Summary
Patients with chronic lung disease are vulnerable to getting severe diseases associated with SARS-CoV-2 infection. Here, we describe protocols for subculturing and differentiating primary normal human bronchial epithelial (NHBE) cells of patients with chronic obstructive lung disease. The differentiation of NHBE cells in air-liquid interface mimics an in vivo airway and provides an in vitro model for studying SARS-CoV-2 infection. We also describe a protocol for detecting proteins in the sectioned epithelium for detailing SARS-CoV-2 infection-induced pathobiology with a vertical view.
Subject areas: Health Sciences, Immunology, Microbiology, Microscopy, Antibody
Graphical Abstract
Highlights
-
•
Culture of COPD primary normal human bronchial epithelial (NHBE) cells
-
•
Differentiation of pseudostratified airway epithelium from primary NHBE cells
-
•
Immunohistochemistry for detection of SARS-CoV-2-induced pathobiology
Patients with chronic lung disease are vulnerable to getting severe diseases associated with SARS-CoV-2 infection. Here, we describe protocols for subculturing and differentiating primary normal human bronchial epithelial (NHBE) cells of patients with chronic obstructive lung disease. The differentiation of NHBE cells in air-liquid interface mimics an in vivo airway and provides an in vitro model for studying SARS-CoV-2 infection. We also describe a protocol for detecting proteins in the sectioned epithelium for detailing SARS-CoV-2 infection-induced pathobiology with a vertical view.
Before you begin
Primary cells
Cryopreserved primary NHBE cells of COPD patients (deidentified, N=3).
Note: The cryopreserved primary cells used for developing this protocol were obtained under a collaboration with Dr. Kristina Bailey at the University of Nebraska Medical Center (UNMC), Omaha, NE with an approved material transfer agreement (MTA).
SARS-CoV-2 infection
The authors recommend infection experiment with SARS-CoV-2 should be performed in a high biocontainment facility.
Note: For developing this protocol, the work with SARS-CoV-2 was performed in the high biocontainment facility at the Rocky Mountain Laboratories (RML), NIAID, NIH in Hamilton, MT (Osan et al., 2020). All infectious works followed standard operating procedures (SOPs) approved by the Institutional Biosafety Committee.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-acetyl-α-tubulin (1:500) | Cell Signaling Technology | Cat # 5335; RRID:AB_10544694 |
| Mouse monoclonal anti-p63 (1:100) | Abcam | Cat # ab735; Clone BC4A4; RRID:AB_305870 |
| Mouse monoclonal Anti-SARS/SARS-CoV-2 Nucleocapsid protein (1:10) | Thermo Fisher Scientific | Cat # MA1-7404; Clone B46F; RRID:AB_1018422 |
| Rabbit polyclonal Anti-SARS/SARS-CoV-2 Coronavirus Spike Protein (1:100) | Thermo Fisher Scientific | Cat # PA5–81795; RRID:AB_2788969 |
| Goat anti-Mouse IgG Antibody, Alexa Fluor 488 (1:100) | Thermo Fisher Scientific | Cat # A-11029; RRID:AB_2534088 |
| Goat anti-Rabbit IgG Antibody, Alexa Fluor 647 (1:100) | Thermo Fisher Scientific | Cat # A-21245; RRID:AB_2535813 |
| Chemicals, peptides, and recombinant proteins | ||
| Airway Epithelial Cell Medium | PromoCell | Cat # C-21060 |
| Airway Epithelial Cell Medium SupplementMix | PromoCell | Cat # C-39165 |
| PureCol, Bovine Collagen | Advanced BioMatrix | Cat # 5005 |
| TrypLE™ Express Enzyme | Thermo Fisher Scientific | Cat # 12604021 |
| Penicillin-Streptomycin | Thermo Fisher Scientific | Cat # 15140122 |
| Amphotericin B | Thermo Fisher Scientific | Cat # 15290026 |
| PneumaCult-ALI Medium | STEMCELL Technologies | Cat # 05001 |
