐UBT indeed improves accuracy of test results. 18 , 60 Accordingly, it appears to be prudent to perform the 13C‐UBT in fasting conditions. 60 In adults, fasting overnight appears reasonable. If this is not feasible, a fasting period of 4–6 h is deemed sufficient. In young children, long fasting periods may be problematic. However, sensitivity of the 13C‐UBT was markedly reduced to about 50% when children were fed a meal immediately before the test. 61 Therefore, a fasting period of 4 h is suggested, including in young children.
Recommendation 2.7
Prior to the 13 C‐urea breath test patients have to abstain from: proton pump inhibitor therapy for ≥2 weeks; antibiotic therapy (including Helicobacter pylori eradication) for ≥ 4 weeks (97%, 0%; Q:A/B S:A).
Recommendation 2.8
Antacids can be allowed before the 13 C‐urea breath (86%, 0%; Q:B S:B).
Proton pump inhibitors (PPI) need to be discontinued because they decrease the load of H. pylori leading to false‐negative results on several tests including 13C‐UBT. 4 , 62 , 63 A 7‐day withdrawal has been shown to be sufficient in most patients; however, as a precaution, 14 days are recommended. 4 H2‐receptor antagonists slightly decrease sensitivity of 13C‐UBT for up to 2 weeks. 64 Topical antacids do not affect sensitivity 62 so that they can be allowed before testing. By contrast, antibiotics, including those used for eradication therapy, and bismuth compounds need to be discontinued for 4 weeks to allow an increase of a detectable bacterial load. 4
How should test results be reported?
Recommendation 2.9
To allow for reliable interpretation of test results, the following parameters should be reported: marker dose and test solution; test result including normal values and interpretation (Helicobacter pylori negative/positive) (95%, 0%; Q:D S:B).
Recommendation 2.10
The test report could be complemented by including clinical characteristics of the patient, last use of proton pump inhibitor and the exact test protocol including equipment used for breath sampling and analysis (84%, 0%; Q:D S:C).
Experts agree that a minimum of information on methodology (marker dose and test solution) and test results (e.g., DOB‐value) including normal values and interpretation (H. pylori positive/negative) are required for medical personnel not involved in the testing to reliably interpret individual findings. Clinical characteristics of the patient including last use of PPI and further methodological information may further facilitate assessment of reliability of test results and choice of clinical consequences.
13C‐GASTRIC EMPTYING BREATH TESTS
Gastric dysmotility can manifest as rapid gastric emptying with dumping syndrome (even in the absence of upper GI surgery) or delayed gastric emptying with symptoms of gastroparesis. The latter applies to the majority of affected patients and is typically associated with nausea, vomiting, early satiety, postprandial fullness, upper abdominal pain, and bloating in adults and children. 6 , 65 , 66 , 67 , 68 , 69 Anorexia and weight loss are further frequent symptoms. Children with gastroparesis experience more vomiting while adolescents with gastroparesis report more nausea and abdominal pain. 68 , 70 There is general consensus that the diagnosis of gastroparesis requires objective evidence of clearly delayed gastric emptying in symptomatic patients. 6 , 66 However, prior to gastric emptying testing, the exclusion of mucosal or structural disorders such as inflammatory or malignant diseases as the underlying cause of symptoms is required. 6 Specific indications for gastric emptying testing in adults as suggested by international guidelines and expert consensus papers are given in Table 5. 6 , 65 , 71 For pediatric patients, there are no generally accepted guideline recommendations on gastric emptying testing. The recommendations given in Table 5 are derived from a recent review of the literature, which shows that nausea, vomiting, and abdominal pain are the most common symptoms in children, while early satiety, postprandial fullness, bloating, and weight loss occur less frequently in pediatric gastroparesis. 72
TABLE 5.
| Grade of recommendation | ||
|---|---|---|
| Adults | High/moderate | Symptoms suggestive of gastroparesis* without evidence of mucosal or structural disease explaining these symptoms (*nausea, vomiting, early satiety, postprandial fullness, bloating, upper abdominal pain) |
| Unexplained impairment of blood glucose control in patients with diabetes mellitus, even in the absence of abdominal symptoms (because of the central role of gastric emptying for regulation of postprandial glycemia) | ||
| Moderate | To support the diagnosis of dumping syndrome | |
| Low | Severe gastroesophageal reflux disease unresponsive to acid suppressants (particularly before fundoplication); systemic sclerosis; after lung transplantation; Parkinson disease; generalized GI motility disorders; patients under consideration for intestinal or colonic surgery or transplantation because of motility disorders | |
| Children | High/moderate | Most common GI symptoms located in the upper GI tract suggestive for gastroparesis: Nausea, vomiting and abdominal pain |
| Low | Less frequent GI symptoms located in the upper GI tract suggestive for gastroparesis: Early satiety, postprandial fullness and bloating | |
Abbreviation: GI, gastrointestinal.
When should 13C‐GEBT be utilized?
Recommendation 3.1
13 C‐gastric emptying breath tests are to be regarded as an established alternative to scintigraphy for measurement of gastric emptying velocity (92%, 0%; Q:A S:A).
Scintigraphy is the reference standard for measurement of gastric emptying. However, while there is a consensus report recommending a standardized protocol in adults in the United States, 73 no European consensus exists on the type of test meal and duration of data acquisition. Likewise, no consensus exists for a standard gastric emptying scintigraphy in pediatrics. However, recent studies have provided confirmation that extending studies from 2 to 4 h increases the diagnostic yield and should be the standard in children and adolescents as it is in adults for measurement of solid gastric emptying. 73 , 74 , 75 For liquids, 2 h are probably sufficient and early gastric emptying has to be accounted for. 76
Several protocols for 13C‐based gastric emptying tests have been successfully validated in comparison with scintigraphy. The medium‐chain fatty acid, 13C‐octanoic acid, 77 , 78 or the edible blue‐green algae, 13C‐Spirulina platensis 79 are typically used to label solids; 13C‐acetate is used for liquids. 80 , 81 On delivery to the duodenum, the 13C‐containing substrate is either absorbed directly (octanoic acid, acetate) or digested and then absorbed (Spirulina). Subsequently, it is metabolized in the liver, and finally excreted by the lungs as 13CO2. 6
The first use of the 13C‐OABT in adults was published by Ghoos et al. in 1993. 77 Gastric half emptying time (T½) was assessed by NLR analysis and corrected for the expected delay caused by postgastric processes (absorption, metabolization, exhalation). The authors observed an excellent correlation between 13C‐OABT parameters and parameters obtained from simultaneous scintigraphy (R = 0.89 for T½ scintigraphy vs. breath test). Sensitivity and specificity, positive and negative predictive values of breath test parameters for delayed gastric emptying were ≥94%. 77 Results of the 13C‐OABT also closely correlate with those of scintigraphy in other studies in adults and children. 78 , 82 , 83 , 84 Following this initial study, all kinds of variation of the test meal and the test protocol have been described, depending on cultural differences and practical considerations (e.g., muffins, pancakes, rolls). In addition, different mathematical analysis methods have been proposed 77 , 80 , 85 , 86 , 87 , 88 (also compare Table 6). Pancakes marked with 13C‐octanoic acid are an acceptable and palatable solid test meal for children but cannot be used in case of allergy to egg, milk or wheat, in coeliac disease or very young children. 78 , 88 , 89
TABLE 6.
Established test protocols for 13C‐GEBT in adults and children a
| Reference | Age | Estimated parameter | Test meal | Breath sampling | Endpoints and normal values | Validity | Remarks |
|---|---|---|---|---|---|---|---|
| Ghoos 1993 77 | Adults | Solid GE | Two slices of white bread, 5 g butter, 200 ml water, omelet made from one egg, yolk doped with 91 µg (= 100 µl) 13 C‐octanoic acid | Samples at baseline (preferentially taken as duplicate), further samples at 15 min intervals up to 4 h pp | T ½ (mean ± 2SD): 28–116 min c |
|
Evaluated against SCINTI in HC (N = 16) and patients with dyspepsia (N = 20), normal values from 42 HC (NLR model), no test kit commercially available, other groups report slightly different T ½ normal values using same protocol: 50–150 min 99 |
| Szarka 2008 90 | Adults | Solid GE | Freeze‐dried scrambled eggs mix containing 100 mg 13 C‐Spirulina platensis, six saltine crackers, and 180 ml of water | Breath samples at baseline, on completion of the meal and at 45, 90, 120, 150, 180, and 240 min pp | kPCD values at 45, 150, and 180 min provide strongest concordance with scintigraphy for accelerated and delayed GE |
|
38 HC and 129 patients with clinically suspected delayed GE, normal T ½ according to SCINTI (10th–90th percentile): 52–86 min,FDA approved, CE marked, commercially available in the United States, only |
| Bertram 2014 100 | Adults | Liquid GE | 150 mg 13 C‐acetate dissolved in 200 ml water with 10 g lacutose | Breath samples at baseline, at 5 min intervals for first hour, at 15 min intervals for second hour pp | Time of maximal 13C‐exhalation b : (P10–P90): 15–40 min | Time of maximal 13C‐exhalation and T ½ SCINTI: R = 0.88, p < 0.005 in validation study by Chew 2003 87 | 22 HC, lactulose used for simultaneous measurement of liquid gastric emptying and small bowel transit by H2‐breath test, time to maximal 13C‐exhalation in HC identical with validation study 87 in 10 HC which used 15 g glucose instead of lactulose |
| Van Den Driessche 1999 101 | 29 healthy premature and term infants gestational age 27–41 weaks, post‐natal age 7–74 days | Liquid GE | Group 1: 50 ml expressed breast milk, Group 2: 50 ml infant formula (Nutrilon premium®) (33.5 kcal), each with 50 µl 13 C‐octanoic acid | Breath sample at baseline, further samples at 5 min intervals for 30 min, then at 10 min intervals up to 4 h pp | T ½ (mean, range): group 1 = 47, 16–86 min c ; Group 2 = 65, 27–98 min c | ‐ | ‐ |
| Hauser 2016 102 | 133 healthy children mean 9 years, range 1–17 years | Liquid GE | 200 ml INZA® milk‐drink (skimmed milk)(112 kcal) with 50 mg (body weight 10–30 kg) or 100 mg (>30 kg) 13 C‐acetate | Breath samples at baseline and at 5 min intervals for 40 min, then at 10 min intervals up to 3 h pp |
|
|
Comparison with scintigraphy in 21 children with upper GI symptoms |
| Eradi 2006 103 | 25 healthy children mean 7.8 years, SD 0.3 years, range 5–10 years | Solid GE | 30 g chocolate crispy cake (147 kcal) with 100 mg 13 C‐octanoic acid | Breath samples at baseline, further samples at 15 min intervals up to 4 h pp | T ½ (mean ± 2SD): 44–155 min b | Comparison 13C‐OABT and gastric emptying scintigraphy:T ½‐OBT and T ½‐SCINTI: R = 0.69 (p < 0.01) | ‐ |
| Hauser 2016 78 | 120 healthy children mean 9 years, SD 4 years, range 1–17 years | Solid GE | One pancake (17 g wheat flour, 7 g sugar, one egg white, one egg yolk, 40 ml semi‐skimmed milk, 5 g margarine) + 5 g sugar + 100 ml water (230 kcal) with 50 µl 13 C‐octanoic acid | Breath sample at baseline further samples at 15 min intervals up to 4 h pp |
|
|
Comparison with scintigraphy in 19 dyspeptic children |
Abbreviations: 13C‐ABT, 13C‐acetate breath test; 13C‐OABT, 13C‐octanoic acid breath test; CV, coefficient of variance; GE, gastric emptying; GEBT, gastric emptying breath test; GI, gastrointestinal; HC, healthy controls; kPCD, percent dose excreted × 1000; NLR, nonlinear regression model; NPV, negative predictive value; P10, 10th percentile; P90, 90th percentile; pp, postprandial; PPV, positive predictive value; SCINTI, scintigraphy; SENS, sensitivity; SPEC, specificity; T½, gastric half emptying time.
Only studies with N ≥ 20.
Calculated breath test data not corrected for scintigraphy.
Scintigraphic equivalent values or breath test data corrected for scintigraphy according to Ghoos et al. 1993. 77
[Corrections added on June 28, 2021 after first online publication: Typos have been corrected in Table 6.]
The lack of standardization is of concern because it makes comparison of values between different laboratories difficult. On the other hand, it allows flexibility in research projects to measure gastric emptying of very different test meals and evaluate the impact of composition on gastric emptying.
Results of the 13C‐Spirulina‐GEBT also show high concordance (R = 0.86) with scintigraphic data. 90 The protocol is exactly defined and has been validated in a large group of healthy volunteers and patients. 90 The test was approved by the FDA for evaluation of gastric emptying in 2015 (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P110015), and it is also CE marked according to the producer's information (https://sercon‐instruments.com/wp‐content/uploads/2017/06/019‐gebt.pdf). However, it has not been marketed in Europe, so far.
13C‐acetate has been used as a marker for acaloric and caloric liquids such as formula diets. 81 , 87 , 91 , 92 , 93 In young children or children dependent on gastrostomy feeding, the test meal often consists of milk or milk‐based formulas. 94 Apple juice has also been used. 32 Results of the test closely correlate with those of scintigraphy in adults and children. 81 , 87 , 91 , 92 , 93 Delayed gastric emptying of solids usually precedes disturbances in gastric emptying of liquids. 95 Therefore, tests of solid gastric emptying are supposed to have a higher sensitivity. However, gastric emptying of liquids can be abnormal in patients with normal gastric emptying of solids. 96 Moreover, liquid test meals are obviously more useful in young children 81 , 94 and probably also for confirmation of rapid gastric emptying in patients with suspected dumping syndrome. 6 , 97
Intra‐individual and inter‐individual variabilities of all 13C‐GEBTs are high in adults and children, but similar to variations observed with scintigraphy 85 , 88 , 89 , 90 , 94 , 98 and, therefore, reflect day‐to‐day physiological variability in gastric emptying. 6
How should 13C‐GEBT be performed?
Recommendation 3.2
13 C‐GEBT (gastric emptying breath test) performed for clinical reasons have to adhere strictly to standardized study protocols adequately validated in a representative patient population. This refers to preparation of the test meal as well as test performance and evaluation of test results (for examples compare Table 6 ) (97%, 0%; Q:C S:A).
