Abstract
Background
Two new antibody–drug conjugates (ADCs) containing a topoisomerase I inhibitor payload have recently emerged in the breast cancer (BC) treatment landscape. Sacituzumab govitecan-hziy (SG) is a first-in-class anti-trophoblast cell-surface antigen 2 ADC approved for pretreated metastatic triple-negative breast cancer (mTNBC) and trastuzumab deruxtecan (T-DXd) gained approval for human epidermal growth factor receptor-2 (HER2)-positive advanced BC (aBC). We aim to provide a contemporary review and the current clinical trial landscape of SG and T-DXd in BC.
Materials and methods
We conducted a literature search from Medline database through PubMed, major conference proceedings [abstracts from European Society for Medical Oncology (Breast) Congress, American Society of Clinical Oncology annual meeting, San Antonio Breast Cancer Symposium] and ClinicalTrials.gov with search terms ‘sacituzumab govitecan’, ‘IMMU-132’, ‘trastuzumab deruxtecan’ and ‘DS-8201a’ up to 21 March 2021.
Results
We assessed 293 records for eligibility, of which 153 were included in this review after screening and exclusion. For SG, efficacy and safety data are available from a phase III trial in pretreated mTNBC and from a phase I/II basket study in mTNBC and hormone receptor-positive/HER2-negative aBC. Thirteen trials with pending primary analysis are ongoing with SG as single agent or in combination, of which 11 are enrolling (2/11 in the early setting). For T-DXd, efficacy/safety data are available as single agent in pretreated HER2-positive (phase Ib and phase II) and in HER2-low aBC (phase Ib), and in combination with nivolumab in HER2-low/positive aBC (phase Ib). Of 23 ongoing trials with T-DXd, 12 are open for enrollment and 3 phase III trials have completed recruitment. The distinct safety profiles of both drugs and their management are discussed.
Conclusion
Given their robust single-agent activity, SG and T-DXd are expected to substantially impact treatment standards, both in and far beyond the currently approved indications. Several trials are investigating new treatment settings for both drugs, including a transition to earlier lines and combinations with other anticancer treatments such as immune checkpoint inhibitors.
Key words: antibody–drug conjugates, sacituzumab govitecan, trastuzumab deruxtecan, HER2-positive breast cancer, triple-negative breast cancer
Highlights
-
•
SG is a new treatment option for previously treated mTNBC.
-
•
T-DXd is approved for HER2-positive aBC after two prior anti-HER2 regimens.
-
•
Both drugs show promising efficacy and are under phase III evaluation in other BC subtypes or treatment settings.
-
•
Interstitial lung disease/pneumonitis is an important identified risk for patients treated with T-DXd.
-
•
SG and T-DXd are expected to substantially impact the BC treatment landscape.
Introduction
Breast cancer (BC) is the most commonly diagnosed malignancy with an estimated 2 261 419 new cases globally in 2020.1 Major advances in early detection, surgery, radiation therapy and systemic treatment increased cure rates of patients diagnosed in stage I-III to ∼70%-80%. However, due to the dismal adverse prognosis for the vast majority of patients with advanced disease combined with its high incidence, BC remains the second most common cause of cancer-related death in women worldwide after lung cancer.1,2
BC molecular subtypes reveal crucial prognostic and therapeutic value and are, together with patient and disease characteristics, the main driver of treatment decision making. The systemic treatment landscape of advanced BC (aBC) has changed significantly over the last two decades, with the main progress situated in the treatment of hormone receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2−) and in HER2-positive (HER2+) BC.2 Triple-negative breast cancer (TNBC), which accounts for ∼15% of all breast malignancies and is more frequent in younger patients, has the worst prognosis of all BC subtypes.3 Until recently, cytotoxic chemotherapy remained the only systemic treatment option for these patients. Between 2018 and 2020, approvals of immune checkpoint inhibitors (ICIs) atezolizumab and pembrolizumab have expanded the treatment options for a subset (∼40%) of patients with programmed death-ligand 1-positive TNBC, while the poly(ADP-ribose) polymerase inhibitors (PARPi) olaparib and talazoparib as single agent are now standard treatment options for advanced HER2− BC in patients with germline BRCA pathogenic variants.4, 5, 6