| Hydrocortisone stock | STEMCELL Technologies | Cat # 07926 |
| Heparin Solution | STEMCELL Technologies | Cat # 07980 |
| NucBlue TM Fixed Cell Stain ReadyProbes | Thermo Fisher Scientific | Cat # R37606 |
| ProLongTM Gold Antifade Mountant | Thermo Fisher Scientific | Cat # P36930 |
| 16% Formaldehyde (methanol free) | Polysciences | Cat # 18814-10 |
| Goat Serum Blocking Solution | Vector Laboratories | Cat # S-1000-20 |
| Rhodamine Phalloidin (Amanita phalloides) | Cytoskeleton, Inc. | Cat # PHDR1 |
| Histo-Clear | Fisher Scientific | Cat # 5032950 |
| Leica-Paraplast REGULAR | Leica Microsystems | Cat # 39601006 |
| Ethanol | N/A | N/A |
| R-buffer A (10×) | Fisher Scientific | Cat # 5031177 |
| 1× PBS pH 7.4 | Prepared in lab | N/A |
| Experimental models: Primary cells | ||
| Normal human bronchial epithelial cells (patients with COPD, deidentified, N=3) | Provided by Dr. Kristina Bailey Laboratory, UNMC, Omaha, NE | N/A |
| Software | ||
| LASX with 2D and 3D Deconvolution | Leica Microsystems | https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/ |
| Other | ||
| Falcon tissue culture dish 100 mm dish | Corning | Cat # 353003 |
| 6.5 mm Transwell with 0.4 μm pore polyester membrane insert | Corning | Cat # 3470 |
| Fisherbrand Superfrost Plus Microscope Slides | Fisher Scientific | Cat # 22-037-246 |
| SuperHT PAP Pen | Research Products International | Cat # 195505 |
| Coverglass 12 mm | Carolina | Cat # 633029 |
| Forceps | N/A | N/A |
| Retriever 2100 | Electron Microscopy Sciences | Cat # 62706 |
| Leica RM2125RTS Paraffin microtome | Leica Microsystems | N/A |
| Leica DMi8 Inverted Microscope | Leica Microsystems | N/A |
Materials and equipment
Complete airway epithelial cell (cAEC) medium
Prepare cAEC medium according to the manufacturer’s instructions and add antibiotics and anti-fungal (see below). Prepare medium in a biological safety cabinet. No filter sterilization is necessary.
| Reagent | Amount |
|---|---|
| Airway Epithelial Cell Growth Medium | 500 mL |
| SupplementMix | 12.3 mL |
| Penicillin-Streptomycin | 5 mL (~1% (V/V)) |
| Amphotericin B | 2.5 mL (~0.5% (V/V)) |
Note: Store the cAEC medium at 4°C for a short-term storage (around six weeks) and bring it to 15°C–25°C before use by warming the medium for several minutes in a water bath set at 37°C.
PenumaCult-ALI Medium
PneumaCult-ALI medium comes with PneumaCult-ALI Basal Medium and PneumaCult ALI 10× Supplement. Thaw 10× supplement (stored at −20°C) in a water bath set at 37°C before use and mix gently. Prepare the PneumaCult-ALI medium by adding following components:
| Reagent | Amount |
|---|---|
| PneumaCult-ALI Basal Medium | 450 mL |
| PneumaCult-ALI 10× Supplement | 50 mL |
Note: For long-term storage (at −20°C, less than six months), the PneumaCult-ALI medium can be aliquoted in 50 mL sterile conical tube without adding additional supplements according to the manufacturer’s instructions. Prepare complete PneumaCult-ALI Maintenance medium according to the manufacturer’s instructions and add antibiotics and anti-fungal (see below).
| Reagent | Amount added to 50 mL medium |
|---|---|
| PneumaCult-ALI Medium | 50 mL |
| PneumaCult-ALI Maintenance Supplement | 0.5 mL |
| Heparin Solution | 0.1 mL |
| Hydrocortisone Solution | 0.25 mL |
| Penicillin-Streptomycin | 1 mL (2% (V/V)) |
| Amphotericin B | 0.5 mL (1% (V/V)) |
Note: PneumaCult-ALI Maintenance Supplement is provided with PneumaCult-ALI Medium kit (catalog: 05001). Keep all the additional supplements at −20°C until use except Heparin solution, which can be kept at 4°C. Bring all medium components to 15°C–25°C before use.