As discussed above, 13C‐GEBT are indirect tests that involve multiple steps and are prone to influences caused by demographic, physiological, and other parameters. 39 , 99 , 104 It has been hypothesized that 13C‐GEBT might be inaccurate in conditions associated with substantial malabsorption, liver, or lung diseases, though this is not substantiated by clinical studies. 39 , 44 , 47 However, it is still important that tests are validated not only in healthy adults and children, but also in the relevant patient population. 77 , 78 , 79 , 81 , 84
Moreover, alterations of marker substance, dose or other components of the test meal markedly influence test results. For instance, a larger labelled test meal will result in higher normal ranges for T½ and gastric lag time. 39 , 82 , 105 For 13C‐acetate an interaction has been demonstrated between the rate of 13C‐delivery to the duodenum and 13C‐recovery in breath. 33
Different mathematical models have been developed for analysis of gastric emptying curves derived from breath tests, in particular the NLR model, the generalized linear regression (GLR) model and the Wagner–Nelson method. 77 , 79 , 106 Cumulative 13CO2‐excretion over time is inversely related but analogous to the scintigraphic gastric emptying curve. However, 13CO2‐excretion does not only depend on gastric emptying velocity but also on postgastric absorption and metabolisation of the substrate and CO2‐exhalation rates. For this reason, Ghoos et al. developed the original NLR model. 77 According to this model, T½ indicates the time at which half of the 13CO2 is excreted, relative to the cumulative excretion when time is infinite. Accordingly, results are determined by the shape of the exhalation curve, independent of absolute 13CO2‐excretion. Measurements are usually performed over 4 h with breath samples at 15 min intervals.
The GLR model published by Lee et al. proposed a minimum number (N = 3) of breath samples at pre‐specified times during the 3 h postprandial period to mathematically predict the gastric emptying endpoints measured by simultaneous scintigraphy. 79 Results reflect absolute 13CO2‐excretion. A similar model with breath samples obtained upon completion of the meal and then at 45, 90, 120, 150, 180, and 240 min postprandially was suggested by the same group 90 and is used for analysis of the test commercially available in the USA.
The Wagner–Nelson method has been suggested for analysis of 13C‐GEBT by Sanaka et al. 106 This method has been developed to describe the entrance of ingested drugs into the venous system based on its urinary excretion data. When applied on breath tests, it describes the manner in which 13CO2 appears in the venous system based on pulmonary 13CO2 excretion data. 106 , 107 It is used less frequently, and, similar to the NLR model, the 13CO2‐exhalation curve must exhibit the decreasing portion during the sampling period for correct estimation of gastric emptying parameters. 108 Accordingly, breath sampling has to be routinely performed for 4 h and potentially longer in gastroparetic patients.
Given that in Europe there is no standardized, well‐validated test kit commercially available, and that tests are usually prepared on site, it is of pivotal importance that clinicians adhere strictly to standardized study protocols including established analysis methods. Especially in children, a large variety of test meals have been explored in accordance with the variable requirements of different age groups. 78 , 101 , 102 , 103 , 109 , 110 , 111 , 112 , 113 , 114 However, several of these studies were performed in small patient groups. Examples of well validated 13C‐GEBT protocols in adults and children (studies with N ≥ 20) are given in Table 6.
How should patients prepare for the test?
Recommendation 3.3
Before and during the test, precautions as described in General Methodology (avoidance of 13 C‐rich food, avoidance of physical activity, and 13 C‐rich infusions during test) have to be observed (100%, 0%; Q:C S:A).
Recommendation 3.4
Drugs with potential influence on gastrointestinal transit should be avoided before the test, unless essential long term medication is concerned or the test is performed to monitor the drug effect on gastric emptying (100%, 0%; Q:C S:B).
Recommendation 3.5
Adult patients, older children, and adolescents have to be fasted overnight (94%, 0%; Q:C S:A).
Recommendation 3.6
A shorter fasting period can be sufficient in very young children (92%, 0%; Q:C S:B).
Dietary and other restrictions, which generally apply before and during 13C‐breath testing, have been explained in General Methodology. Physical activity, in particular, has to be avoided during 13C‐GEBT, not only to standardize CO2‐production but also because physical activity influences gastric emptying velocity. 43 , 115 Tests should preferentially be performed in the sitting position since the supine position may be associated with slower gastric emptying. 116 Experts agree that solid test meals should be consumed within 10–15 min, liquids within 5–10 min.
Adults, adolescents and older children are required to fast overnight prior to breath testing 77 , 79 , 88 ; more precisely, a fasting period of ≥12 h is recommended by experts in adults as questionable results have been obtained in patients eating large meals very late. Fasting duration varies between 8 and 12 h in children and 3–4 h in infants less than 12 months old, depending on the clinical scenario. 81
Drugs which influence gastric motor function should be avoided before the test. This includes established prokinetics as well as drugs with anticholinergic properties (e.g., tricyclic antidepressants), smooth muscle relaxants, and opioids. The duration of withdrawal depends on the half‐life of the drug, 48–72 h are usually sufficient. However, in a patient with dyspeptic symptoms, who requires long‐term medication with, for example, amitriptyline, it is not reasonable to alter the normal clinical situation by discontinuation of the drug before the test.
As 13C‐GEBT are harmless, they can be performed repeatedly and have been used successfully to monitor drug effects in clinical studies and individual patients. 117 , 118 , 119 , 120
How should test results be reported?
Recommendation 3.7
To allow for reliable interpretation of test results the following parameters should be reported: assessment of gastric emptying of solids versus liquids; marker substance; caloric content of the test meal; duration of breath sampling period; test result including normal values; and interpretation (accelerated, normal, delayed gastric emptying) with T½ being the best established and most widely used parameter (94%, 0%; Q:D S:B).
Results can be calculated from breath test data alone, not corrected for scintigraphy 15 , 20 or given as scintigraphic equivalent values (for T½) according to Ghoos et al. 13
Recommendation 3.8
The test report could be complemented by including composition and preparation of the test meal, breath sampling intervals, methods used for analysis, and additional gastric emptying parameters such as T lag and GEC (gastric emptying coefficient) with normal values (86%, 0%; Q:D S:C).
These recommendations are based on the expert consensus that a minimum of information on methodology and test results including normal ranges and interpretation (accelerated/normal/delayed gastric emptying of solids/liquids) is required for reliable interpretation. Further information on methodological aspects and inclusion of several outcome parameters could improve diagnostic gain.
13C‐PANCREATIC FUNCTION TESTS
In adults, chronic pancreatitis, pancreatic cancer, and surgical procedures are the most common causes of PEI, whereas in children, cystic fibrosis is of particular relevance. In these diseases, inflammatory destruction, pancreatic atrophy, ductal obstruction, or resection of pancreatic tissue lead to decreased exocrine secretion. Furthermore, PEI can be caused by regulatory imbalances in the presence of a normal pancreas, such as reduced hormonal and vagal stimulation of pancreatic secretion or inactivation of pancreatic enzymes in the intestinal lumen due to hyperchlorhydria (e.g., Zollinger–Ellison syndrome). 49
Due to the large reserve capacity of the pancreas, mild to moderate exocrine insufficiency is frequently not associated with clinical symptoms, and overt steatorrhea is not expected unless the secretion of pancreatic lipase is reduced to less than 10% of normal (severe PEI). However, patients with “compensated” PEI also have an increased risk of nutritional deficiencies, in particular, of lipid‐soluble vitamins with respective clinical consequences. 7
Indications for pancreatic function testing in adults and children as recommended by international guidelines are given in Table 7. 7 , 121 , 122 , 123 , 124
TABLE 7.
Indications for pancreatic function testing in adults and children 7 , 121 , 122 , 123 , 124 , 125 , 126
| Grade of recommendation | ||
|---|---|---|
| Adults | High | Suspected pancreatic exocrine insufficiency in patients with pancreatic disease/after pancreatic resection |
| Patients with chronic pancreatitis at the time of diagnosis (and annually thereafter if not tested positive for pancreatic exocrine insufficiency) | ||
| Moderate | Monitoring of pancreatic enzyme replacement therapy in patients with an inadequate therapeutic response a | |
| Suspected pancreatic exocrine insufficiency without evidence of pancreatic disease | ||
| Differential diagnosis of chronic diarrhea | ||
| Children | High | Screening for pancreatic exocrine insufficiency of children with chronic pancreatitis every 6–12 months |
| Newly diagnosed CF | ||
| Every 3–12 months (age dependent) in CF patients with pancreatic sufficiency at time of diagnosis | ||
| In CF patient with subnormal weight development | ||
Abbreviation: CF, cystic fibrosis.
only applicable for indirect tests measuring digestion/absorption.
When should 13C‐PFBT be utilized?
Recommendation 4.1
The 13 C‐mixed triglyceride breath test is to be regarded as an established alternative to other non‐invasive and invasive pancreatic function tests (97%, 0%; Q:A S:A).
Several breath tests using 13C‐labeled lipids, proteins, or complex carbohydrates have been developed that indirectly assess pancreatic lipase, protease, and amylase activities. 11 , 12 , 14 , 100 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 Tests investigating lipid digestion and absorption are preferred because, from a clinical point of view, steatorrhea is by far the most important digestive malfunction in PEI: It is generally more severe and develops several years prior to malabsorption of protein or starch 49 and can be associated with decreased absorption of lipid‐soluble vitamins, as mentioned above. Indeed, a 13C‐breath test using naturally enriched maize starch lacks sensitivity and specificity for diagnosis of PEI. 136
Tests with various 13C‐labeled lipids have been investigated, in which triolein, trioctanoin, tripalmitin, cholesteryl‐octanoate, and mixed triglycerides (MTG) are used to generate marker substances. 128 Currently, the original 127 or modified versions 11 , 12 , 14 , 133 of the 13C‐MTGBT developed by Vantrappen et al. are almost exclusively used in the clinic because of practical advantages (e.g., shorter labeled fatty acid allowing for shorter breath sampling period). It is based on the principle that intestinal triglyceride absorption requires prior hydrolysis by pancreatic lipase to produce free fatty acids and mono‐acyl‐glycerol. These metabolites are incorporated into micelles, absorbed, resynthesized, and transported to the liver. Hepatic enzymes subsequently release fatty acids, including 13C‐octanoic acid, that is specifically bound to the Sn‐2 position of 13C‐mixed triglycerides (13C‐MTG: 1,3 distearyl 2[13C‐octanoyl] glycerol). 13C‐octanoic acid then undergoes β‐oxidation, which results in the formation of 13CO2, which is absorbed into the bloodstream, transported to the lung, and exhaled. The increase in 13CO2‐concentration in breath thus correlates with pancreatic lipase secretion.
Direct comparison with the reference standard (determination of pancreatic enzyme and/or bicarbonate output in duodenal aspirates following exogenous stimulation with secretin ± cerulein) demonstrates high sensitivity for severe exocrine insufficiency (90%–100%) with specificity ranging between 80% and 90% in adults. 121 , 137 A modified test using comparably high lipid loads in subjects explicitly avoiding physical activity during testing reached high sensitivity and specificity rates even in mild to moderate PEI (100% and 92%, respectively). 12
However, as evident from the test principle, the 13C‐MTGBT is a test of lipid digestion and absorption. Therefore, the 13C‐MTGBT is accepted as an appropriate alternative to the coefficient of fat absorption, both for the diagnosis of PEI and for evaluating the efficacy of pancreatic enzyme replacement therapy (PERT) in clinical practice. 7 , 11 , 132 , 133 , 138 On the other hand, 13CO2 exhalation is not only decreased by lipase deficiency but also by other causes of lipid malabsorption, for example, celiac disease, short bowel syndrome, or postcibal asynchrony following gastric resection. 139 Thus, the specificity of the test for the differential diagnosis of chronic diarrhea is limited.
Unfortunately, fecal elastase, the other noninvasive test which is predominantly used for pancreatic function testing in the clinic, also has limited specificity in these cases. 137 Direct comparisons between 13C‐MTGBT and fecal elastase favor the 13C‐MTGBT for diagnosis of steatorrhea, 140 and generally in patients with chronic pancreatitis after pancreatic resections. 141
In infants with cystic fibrosis, sensitivity of the 13C‐MTGBT for diagnosis of steatorrhea was high, but specificity was low. 142 Thus, the test has been mainly, though not exclusively, 143 , 144 used to evaluate the efficacy of PERT in children with cystic fibrosis. 145
How should 13C‐PFBT be performed?
Recommendation 4.2
13 C‐MTGBT performed for clinical reasons have to adhere strictly to standardized study protocols adequately validated in a representative patient population. This refers to preparation of the test meal as well as test performance and evaluation of test results (for examples compare Table 8 ) (100%, 0%; Q:C S:A).
TABLE 8.
