Abstract
Objective:
Neuroprosthetics hold tremendous promise to restore function through brain-computer interfaced (BCI) devices. However, clinical applications of implantable microelectrodes remain limited given the challenges of maintaining neuronal signals for extended periods of time and with multiple biological mechanisms negatively affecting electrode performance. Acute and chronic inflammation, oxidative stress, and blood brain barrier (BBB) disruption contribute to inconsistent electrode performance. We hypothesized that therapeutic hypothermia (TH) applied at the microelectrode insertion site will positively modulate both inflammatory and apoptotic pathways, promoting neuroprotection and improved performance in the long-term.
Approach:
A custom device and thermoelectric system were designed to deliver controlled TH locally to the cortical implant site at the time of microelectrode array insertion and immediately following surgery. The TH paradigm was derived from in vivo cortical temperature measurements and finite element modeling of temperature distribution profiles in the cortex. Male Sprague-Dawley rats were implanted with non-functional Utah microelectrodes arrays (UMEA) consisting of 4×4 grid of 1.5mm long parylene-coated silicon shanks. In one group, TH was applied to the implant site for two hours following the UMEA implantation, while the other group was implanted under normothermic conditions without treatment. At 48 hours, 72 hours, 7 days and 14 days post-implantation, mRNA expression levels for genes associated with inflammation and apoptosis were compared between normothermic and hypothermia-treated groups.
Main Results:
The custom system delivered controlled TH to the cortical implant site and the numerical models confirmed that the temperature decrease was confined locally. Furthermore, a one-time application of TH post UMEA insertion significantly reduced the acute inflammatory response with a reduction in the expression of inflammatory regulating cytokines and chemokines.
Significance:
This work provides evidence that acutely applied hypothermia is effective in significantly reducing acute inflammation post intracortical electrode implantation.
Keywords: Therapeutic hypothermia, Utah arrays, microelectrodes, neuroprotection, brain computer interface, neuroprosthesis, inflammation
1. Introduction
In the past decades, intracortical microelectrode arrays have been developed for research, therapeutic and neuroprosthetic applications. These devices working as brain-computer and brain-machine interfaces are intended to benefit patients with severe nervous system injuries (1–4). However, their research and clinical utility has been limited by critical barriers such as long-term electrode stability and variable performance. Multiple groups, including ours, working in this field have demonstrated that the function of intracortical microelectrodes is affected greatly by the host-response to implantation injury (5–7). Several biological mechanisms including acute and chronic inflammation, oxidative stress, and blood-brain barrier (BBB) disruption have been shown to contribute towards the inconsistent chronic electrode performance. Given the complex mechanisms and multiple pathways affecting host response, neuronal survival, and the integrity of BBB, effective interventions to prevent electrode-associated damages need to be developed.
Therapeutic hypothermia (TH) or lowering of body, spinal cord or brain temperature after a central nervous system (CNS) injury has been used successfully used as a neuroprotective intervention for both spinal cord and brain trauma in preclinical models (8–10) and in humans (11, 12). Induction of hypothermia prior or after an injury is known to yield favorable neurological outcomes in both the short- and long-terms and these neuroprotective benefits of early hypothermia application minimize secondary inflammatory responses and injuries due to brain trauma, stroke, and spinal cord injury (11–16). It is also evident that these neuroprotective beneficial effects are enhanced when TH is localized in models of both brain and spinal cord trauma (17–21). Mild to moderate TH has been suggested to affect multiple pathways, modulating inflammatory and apoptotic pathways (22–28) and result in the reduction of free radical production (29, 30). The protective effects of mild hypothermia (MTH) associated with suppression of injury-induced immune responses may be beneficial at countering some of the host responses following electrode implantation. For example, TH inhibits vasogenic edema formation and the release of fibrinogen and fibronectin (31), the suppression of polymorphonucleocyte chemotaxis (8) and shows a reduction in gliosis, leading to greater neuronal and axonal preservation (32). MTH also reduces excitotoxicity by decreasing glutamate release and subsequent NMDA receptor activation leading to cell death (33, 34). Expression levels of several pro-inflammatory cytokines including tumor necrosis factor alpha (TNF-) and apoptotic factors such as caspases are also reduced with TH (27, 28), leading to a significant reduction in the host response. Further, microglial and monocyte activation and infiltration at the injury site is reduced with TH (35, 36). Hypothermia can also minimize BBB-disruption by decreasing vascular permeability, edema, and matrix metalloproteinase (MMPs) expression (9, 37–40), which are known to degrade the extracellular matrix and consequentially increases inflammation (37, 41, 42).
In this study, we investigated whether MTH applied locally at the cortical implant site mitigates the acute inflammatory response that occurs following a microelectrode implantation in the brain tissue. We developed a probe and thermoelectric system that allows delivery of controlled hypothermia to the cortical site of implantation. Temperature distribution profiles in the cortex using this approach were modeled using finite element modeling (FEM) and compared with in vivo temperature measurements carried out during the MTH application. Gene expression levels of several pro- and anti-inflammatory factors were compared at acute time-points (up to 14 days post-implant) in an adult rat model to understand the underlying protective mechanisms associated with MTH following electrode implantation.
2. Methods
2.1. System and approach to deliver hypothermia
A thermoelectric system used to deliver local cooling has been described in detail previously (43, 44). Briefly, a custom-designed Peltier based thermoelectric system and a probe were used to deliver therapeutic hypothermia to the cortical surface. Fluorocarbon (FC-770, 3M Corp.) cooled by the Peltier system was used as the refrigerant and circulated through a semicircular probe made of silicone tubing and two hollow metal cylinders (Figure 1). The hollow copper cylinders which are in contact with the skull, are each 1.6 mm in diameter, 5 mm long and sputter coated with 50 μin (1.27μm) layer of Gold for biocompatibility. The entire probe is designed to serve as a single-use disposable unit. The silicone tubing of the probe is coupled to a peristaltic pump (12V, 5000RPM, adjustable flow rate to 100mL/min) and a custom reservoir attached to air-cooled thermoelectric units (AC-046, TE Technology). The entire system is controlled by a pulse width modulation temperature controller (TE Technology, TC-48–20).
Figure 1.