Other solutions
| Name | Reagent |
|---|---|
| 1× PBS | 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4, pH 7.4 |
| 95% Ethanol | 95 mL 200 proof Ethanol and 5 mL Distilled water |
| 70% Ethanol | 70 mL 200 proof Ethanol and 30 mL Distilled water |
| 10% Goat Serum in PBS | 1 mL goat serum in 10 mL of PBS |
| PBST (0.05% Tween 20) | 0.5 mL of Tween-20 in 1000 mL of PBS |
Step-by-step method details
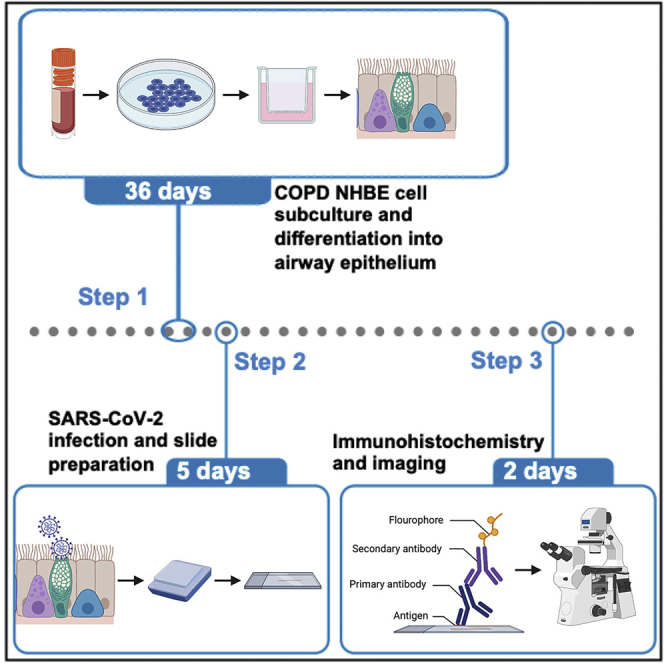
Primary NHBE cell differentiation – ∼5 weeks
Timing: 4–7 days for step 1
Timing: 3–4 weeks for step 3
Primary NHBE cells from COPD patients are grown as a monolayer and they can be cryopreserved for a long-term storage. Passage-4 NHBE cells are differentiated into pseudostratified airway epithelium by using air-liquid interface culture.
-
1.Basic cell culture:
-
a.Coat 100 mm2 cell culture dish with 5 mL PureCol for 20 min at 15°C–25°C.
-
b.Thaw cryopreserved primary early-passage (P0) NHBE cells (around 10ˆ6 cells) in a water bath at 37°C.
-
c.Using a sterile pipette, add 1 mL of new cAEC medium into the cryovial drop by drop.
-
d.Transfer cells from the cryovial into a 15 mL conical tube.
-
e.Spin the cells at 330 × g at 4°C for 5 min in a benchtop centrifuge.
-
f.Remove supernatant and resuspend the cell pellet in 2 mL of cAEC medium.
-
g.Add the cell solution to the precoated 100 mm dish containing 8 mL of cAEC medium and gently swirl the dish for homogeneous distribution of the cells.
-
h.Incubate the cells at 37°C and 5% CO2.
-
i.Replace cAEC medium every 48 h.
-
j.Once cells are fully confluent (around 90%), split the cells at 1 in 3 ratios.
-
a.
CRITICAL: Make sure you mix cells gently while resuspending cell-pellet.
-
2.Subculture for cell propagation (optional):
-
a.Remove the old-medium from confluent NHBE culture in a 100 mm dish and wash with 5 mL of sterile 1× DPBS.
-
b.Add 5 mL of TrypLE express and incubate for 5 min at 37°C.
-
c.Transfer the cells into a 15mL conical tube and spin at 330 × g for 5 min at 4°C.
-
d.Remove supernatant and resuspend cell pellet in cAEC medium.