Established test protocols for 13C‐MTGBT in adults and children
| Age | Test meal | Breath sampling | Endpoints and normal values | Validity | Remarks | |
|---|---|---|---|---|---|---|
| Vantrappen et al. 1989 127 | Adults | 100 g of toast with 0.25 g of butter per kg of body weight, plus 16 mg 13 C ‐MTG per gram of butter | At baseline and at 30 min intervals for 6 h pp | Cumulative 13C‐recovery, normal (estimated from fig 4: Lowest value obtained in HC): >23% of dose |
|
|
| Dominguez‐Munoz et al. 2015 132 | Adults |
|
At baseline and at 30 min intervals for 6 h pp | Cumulative 13C‐recovery, normal >29% of dose (>19% for 4 h test duration) |
|
Developed using quantitative fecal fat (reference standard for steatorrhea) for comparison in healty volunteers (N = 10) and chronic pancreatitis patients with (N = 16) or without (N = 4) PEI, validated in 78 pts with advanced CP, also shown to be of value for monitoring of PERT efficacy, and to correlate with the nutritional status and the severity of chronic pancreatitis |
| Keller et al. 2011 12 | Adults | Two slices of white bread, 20 g butter, 30 g chocolate cream (31 g fat/100 g) mixed with 250 mg 13 C‐MTG (total fat content 26 g) | At baseline and at 30 min intervals for 6 h pp | Cumulative 13C‐recovery, normal >26.8% of dose | SENS 100%, SPEC 92% versus secretin test | Validated using secretin test (reference standard for pancreatic secretion) for comparison in HC and patients with pancreatic disease (N = 19), also detects mild and moderate PEI |
| Keller et al. 2014 14 | Adults | Two slices of white bread, 20 g butter, 30 g chocolate cream (31 g fat/100 g) mixed with 250 mg 13 C‐MTG (total fat content 26 g) | At baseline and at 30 min intervals for 4 h pp | Cumulative 13C‐recovery, normal >13.8% of dose | SENS 88% SPEC 94%, versus 6 h test version | Evaluated in 200 pts undergoing both, 13C‐MTGT and 13C‐GEBT. More convenient, but decreasing duration of the test associated with lower diagnostic accuracy. Tests with less than 4 h duration are markedly influenced by gastric emptying time |
| Van Dijk‐van Aalst et al. 2001 146 | 12 premature infants, 12 full‐term infants (1–6 months), 20 children (3–10 years), 20 teenagers (11–17 years) | Infants: Formula with low 13C content (e.g., NAN1 (Nestlé), Pre‐Aptamil (Milupa) with 100 mg 13 C‐MTG and 1 g polyethylene‐glycol 3350; > 3 years: slice of white bread with 5 g butter and 15 g chocolate paste, mixed with 250 mg 13 C‐MTG, 100 ml whole‐fat milk | Two samples at baseline, further samples at 15 min intervals for 6 h pp |
|
‐ | Mean value for healthy adults: 35.6%, lower limit of normal 22.8% |
Abbreviations: 13C‐GEBT, 13C‐gastric emptying breath test; 13C‐MTG, mixed triglycerides; 13C‐MTGBT, 13C‐mixed triglyceride breath test; ACC, accuracy; HC, healthy controls; PEI, pancreatic exocrine insufficiency; PERT, pancreas enzyme replacement therapy; pp, postprandially; SENS, sensitivity; SPEC, specificity.
[Corrections added on June 28, 2021 after first online publication: Typos have been corrected in Table 8].
Recommendation 4.3
Adult patients, adolescents and older children have to be fasted overnight (97%, 0%; Q:C S:A).
Recommendation 4.4
A shorter fasting period can be sufficient in very young children (95%, 0%; Q:C S:B).
Recommendation 4.5
Before and during the test, precautions as described in general methodology have to be taken into account (avoidance of 13 C‐rich food, avoidance of physical activity and 13 C‐rich infusions during test) (100%, 0%; Q:D S:C).
Compared to 13C‐GEBT, 13C‐MTGBT uses significantly lower amounts of 13C resulting in lower DOB values and lower cumulative 13C‐exhalation rates. Consequently, avoidance of confounders such as 13C‐rich food is of particular importance when conducting the 13C‐MTGBT. Test results were not affected by liver disease in children with cystic fibrosis. 147
While adults and older children are required to fast overnight prior to breath testing, 11 , 12 , 127 , 132 , 133 a shorter period may be sufficient in very young children. In analogy to gastric emptying studies, 3‐4 h of fasting are recommended in infants less than 12 months old, depending on the clinical scenario. 81
A mathematical correction for altered CO2‐production rates in physically active subjects using heart rate recordings improves diagnostic accuracy of the 13C‐MTGBT in non‐resting subjects. 148 However, under clinical conditions, it is pragmatic to request that patients remain seated throughout the test.
Recommendation 4.6
Drugs with potential influence on gastrointestinal transit and/or lipid digestion and absorption should be avoided before the test. The duration of withdrawal depends on the half‐life of the drug, 48–72 h are usually sufficient (91%, 3%; Q:C S:B).
Recommendation 4.7
Pancreatic enzyme replacement therapy should be avoided 2 days before the test, unless the test is performed for monitoring the therapeutic efficacy of pancreatic enzyme replacement therapy (97%, 0%; Q:B S:B).
Intraluminal lipolysis of the marker and lipid absorption require prior delivery of the test meal into the duodenum, adequate mixing of the marker, meal nutrients and digestive secretions and regulated transport of chyme through the small intestine. Therefore, drugs, which accelerate (e.g., prokinetics, potent laxatives) or markedly inhibit (e.g., opioids) GI transit, may alter test results and should be avoided before the test, unless essential long‐term medication is concerned. Depending on the half‐life of the drug, 48–72 h of withdrawal are usually sufficient. However, some test protocols deliberately include administration of metoclopramide in order to accelerate gastric emptying and to shorten the required breath sampling period. 11 , 132 , 149
If the 13C‐MTGBT is performed to assess the presence of PEI, it is obvious that subjects have to stop oral PERT prior to testing. To account for potentially slow GI transit, it is recommended that patients abstain from PERT for 2 days before the test.
On the other hand, 13C‐MTGBT is a suitable and established alternative to fecal fat measurement for monitoring the efficacy of PERT. 11 , 132 To answer this question, subjects are allowed to continue with their usual enzyme replacement therapy. Indeed, it has been shown that PERT can be optimized by repetitive testing with increasing PERT doses until normal 13C‐MTGBT results are achieved. By this, a significant increase of body weight was observed. 11
How should test results be reported?
Recommendation 4.8
To allow for reliable interpretation of test results, the following parameters should be reported: marker substance, test results and interpretation including normal values. Cumulative 13 C‐recovery rate (in % of dose administered) represents the established main outcome parameter (94%, 0%; Q:D S:B).
Recommendation 4.9
The test report can be complemented by including the 13 C‐exhalation curve, composition and preparation of the test meal, breath sampling intervals, methods used for analysis and interpretation of the 13 C‐exhalation with respect to the clinical context (91%, 0%; Q:D S:B).
These recommendations are based on the expert consensus that a minimum of information on test methodology and test results including normal ranges and interpretation (normal/decreased intestinal lipolysis compatible with PEI) is required for reliable interpretation. Further information regarding methodological aspects and interpretation of test results with respect to the individual clinical context could further improve the diagnostic validity.
13C‐LIVER FUNCTION BREATH TESTS
Established parameters for the assessment of liver function under routine clinical conditions are measurement of bilirubin, albumin, liver enzymes and parameters of coagulation factor synthesis in serum or plasma, respectively. Clinical prognostic grading systems (e.g., Child–Pugh score, Model for End‐stage Liver Disease score) combine several of these biochemical parameters including clinical symptoms of advanced liver cirrhosis. 150
In contrast to these “static” liver function tests, “dynamic” quantitative tests measure the elimination of a substance, which is cleared and/or metabolized almost exclusively by the liver via specific metabolic pathways in subcellular compartments, for example, by cytochromes for microsomal liver function or cytosolic or mitochondrial enzymes. 151 Accordingly, these tests constitute a more accurate measure of the specific aspects of liver function. Established dynamic quantitative liver function tests are the indocyanine green clearance test and the galactose elimination capacity test. 150 13C‐LFBT also represent dynamic tests with oral consumption or intravenous application of the marker substance and measurement of the end product of hepatic metabolism, that is exhaled 13CO2. Some modifications have been shown to detect early changes in liver metabolic capacity in patients, prior to the presence of structural damage to the liver (i.e., inflammation, fibrosis), 152 though with limited sensitivities and specificities. 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 Still, the dynamic nature of 13C‐LFBT, their possible versatility in terms of assessing a range of different liver functions, and the ease with which they can be repeated to follow relative changes in liver function with time, generally imply a marked potential for clinical application. 40
When should 13C‐LFBT be utilized?
Recommendation 5.1
13 C‐liver function breath tests could be used for measurement of various aspects of liver function in adults (94%, 0%; Q:A S:C).
Recommendation 5.2
Presently, due to very limited evidence, performance of 13 C‐liver function breath tests for clinical reasons cannot be recommended in children (95%, 0%; Q:D S:B)
Different 13C‐LFBT have been developed for assessment of hepatic mitochondrial (substrates: 13 C‐ketoisocaproate, [methyl‐ 13 C]‐methionine), microsomal (13 C‐methacetin, 13 C‐aminopyrine, [3‐methyl‐ 13 C]‐caffeine) and cytosolic (13 C‐phenylalanine) function. 152 , 157 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 These have been mainly used in patients with liver fibrosis and cirrhosis due to nonalcoholic fatty liver disease, 152 , 157 , 159 , 160 , 161 , 163 , 164 , 169 , 170 chronic hepatitis C, 162 , 165 cirrhosis, 166 , 171 and hepatocellular carcinoma. 172 Additional potential fields of application are in steatohepatitis, 156 , 157 , 159 fatty liver, 160 , 163 , 169 and assessment of prognosis in chronic liver diseases, in general: In a 7‐year prospective follow‐up study in 132 patients with chronic HCV infection, the 4′‐O‐13C‐methacetin breath was not inferior to liver biopsy in predicting liver‐related death and transplantation. 173 Although several studies have reported close correlations between 13C‐LFBT and histological alterations or other established parameters in adults, they have still not entered the mainstream of clinical practice but are used exclusively by highly specialized centers. For instance, the effect of transarterial chemoembolization on liver function has been monitored by 13C‐methacetin test, 174 and several studies suggest that this test could also be used for planning of hepatic resections. 175 , 176 , 177
Given that, as yet, few studies have been performed involving small groups of children with rare diseases, 178 , 179 , 180 , 181 the use of 13C‐LFBT for clinical purposes cannot be recommended in the pediatric population, so far.
How should 13C‐LFBT be performed?
Recommendation 5.3
13 C‐liver function breath tests performed for clinical purposes have to adhere strictly to standardized and adequately validated study protocols. This refers to preparation of the patient, test meal/solution, test performance and evaluation of test results (for examples compare Table 9 ) (100%, 0%; Q:C S:A).
TABLE 9.
Examples of validated test protocols for 13C‐liver function breath tests in adults
| Estimated parameter | Marker and test solution | Breath sampling | Endpoints and normal values | Validity | Remarks | |
|---|---|---|---|---|---|---|
| Afolabi et al 2018 161 | Hepatic mitochondrial function | 1 mg/kg body weight of 13 C‐ketoisocaproate plus 20 mg/kg body weight L‐leucine dissolved in 200 ml of water | At baseline and at 10 min intervals for 60 min pp | Cumulative 13C‐recovery, normal >21% of dose | ‐ | Validated in 11 HC and 77 pts with NAFLD, SENS and SPEC to detect significant fibrosis was not determined |
| Portincasa et al 2006 157 | Hepatic mitochondrial function | 1 mg/kg body weight of 13 C‐ketoisocaproate plus 1 g of L‐leucine dissolved in 200 ml of water | At baseline and at 10 min intervals for 60 min pp | Cumulative 13C‐recovery, normal >14% of dose | Diagnostic accuracy at identifying pts with NASH (cut‐off value 9.6%): SENS 68%, SPEC 94%, PPV 90%, NPV 73% | Validated in 28 HC and 39 pts with NAFLD. The test was also able to discriminate fibrosis stages in patients with NASH |
| Banasch et al 2011 156 | Hepatic mitochondrial function | 2 mg/kg body weight [methyl‐ 13 C]‐methionine dissolved in 100 ml of water, prior oral consumption of 200 ml of orange juice | At baseline and at 10 min intervals for 90 min pp | Cumulative 13C‐recovery, normal >6.1% of dose | Cut‐off value <4.20% of dose for separation of pts with NASH from non‐NASH, SENS 81%; SPEC 76% | Validated in 118 pts with NAFLD and 18 HC. Test predicts higher stages of disease activity |
| Korkmaz et al. 2015 160 | Hepatic mitochondrial function | 2 mg/kg body weight [methyl‐ 13 C]‐methionine dissolved in 100 ml water | At baseline and at 10 min intervals for 90 min pp | Cumulative 13C‐recovery, normal >6.2% of dose | Cut off value <3.71%:SENS 95% SPEC 88% for differentiating advanced liver fibrosis (F2–3) from mild (F0–1) fibrosis | Validated in 164 pts with NAFLD and 56 HC |
| Fierbinteanu‐Braticevici et al.2013 163 | Hepatic microsomal function | Fixed dose of 75 mg 13 C‐methacetin dissolved in 200 ml of water | At baseline and at 10 min intervals for 60 min pp | Cumulative 13C‐recovery, normal >22% of dose | Cut off value <15.2%, SENS 91%, SPEC 82% at detecting significant fibrosis (F ≥ 2) | Validated in 90 pts with NAFLD and 20 HC |
| Park et al 2003 167 | Hepatic microsomal function | 2 mg/kg body weight of [3‐methyl‐ 13 C]‐caffeine dissolved in 30 ml of water, followed by 40 ml water wash of the container | At baseline and at 10 min intervals for 60 min pp | Cumulative 13C‐recovery, normal >2.3% of dose | Cut off value <1.85%: 79% SENS 80% SPEC for detecting NASH | Validated in 48 pts with NAFLD, 48 patients with chronic hepatitis B and 24 HC subjects. Results reflect the extent of hepatic fibrosis |
Abbreviations: HC, healthy controls; NAFLD, non‐alcoholic Fatty Liver disease; NASH, nonalcoholic steatohepatitis; NPV, negative predictive value; PPV, positive predictive value; pp, postprandial; SENS, sensitivity; SPEC, specificity.
[Corrections added on June 28, 2021 after first online publication: In Table 9, typos have been corrected. In the 3rd column, 2nd row, “postprandially” has been deleted from “At baseline and at 10 min intervals postprandially for 60 min pp.”]
For most validated test protocols, the marker substance is dissolved in up to 200 ml of water or unsweetened tea; breath samples are collected before drinking of the test solution and at regular intervals for up to 1.5 h postprandially. As shown in Table 9, cumulative percentage of the marker dose exhaled at the end of the observation period is the primary outcome parameter of the vast majority of tests. The list of tests detailed in the table is incomplete but concentrates on examples for test protocols using different marker substances, which have been validated in an adequate patient population and have established normal or cut off values for specific diagnoses. Apart from tests with oral marker application, the LIMAx test 177 is commercially available in some European countries, which uses intravenous application of 4′‐O‐13C‐methacetin. For this test, the manufacturer's instructions have to be followed exactly.
Recommendation 5.4
Before and during the test precautions as described in general methodology have to be observed (avoidance of 13 C‐rich food, of physical activity, and of 13 C‐rich infusions) (100%, 0%; Q:C S:A).