Paul Ehrlich's work on standardization of sera for antibody concentration and his historical manuscript published in 1897 on the ‘side-chain theory of immunity’ evolved into what later became known as Ehrlich's ‘magic bullet concept’.7 This concept led to the development of the first technology for the production of monoclonal antibodies (mAbs) in 1975,8,9 while the idea of attaching toxins to antibodies gave rise to a new class of targeted anticancer treatment, namely antibody–drug conjugates (ADCs).10,11 ADCs are composed of a mAb linked to a cytotoxic drug, also called a payload. They contribute to higher efficacy of anticancer therapy by targeted delivery of the cytotoxic agent to antigen (Ag)-expressing cells, minimizing cytotoxic exposure to healthy cells. The ADC binds to the surface Ag of an Ag-presenting cell. The ADC–Ag complex is internalized and incorporated into endosomes or lysosomes, where the payload is released through proteolytic degradation of the entire ADC molecule or due to cleavage of the linker, which can be provoked by extracellular or intracellular conditions.12 The payload then binds to its intracellular target such as tubulin, DNA or topoisomerase 1. Membrane permeability for the payload and linker instability can cause off-target effects on nearby Ag-positive and Ag-negative cells, which is called the bystander effect.13 The drug class of ADCs is rapidly expanding and is expected to become the next drug wave in oncology.9,12,13
In 2013, ADCs made their introduction as treatment option for advanced solid tumors with the Food and Drug Administration (FDA) approval of ado-trastuzumab emtansine (T-DM1, Kadcyla®) for metastatic HER2+ BC pretreated with trastuzumab and a taxane, based on results from the phase III EMILIA trial.14,15 T-DM1 also proved its value in later-line advanced setting and in the adjuvant setting in patients with residual disease after neoadjuvant trastuzumab and taxanes.16,17 The promising results of T-DM1 and this innovative approach sparked interest in the development of several other ADCs for breast and other solid cancers, but with varying degrees of success.11
More recently, two new ADCs have emerged in the treatment landscape of aBC. Trastuzumab deruxtecan (T-DXd) showed promising results in heavily pretreated advanced HER2+ BC in the phase II DESTINY-Breast01 trial.18 Sacituzumab govitecan-hziy (SG) demonstrated high activity in pretreated metastatic TNBC (mTNBC) in a phase I/II basket trial, which was later confirmed in a randomized phase III trial versus single-agent chemotherapy of physician's choice.19
Given the impressive single-agent activity of both drugs, their introduction in the treatment landscape is expected to substantially impact standard of care in advanced HER2+ and TNBC. Based on promising findings in early clinical trials in estrogen receptor-positive/HER2− and HER2-low BC for SG and T-DXd, respectively, the impact of these new ADCs may reach far beyond current indications. Several clinical trials are ongoing with both drugs in earlier treatment settings and other BC subtypes, both as single agent and in combination with other anticancer treatments such as ICIs or PARPi.12 The aim of this review is to provide a contemporary overview of current data and ongoing trials of T-DXd and SG in BC.
Materials and methods
We conducted a literature search from Medline database through PubMed to retrieve published articles concerning SG or T-DXd in BC. We applied the following Medical Subject Headings terms: ‘sacituzumab govitecan’/‘IMMU-132’ and ‘trastuzumab deruxtecan’/‘DS-8201a’. A search was conducted in ClinicalTrials.gov with the same search terms to obtain an overview of the ongoing clinical trials with both drugs. Furthermore, we reviewed the major conference proceedings including abstracts from the European Society for Medical Oncology (ESMO) Congress up to 2019 and ESMO Virtual Congress 2020, ESMO Breast Congress 2019 and ESMO Breast Virtual Congress 2020, American Society of Clinical Oncology (ASCO) Annual Meeting up to 2019 and ASCO Virtual Annual Meeting 2020 and San Antonio Breast Cancer Symposium (SABCS) up to 2019 and Virtual SABCS 2020. Records that met at least one of the following criteria were excluded: (i) records evaluating SG or T-DXd in other cancer types, (ii) records not evaluating SG or T-DXd, (iii) records on animal studies, (iv) expert opinions, (v) withdrawn clinical trials, (vi) ongoing trial posters, (vii) records written in languages other than English, (viii) duplicate search results. These searches were carried out up to 21 March 2021 with no date restrictions. References from included records were not considered for inclusion in this review.