-
e.Seed cells into a new 100 mm dish at desired cell concentration (1:3 ratio) for subculture.
-
a.
Note: First, do not neutralize TrypLE express treatment by adding Trypsin neutralizing reagent. Second, subculture of the cells can be done more than four times; however, the differentiation of the cells into airway epithelium has been tested up to passage 4. For differentiation, we do not recommend using more than four-passaged cells. Third, splitting cells is required while the cell monolayer is at 90% confluency. Cell split ratio at 1:3 in a 100 mm culture dish has been found optimal for cell growth.
-
3.Differentiation:
-
a.Coat Transwells (e.g., 6.5 mm) at the apical side with 100 μL PureCol for 20 min at 15°C–25°C.
-
b.Remove the old-medium from a confluent NHBE culture (Passage 4) in a 100 mm dish and wash with 5 mL of sterile 1× DPBS.
-
c.Add 5 mL of TrypLE express and incubate for 5 min at 37°C.
-
d.Take Transfer the cells into a 15 mL conical tube and spin at 330 × g for 5 min at 4°C.
-
e.Remove supernatant and resuspend cell pellet in 2 mL of cAEC medium.
-
f.Count cells, e.g., manual count using trypan blue dye and hematocytometer.
-
g.Remove PureCol and seed 50,000 cells in 100 μL of cAEC medium on the apical side of a Transwell. Add 500 μL of cAEC medium on the basal side.
-
h.After 24–48 h, cells form a 100% confluent monolayer. Remove the cAEC medium from both apical and basal sides of the Transwell. Add 500 μL complete PneumaCult-ALI medium on the basal side of the Transwell.
-
i.Replace complete PneumaCult-ALI medium every 48 h on basal sides for differentiation for 3–4 weeks.
-
j.Airway epithelium starts producing mucus from around the 14th day of differentiation, and ciliary function is also visible around that time. 200 μL of sterile 1× DPBS was used to wash the mucus once a week since day 14 differentiation. This was increased to every other day when excessive mucus production was observed.
-
k.After 3–4 weeks of differentiation, the airway epithelium can be verified for
-
i.Ciliary function by quantifying ciliary beat frequency (CBF) using a high-speed live-cell imaging followed by image quantification using SAVA-system (Ammons Engineering)
-
ii.Membrane integrity by measuring transepithelial electrical resistance (TEER) using an EVOM2 voltmeter (World Precision Instrument) (not described).
-
i.
-
a.
Immunohistochemistry/immunofluorescence – ∼3–4 days
Timing: 24–26 h for step 4
Timing: 5–6 h for step 5
Timing: 3–5 h for step 6
Timing: 3–4 h for step 7
Timing: 4–5 h for step 8
SARS-CoV-2 infected COPD airway epithelium needs PFA-fixation, paraffin-embedding and sectioning for staining. Here, the sectioned slides are stained for SARS-CoV-2 S or N.
-
4.PFA-fixation of SARS-CoV-2 infected COPD airway epithelium:
-
a.SARS-CoV-2 infection should be performed at a high-containment facility. Infect four-week differentiated COPD epithelium in Transwells with SARS-CoV-2 (nCoV-WA1–2020 (MN985325.1) at a multiplicity of infection (MOI) of 0.1 for four days at 37°C and in a 5% CO2 humidified incubator.Note: For virus inactivation, use freshly prepared 1% sodium hypochlorite (chlorine bleach, e.g., Clorox). For laboratory waste disposal, follow institutional biosafety committee recommended standard operating procedures.
-
b.At four days post-infection, wash apical and basal sides of the Transwells twice with 1× DPBS (200 μL for apical and 500 μL for basal).
-
c.Add freshly prepared 4% paraformaldehyde (PFA) solution to both apical and basal sides of the Transwells (200 μL for apical and 500 μL for basal).
-
d.Incubate the Transwells for 18–24 h at 15°C–25°C.
-
e.The virus-infected Transwells should be PFA-fixed over night before transporting them safely from the high biocontainment facility. Remove PFA and wash the Transwells twice with 1× DPBS on both apical and basal sides (200 μL for apical and 500 μL for basal).