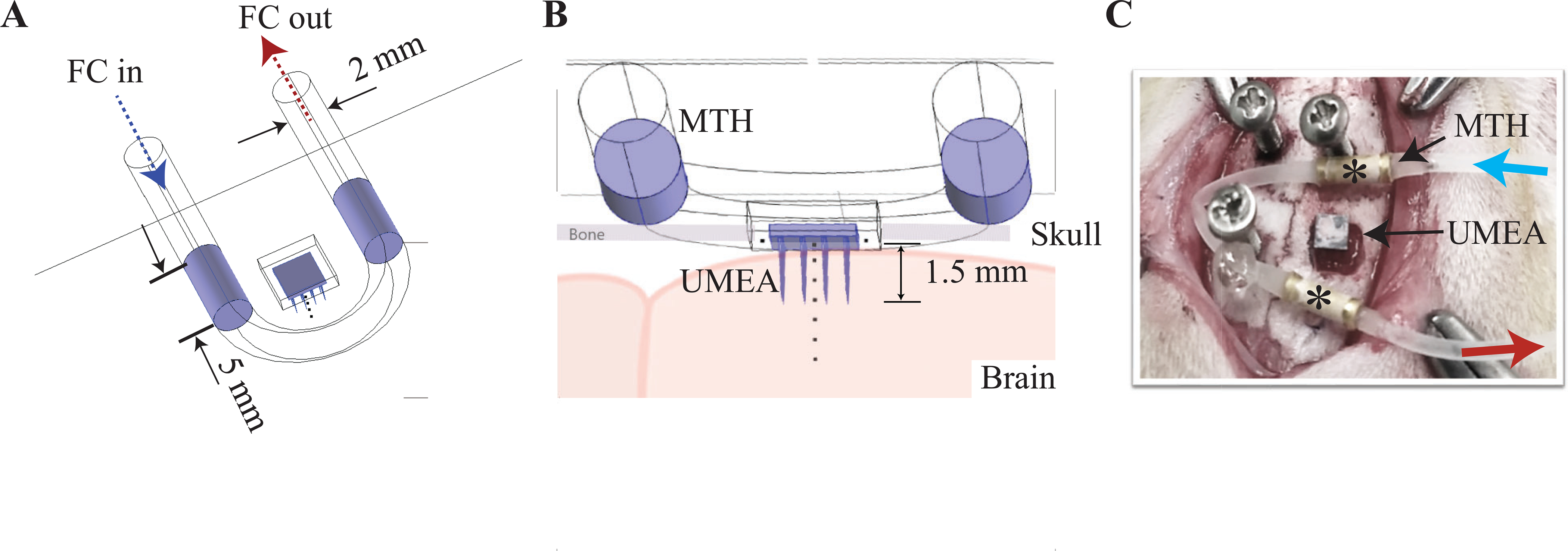
(A) shows the three-dimensional geometry of the MTH probe designed and used in the numerical model as well as the experiments. The probe dimensions are highlighted along with its positioning relative to the craniotomy site and UMEA placement. FC: fluorocarbon (FC-777, 3M Corp.) was used as a refrigerant and circulated through the MTH probe made of 2 mm silicone tubing with two in-line 5 mm long gold-coated hollow metallic (copper) cylinders. (B) shows a side-view depiction of the probe and the 1.5 mm long UMEA (16 channels) inserted into the brain tissue. Temperature profiles over time were numerical calculated at multiple sites along the center of the electrode (dotted line, every 0.5 mm and are described in Figure 2). C) Placement of MTH probe and inserted UMEA from one of the rat surgeries is shown. Fluorocarbon cooled by an external Peltier element is circulated (blue and red arrows) through a loop of silicone tubing complete with two gold-plated copper tips (*).
2.2. Numerical model of MTH
To study the temporal and spatial temperature distributions within the cerebral cortex as a result of the MTH system and probe, a finite element model utilizing modified bio-heat equation (eq. 1) with blood perfusion was developed using the bio-heat transfer application mode with time dependency in COMSOL similar to one we have published previously (45). Figure 1 shows the model setup.
| Eq. 1 |
Here, T is the temperature; ρm, Cm and km, are respectively the density, heat capacity and the thermal conductivity of the material for bone or water. Q is the boundary heat source coefficient of the hypothermia probe and Qbio is the blood metabolic heat source:
| Eq. 2 |
where ρb is the density for blood, Cb is the specific heat capacity, ωb is the perfusion rate, Tb is the arterial temperature, T is temperature of the surrounding tissue and Qmet is the metabolic heat source. These properties are listed in Table 1 and derived from previous studies (46–51). Perfusion was assumed to be homogeneous and isotropic. We used a simplified representation of the brain, dura, cooling probe and electrode array components in this model, which are not based on 3D physical models of human head and did not consider extensive blood perfusions. Thus, it may overestimate the temperature change as discussed in detail below. The design, analysis and simulation were performed on a commercially available software package COMSOL (Comsol Inc., Burlington, MA). The initial geometry is displayed in Figure 1a and shows the placement of the 2 mm tubing and two 5 mm gold-coated contacts. The temperature of the brain and bone was set initially to 36 °C. We discounted both radiation and convection heat due to their very small contribution to overall temperature change (discussed in detail previously (45)). Utilizing the calculated temperature at the measurement site (near array), the temperature distribution was calculated in vertical and horizontal planes parallel to the probe starting at the center of the array and at every 0.5 mm into the brain up to a depth of 3 mm (double the length of the electrodes in the array). The locations of the calculation sites are marked in Figure 1B.
Table 1.
Physiological properties used in model computations. Density, specific heat, thermal conductivity, blood perfusion, metabolic heat generation and thickness are shown for brain, bone and blood.
| Type | Density (kg/m3) | Specific Heat (J/kg∙K) | Thermal Conductivity (W/m∙K) | Blood Perfusion Rate (1/s) | Metabolic Heat Generation (W/m3) | Thickness (mm) |
|---|---|---|---|---|---|---|
| Bone | 1,908 | 1313 | 0.32 | - | - | 0.75 |
| Brain | 994 | 4178 | 0.60 | - | - | 20 |
| Blood | 1,050 | 3840 | 0.52 | .0097 | 1,100 | - |
2.3. In vivo experiments and surgical procedures
All experimental procedures were reviewed and approved by the University of Miami Animal Care and Use Committee and followed the recommendations of the Guide for the Care and Use of Laboratory Animals (National Research Council). Adult, male Sprague-Dawley rats (250–300g) were used for the experiment (total n=48). Animals were randomly assigned to one of the following experimental groups: acute, in vivo MTH temperature recordings (n=3), UMEA insertion under normothermic conditions (n=20), UMEA insertion with MTH treatment (n=20), and un-implanted, un-treated control (n=5).
2.3.1. MTH Temperature Recordings in the Rat Cortex:
Acute experiments were performed to determine the efficacy of our MTH device and system to lower, maintain and rewarm the local cortical temperature. Animals (n=3) were anesthetized with 1–3% isoflurane (1.5 L Oxygen) and placed in a stereotaxic head frame. A midline incision was made along the rostro-caudal axis of the skull, the periosteum was scraped, and the skull was cleaned with hydrogen peroxide. A 4 × 4mm area was marked on the skull over the sensorimotor cortex (1mm lateral and 2mm posterior to the bregma) to ensure proper placement for the craniotomy. Once the craniotomy was completed, a 25-gauge needle angled at ~45 degrees was used to pierce the dura which was then cut and reflected on the sides to expose the cortex. MTH was applied as shown in Figure 1C. The MTH probe tubing was secured with the support screws with the 2 probes positioned rostral and caudal to the craniotomy. A thermistor (not shown, Omega, 5SC-TT-T-40–36) was then placed into the cortex ~1 mm from the probe and ~2 mm deep and temperature readings were measured every 2 minutes during the application of MTH. We reduced the temperature of the circulating fluorocarbon by 5 °C every 2 minutes until the measured cortex temperature was lowered by 3–5 °C below baseline of ~37 °C, or to 32–34 °C. The lowered temperature was maintained for 50 minutes followed by a rewarming phase where the probe temperature was increased by 5 °C every 2 minutes.
2.3.2. Electrode Implantation in the rat cortex:
Non-functional UMEAs consisting of 4 × 4 grid of 1.5 mm long parylene-coated silicon shanks with Pt metallization that were 400 mm apart were used. Detailed microelectrode surgical procedures have been described previously (7, 52). Under isoflurane anesthesia, a craniotomy (~4 × 4mm) was drilled over the sensorimotor cortex (1mm lateral and 2mm posterior to the bregma). The UMEA was carefully inserted (Figure 1C, “UMEA”) using a pneumatic inserter (Blackrock Microsystems, Inc, UT). After UMEA insertion, the craniotomy was covered by a sterile silastic sheet (25 μm thickness). Dental acrylic was placed over the craniotomy and the skull surface. Animals were implanted using similar procedures for either normothermic (n=20) or MTH (n=20) groups.