Recommendation 5.5
Drugs with potential influence on gastrointestinal transit and/or cytochrome p450 metabolism should be avoided before and during the test (96%, 0%; Q:C S:B).
Recommendation 5.6
As far as essential long‐term medication is concerned, it can be necessary to perform 13 C‐liver function breath tests despite ongoing medication as long as these medications are not administered during the test period (93%, 0%; Q:B S:B).
As discussed before, potential confounders of test results detailed in general methodology have to be avoided. For 13C‐LFBT that investigate cytochrome P450 enzymes, drugs with potential influence on cytochrome P450 metabolism are of particular relevance, 182 , 183 , 184 and it may be necessary to perform a test despite ongoing medication.
Especially for the most frequently applied test substrate 13C‐methacetin, strong CYP1A2 inhibitors (e.g., ciprofloxacin, fluvoxamin) will have an influence on hepatic methacetin metabolism, although no interaction studies have been systematically performed. Influences of weak CYP1A2 inhibitors (e.g., norfloxacin, propranolol) have not been reported. However, due to the rather short duration of the tests (no longer than 2 h for most protocols), administration during the test period can usually be circumvented, even if essential long‐term medication is concerned.
How should test results be reported?
Recommendation 5.7
To allow for reliable interpretation of test results the following parameters should be reported: marker substance; test results and interpretation including normal values (93%, 0%; Q:D S:B).
Recommendation 5.8
The test report could be complemented by including the 13 C‐exhalation curve, composition and preparation of the test meal/solution, breath sampling intervals, methods used for analysis, and interpretation of test results with respect to the clinical context (82%, 0%; Q:D S:C).
As for other 13C‐breath tests, these recommendations are based on the expert consensus that a minimum of information on methodology and test results including normal ranges and interpretation is required for reliable interpretation. Further information regarding methodological aspects and interpretation of test results with respect to the individual clinical context could further improve the diagnostic validity.
OTHER 13C‐BREATH TESTS
The 13C‐breath tests discussed in the previous chapters have gastroenterological indications and are either clinically established or at least used regularly by specialized centers. Apart from these, there is a multitude of other test options at various developmental stages for gastroenterological indications and 13C‐breath tests, which are used by other medical specialties. Examples for these are detailed in Table 10 and demonstrate the very broad potential applicability of 13C‐breath test technology.
TABLE 10.
Examples of other 13C‐breath tests
| References | Estimated parameter | Remarks |
|---|---|---|
| (185, 186, 187, 188, 189, 190, 191, 192, 193, 194) | Insulin resistance, metabolic syndrome | 13C‐glucose + unmarked glucose (e.g., 75 g) dissolved in water, outcome parameters: Cumulative 13CO2‐exhalation, maximal DOB‐value, 13C‐exhalation at specific time point |
| 13C‐exhalation is significantly decreased in patients with impaired insulin resistance/metabolic syndrome, site of labeled carbon atom in glucose molecule is relevant for metabolic results | ||
| Breath test described as valid surrogate index of clamp‐derived measures of insulin resistance, with good accuracy and precision, but controversial findings regarding applicability as screening tool (insufficient accuracy) in minority of studies | ||
| Non‐invasive tool for early diagnosis and follow up of patients in high‐risk groups | ||
| (195, 196, 197, 198) | SIBO, OCTT | Lactose‐[13C]ureide, moderate sensitivity (66.7%), high specificity (100%) for SIBO in comparison with culture from jejunal aspirates, glucose‐[13C]ureide could be used alternatively |
| Bacterial metabolism of marker is prerequisite for 13CO2‐exhalation, increase caused by SIBO or arrival of the marker substance in the colon | ||
| Predosing with unlabelled ureide is frequently part of the protocol, induces enzyme induction, increases rate of 13CO2‐recovery but complicates procedure | ||
| (136, 199, 200, 201) |
|
Maize starch is naturally enriched with 13C, 50–100 g used as pancreatic function test, but limited sensitivity (up to 77%) and specificity (up to 74%) in comparison with “gold standard” (secretin cerulein test) |
| 13CO2 release following labelled starch or sucrose is decreased in patients with sucrase‐isomaltase deficiency | ||
| (202, 203, 204, 205, 206, 207, 208, 209, 210) | Activity of drug metabolizing enzymes | 13C‐erythromycin applied iv (or orally) → hepatic (and intestinal) cytochrome P450 3A4 and 3A5 (CYP3A4/5) activity in vivo, pilot studies |
| 13C‐pantoprazole → CYP2C19 activity, can predict the anti‐platelet efficacy of clopidogrel (which is metabolized by CYP2C19 to its active metabolite) and high metabolization rate of PPI | ||
| 13C‐dextromethorphan → CYP2D6 activity (e.g., relevant for activation of the prodrug tamoxifen), [2‐(13)C]uracil → dihydropyrimidine dehydrogenase (DPD) deficiency to predict 5‐fluorouracil dose‐related toxicity |
Abbreviations: DOB, δ over baseline; OCTT, oro‐cecal transit time; PEI, pancreatic exocrine insufficiency; PPI, proton pump inhibitor; SIBO, small intestinal bacterial overgrowth.
CONCLUSIONS AND FUTURE PERSPECTIVES
This consensus‐based clinical practice guideline aims to assist physicians and provide them with the information required to perform high quality 13C‐breath tests for patients with various GI symptoms and diseases. In a consensus process, in which representatives from all regions of Europe and specialists representing European scientific societies participated, the available evidence was evaluated, taking account of local facilities, diverse clinical practice, and health care environments. Patient involvement was not covered in our guideline because of its focus on diagnostic recommendations. However, after publication of the guideline, a qualitative and quantitative assessment of guideline adoption into clinical practice is planned, including patient and public involvement.
The guideline gives an overview over general methodology of 13C‐breath testing and provides recommendations for the use of 13C‐breath tests to diagnose H. pylori infection, measure gastric emptying time and monitor pancreatic exocrine and liver function in adult and pediatric patients. Other potential applications of 13C‐breath testing are summarized briefly. The recommendations specifically detail when and how individual 13C‐breath tests should be performed including examples for well‐established test protocols.
13C‐breath tests are indirect tests and mostly require several digestive and metabolic steps that ultimately lead to the exhalation of 13C‐enriched breath, including intestinal absorption, hepatic metabolization, and pulmonary excretion. Therefore, demographic parameters, concomitant diseases, and/or medication affecting these processes may confound breath test results. In fact, studies have shown that the influence of concomitant diseases on many 13C‐breath tests is small or can be prevented by adaptation of the breath test protocol. Information on general 13C‐breath test methodology as detailed in the guideline will help clinicians to optimize patient selection and preparation and to better understand and interpret breath test results for individual patients.
As depicted in Figure 2, 13C‐UBT has excellent sensitivity and specificity for diagnosis of H. pylori infection, superior to most other tests, it is highly standardized and well established in the clinic. 13C‐GEBT and 13C‐PFBT are validated and accepted tests for diagnosis of gastric emptying disturbances and PEI, respectively. So far, their wide spread use has been hampered by the lack of standardization among centers, which we aim to improve by this guideline. Moreover, the tests are currently not commercially available in Europe, so that test meals need to be prepared on site. 13C‐LFBT are currently used by highly specialized centers, only. They have the potential to measure various aspects of liver function, including assessment of prognosis in chronic liver disease. However, their clinical role, in general, and applicability in pediatric patients, in particular, still need to be established.
FIGURE 2.
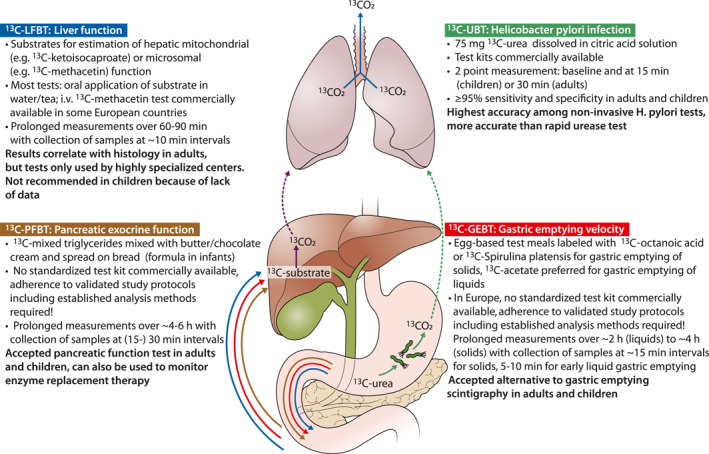
General principle, performance and clinical role of 13C‐breath tests used in gastroenterology. 13C‐urea used for detection of H. pylori (13C‐UBT, marked in green) is metabolized by bacterial urease to produce 13CO2, which is absorbed, transported to the lung (broken green arrow) and exhaled. For 13C‐gastric emptying breath tests (13C‐GEBT, marked in red), 13C‐pancreatic function breath test (13C‐PFBT, marked in brown), and 13C‐liver function breath tests (13C‐LFBT, marked in blue), orally applied substrates or their metabolites are absorbed in the small intestine. Subsequently, they are transported to the liver where they undergo further metabolization with production of 13CO2, which is transported to the lung and exhaled[Corrections added on June 28, 2021 after first online publication: Figure 3 (image and caption) has been revised.]
Apart from identification of this and other areas of future research, the guideline should improve pan‐European harmonization of diagnostic approaches to symptoms and disorders, which are very common in specialist and primary care gastroenterology practice, both in adult and pediatric patients.
CONFLICT OF INTEREST
Oliver Goetze received financial support/honoraria for clinical studies/lectures from Kibion, Mayoly Spindler Laboratories. Stephan L. Haas received honoraria by Mylan for oral presentations. The other authors have nothing to disclose.
ACKNOWLEDGMENTS
This guideline was developed with the support of an UEG Activity Grant.
Members of European 13C‐Breath Test Group: Afolabi Paul; Altorjay, Istvan; Barbara, Giovanni; Basilisco, Guido; Baumann‐Durchschein, Franziska; Belei, Oana; Benninga, Marc; Borrelli, Osvaldo; Churchev, Stanislav; Dominguez‐Munoz, Enrique; Dumitrascu, Dan; Effenberger, Maria; Fox, Mark; Fürst, Stefan; Gasbarrini, Antonio; Goetze, Oliver; Haas, Stephan; Hammer, Heinz; Hammer, Johann; Hammer, Karin; Hauser, Bruno; Herszenyi, Laszlo; Keller, Jutta; Krznaric, Zeljko; Leja, Marcis; Lopetuso, Loris; Mion, Francois; Mulak, Agata; Nakov, Radislav; Nakov, Ventsislav; Pohl, Daniel; Reinisch, Sieglinde; Salvatore, Silvia; Shvet, Oleg; Simren, Magnus; Surdea‐Blaga, Teodora; Tepes, Bojan; Thapar, Nikhil; Törnblom, Hans; Tutuian Radu; Verbeke, Kristin; Vogelsang, Harald; Vranesic‐Bender, Darija; Wilder‐Smith, Clive
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Sonyi M, Keller J, Fox M, Hammer HF. Development of a multinational clinical practice guideline: a practical structured procedure. Basel: Digestive diseases; 2020. [DOI] [PubMed] [Google Scholar]
- 2. Keller J, Franke A, Storr M, Wiedbrauck F, Schirra J. Clinically relevant breath tests in gastroenterological diagnostics‐‐recommendations of the German Society for Neurogastroenterology and Motility as well as the German Society for Digestive and Metabolic Diseases. Z Gastroenterol. 2005;43 (9):1071–90. [DOI] [PubMed] [Google Scholar]
- 3. Schoeller DA, Schneider JF, Solomons NW, Watkins JB, Klein PD. Clinical diagnosis with the stable isotope 13C in CO2 breath tests: methodology and fundamental considerations. J Lab Clin Med. 1977;90 (3):412–21. [PubMed] [Google Scholar]
- 4. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection‐the Maastricht V/Florence consensus report. Gut. 2017;66 (1):6–30. [DOI] [PubMed] [Google Scholar]
- 5. Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016). J Pediatr Gastroenterol Nutr. 2017;64 (6):991–1003. [DOI] [PubMed] [Google Scholar]
- 6. Keller J, Bassotti G, Clarke J, Dinning P, Fox M, Grover M, et al. Expert consensus document: advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018;15 (5):291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lohr JM, Dominguez‐Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, et al. United European Gastroenterology evidence‐based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5 (2):153–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones PJ, Leatherdale ST. Stable isotopes in clinical research: safety reaffirmed. Clin Sci (Lond). 1991;80 (4):277–80. [DOI] [PubMed] [Google Scholar]
- 9. Klein PD, Klein ER. Stable isotopes: origins and safety. J Clin Pharmacol. 1986;26 (6):378–82. [DOI] [PubMed] [Google Scholar]
- 10. Gregg CT, Hutson JY, Prine JR, Ott DG, Furchner JE. Substantial replacement of mammalian body carbon with carbon‐13. Life Sci. 1973;13 (7):775–82. [DOI] [PubMed] [Google Scholar]
- 11. Dominguez‐Munoz JE, Iglesias‐Garcia J, Vilarino‐Insua M, Iglesias‐Rey M. 13C‐mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2007;5 (4):484–8. [DOI] [PubMed] [Google Scholar]
- 12. Keller J, Bruckel S, Jahr C, Layer P. A modified (1)(3)C‐mixed triglyceride breath test detects moderate pancreatic exocrine insufficiency. Pancreas. 2011;40 (8):1201–5. [DOI] [PubMed] [Google Scholar]
- 13. Jonderko K, Dus Z, Szymszal M, Kasicka‐Jonderko A, Blonska‐Fajfrowska B. Normative values for the 13C‐mixed triglyceride breath test in two age groups. Med Sci Mon Int Med J Exp Clin Res. 2009;15 (5): Cr255‐9. [PubMed] [Google Scholar]