-
a.
Pause point: Keep the Transwells submerged in 1× DPBS for storage at 4°C. We recommend processing Transwells at the earliest convenience; however, the Transwells can be kept in PBS at 4°C for short-term storage (less than a month).
-
5.Paraffin-embedding and sectioning:
-
a.Carefully take out polycarbonate membrane containing the epithelium from the Transwell using a sterile scalpel and place the membrane in a petri dish containing PBS.
-
b.Cut the membrane into at least two small pieces to fit into a Paraffin cassette, which has a smaller diameter than the membrane.
-
c.Place the membrane into a Paraffin cassette containing PBS.
 Pause point: The membrane can be stored in PBS for several days (less than a week) at 4°C before processing. The membrane stays in PBS till further processing.
Pause point: The membrane can be stored in PBS for several days (less than a week) at 4°C before processing. The membrane stays in PBS till further processing. -
d.For processing, the membrane in the cassette undergoes following incubation steps:
Step Reagent Time 1 PBS 5 min 2 50% Ethanol 20 min 3 70% Ethanol 20 min 4 95% Ethanol 20 min 5 95% Ethanol 20 min 6 100% Ethanol 10 min 7 100% Ethanol 10 min 8 Histo-Clear I 20 min 9 Histo-Clear II 20 min -
e.Three, 30-min paraffin infiltration steps are required in a vacuum oven with a temperature setting of 58°C +/− 2°C under pressure for enhanced paraffin infiltration.
-
f.Embed the membrane on the edge and the paraffin block can be stored at 4°C.
 Pause point: The block can be stored at 4°C before sectioning for slide preparation.
Pause point: The block can be stored at 4°C before sectioning for slide preparation. -
g.Cut sections about 5 μM thick using a rotary microtome and mount onto superfrost plus slides.
-
h.Sectioned slides can be stored at 4°C for several months.
-
a.
-
6.Deparaffinization and antigen retrieval process:
-
a.Day 1: Deparaffinize membrane on section slides by incubating them in following solutions:
Step Reagent Time 1 Histo-Clear I 5 min 2 Histo-Clear II 5 min 3 100% Ethanol 5 min 4 100% Ethanol 5 min 5 100% Ethanol 5 min 6 95% Ethanol 5 min 7 70% Ethanol 5 min 8 Tap water 5 min 9 Tap water 5 min -
b.Transfer slides into a slide chamber and submerge them in R-Buffer A 10× solution for antigen retrieval using the 2100 Retriever.
-
c.Fill the 2100 Retriever’s body with 750 mL of deionized water and place the slide chamber rack into the body.
-
d.Secure the lid and make sure the depressurizing valve is closed.
-
e.Push the Start button and run for 20 min. Let the unit cool down for 2 h or longer (around 12 h) at 15°C–25°C before the slide chambers are removed from 2100 Retriever.
-
a.
Pause point: The slides can be cooled down around 12 h at 15°C–25°C in the slide holder containing R-buffer
-
7.Blocking and primary antibody incubation:
-
a.Place slides in a coplin jar and wash 3 times for 10-min in 1× PBST. The Coplin jar is placed on the belly button shaker on medium speed for efficient washing.
-
b.After final wash, using a fresh KimWipe gently wick away as much PBS as possible without disturbing the tissue.
-
c.Using a PAP pen, draw a circle around each tissue to create a hydrophobic barrier to keep staining reagents localized on the individual tissue section.
-
d.Add 300 ul of blocking buffer (10% goat serum in 1× PBS). Incubate at 15°C–25°C for 2 h in a humidified, light-protected chamber.
-
e.Prepare solution for primary antibody incubation (more than one antibody can be used) in antibody diluent (10% goat serum in 1× PBS). For examples, combination 1: SARS-CoV-2 N specific mouse monoclonal (1:10 dilution) and Acetyl-alpha-tubulin specific rabbit polyclonal (1:500 dilution) and combination 2: SARS-CoV-2 S specific rabbit polyclonal (1:100 dilution) and specific mouse monoclonal p63 (1: 100 dilution).