2.3.3. Hypothermia delivery:
Animals receiving MTH treatment had the cooling probe secured around the craniotomy site as described above (Figure 1C) prior to the placement and insertion of the UMEA. A slow cooling phase was initiated to decrease the site temperature by ~4°C over 10 minutes prior to the implantation. With the cortical temperature reduced, a non-functional UMEA was placed on the exposed cortex and pneumatically inserted as described above. The craniotomy was covered by a silastic sheet and dental acrylic was used to cover the craniotomy, hypothermia tubing and probe, and the skull surface. MTH continued to be maintained at the cortex for an additional 120 minutes followed by a 10-minute rewarming phase. Once the MTH protocol was completed, the hypothermia tubing was disconnected from the external cooling system and the tubing sealed with super glue to avoid potential infection. Probes and tubing were sterilized prior to use and we did not observe any issues with infection even with the probes being left in place for up to 14 days.
2.4. Tissue Processing and quantitative real-time PCR
The two UMEA implanted animals divided into two groups – normothermic or hypothermia-treated – were examined at 48-hours, 72-hours, 7 days, and 14 days post-surgery (n =5 animals for each time-point for each group) to study the effects of MTH on inflammation. At respective end time-points, animals were deeply anesthetized and euthanized using an overdose of ketamine-xylazine cocktail. The head cap was removed, and the electrode array was gently extracted from the tissue. A 4mm x 2mm piece of tissue harboring the electrode was measured using a rat brain matrix (Kent Scientific), weighed, immediately flash-frozen in liquid nitrogen, and stored at −80°C. Age and weight-matched non-implanted, non-treated control animals were euthanized in a similar manner, extracting a similar size of the brain tissue for the molecular analysis (n=5).
The samples were dissected in RNAse-free phosphate buffered saline (PBS) before RNA isolation. A protocol was developed and followed for RNA extraction, reverse transcriptase, and cDNA amplification (53, 54). Tissue samples were weighed and homogenized with 1 ml of PureZol (BioRad, CA). The lysate sample was allowed to rest for 5 min at room temperature for complete dissociation of nucleoprotein complexes to occur. RNA purity and concentration were determined by the absorbance at 260 nm and 280 nm using Nano Drop ND-1000 (Thermofisher Scientific). After extraction, 0.5 mg RNA was used for reverse transcriptase using Superscript First-Strand Synthesis System. Collected cDNA was stored at −80°C until used for real-time quantitative PCR (qRT-PCR). A solution consisting of 10 μl of SsoAdvanced Universal Probes Supermix (BioRad Laboratories, Inc.), 3μl of cDNA, 1μl of primer, and 6μl of H2O was used for each well on the PCR plate. Each gene was run in duplicate wells and GAPDH was used as the housekeeping gene for all samples. All experimental (normothermic and MHT) tissue samples were normalized with respect to control tissue samples from non-operated animals, which served as the baseline values for gene transcription. All control and experimental samples were run in duplicate for each primer tested. The control and experimental sample Cq values were first normalized with respect to the sample GAPDH Cq values, producing ΔCq (ΔCq ¼ Cq (a target gene) e Cq (a reference gene i.e. GAPDH)). The ΔΔCq value was calculated by normalizing each experimental ΔCq to its control sample ΔCq value (ΔΔCq ¼ ΔCq (a target gene) ΔCq (a control sample)). Finally, the relative fold changes in mRNA levels of genes with respect to control (non-operated) animals was calculated using the 2(ΔΔCq) method (55). A two-fold or higher change in gene expression was considered significant (56). The combination criteria of setting a specific fold change in gene expression (1.3 to 2-fold) and p-value (p<0.05 or 0.01) to assess significant difference is more biologically meaningful than p-values alone (57–59).
2.5. Statistics
Fold change gene regulation values from each group (“UMEA + normothermia” and “UMEA + MTH”) and time-points was tested for normality using the Shapiro-Wilk test before applying analysis of variance (ANOVA). For each gene (all time-points included), we combined groups across time and used 2-way ANOVA to determine whether there was a difference with the MTH treatment between the two groups or across time. If there was a significant difference (p < 0.05) in either row or column, one-way ANOVA followed by Tukey post-hoc comparison test was used to find which groups were significantly different.
3. Results
3.1. Numerical modeling and in vivo measurements suggest spatial localization of MTH
Figure 2A1–F2 show geometric mesh of the brain along with the MTH probe placed over the skull used in the numerical analysis over time during the cooling phase. The temperature contours are shown in two orientations to highlight spatial localization of temperature. The temperature contours across the cooling probe, electrode array and surrounding tissue are shown at the beginning of the cooling phase (A1-A2) up to 16 minutes into the cooling phase (F1-F2) and were measured every 0.5 mm along the center of the electrode (Figure 3A). The temperatures at the metal contacts lowered from 37 °C to 24 °C over the 16-minute period. The brain and skull areas surrounding MTH probe tubing remained near 37 °C, while the brain volume that includes the electrodes cooled between 31–33 °C uniformly. The temperature of the brain tissue at the center of the electrode reached 31 °C at 16 minutes with continuous application of MTH. At the same time, the temperature at 3 mm depth along the center of the electrode reached 33 °C or 4 °C below body temperature.
Figure 2.

Temperature contours at different time points along the cooling probe, at the site of UMEA array and surrounding brain tissue calculated numerically are shown during the cooling phase. The temperature distribution was calculated in vertical and horizontal planes parallel to the probe starting at the center of the array and at every 0.5 mm into the brain up to a depth of 3 mm (double the length of the UMEA electrodes used in this study). (A1-A2) show the contours in two different orientations at baseline. (B1-B2) show the same at 2 minutes, (C1-C2) at 4 minutes, (D1-D2) at 6 minutes, (E1-E2) at 8 minutes and (F1-F2) at 16 minutes into the cooling phase.
Figure 3.
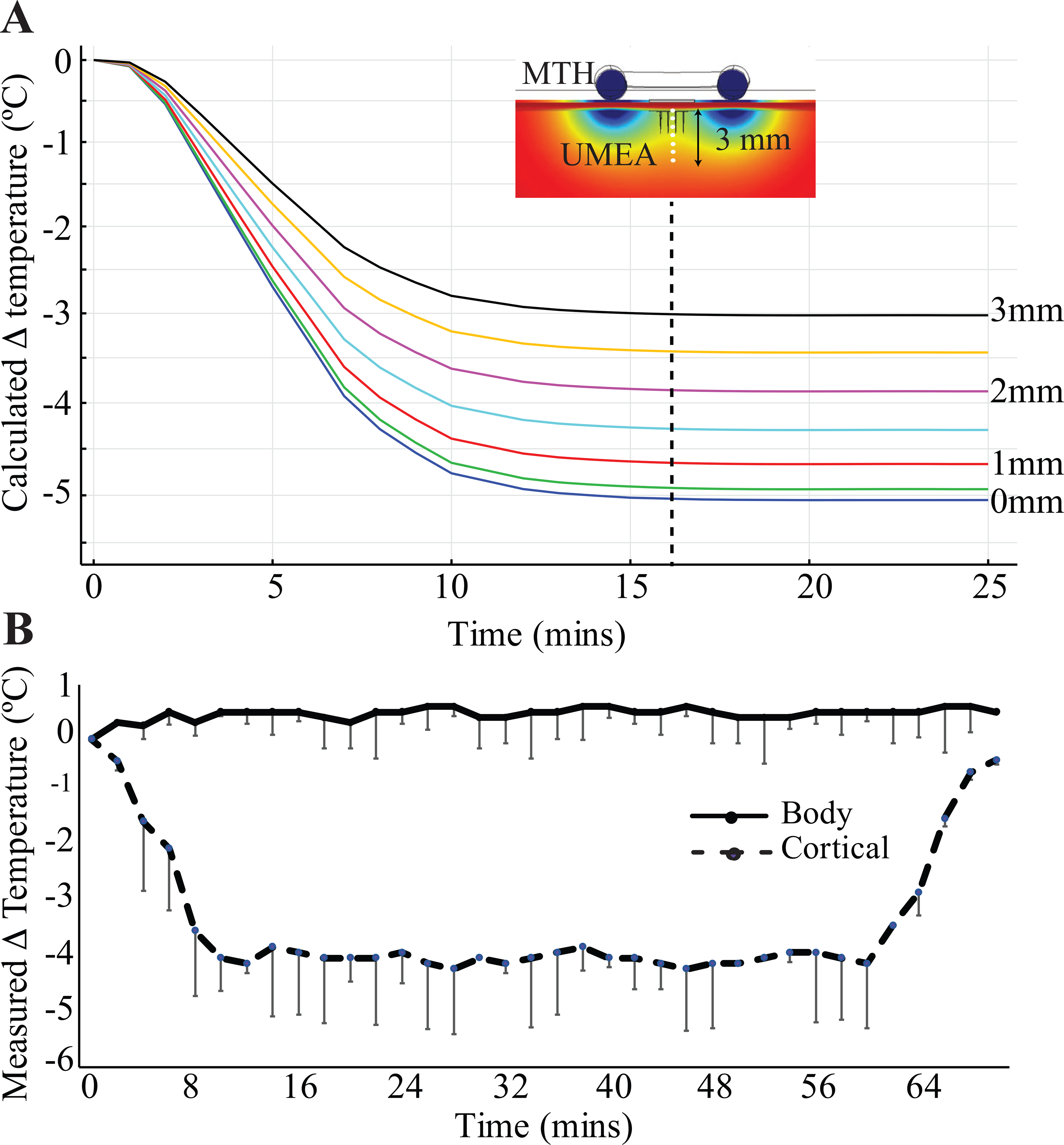
A) shows temperature profiles calculated numerically at every 0.5 mm along the center of the electrode over a duration of 25 minutes. B) shows in vivo temperature measured in acute experiments (n=3, mean ± s.d.) during MTH recorded every 2 minutes. The experimental temperature of the brain tissue shows a close agreement with the numerical results for the custom-designed MTH device and system.