- 14. Keller J, Meier V, Wolfram KU, Rosien U, Layer P. Sensitivity and specificity of an abbreviated (13)C‐mixed triglyceride breath test for measurement of pancreatic exocrine function. United European Gastroenterol J. 2014;2 (4):288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leodolter A, Domínguez‐Muñoz JE, von Arnim U , Kahl S, Peitz U, Malfertheiner P. Validity of a modified 13C‐urea breath test for pre‐ and posttreatment diagnosis of Helicobacter pylori infection in the routine clinical setting. Am J Gastroenterol. 1999;94 (8):2100–4. [DOI] [PubMed] [Google Scholar]
- 16. Savarino V, Vigneri S, Celle G. The 13C urea breath test in the diagnosis of Helicobacter pylori infection. Gut. 1999;45 (Suppl 1):I18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savarino V, Mela GS, Zentilin P, Bisso G, Pivari M, Mansi C, et al. Comparison of isotope ratio mass spectrometry and nondispersive isotope‐selective infrared spectroscopy for 13C‐urea breath test. Am J Gastroenterol. 1999;94 (5):1203–8. [DOI] [PubMed] [Google Scholar]
- 18. Mana F, Franken PR, Ham HR, Reynaert H, Urbain D. 13C urea breath test with nondispersive isotope‐selective infrared spectrometry: reproducibility and importance of the fasting status. Helicobacter. 2000;5 (2):104–8. [DOI] [PubMed] [Google Scholar]
- 19. Gisbert JP, Gomollon F, Dominguez‐Munoz JE, Borda F, Jimenez I, Vazquez MA, et al. [Comparison between two 13C‐urea breath tests for the diagnosis of Helicobacter pylori infection: isotope ratio mass spectrometer versus infrared spectrometer]. Gastroenterol Hepatol. 2003;26 (3):141–6. [DOI] [PubMed] [Google Scholar]
- 20. Plavnik RG, Nevmerzhitsky VI, Butorova LI, Plavnik TE. [C assessment of mass‐spectrometry and infrared specrtrometry used in 13C‐urea breath test for helicobacter pylori]. Klin Med (Mosk). 2015;93 (9):42–5. [PubMed] [Google Scholar]
- 21. Kato M, Saito M, Fukuda S, Kato C, Ohara S, Hamada S, et al. 13C‐Urea breath test, using a new compact nondispersive isotope‐selective infrared spectrophotometer: comparison with mass spectrometry. J Gastroenterol. 2004;39 (7):629–34. [DOI] [PubMed] [Google Scholar]
- 22. Ferwana M, Abdulmajeed I, Alhajiahmed A, Madani W, Firwana B, Hasan R, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta‐analysis. World J Gastroenterol. 2015;21 (4):1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schadewaldt P, Schommartz B, Wienrich G, Brosicke H, Piolot R, Ziegler D. Application of isotope‐selective nondispersive infrared spectrometry (IRIS) for evaluation of [13C]octanoic acid gastric‐emptying breath tests: comparison with isotope ratio‐mass spectrometry (IRMS). Clin Chem. 1997;43 (3):518–22. [PubMed] [Google Scholar]
- 24. Goetze O, Selzner N, Fruehauf H, Fried M, Gerlach T, Mullhaupt B. 13C‐methacetin breath test as a quantitative liver function test in patients with chronic hepatitis C infection: continuous automatic molecular correlation spectroscopy compared to isotopic ratio mass spectrometry. Aliment Pharmacol Ther. 2007;26 (2):305–11. [DOI] [PubMed] [Google Scholar]
- 25. Boedeker C, Goetze O, Pfaffenbach B, Luypaerts A, Geypens B, Adamek RJ. 13C mixed‐triglyceride breath test: isotope selective non‐dispersive infrared spectrometry in comparison with isotope ratio mass spectrometry in volunteers and patients with chronic pancreatitis. Scand J Gastroenterol. 1999;34 (11):1153–6. [DOI] [PubMed] [Google Scholar]
- 26. Craig H. Isotopic standards for carbon and oxygen and correction factors for mass spectrometric analysis of carbon dioxide Geochemica. Cosmochimica Acta. 1957;12:181–6. [Google Scholar]
- 27. van der Merwe N. Carbon isotopes, photosynthesis, and archaeology. Am Sci. 1982;70:596–606. [Google Scholar]
- 28. Colaiocco Ferrante L, Papponetti M, Marcuccitti J, Neri M, Festi D. 13C‐urea breath test for helicobacter pylori infection: stability of samples over time. Scand J Gastroenterol. 1999;34 (9):942–3. [DOI] [PubMed] [Google Scholar]
- 29. Irving CS, Wong WW, Shulman RJ, Smith EO, Klein PD. [13C]bicarbonate kinetics in humans: intra‐ vs. interindividual variations. Am J Physiol. 1983;245 (2):R190–202. [DOI] [PubMed] [Google Scholar]
- 30. Kindermann A, Demmelmair H, Koletzko B, Krauss‐Etschmann S, Wiebecke B, Koletzko S. Influence of age on 13C‐urea breath test results in children. J Pediatr Gastroenterol Nutr. 2000;30 (1):85–91. [DOI] [PubMed] [Google Scholar]
- 31. Caubet MS, Laplante A, Caille J, Brazier JL. [13C]aminopyrine and [13C]caffeine breath test: influence of gender, cigarette smoking and oral contraceptives intake. Isot Environ Health Stud. 2002;38 (2):71–7. [DOI] [PubMed] [Google Scholar]
- 32. Hellmig S, Von Schoning F , Gadow C, Katsoulis S, Hedderich J, Folsch UR, et al. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol. 2006;21 (12):1832–8. [DOI] [PubMed] [Google Scholar]
- 33. Goetze O, Fox M, Kwiatek MA, Treier R, Schwizer W, Thumshirn M, et al. Effects of postgastric 13C‐acetate processing on measurement of gastric emptying: a systematic investigation in health. Neuro Gastroenterol Motil. 2009;21 (10):1047‐e85. [DOI] [PubMed] [Google Scholar]
- 34. Sonko BJ, Prentice AM, Coward WA, Murgatroyd PR, Goldberg GR. Dose‐response relationship between fat ingestion and oxidation: quantitative estimation using whole‐body calorimetry and 13C isotope ratio mass spectrometry. Eur J Clin Nutr. 2001;55 (1):10–8. [DOI] [PubMed] [Google Scholar]
- 35. McCue MD, Passement CA, Rodriguez M. The magnitude of the naturally occurring isotopic enrichment of 13C in exhaled CO2 is directly proportional to exercise intensity in humans. Comp Biochem Physiol Mol Integr Physiol. 2015;179:164–71. [DOI] [PubMed] [Google Scholar]
- 36. Keller J, Fliegner‐Baia M, Layer P. Physical activity alters normal values of the "European standard" 13C‐octanoic acid breath test. Gut. 2002;51 (Suppl III):A136. [Google Scholar]
- 37. Bammens B, Evenepoel P, Rutgeerts P, Ghoos Y, Maes B. 13C‐breath tests in peritoneal dialysis patients: influence of dialysis fluids. Eur J Gastroenterol Hepatol. 2003;15 (8):931–2. [DOI] [PubMed] [Google Scholar]
- 38. Eaton S, Pacilli M, Wood J, McHoney M, Corizia L, Kingsley C, et al. Factors affecting 13C‐natural abundance measurement of breath carbon dioxide during surgery: absorption of carbon dioxide during endoscopic procedures. Rapid Commun Mass Spectrom. 2008;22 (11):1759–62. [DOI] [PubMed] [Google Scholar]
- 39. Keller J, Andresen V, Wolter J, Layer P, Camilleri M. Influence of clinical parameters on the results of 13C‐octanoic acid breath tests: examination of different mathematical models in a large patient cohort. Neuro Gastroenterol Motil. 2009;21 (10):1039‐e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Afolabi P, Wright M, Wootton SA, Jackson AA. Clinical utility of 13C‐liver‐function breath tests for assessment of hepatic function. Dig Dis Sci. 2013;58 (1):33–41. [DOI] [PubMed] [Google Scholar]
- 41. Westerterp KR, Plasqui G. Physical activity and human energy expenditure. Curr Opin Clin Nutr Metab Care. 2004;7 (6):607–13. [DOI] [PubMed] [Google Scholar]
- 42. Keim NL, Blanton CA, Kretsch MJ. America's obesity epidemic: measuring physical activity to promote an active lifestyle. J Am Diet Assoc. 2004;104 (9):1398–409. [DOI] [PubMed] [Google Scholar]
- 43. Costa RJS, Snipe RMJ, Kitic CM, Gibson PR. Systematic review: exercise‐induced gastrointestinal syndrome‐implications for health and intestinal disease. Aliment Pharmacol Ther. 2017;46 (3):246–65. [DOI] [PubMed] [Google Scholar]
- 44. van de Casteele M , Luypaerts A, Geypens B, Fevery J, Ghoos Y, Nevens F. Oxidative breakdown of octanoic acid is maintained in patients with cirrhosis despite advanced disease. Neuro Gastroenterol Motil. 2003;15 (2):113–20. [DOI] [PubMed] [Google Scholar]
- 45. Mawatari H, Inamori M, Fujita K, Yoneda M, Iida H, Endo H, et al. The continuous real‐time 13C‐octanoate breath test for patients with nonalcoholic steatohepatitis using the BreathID system. Hepato‐gastroenterology. 2009;56 (94‐95):1436–8. [PubMed] [Google Scholar]
- 46. Schneider AR, Kraut C, Lindenthal B, Braden B, Caspary WF, Stein J. Total body metabolism of 13C‐octanoic acid is preserved in patients with non‐alcoholic steatohepatitis, but differs between women and men. Eur J Gastroenterol Hepatol. 2005;17 (11):1181–4. [DOI] [PubMed] [Google Scholar]
- 47. Chapman MJ, Besanko LK, Burgstad CM, Fraser RJ, Bellon M, O'Connor S, et al. Gastric emptying of a liquid nutrient meal in the critically ill: relationship between scintigraphic and carbon breath test measurement. Gut. 2011;60 (10):1336–43. [DOI] [PubMed] [Google Scholar]
- 48. Maes BD, Ghoos YF, Geypens BJ, Hiele MI, Rutgeerts PJ. Relation between gastric emptying rate and rate of intraluminal lipolysis. Gut. 1996;38 (1):23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54 (Suppl 6):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Best LM, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, et al. Non‐invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3 (3):Cd012080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102 (8):1808–25. [DOI] [PubMed] [Google Scholar]
- 52. Leal YA, Flores LL, Fuentes‐Panana EM, Cedillo‐Rivera R, Torres J. 13C‐urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta‐analysis. Helicobacter. 2011;16 (4):327–37. [DOI] [PubMed] [Google Scholar]
- 53. Fischbach W, Malfertheiner P, Lynen Jansen P, Bolten W, Bornschein J, Buderus S, et al. S2k‐Leitlinie Helicobacter pylori und gastroduodenale Ulkuskrankheit. Z Gastroenterol. 2017;54 (4):327–63. [DOI] [PubMed] [Google Scholar]
- 54. Leodolter A, Domínguez‐Muñoz JE, Von Arnim U , Malfertheiner P. Citric acid or orange juice for the 13C‐urea breath test: the impact of pH and gastric emptying. Aliment Pharmacol Ther. 1999;13 (8):1057–62. [DOI] [PubMed] [Google Scholar]
- 55. Domínguez‐Muñoz JE, Leodolter A, Sauerbruch T, Malfertheiner P. A citric acid solution is an optimal test drink in the 13C‐urea breath test for the diagnosis of Helicobacter pylori infection. Gut. 1997;40 (4):459–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Elitsur Y, Tolia V, Gilger MA, Reeves‐Garcia J, Schmidt‐Sommerfeld E, Opekun AR, et al. Urea breath test in children: the United States prospective, multicenter study. Helicobacter. 2009;14 (2):134–40. [DOI] [PubMed] [Google Scholar]
- 57. Graham DY, Klein PD, Evans DJ Jr., Evans DG, Alpert LC, Opekun AR, et al. Campylobacter pylori detected noninvasively by the 13C‐urea breath test. Lancet (London, England). 1987;1 (8543):1174–7. [DOI] [PubMed] [Google Scholar]
- 58. Di Rienzo TA , D'Angelo G, Ojetti V, Campanale MC, Tortora A, Cesario V, et al. 13C‐Urea breath test for the diagnosis of Helicobacter pylori infection. Eur Rev Med Pharmacol Sci. 2013;17 (Suppl 2):51–8. [PubMed] [Google Scholar]
- 59. Logan RP, Polson RJ, Misiewicz JJ, Rao G, Karim NQ, Newell D, et al. Simplified single sample 13Carbon urea breath test for Helicobacter pylori: comparison with histology, culture, and ELISA serology. Gut. 1991;32 (12):1461–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gisbert JP, Pajares JM. Review article: 13C‐urea breath test in the diagnosis of Helicobacter pylori infection ‐‐ a critical review. Aliment Pharmacol Ther. 2004;20 (10):1001–17. [DOI] [PubMed] [Google Scholar]
- 61. Rowland M, Lambert I, Gormally S, Daly LE, Thomas JE, Hetherington C, et al. Carbon 13‐labeled urea breath test for the diagnosis of Helicobacter pylori infection in children. J Pediatr. 1997;131 (6):815–20. [DOI] [PubMed] [Google Scholar]
- 62. Gatta L, Vakil N, Ricci C, Osborn JF, Tampieri A, Perna F, et al. Effect of proton pump inhibitors and antacid therapy on 13C urea breath tests and stool test for Helicobacter pylori infection. Am J Gastroenterol. 2004;99 (5):823–9. [DOI] [PubMed] [Google Scholar]
- 63. Graham DY, Opekun AR, Hammoud F, Yamaoka Y, Reddy R, Osato MS, et al. Studies regarding the mechanism of false negative urea breath tests with proton pump inhibitors. Am J Gastroenterol. 2003;98 (5):1005–9. [DOI] [PubMed] [Google Scholar]
- 64. Savarino V, Tracci D, Dulbecco P, Mele MR, Zentilin P, Mansi C, et al. Negative effect of ranitidine on the results of urea breath test for the diagnosis of Helicobacter pylori. Am J Gastroenterol. 2001;96 (2):348–52. [DOI] [PubMed] [Google Scholar]
- 65. Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108 (1):18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Parkman HP, Camilleri M, Farrugia G, McCallum RW, Bharucha AE, Mayer EA, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neuro Gastroenterol Motil. 2010;22 (2):113–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vijayvargiya P, Jameie‐Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta‐analysis. Gut. 2019;68 (5):804–13. [DOI] [PubMed] [Google Scholar]
- 68. Rodriguez L, Irani K, Jiang H, Goldstein AM. Clinical presentation, response to therapy, and outcome of gastroparesis in children. J Pediatr Gastroenterol Nutr. 2012;55 (2):185–90. [DOI] [PubMed] [Google Scholar]
- 69. Waseem S, Islam S, Kahn G, Moshiree B, Talley NJ. Spectrum of gastroparesis in children. J Pediatr Gastroenterol Nutr. 2012;55 (2):166–72. [DOI] [PubMed] [Google Scholar]
- 70. Saliakellis E, Fotoulaki M. Gastroparesis in children. Ann Gastroenterol. 2013;26 (3):204–11. [PMC free article] [PubMed] [Google Scholar]