-
f.After blocking, remove the blocking buffer by gently wicking away with a KimWipe.
-
g.Add 100 μL (2–3 drops) of primary antibody solution on the slides.
-
h.Incubate the slides around 12 h at 4°C in a humidified light-protectant chamber.
-
a.
CRITICAL: Do not allow tissue to dry out.
Note: Do not wash the slides in between blocking and primary antibody incubation steps.
Pause point: Slides can be incubated around 12 h with primary antibody at 4°C.
-
8.Secondary antibody incubation followed by Phalloidin and DAPI staining:
-
a.Remove primary antibody solution from the slides and wash three times with 1× PBST. For washing, follow the steps mentioned above in a coplin jar and on the shaker.
-
b.Prepare secondary antibody solution (secondary antibodies should correspond to the primary antibodies) at 1:100 in antibody diluent (PBS with 10% goat serum). For example, combination 1: goat anti-mouse AF488 and goat anti-rabbit AF647 and combination 2: goat anti-rabbit AF647 and goat anti-mouse AF488.
-
c.After the final wash step, using a fresh KimWipe, gently wick away as much PBS as possible, then add 100 μL (2–3 drops) per section of the appropriate secondary antibody solution.
-
d.Incubate slides in secondary antibody solution for 45 min at 15°C–25°C in a humidified, light-protected chamber.
-
e.After 45 min, perform three 10 min washes with PBST as mentioned above in a coplin jar and on the shaker.
-
f.Prepare rhodamine phalloidin soulution 1:100 in antibody diluent (PBS with 10% goat serum).
-
g.Wipe with KimWipe to remove excess liquid. Add 100 ul of rhodamine phalloidin solution and incubate slides for 30 min at 15°C–25°C in a humidified, light-protected chamber.
-
h.Perform three 10-min washes with PBST as mentioned above in a coplin jar and on the shaker.
-
i.Add 1 drop of DAPI (NucBlue TM fixed cell stain ReadyProbes) to each slide.
-
j.Incubate for 5 min at 15°C–25°C in a humidified, light-protected chamber.
-
k.Perform two 10 min washes in PBST in a coplin jar using a shaker. Add one water wash for 10 min.
-
l.After the final wash, mount slide with a coverslip using gold antifade reagent
 Pause point: The slides can be dried around 12 h at 15°C–25°C in the dark.
Pause point: The slides can be dried around 12 h at 15°C–25°C in the dark. -
m.The next day clear nail polish is placed around the coverslip allowed dry for an hour.
-
n.Image the slides immediately. Slides can be kept at 4°C for longer term storage (several months).
-
a.
-
9.Image acquisition by using Leica DMi8 inverted epifluorescence microscope:
-
a.Turn on the microscope and Leica LASX software on the computer.
-
b.Add all the channels on the software. We used four channels: DAPI: DAPI (EX: 325–375 nm and EM: 435–485 nm), Phalloidin: TexRed (EX: 540–580 nm and EM: 592–668 nm), AF488: Cy3/FITC (EX: 460–500 nm and EM: 512–542 nm) and AF647: Cy5 (EX: 590–650 nm and EM: 662–738 nm)
-
c.The membrane section is like a hair strand, so first find it at a lower magnification by using the 5× objective.
-
d.Take images at 63× oil objective for higher resolution.
-
e.Acquired images can be further processed by using 2D- or 3D-deconvulation option available in the LASX software to get sharper images.
-
a.
Expected outcomes
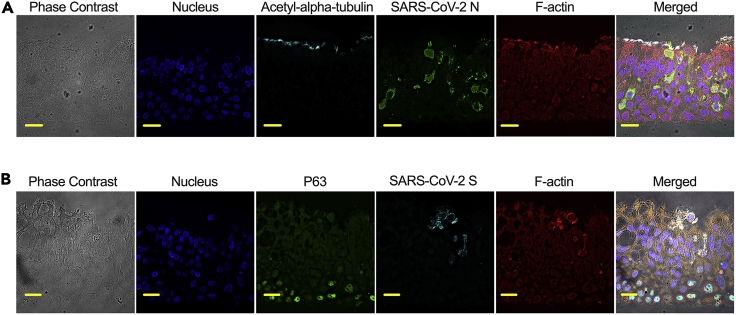
Detection of SARS-CoV-2 N or S protein in the SARS-CoV-2 infected COPD patient derived airway epithelium (in vitro) (Figure 1). Multifluorescent labeling allows simultaneous detection of viral and cellular proteins.