We used the numerical model to compute the temperature change at along the central axis of the electrode for a period of 25 minutes (Figure 3A). Within the first 10 minutes, the temperature at the surface (0 mm) reduced to ~33 °C and remained steady for the duration. The temperature along this axis reached between 31–33°C – the range for MTH – and remained steady. For direct comparison, in vivo temperature measurements were carried out. The temperature in acute rat preparations were recorded every 2 minutes and measured from the cortex at the craniotomy site. The results are shown in Figure 3B (n=3, a total of 3 repetitions, mean ± s.d.). The in vivo measurements showed similar reductions of ~4 °C during a slow initial cooling phase lasting 10 minutes. The decrease in cortical temperature was maintained steady within ±0.5°C for 60 minutes in these acute experiments confirming that our custom-designed MTH probe could be used to maintain temperature of the brain tissue steady over a long duration. The 10-minute rewarming phase followed a similar time constant to the initial cooling phase, with the brain tissue rewarmed to 37 °C (basal body temperature). In each of the rats tested in our acute study, the application of MTH did not alter core body temperature throughout the experiment (Figure 3B). This was also true for all rats (data not shown).
3.2. MTH regulates mRNA expression of pro-inflammatory cytokines in UMEA implanted rats:
Cytokines such as tumor necrosis factor alpha (TNFα) and interleukin 1a and interleukin 6 (IL1a and IL6) are important cell signaling molecules that can trigger systemic inflammation. They are often produced by activated microglia and are released as part of the host response to injury. Binding of the pro-inflammatory molecules cell surface receptors can then lead to necroptosis and apoptosis of the bound cell. For example, TNFα can initiate cellular cascades that then lead to apoptosis through caspase signaling. MMPs, such as MMP-9 degrade the extracellular matrix of of the BBB, making the barrier more permeable and further increasing neuroinflammation. Therefore, to examine the effects of MTH on inflammation at the implant site following electrode insertions, we compared mRNA expression levels for pro-inflammatory genes at 48 hours, 72 hours, 7 days and 14 days post-implant between normothermic and hypothermia-treated groups. UMEA insertion increased mRNA expression of TNFα at 48- and 72-hours and 7-days over control levels (P<0.05, df=4) while MTH significantly reduced expression levels these same time points (P<0.05, df=4 compared to normothermia groups) (Figure 4A). UMEA insertion also increased mRNA expression of IL1a at all time points examined and that of IL6 at 24- and 72- hours, respectively (Figure 4B and 4C). In contrast, the treatment with MTH significantly reduced mRNA expression of IL1a at 48-hours and 7-days (p<0.05, df=4) when compared to normothermia group while mRNA expression of IL-6 was reduced at 48-hours (P<0.05, df=4). Expression levels of IL6 normalized for both experimental groups at 7- and 14-days post-implant. MMP-9 expression was increased with UMEA insertion under normothermic conditions at 24 and 48-hours as well as up to 7-days post-implant while application of MTH significantly reduced expression levels at all four time points examined (Figure 4D, p<0.05, df=4) when compared to the normothermic group.
Figure 4.
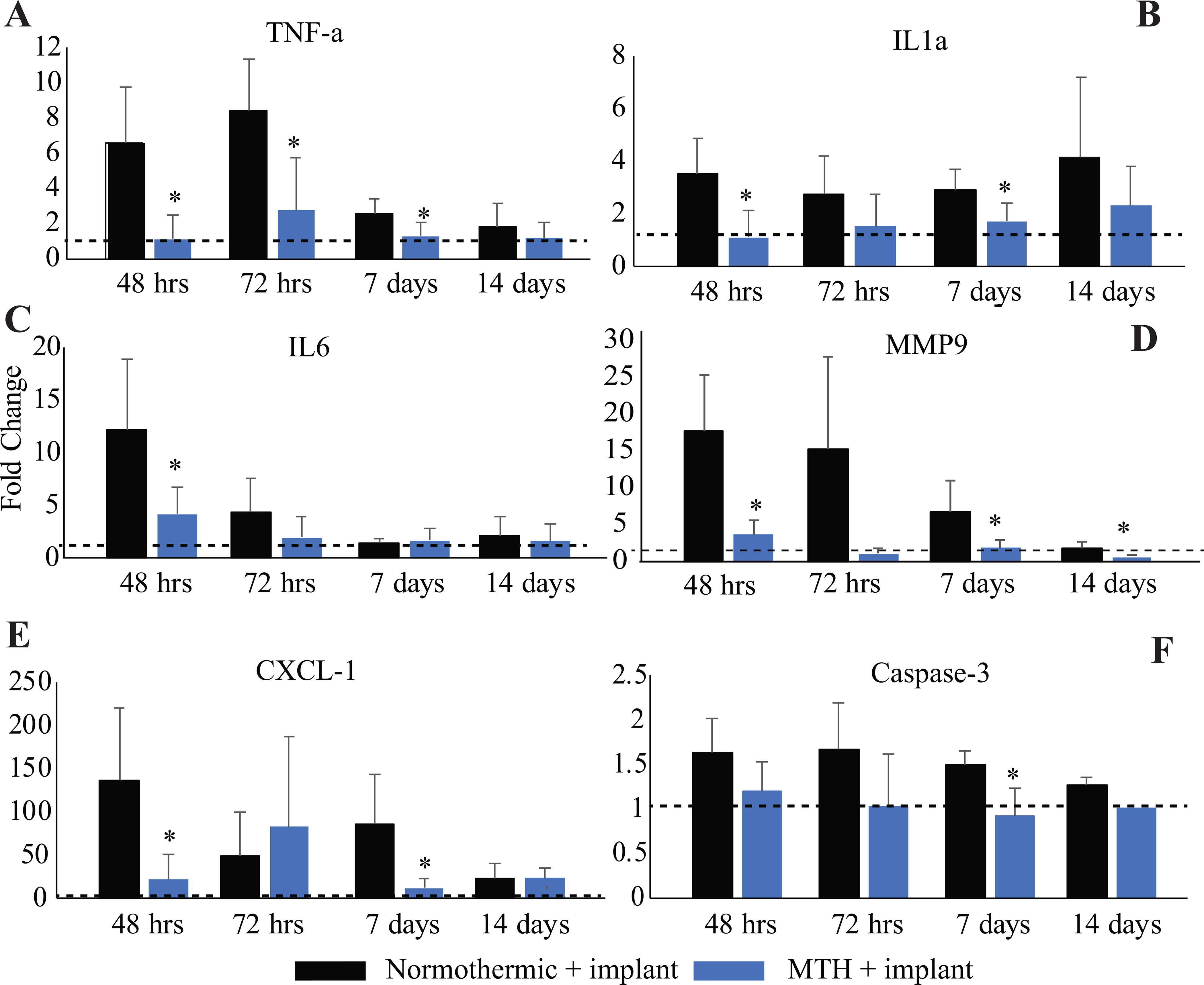
mRNA expression levels of the pro-inflammatory cytokines (A) TNFα, (B) IL1a, (C) IL6, (D) MMP-9, (E) CXCL1, and pro-apoptotic factor (F) Caspase-3, were measured at multiple time points (48 hours, 72 hours, 7 days and 14 days) following UMEA implantation. The fold changes in gene expression in two groups of animals receiving UMEA implants (normothermic and MTH-treated) relative to non-implanted animals (dashed black lines) are shown. n=5/group. *p<0.05.