- 71. Kempler P, Amarenco G, Freeman R, Frontoni S, Horowitz M, Stevens M, et al. Management strategies for gastrointestinal, erectile, bladder, and sudomotor dysfunction in patients with diabetes. Diabetes Metab Res Rev. 2011;27 (7):665–77. [DOI] [PubMed] [Google Scholar]
- 72. Kovacic K, Elfar W, Rosen JM, Yacob D, Raynor J, Mostamand S, et al. Update on pediatric gastroparesis: a review of the published literature and recommendations for future research. Neuro Gastroenterol Motil. 2020;32 (3): e13780. [DOI] [PubMed] [Google Scholar]
- 73. Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103 (3):753–63. [DOI] [PubMed] [Google Scholar]
- 74. Edwards ST, Cocjin J, Theut SB, Rivard D, Sherman AK, Friesen CA. A comparison of the diagnosis of gastroparesis in 4 h pediatric gastric emptying studies versus 2 h studies. BMC Gastroenterol. 2019;19 (1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chogle A, Saps M. Gastroparesis in children: the benefit of conducting 4‐hour scintigraphic gastric‐emptying studies. J Pediatr Gastroenterol Nutr. 2013;56 (4):439–42. [DOI] [PubMed] [Google Scholar]
- 76. Lin E, Connolly LP, Drubach L, Zurakowski D, DiCanzio J, Mitchell K, et al. Effect of early emptying on quantitation and interpretation of liquid gastric emptying studies of infants and young children. J Nucl Med. 2000;41 (4):596–9. [PubMed] [Google Scholar]
- 77. Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, et al. Measurement of gastric emptying rate of solids by means of a carbon‐labeled octanoic acid breath test. Gastroenterology. 1993;104 (6):1640–7. [DOI] [PubMed] [Google Scholar]
- 78. Hauser B, Roelants M, De Schepper J , Veereman G, Caveliers V, Devreker T, et al. Gastric emptying of solids in children: reference values for the (13) C‐octanoic acid breath test. Neuro Gastroenterol Motil. 2016;28 (10):1480–7. [DOI] [PubMed] [Google Scholar]
- 79. Lee JS, Camilleri M, Zinsmeister AR, Burton DD, Choi MG, Nair KS, et al. Toward office‐based measurement of gastric emptying in symptomatic diabetics using [13C]octanoic acid breath test. Am J Gastroenterol. 2000;95 (10):2751–61. [DOI] [PubMed] [Google Scholar]
- 80. Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, et al. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108 (4):1048–55. [DOI] [PubMed] [Google Scholar]
- 81. Gatti C, di Abriola FF , Dall'Oglio L, Villa M, Franchini F, Amarri S. Is the 13C‐acetate breath test a valid procedure to analyse gastric emptying in children? J Pediatr Surg. 2000;35 (1):62–5. [DOI] [PubMed] [Google Scholar]
- 82. Delbende B, Perri F, Couturier O, Leodolter A, Mauger P, Bridgi B, et al. 13C‐octanoic acid breath test for gastric emptying measurement. Eur J Gastroenterol Hepatol. 2000;12 (1):85–91. [DOI] [PubMed] [Google Scholar]
- 83. Dickman R, Steinmetz A, Bernnstine H, Groshar D, Niv Y. A novel continuous breath test versus scintigraphy for gastric emptying rate measurement. J Clin Gastroenterol. 2011;45 (1):22–5. [DOI] [PubMed] [Google Scholar]
- 84. Zahn A, Langhans CD, Hoffner S, Haberkorn U, Rating D, Haass M, et al. Measurement of gastric emptying by 13C‐octanoic acid breath test versus scintigraphy in diabetics. Z Gastroenterol. 2003;41 (5):383–90. [DOI] [PubMed] [Google Scholar]
- 85. Rao SS, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neuro Gastroenterol Motil. 2011;23 (1):8–23. [DOI] [PubMed] [Google Scholar]
- 86. Odunsi ST, Camilleri M, Szarka LA, Zinsmeister AR. Optimizing analysis of stable isotope breath tests to estimate gastric emptying of solids. Neuro Gastroenterol Motil. 2009;21 (7):706‐e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chew CG, Bartholomeusz FD, Bellon M, Chatterton BE. Simultaneous 13C/14C dual isotope breath test measurement of gastric emptying of solid and liquid in normal subjects and patients: comparison with scintigraphy. Nucl Med Rev Cent East Eur. 2003;6 (1):29–33. [PubMed] [Google Scholar]
- 88. Hauser B, De Schepper J , Caveliers V, Salvatore S, Salvatoni A, Vandenplas Y. Variability of the 13C‐octanoic acid breath test for gastric emptying of solids in healthy children. Aliment Pharmacol Ther. 2006;23 (9):1315–9. [DOI] [PubMed] [Google Scholar]
- 89. Van Den Driessche M . Study of gastrointestinal motility in infants and children using 13C breath tests. Leuven University Press; 2001. [Google Scholar]
- 90. Szarka LA, Camilleri M, Vella A, Burton D, Baxter K, Simonson J, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol. 2008;6 (6):635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Braden B, Peterknecht A, Piepho T, Schneider A, Caspary WF, Hamscho N, et al. Measuring gastric emptying of semisolids in children using the 13C‐acetate breath test: a validation study. Dig Liver Dis. 2004;36 (4):260–4. [DOI] [PubMed] [Google Scholar]
- 92. Barbosa L, Vera H, Moran S, Del Prado M , Lopez‐Alarcon M. Reproducibility and reliability of the 13C‐acetate breath test to measure gastric emptying of liquid meal in infants. Nutrition. 2005;21 (3):289–94. [DOI] [PubMed] [Google Scholar]
- 93. Sanaka M, Urita Y, Sugimoto M, Yamamoto T, Kuyama Y. Comparison between gastric scintigraphy and the [13C]‐acetate breath test with Wagner‐Nelson analysis in humans. Clin Exp Pharmacol Physiol. 2006;33 (12):1239–43. [DOI] [PubMed] [Google Scholar]
- 94. Hauser B, De Schepper J , Caveliers V, Salvatore S, Salvatoni A, Vandenplas Y. Variability of the 13C‐acetate breath test for gastric emptying of liquids in healthy children. J Pediatr Gastroenterol Nutr. 2006;42 (4):392–7. [DOI] [PubMed] [Google Scholar]
- 95. Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356 (8):820–9. [DOI] [PubMed] [Google Scholar]
- 96. Ziessman HA, Chander A, Clarke JO, Ramos A, Wahl RL. The added diagnostic value of liquid gastric emptying compared with solid emptying alone. J Nucl Med. 2009;50 (5):726–31. [DOI] [PubMed] [Google Scholar]
- 97. van Beek AP , Emous M, Laville M, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. 2017;18 (1):68–85. [DOI] [PubMed] [Google Scholar]
- 98. Bharucha AE, Camilleri M, Veil E, Burton D, Zinsmeister AR. Comprehensive assessment of gastric emptying with a stable isotope breath test. Neuro Gastroenterol Motil. 2013;25 (1):e60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Keller J, Binnewies U, Rosch M, Juul Holst J, Beglinger C, Andresen V, et al. Gastric emptying and disease activity in inflammatory bowel disease. Eur J Clin Invest. 2015;45 (12):1234–42. [DOI] [PubMed] [Google Scholar]
- 100. Bertram F, Andresen V, Layer P, Keller J. Simultaneous non‐invasive measurement of liquid gastric emptying and small bowel transit by combined 13C‐acetate and H2‐lactulose breath test. J Breath Res. 2014;8 (4):046007. [DOI] [PubMed] [Google Scholar]
- 101. Van Den Driessche M , Peeters K, Marien P, Ghoos Y, Devlieger H, Veereman‐Wauters G. Gastric emptying in formula‐fed and breast‐fed infants measured with the 13C‐octanoic acid breath test. J Pediatr Gastroenterol Nutr. 1999;29 (1):46–51. [DOI] [PubMed] [Google Scholar]
- 102. Hauser B, Roelants M, De Schepper J, Veereman G, Caveliers V, Devreker T, et al. Gastric emptying of liquids in children. J Pediatr Gastroenterol Nutr. 2016;62 (3):403–8. [DOI] [PubMed] [Google Scholar]
- 103. Eradi B, Wright J, Gibbons NJ, Blackshaw PE, Perkins AC, Wakefield J, et al. Validity of 13C octanoic acid breath test for measurement of solid meal gastric emptying time in children. J Pediatr Surg. 2006;41 (12):2062–5. [DOI] [PubMed] [Google Scholar]
- 104. Keller J, Beglinger C, Holst JJ, Andresen V, Layer P. Mechanisms of gastric emptying disturbances in chronic and acute inflammation of the distal gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;297 (5):G861–8. [DOI] [PubMed] [Google Scholar]
- 105. Gonlachanvit S, Chey WD, Goodman KJ, Parkman HP. Effect of meal size and test duration on gastric emptying and gastric myoelectrical activity as determined with simultaneous [13C]octanoate breath test and electrogastrography in normal subjects using a muffin meal. Dig Dis Sci. 2001;46 (12):2643–50. [DOI] [PubMed] [Google Scholar]
- 106. Sanaka M, Yamamoto T, Ishii T, Kuyama Y. The Wagner‐Nelson method can generate an accurate gastric emptying flow curve from CO2 data obtained by a 13C‐labeled substrate breath test. Digestion. 2004;69 (2):71–8. [DOI] [PubMed] [Google Scholar]
- 107. Verbeke K. Will the 13C‐octanoic acid breath test ever replace scintigraphy as the gold standard to assess gastric emptying? Neuro Gastroenterol Motil. 2009;21 (10):1013–6. [DOI] [PubMed] [Google Scholar]
- 108. Sanaka M, Yamamoto T, Osaki Y, Kuyama Y. Assessment of the gastric emptying velocity by the 13C‐octanoate breath test: deconvolution versus a Wagner‐Nelson analysis. J Gastroenterol. 2006;41 (7):638–46. [DOI] [PubMed] [Google Scholar]
- 109. Maes BD, Ghoos YF, Geypens BJ, Hiele MI, Rutgeerts PJ. Relation between gastric emptying rate and energy intake in children compared with adults. Gut. 1995;36 (2):183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Maes BD, Ghoos YF, Geypens BJ, Hiele MI, Rutgeerts PJ. Influence of octreotide on the gastric emptying of solids and liquids in normal healthy subjects. Aliment Pharmacol Ther. 1995;9 (1):11–8. [DOI] [PubMed] [Google Scholar]
- 111. Perri F, Pastore M, Zicolella A, Annese V, Quitadamo M, Andriulli A. Gastric emptying of solids is delayed in celiac disease and normalizes after gluten withdrawal. Acta Paediatrica (Oslo). 2000;89 (8):921–5. [DOI] [PubMed] [Google Scholar]
- 112. Staelens S, Van den Driessche M , Barclay D, Carrié‐Faessler AL, Haschke F, Verbeke K, et al. Gastric emptying in healthy newborns fed an intact protein formula, a partially and an extensively hydrolysed formula. Clin Nutr. 2008;27 (2):264–8. [DOI] [PubMed] [Google Scholar]
- 113. Machado RS, Yamamoto E, da Silva Patrício FR , Reber M, Kawakami E. Gastric emptying evaluation in children with erosive gastroesophageal reflux disease. Pediatr Surg Int. 2010;26 (5):473–8. [DOI] [PubMed] [Google Scholar]
- 114. Perano SJ, Rayner CK, Kritas S, Horowitz M, Donaghue K, Mpundu‐Kaambwa C, et al. Gastric emptying is more rapid in adolescents with type 1 diabetes and impacts on postprandial glycemia. J Clin Endocrinol Metab. 2015;100 (6):2248–53. [DOI] [PubMed] [Google Scholar]
- 115. Lipp RW, Schnedl WJ, Hammer HF, Kotanko P, Leb G, Krejs GJ. Effects of postprandial walking on delayed gastric emptying and intragastric meal distribution in longstanding diabetics. Am J Gastroenterol. 2000;95 (2):419–24. [DOI] [PubMed] [Google Scholar]
- 116. Ikeda T, Inamori M, Fujisawa N, Iwasaki T, Akiyama T, Akimoto K, et al. Effects of body positions on gastric emptying with enteral nutrition: a crossover study using a continuous real time 13C breath test (BreathID system). Hepato‐gastroenterology. 2008;55 (86‐87):1905–7. [PubMed] [Google Scholar]
- 117. Akimoto K, Inamori M, Iida H, Endo H, Akiyama T, Ikeda T, et al. Does postprandial coffee intake enhance gastric emptying?: a crossover study using continuous real time 13C breath test (BreathID system). Hepato‐gastroenterology. 2009;56 (91‐92):918–20. [PubMed] [Google Scholar]
- 118. Anjiki H, Sanaka M, Kuyama Y. Dual effects of rabeprazole on solid‐phase gastric emptying assessed by the 13C‐octanoate breath test. Digestion. 2005;72 (2‐3):189–94. [DOI] [PubMed] [Google Scholar]
- 119. DiBaise JK, Park FL, Lyden E, Brand RE, Brand RM. Effects of low doses of erythromycin on the 13C Spirulina platensis gastric emptying breath test and electrogastrogram: a controlled study in healthy volunteers. Am J Gastroenterol. 2001;96 (7):2041–50. [DOI] [PubMed] [Google Scholar]
- 120. Kulik W, van Weissenbruch MM , Menelik N, Cranendonk A, Kneepkens CM, Lafeber HN. Improved use of the [13C]octanoic acid breath test as intra‐individual parameter to study the effect of a prokinetic drug on gastric emptying in preterm infants with oral feeding intolerance. J Chromatogr B Biomed Sci Appl. 2001;750 (1):147–53. [DOI] [PubMed] [Google Scholar]
- 121. Hoffmeister A, Mayerle J, Beglinger C, Büchler MW, Bufler P, Dathe K, et al. English language version of the S3‐consensus guidelines on chronic pancreatitis: definition, aetiology, diagnostic examinations, medical, endoscopic and surgical management of chronic pancreatitis. Z Gastroenterol. 2015;53 (12):1447–95. [DOI] [PubMed] [Google Scholar]
- 122. Thomas PD, Forbes A, Green J, Howdle P, Long R, Playford R, et al. Guidelines for the investigation of chronic diarrhoea, 2nd edition. Gut. 2003;52 (Suppl 5):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. American Gastroenterological Association medical position statement: guidelines for the evaluation and management of chronic diarrhea. Gastroenterology. 1999;116(6):1461‐3. [DOI] [PubMed] [Google Scholar]
- 124. Abu‐El‐Haija M, Uc A, Werlin SL, Freeman AJ, Georgieva M, Jojkić‐Pavkov D, et al. Nutritional considerations in pediatric pancreatitis: a position paper from the NASPGHAN Pancreas Committee and ESPGHAN Cystic Fibrosis/Pancreas Working Group. J Pediatr Gastroenterol Nutr. 2018;67 (1):131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Stern MEH, Palm B, Posselt H‐G, Smaczny C. Leitlinien der Gesellschaft für Pädiatrische Gastroenterologie und Ernährung (GPGE): Mukoviszidose (Cystische Fibrose): Ernährung und exokrine Pankreasinsuffizienz. AWMF; 2011. https://www.muko.info/fileadmin/user_upload/angebote/qualitaetsmanagement/LL_S1_mukoviszidose_ernaehrung_exokrine_pankreasinsuffizienz.pdf.