Results
The flow diagram of the record identification and selection process is available in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100204. From 297 records initially identified, 292 were screened after duplicate removal. During screening, we excluded 142 records for the following reasons: articles about other cancer types, other treatment, animal studies, expert opinions or language other than English (n = 85), conference abstracts about other cancer types or ongoing trial posters (n = 28) or records from ClinicalTrials.gov database not recruiting BC patients or withdrawn trials (n = 29). A total of 150 records were included in this review: 64 for SG (32 full-text articles, 14 ClinicalTrials.gov records, 18 conference abstracts and poster presentations) and 86 for T-DXd (40 full-text articles, 23 ClinicalTrials.gov records, 23 conference abstracts and poster presentations).
Sacituzumab govitecan
The trophoblast cell-surface Ag-2 (Trop-2) is a transmembrane glycoprotein calcium signal transducer that is overexpressed in several epithelial tumors. The Ag stimulates cancer growth by raising cellular proliferation and invasion. High amount of Trop-2 expression is seen in different solid tumors, including breast, urothelial, colon, prostate, pancreatic and lung cancer. Trop-2 expression is detected in all BC subtypes and membrane localization has been associated with poor prognosis.20, 21, 22, 23, 24
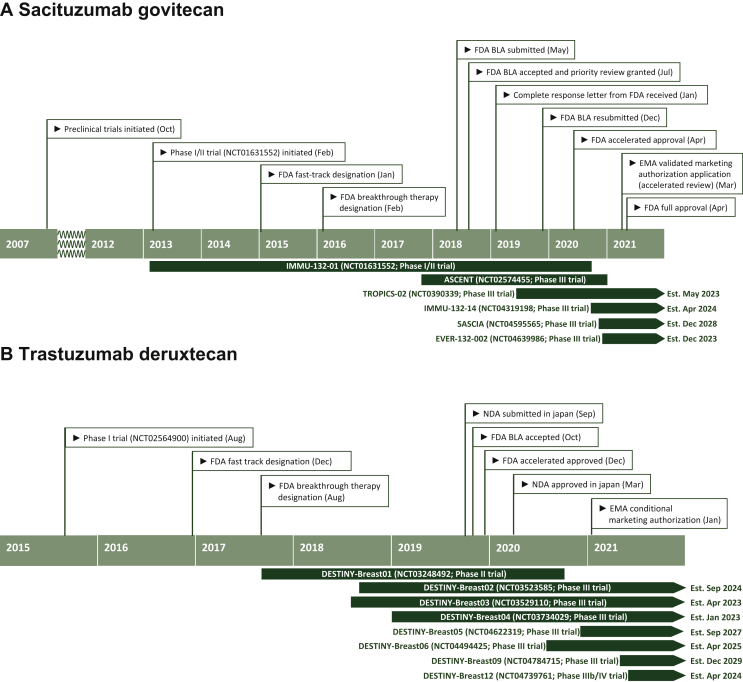
SG (IMMU-132, Trodelvy®) is a first-in-class anti-Trop-2 ADC. It consists of the humanized mAb (hRS7) and SN-38 as cytotoxic payload (SN-38), which is the active metabolite of irinotecan (CPT-11) and functions as a topoisomerase I inhibitor. Both are coupled via a hydrolysable linker (CL2A) that contributes to the bystander effect. The ADC has a high drug-to-antibody ratio of 7.6 SN-38 molecules per antibody.20 In April 2020, SG received accelerated FDA approval for mTNBC in patients who received two prior regimens for metastatic disease based on efficacy and safety data from a phase I/II basket study, IMMU-132-01.19,22,25,26 The primary outcome analysis of the confirmatory phase III ASCENT study was presented in September 2020 and has recently been published.24 Recently, SG received regular approval by FDA for patients with unresectable locally advanced or mTNBC who have received at least two prior therapies, including at least one prior therapy for metastatic disease, reflecting the ASCENT inclusion criteria of recruiting patients after one prior regimen for advanced disease progressing within 12 months of (neo)adjuvant therapy.24,27 In March 2021, the European Medicines Agency (EMA) validated the marketing authorization application and granted SG accelerated review for a similar indication as the full FDA approval, while regulatory reviews of SG for mTNBC are also ongoing in the UK, Canada, Switzerland, Australia and Singapore.28 A timeline of development and regulatory milestones of SG in BC is shown in Figure 1A.