We also measured the relative expression of the inflammation-related chemokine (C-X-C motif) ligand (CxCl1) and another pro-apoptotic factor, Caspase-3, in brains that underwent UMEA implantations under normothermic conditions or in animals that received local MTH. MTH showed significant beneficial effects by down-regulating both of these factors up to 14 days. UMEA insertion significantly increased mRNA expression of CXCL-1 at all time poits measured while MTH reduced expression at 48-hours and 7 days (Figure 4E, p<0.05, df=4) when compared to the normothermia group. UMEA insertion increased mRNA expression of caspase-3 up to 7 days post-implant when compared with controls while MTH normalized or reduced expression levels at the same time points (Figure 4F, P<0.01, df=4). Expression levels of Caspase-3 normalized for both experimental groups by 14-days. A summary of the temporal changes of all genes examined is shown in Table 2.
Table 2.
Summary of temporal changes in Pro-Inflammatory and Anti-inflammatory mRNA expression associated with mTH. Up-arrow: gene expression level was upregulated. Down-arrow: gene expression level was downregulated.
| Pro-inflammatory | Early | Mid (7 days) | Late (14 days) |
|---|---|---|---|
| TNFa | ↓ (48 hours) ↓ (72 hours) | ↓ | X |
| Caspase 3 | X | ↓ | X |
| IL1a | ↓ (48 hours) | ↓ | X |
| IL6 | ↓ (48 hours) | X | X |
| CXCL1 | ↓ (48 hours) | ↓ | X |
| MMP-9 | ↓ (48 hours) ↓ (72 hours) | ↓ | ↓ |
| Anti-inflammatory | Early | Mid (7 days) | Late (14 days) |
| IL1RN | ↓ (48 hours) | X | X |
| BCL-2 | ↓ (48 hours) ↑ (72 hours) | X | X |
3.3. MTH regulates mRNA expression of anti-inflammatory cytokines in UMEA implanted rats:
Both IL1RN and Bcl-2, in contrast to the effects of pro-inflammatory cytokines, can reduce inflammation and apoptosis. UMEA insertion increased IL1RN mRNA expression at 48-hours and 72-hours (Figure 5A, P<0.05, df=4), which was reduced following MTH at 48-hours (P<0.05, df=4, compared to normothermia groups). Following an initial decrease in expression (48 hours; P<0.05, df=4, compared to normothermia groups), MTH promoted upregulated expression of the anti-apoptotic gene Bcl-2 at 72-hours (P<0.05, df=4) compared to normothermic groups (Figure 5B). Expression levels of Bcl-2 normalized for both experimental groups by 7-days after implant.
Figure 5.
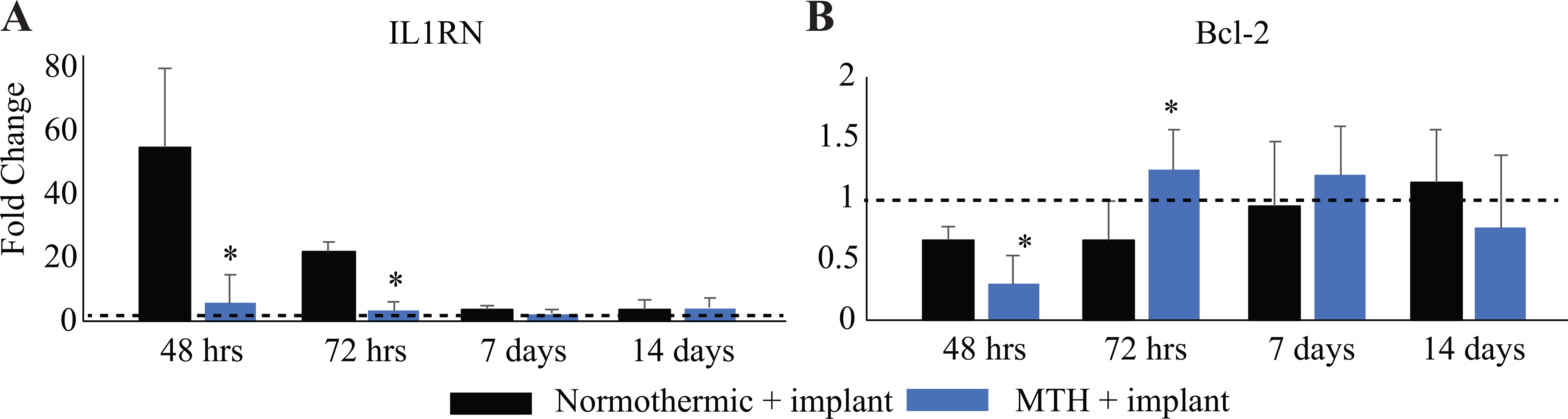
mRNA expression levels of two anti-inflammatory cytokines (A) IL1RN, and (B) Bcl-2 were measured at multiple time points (48 hours, 72 hours, 7 days and 14 days) following UMEA implantation. Shown above are the fold changes in gene expression in two groups of animals receiving UMEA implants (normothermic and MTH-treated) relative to non-implanted animals (dashed black lines). n=5/group. *p<0.05.
4. Discussion
4.1. Mild therapeutic hypothermia as an intervention to reduce foreign body response:
Localized MTH has been widely studied for neuroprotection against secondary injuries due to brain trauma, strokes, and spinal cord injuries. Several clinical trials using MTH have been completed or are currently underway (60–62) and in single institutional studies, hypothermia has consistently been shown to improve outcome after traumatic brain injury and spinal cord injury. The present work identified a novel application of local and transient MTH during microelectrode array implantation that maybe counter some of the pathological processes associated with the electrode insertion and surgical trauma. In this study, we describe a device and protocol to safely deliver controlled MTH to the cortex during pneumatic electrode insertion of UMEAs. This non-pharmacological approach does not require modification of the electrode arrays or surgical approach and can be extended to a clinical setting. Theoretical modeling and in vivo experiments confirmed that with our MTH device and probe, the cortical temperature can be lowered and maintained around 33–35 °C. The MTH effect was observed even at a depth of up to 3mm in the cortex, twice as deep as the UMEA implant.