- 126. Turck D, Braegger CP, Colombo C, Declercq D, Morton A, Pancheva R, et al. ESPEN‐ESPGHAN‐ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin Nutr. 2016;35 (3):557–77. [DOI] [PubMed] [Google Scholar]
- 127. Vantrappen GR, Rutgeerts PJ, Ghoos YF, Hiele MI. Mixed triglyceride breath test: a noninvasive test of pancreatic lipase activity in the duodenum. Gastroenterology. 1989;96 (4):1126–34. [DOI] [PubMed] [Google Scholar]
- 128. Perri F, Andriulli A. Mixed" triglyceride breath test: methodological problems and clinical applications. Rev Med Univ Navarra. 1998;42 (2):99–103. [PubMed] [Google Scholar]
- 129. Sun DY, Jiang YB, Rong L, Jin SJ, Xie WZ. Clinical application of 13C‐Hiolein breath test in assessing pancreatic exocrine insufficiency. Hepatobiliary & pancreatic diseases international. HBPD Int. 2003;2 (3):449–52. [PubMed] [Google Scholar]
- 130. Ritz MA, Fraser RJ, Di Matteo AC , Greville H, Butler R, Cmielewski P, et al. Evaluation of the 13C‐triolein breath test for fat malabsorption in adult patients with cystic fibrosis. J Gastroenterol Hepatol. 2004;19 (4):448–53. [DOI] [PubMed] [Google Scholar]
- 131. Löser C, Brauer C, Aygen S, Hennemann O, Fölsch UR. Comparative clinical evaluation of the 13C‐mixed triglyceride breath test as an indirect pancreatic function test. Scand J Gastroenterol. 1998;33 (3):327–34. [DOI] [PubMed] [Google Scholar]
- 132. Domínguez‐Muñoz JE, Nieto L, Vilariño M, Lourido MV, Iglesias‐García J. Development and diagnostic accuracy of a breath test for pancreatic exocrine insufficiency in chronic pancreatitis. Pancreas. 2016;45 (2):241–7. [DOI] [PubMed] [Google Scholar]
- 133. Keller J, Layer P, Bruckel S, Jahr C, Rosien U. 13C‐mixed triglyceride breath test for evaluation of pancreatic exocrine function in diabetes mellitus. Pancreas. 2014;43 (6):842–8. [DOI] [PubMed] [Google Scholar]
- 134. Evenepoel P, Hiele M, Geypens B, Geboes KP, Rutgeerts P, Ghoos Y. 13C‐egg white breath test: a non‐invasive test of pancreatic trypsin activity in the small intestine. Gut. 2000;46 (1):52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ishii Y, Kohno T, Ito A, Suzuki S, Kohno T, Takayama T, et al. Evaluation of pancreatic exocrine secretion using 13C‐dipeptide (benzoyl‐L‐tyrosyl‐[1‐(13)C]alanine) breath test: focusing on pancreatoduodenectomy cases. Pancreas. 2007;35 (4):313–9. [DOI] [PubMed] [Google Scholar]
- 136. Löser C, Möllgaard A, Aygen S, Hennemann O, Fölsch UR. 13C‐starch breath test‐‐comparative clinical evaluation of an indirect pancreatic function test. Z Gastroenterol. 1997;35 (3):187–94. [PubMed] [Google Scholar]
- 137. Siegmund E, Löhr JM, Schuff‐Werner P. [The diagnostic validity of non‐invasive pancreatic function tests‐‐a meta‐analysis]. Z Gastroenterol. 2004;42 (10):1117–28. [DOI] [PubMed] [Google Scholar]
- 138. Wejnarska K, Kolodziejczyk E, Ryzko J, Oracz G. Comparison of 72‐hour fecal fat quantification and the 13C‐mixed triglyceride breath test in assessing pancreatic exocrine sufficiency in children with chronic pancreatitis. Dev Period Med. 2016;20 (3):222–7. [PubMed] [Google Scholar]
- 139. Nakamura H, Murakami Y, Morifuji M, Uemura K, Hayashidani Y, Sudo T, et al. Analysis of fat digestive and absorptive function after subtotal gastrectomy by a 13C‐labeled mixed triglyceride breath test. Digestion. 2009;80 (2):98–103. [DOI] [PubMed] [Google Scholar]
- 140. Nakamura H, Morifuji M, Murakami Y, Uemura K, Ohge H, Hayashidani Y, et al. Usefulness of a 13C‐labeled mixed triglyceride breath test for assessing pancreatic exocrine function after pancreatic surgery. Surgery. 2009;145 (2):168–75. [DOI] [PubMed] [Google Scholar]
- 141. González‐Sánchez V, Amrani R, González V, Trigo C, Picó A, de‐Madaria E. Diagnosis of exocrine pancreatic insufficiency in chronic pancreatitis: (13)C‐mixed triglyceride breath test versus fecal elastase. Pancreatology. 2017;17 (4):580–5. [DOI] [PubMed] [Google Scholar]
- 142. Kent DS, Remer T, Blumenthal C, Hunt S, Simonds S, Egert S, et al. 13C‐Mixed triglyceride breath test and fecal elastase as an indirect pancreatic function test in cystic fibrosis infants. J Pediatr Gastroenterol Nutr. 2018;66 (5):811–5. [DOI] [PubMed] [Google Scholar]
- 143. Suzuki M, Tanaka K, Ohtani K, Kitamura K, Kudo T, Shoji H, et al. Estimation of postoperative fat absorption using the 13C mixed‐triglyceride breath test in children with choledochal cyst. Eur J Pediatr. 2009;168 (1):35–8. [DOI] [PubMed] [Google Scholar]
- 144. Lisowska A, Pogorzelski A, Oracz G, Siuda K, Skorupa W, Rachel M, et al. Oral antibiotic therapy improves fat absorption in cystic fibrosis patients with small intestine bacterial overgrowth. J Cyst Fibros. 2011;10 (6):418–21. [DOI] [PubMed] [Google Scholar]
- 145. Herzog DC, Delvin EE, Albert C, Marcotte JE, Pelletier VA, Seidman EG. 13C‐labeled mixed triglyceride breath test (13C MTG‐BT) in healthy children and children with cystic fibrosis (CF) under pancreatic enzyme replacement therapy (PERT): a pilot study. Clin Biochem. 2008;41 (18):1489–92. [DOI] [PubMed] [Google Scholar]
- 146. van Dijk‐van Aalst K , Van Den Driessche M , van Der Schoor S, Schiffelers S, van't Westeinde T, Ghoos Y, et al. 13C mixed triglyceride breath test: a noninvasive method to assess lipase activity in children. J Pediatr Gastroenterol Nutr. 2001;32 (5):579–85. [DOI] [PubMed] [Google Scholar]
- 147. Ling SC, Amarri S, Slater C, Hollman AS, Preston T, Weaver LT. Liver disease does not affect lipolysis as measured with the 13C‐mixed triacylglycerol breath test in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2000;30 (4):368–72. [DOI] [PubMed] [Google Scholar]
- 148. Slater C, Preston T, Weaver LT. Improving the specificity of the [13C]mixed triacylglycerol breath test by estimating carbon dioxide production from heart rate. Eur J Clin Nutr. 2006;60 (11):1245–52. [DOI] [PubMed] [Google Scholar]
- 149. Domínguez‐Muñoz JE, Malfertheiner P. Optimized serum pancreolauryl test for differentiating patients with and without chronic pancreatitis. Clin Chem. 1998;44 (4):869–75. [PubMed] [Google Scholar]
- 150. Hoekstra LT, de Graaf W , Nibourg GA, Heger M, Bennink RJ, Stieger B, et al. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257 (1):27–36. [DOI] [PubMed] [Google Scholar]
- 151. Dietrich CG, Götze O, Geier A. Molecular changes in hepatic metabolism and transport in cirrhosis and their functional importance. World J Gastroenterol. 2016;22 (1):72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Afolabi P, Wright M, Wootton SA, Jackson AA. 13C‐aminopyrine demethylation is decreased in cirrhotic patients with normal biochemical markers. Isot Environ Health Stud. 2013;49 (3):346–56. [DOI] [PubMed] [Google Scholar]
- 153. Saadeh S, Behrens PW, Parsi MA, Carey WD, Connor JT, Grealis M, et al. The utility of the 13C‐galactose breath test as a measure of liver function. Aliment Pharmacol Ther. 2003;18 (10):995–1002. [DOI] [PubMed] [Google Scholar]
- 154. Giannini EG, Fasoli A, Borro P, Botta F, Malfatti F, Fumagalli A, et al. 13C‐galactose breath test and 13C‐aminopyrine breath test for the study of liver function in chronic liver disease. Clin Gastroenterol Hepatol. 2005;3 (3):279–85. [DOI] [PubMed] [Google Scholar]
- 155. Braden B, Faust D, Sarrazin U, Zeuzem S, Dietrich CF, Caspary WF, et al. 13C‐methacetin breath test as liver function test in patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2005;21 (2):179–85. [DOI] [PubMed] [Google Scholar]
- 156. Banasch M, Ellrichmann M, Tannapfel A, Schmidt WE, Goetze O. The non‐invasive (13)C‐methionine breath test detects hepatic mitochondrial dysfunction as a marker of disease activity in non‐alcoholic steatohepatitis. Eur J Med Res. 2011;16 (6):258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Portincasa P, Grattagliano I, Lauterburg BH, Palmieri VO, Palasciano G, Stellaard F. Liver breath tests non‐invasively predict higher stages of non‐alcoholic steatohepatitis. Clin Sci (Lond). 2006;111 (2):135–43. [DOI] [PubMed] [Google Scholar]
- 158. Rocco A, de Nucci G , Valente G, Compare D, D'Arienzo A, Cimino L, et al. 13C‐aminopyrine breath test accurately predicts long‐term outcome of chronic hepatitis C. J Hepatol. 2012;56 (4):782–7. [DOI] [PubMed] [Google Scholar]
- 159. Tribonias G, Margariti E, Tiniakos D, Pectasides D, Papatheodoridis GV. Liver function breath tests for differentiation of steatohepatitis from simple fatty liver in patients with nonalcoholic Fatty liver disease. J Clin Gastroenterol. 2014;48 (1):59–65. [DOI] [PubMed] [Google Scholar]
- 160. Korkmaz H, Unler GK, Gokturk HS, Schmidt WE, Kebapcilar L. Noninvasive estimation of disease activity and liver fibrosis in nonalcoholic fatty liver disease using anthropometric and biochemical characteristics, including insulin, insulin resistance, and 13C‐methionine breath test. Eur J Gastroenterol Hepatol. 2015;27 (10):1137–43. [DOI] [PubMed] [Google Scholar]
- 161. Afolabi PR, Scorletti E, Smith DE, Almehmadi AA, Calder PC, Byrne CD. The characterisation of hepatic mitochondrial function in patients with non‐alcoholic fatty liver disease (NAFLD) using the (13)C‐ketoisocaproate breath test. J Breath Res. 2018;12 (4):046002. [DOI] [PubMed] [Google Scholar]
- 162. Banasch M, Emminghaus R, Ellrichmann M, Schmidt WE, Goetze O. Longitudinal effects of hepatitis C virus treatment on hepatic mitochondrial dysfunction assessed by C‐methionine breath test. Aliment Pharmacol Ther. 2008;28 (4):443–9. [DOI] [PubMed] [Google Scholar]
- 163. Fierbinteanu‐Braticevici C, Plesca DA, Tribus L, Panaitescu E, Braticevici B. The role of 1³C‐methacetin breath test for the non‐invasive evaluation of nonalcoholic fatty liver disease. J Gastrointestin Liver Dis. 2013;22 (2):149–56. [PubMed] [Google Scholar]
- 164. Kempinski R, Neubauer K, Wieczorek S, Dudkowiak R, Jasinska M, Poniewierka E. 13C‐Methacetin breath testing in patients with non‐alcoholic fatty liver disease. Adv Clin Exp Med. 2016;25 (1):77–81. [DOI] [PubMed] [Google Scholar]
- 165. Dinesen L, Caspary WF, Chapman RW, Dietrich CF, Sarrazin C, Braden B. 13C‐methacetin‐breath test compared to also noninvasive biochemical blood tests in predicting hepatic fibrosis and cirrhosis in chronic hepatitis C. Digestive and liver disease. Dig Liver Dis. 2008;40 (9):743–8. [DOI] [PubMed] [Google Scholar]
- 166. Giannini EG, Savarino V. Relationship between 13C‐aminopyrine breath test and the MELD score and its long‐term prognostic use in patients with cirrhosis. Dig Dis Sci. 2013;58 (10):3024–8. [DOI] [PubMed] [Google Scholar]
- 167. Park GJ, Katelaris PH, Jones DB, Seow F, Le Couteur DG , Ngu MC. Validity of the 13C‐caffeine breath test as a noninvasive, quantitative test of liver function. Hepatology. 2003;38 (5):1227–36. [DOI] [PubMed] [Google Scholar]
- 168. Ishii T, Furube M, Hirano S, Takatori K, Iida K, Kajiwara M. Evaluation of 13C‐phenylalanine and 13C‐tyrosine breath tests for the measurement of hepatocyte functional capacity in patients with liver cirrhosis. Chem Pharm Bull. 2001;49 (12):1507–11. [DOI] [PubMed] [Google Scholar]
- 169. Lykke Eriksen P, Sørensen M, Grønbæk H, Hamilton‐Dutoit S, Vilstrup H, Thomsen KL. Non‐alcoholic fatty liver disease causes dissociated changes in metabolic liver functions. Clin Res Hepatol Gastroenterol. 2019;43 (5):551–60. [DOI] [PubMed] [Google Scholar]