Figure 1.
Timeline of development and regulatory milestones of sacituzumab govitecan for the treatment of advanced TNBC (A) and trastuzumab deruxtecan for the treatment of advanced HER2-positive breast cancer (B).
Est, estimated; BLA, Biologics License Application; TNBC, triple-negative breast cancer; FDA, Food and Drug Administration; NDA, new drug application; EMA, European Medicines Agency.
In IMMU-132-01, 108 patients with mTNBC progressing after at least two previous anticancer therapies for metastatic disease received SG at a starting dose of 10 mg/kg intravenously on days 1 and 8 of a 21-day treatment cycle. Included patients received a median of three previous anticancer regimens, including previous use of taxanes and anthracyclines, in 98.1%, 86.1% and 68.5% of patients, respectively. The investigator-assessed objective response rate (ORR) was 33.3%, with a median duration of response of 7.7 months. The median progression-free survival (PFS) and overall survival (OS) were 5.5 months and 13 months, respectively. Grade 3/4 adverse events (AEs) occurred in 92 of 108 patients (85%). Nausea, diarrhea, fatigue, neutropenia and anemia were the most common AEs. Grade ≥3 febrile neutropenia and diarrhea were both observed in 8% of patients.
The confirmatory ASCENT study was an open-label, randomized, controlled phase III trial investigating the efficacy and safety of SG versus single-agent chemotherapy of physician's choice [treatment of physician's choice (TPC); capecitabine, eribulin, vinorelbine or gemcitabine] in patients with mTNBC after ≥2 prior regimens in the advanced setting (including a taxane).24 The primary endpoint was PFS as evaluated by blinded independent central review (ICR) in the patients without baseline brain metastases (n = 468). Eligibility criteria did not define an upper limit of prior regimens. Patients with a disease-free interval after last (neo)adjuvant therapy of <12 months were eligible for ASCENT after one prior regimen in the advanced setting; this line in the early setting was then counted as the second prior regimen for eligibility assessment and stratification. This resulted in a heavily pretreated patient population with a median number of 4 prior treatment lines (range, 2-17), including carboplatin and ICIs in 65.5% and 27.1% of patients in the primary outcome population, respectively. Compared to patients randomized to TPC, patients treated with SG experienced a statistically significant and clinically relevant improvement in PFS [hazard ratio (HR) 0.41; median PFS 5.6 versus 1.7 months; P < 0.0001] and OS (HR 0.48; median PFS 12.1 versus 6.7 months; P < 0.0001). The ORR improved from 5% in patients randomized to TPC to 35% for patients randomized to SG (P < 0.0001), while the median duration of response was 6.3 months and 3.6 months in the SG and TPC arms, respectively. ASCENT was halted early due to compelling evidence of efficacy. In an exploratory biomarker analysis, SG outperforms TPC regardless of Trop-2 expression and germline BRCA mutations. The magnitude of benefit seems lower in patients with low Trop-2 H-scores (20.3% of the biomarker-evaluable population), but the low numbers warrant caution in the interpretation.29,30 A subgroup analysis of patients with baseline brain metastases (n = 61) showed numerically better PFS in patients randomized to SG versus TPC but no improvement in OS, but the small sample size limits interpretation.31
Treatment-related adverse events (TRAEs) observed in ASCENT were reported for all patients (including those with brain metastases at diagnosis) who received ≥1 dose of study treatment (n = 482) and are summarized in Table 1. The most reported TRAEs in patients treated with SG are neutropenia, diarrhea, nausea, alopecia, fatigue and anemia.24 Treatment-related alopecia is reported in 46%, but the proportions of patients with grade 1 versus grade 2 alopecia are currently unavailable. The proportion of patients with prior alopecia may have been high and/or underreporting of alopecia may have occurred. Alopecia is as such to be expected in