Previous studies have identified hypothermia systems (neckbands or torso-cooling pads) as efficient devices for lowering temperature of the brain and/or spinal cord locally (63, 64). We have previously shown that a local MTH approach is feasible in preclinical models of cochlear implant insertion trauma without wide systemic effects and confirmed the spatial localization in human cadaver bones locally (44, 45). MTH in these pre-clinical studies showed significant neuroprotective effects preserving residual hearing post-cochlear implant insertion trauma. Here, we have extended the approach and provided a simplified numerical model based on first principles and validated it experimentally to investigate the translational potential of therapeutic hypothermia application. The numerical model showed that hypothermia in the range of MTH (~33°C) was achieved in cortical tissue surrounding the UMEA. The temperature distribution profiles showed a symmetric distribution of temperature below the cooling probe(s) and that the rate of the cooling can be controlled using such an approach. While the simplified model presented here does not account for blood flow and other complex biological mechanisms, a comparison between the theoretically predicted temperatures and the in vivo experimental measurements in a rat model were consistent. With the approach and device discussed, we achieved MTH induction at ~0.5 °C per minute, maintain it within ±0.2 °C and rewarm it along similar rates in vivo in the rat cortex. There are several advantages of the system and approach described here. Studies of mechanisms underlying hypothermia’s protective effects suggest that speed of induction of hypothermia and rewarming are critical along with the duration of the hypothermia (65). With the microelectrode implants, the outcomes are complicated by the fact that there are contributions of multiple mechanisms to the ongoing injury (for biological mechanisms and failure modes, readers are referred to detailed reviews by (5, 6)). Here, local MTH applied at the time of injury and acutely can be effective at addressing some of these mechanisms to provide neuroprotection. The rewarming phase is another challenge for homoeothermic organisms as a rise in temperature implies an increase in metabolism and oxygen demand by tissues. Therefore, our protocol strictly controls the rate of rewarming and was informed by our experimental results and extensive literature on hypothermia (15, 66–71). In addition, application of MTH at 31°C in a model of oxygen-glucose deprivation in hippocampal cultures, (72) also demonstrated neuroprotection that may be achieved using our protocol and temperature range of MTH.
4.2. Mechanisms of action:
Implant-induced trauma has been shown to initiate an inflammatory response. Our results suggest that MTH reduced several important inflammatory factors that have been shown to initiate multiple secondary cellular processes leading to cellular decay. For example, following brain injury inflammation contributes to further tissue damage by initiating cellular cascades for necroptosis and apoptosis. TNFα signals through the TRFR1/2 receptors which produces a signal cascade that can lead to caspase-8 mediated necroptosis and caspase-3 mediated apoptosis (73, 74). Following injury, pro-inflammatory transcription is regulated in part by the binding of pro-inflammatory cytokines. For example, IL1a signals through the type IL-1 receptor/IL-1 accessory protein complex while CXCL-1 signals through the receptor CXC2R and TNFα through LT-b receptor. These pro-inflammatory cytokines can lead to NFκB-dependent transcription of hundreds of genes that are associated with the inflammatory responses (75, 76). For example, MMPs are transcriptionally regulated by several inflammatory cytokines, including TNFα and IL1 (77). An upregulation of MMP-9 can degrade some properties of the BBB such as the basal lamina of cerebral blood vessels making the barrier more permeable and increasing inflammation (78–81). MTH reduced the expression of several of these factors (TNFα, IL1a, IL6, Caspase-3, MMP-9, Figure 4) suggesting an effect on the early inflammatory process associated with the trauma of the electrode insertion (for a summary see Table 2). Studies examining electrode stability have shown early macrophage infiltration influx correlates with long term function suggesting that targeting these processes early after injury may be beneficial.
Aside from reducing pro-inflammatory factors, MTH promoted positive modulation of anti-inflammatory factors interleukin 1 receptor antagonist (IL1RN) and b-cell lymphoma 2 (Bcl-2). Several structurally related proteins such as IL1-α, interleukin 1, beta (IL-1β), and IL1RN are part of the IL-1 family and bind to the same receptor. However, IL1RN is a competitive inhibitor of IL-1α and IL-1β. The IL1RN gene encodes the IL1RA protein which inhibits the activities of IL1a and IL1b, and modulates a variety of IL1 related immune and inflammatory responses. Similarly, Bcl-2, is known to play an important role in promoting cellular survival and it inhibits apoptosis by inhibiting free radical production, preventing the release of cytochrome c from mitochondria, blocking caspase activation, regulating calcium sequestration; important signals in the apoptosis cascade (82, 83). These protective effects of Bcl-2 include increased cellular survival by inhibiting the pro-apoptotic actions of Bax and Bak, other members of the Bcl-2 family (84). Studies in a rat model of transient global ischemia have shown that hypothermia related protective effects are correlated with a significant increase in expression of Bcl-2 (82, 83). Disruption of the Bcl-2 gene in mice can exacerbate focal ischemic brain injury (85), while overexpression of Bcl-2 plays a protective role against neurological insults (86, 87). In this study, mRNA expression levels of IL1RN were reduced with MTH application, while Bcl-2 was upregulated at 72 hours. Taken together, the reduction in pro-inflammatory cytokines and an increase in anti-inflammatory and anti-apoptotic factors suggest a significant benefit of MTH to reduce the host response and provide neuroprotection following electrode implantation.
5. Conclusion
In summary, we demonstrate of a custom-designed MTH system and probe to deliver controlled, local cooling to the cortical structures surrounding UMEA in a preclinical rat model. Our results showed positive modulation of molecular responses and signaling changes in the cytokines and chemokines involved in neuroinflammation following MTH. While we controlled for the time of day, i.e. potential effects of circadian-clock control of global gene expression and limited the current study to male rats only, we did not directly measure protein levels to correlate or validate the gene expression. This study also did not address functional outcomes, which will require long-term electrophysiological studies and evaluations of the electrode stability. While this is clearly a limitation of this study, MTH during cortical electrode implantation may just provide a much-needed new strategy of therapeutic interventions and lead to a new generation of electrodes featuring thermoelectric elements to reduce acute and chronic damage.
Funding and Acknowledgments
This work was supported by NIH R01DC01379801A1, NIH 1DP2EB022357, a pilot award from National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002736, Miami Clinical and Translational Science Institute, and the Wallace H. Coulter Center for Translational Research Neural Engineering SEED Grants. The authors sincerely thank Drs. Florian Solzbacher and Rohit Sharma at the University of Utah for providing the non-functional Utah microelectrode arrays for this study.