- 170. Banasch M, Goetze O, Hollborn I, Hochdorfer B, Bulut K, Schlottmann R, et al. 13C‐methionine breath test detects distinct hepatic mitochondrial dysfunction in HIV‐infected patients with normal serum lactate. J Acquir Immune Defic Syndr. 2005;40 (2):149–54. [DOI] [PubMed] [Google Scholar]
- 171. Forestier J, Dumortier J, Guillaud O, Ecochard M, Roman S, Boillot O, et al. Noninvasive diagnosis and prognosis of liver cirrhosis: a comparison of biological scores, elastometry, and metabolic liver function tests. Eur J Gastroenterol Hepatol. 2010;22 (5):532–40. [DOI] [PubMed] [Google Scholar]
- 172. Palmieri VO, Grattagliano I, Minerva F, Pollice S, Palasciano G, Portincasa P. Liver function as assessed by breath tests in patients with hepatocellular carcinoma. J Surg Res. 2009;157 (2):199–207. [DOI] [PubMed] [Google Scholar]
- 173. Goetze O, Breuer M, Geier A, Fried M, Weber A, Jochum W, et al. The 13C‐methactin breath test is non‐inferior to liver biopsy in predicting liver‐related death and transplantation: a 7‐year prospective follow‐up study in 132 patients with chronic hepatitis C infection. GastroHep. 2020;2 (6):344–50. [Google Scholar]
- 174. Barzakova ES, Schulze‐Hagen M, Zimmermann M, Lurje G, Bednarsch J, Pedersoli F, et al. Monitoring liver function of patients undergoing transarterial chemoembolization (TACE) by a 13C breath test (LiMAx). Cardiovasc Intervent Radiol. 2019;42 (12):1702–8. [DOI] [PubMed] [Google Scholar]
- 175. Makridis G, Oldhafer KJ. First intraoperative measurement of liver functional capacity during liver surgery using the (13) C‐methacetin breath test: early results of a pilot study. J Hepato‐Biliary‐Pancreatic Sci. 2020;27 (5):280–1. [DOI] [PubMed] [Google Scholar]
- 176. Jara M, Reese T, Malinowski M, Valle E, Seehofer D, Puhl G, et al. Reductions in post‐hepatectomy liver failure and related mortality after implementation of the LiMAx algorithm in preoperative work‐up: a single‐centre analysis of 1170 hepatectomies of one or more segments. HPB (Oxford). 2015;17 (7):651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Stockmann M, Lock JF, Malinowski M, Niehues SM, Seehofer D, Neuhaus P. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB (Oxford). 2010;12 (2):139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Duro D, Fitzgibbons S, Valim C, Yang CF, Zurakowski D, Dolan M, et al. [13C]Methionine breath test to assess intestinal failure‐associated liver disease. Pediatr Res. 2010;68 (4):349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Shteyer E, Lalazar G, Hemed N, Pappo O, Granot E, Yerushalmi B, et al. Continuous 13C‐methacetin breath test differentiates biliary atresia from other causes of neonatal cholestasis. J Pediatr Gastroenterol Nutr. 2013;56 (1):60–5. [DOI] [PubMed] [Google Scholar]
- 180. Wada M, Wada Y, Uchiyama M, Kajiwara M, Takatori K. (13)C‐phenylalanine breath test correlates with liver fibrosis in postoperative biliary atresia. Pediatr Int. 2007;49 (6):836–41. [DOI] [PubMed] [Google Scholar]
- 181. Turki A, Murthy G, Ueda K, Cheng B, Giezen A, Stockler‐Ipsiroglu S, et al. Minimally invasive (13)C‐breath test to examine phenylalanine metabolism in children with phenylketonuria. Mol Genet Metabol. 2015;115 (2‐3):78–83. [DOI] [PubMed] [Google Scholar]
- 182. Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007;76 (3):391–6. [PubMed] [Google Scholar]
- 183. Herold C, Ganslmayer M, Ocker M, Zopf S, Gailer B, Hahn EG, et al. Inducibility of microsomal liver function may differentiate cirrhotic patients with maintained compared with severely compromised liver reserve. J Gastroenterol Hepatol. 2003;18 (4):445–9. [DOI] [PubMed] [Google Scholar]
- 184. Jonderko K, Skałba P, Kasicka‐Jonderko A, Kamińska M, Bizior‐Frymus D, Dyja R. Impact of combined oral contraceptives containing ethinylestradiol on the liver microsomal metabolism. Eur J Contracept Reprod Health Care. 2013;18 (4):284–92. [DOI] [PubMed] [Google Scholar]
- 185. Banerjee D, Vikram N, Mishra P, Bhatt R, Prakash S, Misra A. Correlation of a [13C]glucose breath test with surrogate markers of insulin resistance in urban and rural Asian Indians. Metab Syndr Relat Disord. 2009;7 (3):215–9. [DOI] [PubMed] [Google Scholar]
- 186. Ghosh C, Maity A, Banik GD, Som S, Chakraborty A, Selvan C, et al. Non‐invasive 13C‐glucose breath test using residual gas analyzer‐mass spectrometry: a novel tool for screening individuals with pre‐diabetes and type 2 diabetes. J Breath Res. 2014;8 (3): 036001. [DOI] [PubMed] [Google Scholar]
- 187. Hussain M, Jangorbhani M, Schuette S, Considine RV, Chisholm RL, Mather KJ. [13C]glucose breath testing provides a noninvasive measure of insulin resistance: calibration analyses against clamp studies. Diabetes Technol Therapeut. 2014;16 (2):102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Jetha MM, Nzekwu U, Lewanczuk RZ, Ball GD. A novel, non‐invasive 13C‐glucose breath test to estimate insulin resistance in obese prepubertal children. J Pediatr Endocrinol Metab. 2009;22 (11):1051–9. [DOI] [PubMed] [Google Scholar]
- 189. Kawagoe N, Kano O, Kijima S, Tanaka H, Takayanagi M, Urita Y. Investigation of metabolism of exogenous glucose at the early stage and onset of diabetes mellitus in Otsuka long‐evans Tokushima fatty rats using [1, 2, 3‐13C]glucose breath tests. PloS One. 2016;11 (8):e0160177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lewanczuk RZ, Paty BW, Toth EL. Comparison of the [13C]glucose breath test to the hyperinsulinemic‐euglycemic clamp when determining insulin resistance. Diabetes Care. 2004;27 (2):441–7. [DOI] [PubMed] [Google Scholar]
- 191. Maldonado‐Hernandez J, Martinez‐Basila A, Salas‐Fernandez A, Navarro‐Betancourt JR, Pina‐Aguero MI, Bernabe‐Garcia M. The 13C‐glucose breath test for insulin resistance assessment in adolescents: comparison with fasting and post‐glucose stimulus surrogate markers of insulin resistance. J Clin Res Pediatr Endocrinol. 2016;8 (4):419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Mizrahi M, Lalazar G, Adar T, Raz I, Ilan Y. Assessment of insulin resistance by a 13C glucose breath test: a new tool for early diagnosis and follow‐up of high‐risk patients. Nutr J. 2010;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Salas‐Fernandez A, Maldonado‐Hernandez J, Martinez‐Basila A, Martinez‐Razo G, Jasso‐Saavedra F. The 13C‐glucose breath test is a valid non‐invasive screening tool to identify metabolic syndrome in adolescents. Clin Chem Lab Med. 2015;53 (1):133–8. [DOI] [PubMed] [Google Scholar]
- 194. Takemoto I, Kawagoe N, Kijima S, Sasaki Y, Watanabe T, Urita Y. (13)C‐glucose breath tests: a non‐invasive method for detecting early clinical manifestations of exogenous glucose metabolism in type 2 diabetic patients. Acta Diabetol. 2019;56 (4):449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Berthold HK, Schober P, Scheurlen C, Marklein G, Horre R, Gouni‐Berthold I, et al. Use of the lactose‐[13C]ureide breath test for diagnosis of small bowel bacterial overgrowth: comparison to the glucose hydrogen breath test. J Gastroenterol. 2009;44 (9):944–51. [DOI] [PubMed] [Google Scholar]
- 196. Wutzke KD, Glasenapp B. The use of 13C‐labelled glycosyl ureides for evaluation of orocaecal transit time. Eur J Clin Nutr. 2004;58 (4):568–72. [DOI] [PubMed] [Google Scholar]
- 197. Morrison DJ, Dodson B, Preston T, Weaver LT. Gastrointestinal handling of glycosyl [13C]ureides. Eur J Clin Nutr. 2003;57 (8):1017–24. [DOI] [PubMed] [Google Scholar]
- 198. Van Den Driessche M , Van Malderen N , Geypens B, Ghoos Y, Veereman‐Wauters G. Lactose‐[13C]ureide breath test: a new, noninvasive technique to determine orocecal transit time in children. J Pediatr Gastroenterol Nutr. 2000;31 (4):433–8. [DOI] [PubMed] [Google Scholar]
- 199. Jonderko K, Kasicka‐Jonderko A, Syrkiewicz‐Trepiak D, Blonska‐Fajfrowska B. Feasibility of a breath test with a substrate of natural 13C‐abundance and isotope‐selective non‐dispersive infrared spectrometry: a preliminary study. J Gastroenterol Hepatol. 2005;20 (8):1228–34. [DOI] [PubMed] [Google Scholar]
- 200. Robayo‐Torres CC, Diaz‐Sotomayor M, Hamaker BR, Baker SS, Chumpitazi BP, Opekun AR, et al. 13C‐Labeled‐Starch breath test in congenital sucrase‐isomaltase deficiency. J Pediatr Gastroenterol Nutr. 2018;66 (Suppl 3):S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Robayo‐Torres CC, Opekun AR, Quezada‐Calvillo R, Villa X, Smith EO, Navarrete M, et al. 13C‐breath tests for sucrose digestion in congenital sucrase isomaltase‐deficient and sacrosidase‐supplemented patients. J Pediatr Gastroenterol Nutr. 2009;48 (4):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. de Wildt SN , Berns MJ, van den Anker JN . 13C‐erythromycin breath test as a noninvasive measure of CYP3A activity in newborn infants: a pilot study. Ther Drug Monit. 2007;29 (2):225–30. [DOI] [PubMed] [Google Scholar]
- 203. Furuta T, Iwaki T, Umemura K. [13C]pantoprazole breath test as a predictor of the anti‐platelet function of clopidogrel. Eur J Clin Pharmacol. 2010;66 (5):457–63. [DOI] [PubMed] [Google Scholar]
- 204. Furuta T, Kodaira C, Nishino M, Yamade M, Sugimoto M, Ikuma M, et al. [13C]‐pantoprazole breath test to predict CYP2C19 phenotype and efficacy of a proton pump inhibitor, lansoprazole. Aliment Pharmacol Ther. 2009;30 (3):294–300. [DOI] [PubMed] [Google Scholar]
- 205. Modak AS, Klyarytska I, Kriviy V, Tsapyak T, Rabotyagova Y. The effect of proton pump inhibitors on the CYP2C19 enzyme activity evaluated by the pantoprazole‐(13)C breath test in GERD patients: clinical relevance for personalized medicine. J Breath Res. 2016;10 (4):046017. [DOI] [PubMed] [Google Scholar]
- 206. Leeder JS, Pearce RE, Gaedigk A, Modak A, Rosen DI. Evaluation of a [13C]‐dextromethorphan breath test to assess CYP2D6 phenotype. J Clin Pharmacol. 2008;48 (9):1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Opdam FL, Dezentje VO, den Hartigh J, Modak AS, Vree R, Batman E, et al. The use of the 13C‐dextromethorphan breath test for phenotyping CYP2D6 in breast cancer patients using tamoxifen: association with CYP2D6 genotype and serum endoxifen levels. Canc Chemother Pharmacol. 2013;71 (3):593–601. [DOI] [PubMed] [Google Scholar]
- 208. Safgren SL, Suman VJ, Kosel ML, Gilbert JA, Buhrow SA, Black JL, et al. Evaluation of CYP2D6 enzyme activity using a 13C‐dextromethorphan breath test in women receiving adjuvant tamoxifen. Pharmacogenetics Genom. 2015;25 (4):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mattison LK, Ezzeldin H, Carpenter M, Modak A, Johnson MR, Diasio RB. Rapid identification of dihydropyrimidine dehydrogenase deficiency by using a novel 2‐13C‐uracil breath test. Clin Canc Res. 2004;10 (8):2652–8. [DOI] [PubMed] [Google Scholar]
- 210. Mattison LK, Fourie J, Hirao Y, Koga T, Desmond RA, King JR, et al. The uracil breath test in the assessment of dihydropyrimidine dehydrogenase activity: pharmacokinetic relationship between expired 13CO2 and plasma [2‐13C]dihydrouracil. Clin Canc Res. 2006;12 (2):549–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