Figure 1.
Immunohistochemistry for SARS-CoV-2 Nucleoprotein (N) and spike (S) detection in the infected PFA-fixed, paraffin-embedded, sectioned COPD derived airway epithelium (in vitro)
SARS-CoV-2 N (A, shown in green) or S (B, shown in cyan) was detected by immunostaining using N- or S-specific primary antibodies and corresponding fluorescent-labeled secondary antibodies, respectively. Acetyl-alpha tubulin (A, shown in cyan) and P63 (B, shown in green) were similarly detected by immunostaining. Here, F-actin (red) and nucleus (blue) were stained with rhodamine phalloidin and DAPI, respectively. The images were taken under a Leica DMI8 modular epifluorescence microscope with a 63× oil objective. The scale bar is 20 μM.
Limitations
One of the limitations is difficulty in detecting SARS-CoV-2 cell surface receptor ACE2 or its co-factor TMPRSS2. The second limitation is not able to detect tight-junction protein, e.g., E-cadherin or ZO-1. A possible solution is using a thicker section of airway epithelium instead of the 5μM section for staining.
Troubleshooting
Problem 1
The differentiated NHBE cells are a thin layer tissue-like airway epithelium (ranging from 40 – 60 μm) on a Transwell. There is a chance of potential damage of the layer during the paraffin-embedding procedure and followed by sectioning (step – 5. Paraffin embedding and sectioning).
Potential solution
Section with a higher thickness for the paraffin-embedding step would help.
Problem 2
Harsh antigen retrieval steps may damage the paraffin-embedded section (step – 6. Deparaffinization and antigen retrieval process).
Potential solution
Milder antigen retrieval steps or an alternative approach, e.g., deparaffinization is followed by a permeabilization step (0.5% TritonX-100 treatment) instead of antigen retrieval treatment.
Problem 3
Antigen retrieval steps may quench fluorescent from the fluorescently-labeled virus, which may hamper successful detection of the infected cells.
Potential solution
Deparaffinization is followed by a permeabilization step (0.5% TritonX-100 treatment) before staining.
Problem 4
Difficulties in antibody-based detection of two or more proteins.
Potential solution
Primary and secondary antibodies selection based on specificity, consistency, and sensitivity are crucial.
Problem 5
Difficulties in differentiating airway epithelium and maintaining COPD NHBE cells.
Potential solution
Higher cell density is required for maintaining NHBE cells while subculturing. For differentiation, the cells need to be fully confluent on Transwell before changing the cAEC medium with complete PneumaCult-ALI medium.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Masfique Mehedi (masfique.mehedi@und.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate any unique datasets or code.
Acknowledgments
We are grateful to Dr. Kristina Bailey at the UNMC for providing primary cells. We are also grateful to Dr. Heinz Feldmann and Freideric Feldmann at the Rocky Mountain Laboratories, Hamilton, MT, for performing the SARS-CoV-2 infection experiment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Histological services were provided by the UND Histology Core, supported by NIH/NIGMS awards P20GM113123, U54GM128729, and UND SMHS funds. This work was funded by NIH/NIGMS award P20GM113123.
Author contributions
Protocol development, J.K.O., B.A.D., and M.M.; paraffin-embedding, IHC, and imaging, J.K.O., B.A.D., and M.M.; writing and editing, J.K.O. and M.M.
Declaration of interests
The authors declare no competing interests.
References
- Osan J.K., Talukdar S.N., Feldmann F., Ann Demontigny B., Jerome K., Bailey K.L., Feldmann H., Mehedi M. Goblet cell hyperplasia increases SARS-CoV-2 infection in COPD. bioRxiv. 2020 doi: 10.1101/2020.11.11.379099v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unique datasets or code.