References
- 1.Brandman DM, Cash SS, Hochberg LR. Review: Human Intracortical Recording and Neural Decoding for Brain-Computer Interfaces. IEEE Trans Neural Syst Rehabil Eng. 2017;25(10):1687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilja V, Pandarinath C, Blabe CH, Nuyujukian P, Simeral JD, Sarma AA, et al. Clinical translation of a high-performance neural prosthesis. Nature medicine. 2015;21(10):1142–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485(7398):372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homer ML, Nurmikko AV, Donoghue JP, Hochberg LR. Sensors and decoding for intracortical brain computer interfaces. Annu Rev Biomed Eng. 2013;15:383–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermann JK, Capadona JR. Understanding the Role of Innate Immunity in the Response to Intracortical Microelectrodes. Crit Rev Biomed Eng. 2018;46(4):341–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013;10(6):066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad A, Xue QS, Dieme R, Sankar V, Mayrand RC, Nishida T, et al. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front Neuroeng. 2014;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatzipanteli K, Alonso OF, Kraydieh S, Dietrich WD. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J Cereb Blood Flow Metab. 2000;20(3):531–42. [DOI] [PubMed] [Google Scholar]
- 9.Truettner JS, Alonso OF, Dietrich WD. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2005;25(11):1505–16. [DOI] [PubMed] [Google Scholar]
- 10.Truettner JS, Suzuki T, Dietrich WD. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res Mol Brain Res. 2005;138(2):124–34. [DOI] [PubMed] [Google Scholar]
- 11.Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine (Phila Pa 1976). 2010;35(2):E57–62. [DOI] [PubMed] [Google Scholar]
- 12.Levi AD, Casella G, Green BA, Dietrich WD, Vanni S, Jagid J, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670–7. [DOI] [PubMed] [Google Scholar]
- 13.Lo TP Jr., Cho KS, Garg MS, Lynch MP, Marcillo AE, Koivisto DL, et al. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J Comp Neurol. 2009;514(5):433–48. [DOI] [PubMed] [Google Scholar]
- 14.Kawai N, Okauchi M, Morisaki K, Nagao S. Effects of delayed intraischemic and postischemic hypothermia on a focal model of transient cerebral ischemia in rats. Stroke. 2000;31(8):1982–9; discussion 9. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich WD, Levi AD, Wang M, Green BA. Hypothermic treatment for acute spinal cord injury. Neurotherapeutics. 2011;8(2):229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purdy PD, Novakovic RL, Giles BP, Miller SL, Riegel MS. Spinal cord hypothermia without systemic hypothermia. AJNR American journal of neuroradiology. 2013;34(1):252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzen YT, Brienza DM, Karg PE, Loughlin PJ. Effectiveness of local cooling for enhancing tissue ischemia tolerance in people with spinal cord injury. The journal of spinal cord medicine. 2013;36(4):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clifton GL, Jiang JY, Lyeth BG, Jenkins LW, Hamm RJ, Hayes RL. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. 1991;11(1):114–21. [DOI] [PubMed] [Google Scholar]
- 20.Inamasu J, Ichikizaki K. Mild hypothermia in neurologic emergency: an update. Ann Emerg Med. 2002;40(2):220–30. [DOI] [PubMed] [Google Scholar]
- 21.Inamasu J, Nakamura Y, Ichikizaki K. Induced hypothermia in experimental traumatic spinal cord injury: an update. J Neurol Sci. 2003;209(1–2):55–60. [DOI] [PubMed] [Google Scholar]
- 22.Wang GJ, Deng HY, Maier CM, Sun GH, Yenari MA. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002;114(4):1081–90. [DOI] [PubMed] [Google Scholar]
- 23.Toyoda T, Suzuki S, Kassell NF, Lee KS. Intraischemic hypothermia attenuates neutrophil infiltration in the rat neocortex after focal ischemia-reperfusion injury. Neurosurgery. 1996;39(6):1200–5. [DOI] [PubMed] [Google Scholar]
- 24.Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29(10):2171–80. [DOI] [PubMed] [Google Scholar]
- 25.Inamasu J, Suga S, Sato S, Horiguchi T, Akaji K, Mayanagi K, et al. Postischemic hypothermia attenuates apoptotic cell death in transient focal ischemia in rats. Acta neurochirurgica Supplement. 2000;76:525–7. [DOI] [PubMed] [Google Scholar]
- 26.Lee SM, Zhao H, Maier CM, Steinberg GK. The protective effect of early hypothermia on PTEN phosphorylation correlates with free radical inhibition in rat stroke. J Cereb Blood Flow Metab. 2009;29(9):1589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JS, Park J, Suk K, Moon C, Park YK, Han HS. Mild Hypothermia Attenuates Intercellular Adhesion Molecule-1 Induction via Activation of Extracellular Signal-Regulated Kinase-1/2 in a Focal Cerebral Ischemia Model. Stroke Res Treat. 2011;2011:846716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairchild KD, Singh IS, Carter HC, Hester L, Hasday JD. Hypothermia enhances phosphorylation of I{kappa}B kinase and prolongs nuclear localization of NF-{kappa}B in lipopolysaccharide-activated macrophages. American journal of physiology Cell physiology. 2005;289(5):C1114–21. [DOI] [PubMed] [Google Scholar]
- 29.Belch JJ. Free radicals and their scavenging in stroke. Scottish medical journal. 1992;37(3):67–8. [DOI] [PubMed] [Google Scholar]
- 30.Hall ED. Brain attack. Acute therapeutic interventions. Free radical scavengers and antioxidants. Neurosurg Clin N Am. 1997;8(2):195–206. [PubMed] [Google Scholar]
- 31.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med. 2008;168(14):1522–30. [DOI] [PubMed] [Google Scholar]
- 32.Oh SH, Yu WS, Song BH, Lim D, Koo JW, Chang SO, et al. Expression of heat shock protein 72 in rat cochlea with cisplatin-induced acute ototoxicity. Acta Otolaryngol. 2000;120(2):146–50. [DOI] [PubMed] [Google Scholar]
- 33.Rokkas CK, Cronin CS, Nitta T, Helfrich LR Jr., Lobner DC, Choi DW, et al. Profound systemic hypothermia inhibits the release of neurotransmitter amino acids in spinal cord ischemia. J Thorac Cardiovasc Surg. 1995;110(1):27–35. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke. 1996;27(5):913–8. [DOI] [PubMed] [Google Scholar]
- 35.Deng H, Han HS, Cheng D, Sun GH, Yenari MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke. 2003;34(10):2495–501. [DOI] [PubMed] [Google Scholar]
- 36.Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci. 2002;22(10):3921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JE, Yoon YJ, Moseley ME, Yenari MA. Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. J Neurosurg. 2005;103(2):289–97. [DOI] [PubMed] [Google Scholar]
- 38.Liu YC, Lee YD, Wang HL, Liao KH, Chen KB, Poon KS, et al. Anesthesia-Induced Hypothermia Attenuates Early-Phase Blood-Brain Barrier Disruption but Not Infarct Volume following Cerebral Ischemia. PLoS One. 2017;12(1):e0170682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagel S, Su Y, Horstmann S, Heiland S, Gardner H, Koziol J, et al. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 2008;1188:198–206. [DOI] [PubMed] [Google Scholar]
- 40.Wagner S, Nagel S, Kluge B, Schwab S, Heiland S, Koziol J, et al. Topographically graded postischemic presence of metalloproteinases is inhibited by hypothermia. Brain Res. 2003;984(1–2):63–75. [DOI] [PubMed] [Google Scholar]
- 41.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. American journal of physiology Heart and circulatory physiology. 2005;289(2):H558–68. [DOI] [PubMed] [Google Scholar]
- 42.Justicia C, Panes J, Sole S, Cervera A, Deulofeu R, Chamorro A, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2003;23(12):1430–40. [DOI] [PubMed] [Google Scholar]
- 43.Perez E, Viziano A, Al-Zhagal Z, Telischi FF, Sangaletti R, Jiang W, et al. Anatomical correlates and surgical considerations for localized therapeutic hypothermia application in cochlear implantation surgery. Otology & Neurotology. 2019;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamames I, King C, Bas E, Dietrich WD, Telischi F, Rajguru SM. A cool approach to reducing electrode-induced trauma: Localized therapeutic hypothermia conserves residual hearing in cochlear implantation. Hear Res. 2016;339:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamames I, King C, Huang CY, Telischi FF, Hoffer ME, Rajguru SM. Theoretical Evaluation and Experimental Validation of Localized Therapeutic Hypothermia Application to Preserve Residual Hearing After Cochlear Implantation. Ear Hear. 2018;39(4):712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dexter F, Hindman BJ. Computer simulation of brain cooling during cardiopulmonary bypass. Ann Thorac Surg. 1994;57(5):1171–8; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 47.Diao C, Zhu L, Wang H. Cooling and rewarming for brain ischemia or injury: theoretical analysis. Annals of biomedical engineering. 2003;31(3):346–53. [DOI] [PubMed] [Google Scholar]
- 48.Kim S, Tathireddy P, Normann RA, Solzbacher F. Thermal impact of an active 3-D microelectrode array implanted in the brain. IEEE Trans Neural Syst Rehabil Eng. 2007;15(4):493–501. [DOI] [PubMed] [Google Scholar]
- 49.Tamames I, King C, Huang CY, Telischi FF, Hoffer ME, Rajguru SM. Theoretical Evaluation and Experimental Validation of Localized Therapeutic Hypothermia Application to Preserve Residual Hearing After Cochlear Implantation. Ear Hear. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Zhu L. Targeted brain hypothermia induced by an interstitial cooling device in human neck: theoretical analyses. Eur J Appl Physiol. 2007;101(1):31–40. [DOI] [PubMed] [Google Scholar]
- 51.Yin L, Jiang H, Zhao W, Li H. Inducing therapeutic hypothermia via selective brain cooling: a finite element modeling analysis. Med Biol Eng Comput. 2019;57(6):1313–22. [DOI] [PubMed] [Google Scholar]
- 52.Prasad A, Xue QS, Sankar V, Nishida T, Shaw G, Streit WJ, et al. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J Neural Eng. 2012;9(5):056015. [DOI] [PubMed] [Google Scholar]
- 53.Bennett C, Mohammed F, Alvarez-Ciara A, Nguyen M, Dietrich WD, Rajguru SM, et al. Neuroinflammation, oxidative stress, and blood-brain barrier (BBB) disruption in acute Utah electrode array implants and the effect of deferoxamine as an iron chelator on acute foreign body response. Biomaterials. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett C, Samikkannu M, Mohammed F, Dietrich WD, Rajguru SM, Prasad A. Blood brain barrier (BBB)-disruption in intracortical silicon microelectrode implants. Biomaterials. 2018;164:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 56.Butte AJ, Ye J, Haring HU, Stumvoll M, White MF, Kohane IS. Determining significant fold differences in gene expression analysis. Pac Symp Biocomput. 2001:6–17. [DOI] [PubMed] [Google Scholar]
- 57.Huggins CE, Domenighetti AA, Ritchie ME, Khalil N, Favaloro JM, Proietto J, et al. Functional and metabolic remodelling in GLUT4-deficient hearts confers hyper-responsiveness to substrate intervention. J Mol Cell Cardiol. 2008;44(2):270–80. [DOI] [PubMed] [Google Scholar]
- 58.Peart MJ, Smyth GK, van Laar RK, Bowtell DD, Richon VM, Marks PA, et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102(10):3697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raouf A, Zhao Y, To K, Stingl J, Delaney A, Barbara M, et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell. 2008;3(1):109–18. [DOI] [PubMed] [Google Scholar]
- 60.Therapeutic Hypothermia for Severe Traumatic Brain Injury in Japan [Internet]. ClinicalTrials.gov Identifier: NCT00134472. 2012.
- 61.Randomized Controlled Trial of Long-term Mild Hypothermia for Severe Traumatic Brain Injury (LTH-Ⅰ) [Internet]. 2017.
- 62.The Prophylactic Hypothermia Trial to Lessen Traumatic Brain Injury (POLAR-RCT) [Internet]. 2017. [DOI] [PMC free article] [PubMed]
- 63.Keller E, Mudra R, Gugl C, Seule M, Mink S, Frohlich J. Theoretical evaluations of therapeutic systemic and local cerebral hypothermia. J Neurosci Methods. 2009;178(2):345–9. [DOI] [PubMed] [Google Scholar]
- 64.Smith KD. Experimental study and model validation of selective spinal cord and brain hypothermia induced by a simple torso-cooling pad. Proc Inst Mech Eng H. 2011;225(6):533–47. [DOI] [PubMed] [Google Scholar]
- 65.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Critical care medicine. 2009;37(7 Suppl):S186–202. [DOI] [PubMed] [Google Scholar]
- 66.Alva N, Carbonell T, Palomeque J. A model of deep experimental hypothermia and rewarming in rat. Journal of Thermal Biology. 2004;29(4–5):259–64. [Google Scholar]
- 67.Alva N, Palomeque J, Carbonell T. Oxidative stress and antioxidant activity in hypothermia and rewarming: can RONS modulate the beneficial effects of therapeutic hypothermia? Oxid Med Cell Longev. 2013;2013:957054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dietrich WD, Bramlett HM. Therapeutic hypothermia and targeted temperature management for traumatic brain injury: Experimental and clinical experience. Brain Circ. 2017;3(4):186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polderman KH. Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: Indications and evidence. Intensive Care Med. 2004;30(4):556–75. [DOI] [PubMed] [Google Scholar]
- 70.Polderman KH, Callaghan J. Equipment review: cooling catheters to induce therapeutic hypothermia? Crit Care. 2006;10(6):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37(3):1101–20. [DOI] [PubMed] [Google Scholar]
- 72.McManus T, Sadgrove M, Pringle AK, Chad JE, Sundstrom LE. Intraischaemic hypothermia reduces free radical production and protects against ischaemic insults in cultured hippocampal slices. J Neurochem. 2004;91(2):327–36. [DOI] [PubMed] [Google Scholar]
- 73.Cohen GM. Caspases: the executioners of apoptosis. The Biochemical journal. 1997;326 (Pt 1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gamen S, Anel A, Pineiro A, Naval J. Caspases are the main executioners of Fas-mediated apoptosis, irrespective of the ceramide signalling pathway. Cell Death Differ. 1998;5(3):241–9. [DOI] [PubMed] [Google Scholar]
- 75.Hang CH, Shi JX, Li JS, Li WQ, Wu W. Expressions of intestinal NF-kappaB, TNF-alpha, and IL-6 following traumatic brain injury in rats. J Surg Res. 2005;123(2):188–93. [DOI] [PubMed] [Google Scholar]
- 76.Shih RH, Wang CY, Yang CM. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol Neurosci. 2015;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. [DOI] [PubMed] [Google Scholar]
- 78.Choi CH, Jang CH, Cho YB, Jo SY, Kim MY, Park BY. Matrix metalloproteinase inhibitor attenuates cochlear lateral wall damage induced by intratympanic instillation of endotoxin. Int J Pediatr Otorhinolaryngol. 2012;76(4):544–8. [DOI] [PubMed] [Google Scholar]
- 79.Mirzaie M, Karimi M, Fallah H, Khaksari M, Nazari-Robati M. Downregulation of Matrix Metalloproteinases 2 and 9 is Involved in the Protective Effect of Trehalose on Spinal Cord Injury. Int J Mol Cell Med. 2018;7(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenberg GA. Matrix metalloproteinases and neuroinflammation in multiple sclerosis. Neuroscientist. 2002;8(6):586–95. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H, Stark G, Reiss L. Changes in Gene Expression and Hearing Thresholds After Cochlear Implantation. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2015;36(7):1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H, Xu G, Zhang J, Murong S, Mei Y, Tong E. Mild hypothermia reduces ischemic neuron death via altering the expression of p53 and bcl-2. Neurol Res. 2010;32(4):384–9. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Z, Sobel RA, Cheng D, Steinberg GK, Yenari MA. Mild hypothermia increases Bcl-2 protein expression following global cerebral ischemia. Brain Res Mol Brain Res. 2001;95(1–2):75–85. [DOI] [PubMed] [Google Scholar]
- 84.Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–67. [DOI] [PubMed] [Google Scholar]
- 85.Hata R, Gillardon F, Michaelidis TM, Hossmann KA. Targeted disruption of the bcl-2 gene in mice exacerbates focal ischemic brain injury. Metab Brain Dis. 1999;14(2):117–24. [DOI] [PubMed] [Google Scholar]
- 86.Lawrence MS, Ho DY, Sun GH, Steinberg GK, Sapolsky RM. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J Neurosci. 1996;16(2):486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13(4):1017–30. [DOI] [PubMed] [Google Scholar]