patients treated with SG, while the efficacy of scalp cooling for prevention of SG-induced alopecia is unknown. Most frequent grade 3/4 TRAEs in patients receiving SG are neutropenia, diarrhea, anemia and febrile neutropenia, occurring in 46%, 10%, 8% and 6% of patients, respectively. Neutropenia and diarrhea are boxed warnings in the FDA highlights of prescribing information,24 but are manageable with active monitoring, early intervention and dose reductions/interruptions. Granulocyte colony-stimulating factor (G-CSF) use was reported in 49% of patients in the SG arm versus 23% in the TPC arm. There were no treatment-related deaths and no grade >2 neuropathy or grade >3 interstitial lung disease (ILD) in patients treated with SG. Treatment discontinuations due to AE were infrequent both for SG and TPC (4.7% and 5.4%, respectively), while dose reductions and interruptions occurred in 26% versus 22% of patients and in 61% versus 33% in the SG versus TPC arms, respectively.30,32 The efficacy of SG was not impaired by dose reductions. A dose reduction of 25% and administration of G-CSF is recommended in patients treated with SG experiencing grade 4 neutropenia ≥7 days, grade 3 febrile neutropenia or a delay of the next scheduled dose because of grade 3-4 neutropenia by 2 or 3 weeks before recovery to grade ≤1. At onset of severe diarrhea, infectious causes should be excluded, and if negative, loperamide should be initiated. Nausea and vomiting with SG can typically occur up to 3 weeks after treatment initiation. Effective antiemetic pre- and/or concomitant medication is important and was used in 86% and 63% of patients in the SG and TPC arms of ASCENT, respectively.32 Infusion reactions with hypersensitivity including anaphylactic reactions may occur for which pre-infusion medication with antipyretics and antihistamines is recommended, while atropine premedication can be considered in patients who experience excessive cholinergic reactions after administration such as abdominal cramping or early diarrhea.33 Loperamide should be promptly initiated at the onset of late diarrhea after evaluation for infectious causes. If severe diarrhea occurs, SG should be witheld until resolved to grade ≤1 and subsequent doses should be reduced. Given that reduced uridine diphosphate glucuronosyltransferase enzymatic activity prevents SN-38 glucuronidation and inactivation and is associated with hematological toxicity, an exploratory analysis investigated whether patients with UGT1A1 polymorphisms are at increased toxicity risk.32 Patients with UGT1A1 homozygous ∗28/∗28 genotype (34/250; 13.6%) were only modestly at higher risk for neutropenia with SG and should be monitored closely. This increased risk for neutropenia in patients with UGT1A1 homozygous ∗28/∗28 genotype was confirmed in an analysis of other solid cancer types treated with SG in the IMMU-132-01 basket trial.34 However, diarrhea did not appear to be increased in patients homozygous for UGT1A1 ∗28, and the magnitude of impact of this homozygous polymorphism on the overall safety profile was limited in ASCENT and IMMU-132-01. The UGT1A1 status does not alter recommendations for treatment or toxicity management, but patients with known UGT1A1 ∗28 homozygosity should be monitored closely.32
Table 1.
Treatment-related adverse events of sacituzumab govitecan-hziy (all grade >20%, grade 3 or 4 >5% of patients)
| TRAE | All grade (%) | Grade 3 (%) | Grade 4 (%) | |
|---|---|---|---|---|
| Hematologic | Neutropenia | 63 | 46 | 17 |
| Anemia | 34 | 8 | 0 | |
| Leukopenia | 16 | 10 | 1 | |
| Febrile neutropenia | 6 | 5 | 1 | |
| Gastrointestinal | Diarrhea | 59 | 10 | 0 |
| Nausea | 57 | 2 | <1 | |
| Vomiting | 29 | 1 | <1 | |
| Other | Fatigue | 45 | 3 | 0 |
| Alopecia | 46 | 0 | 0 |
Data based on the ASCENT clinical trial.25
TRAE, treatment-related adverse event.
SG also is under investigation in BC settings beyond advanced TNBC. In the IMMU-132-01 study, 54 patients with HR+/HER2− metastatic BC and at least two prior lines of therapy who received SG had an ORR of 31.5% and a median PFS of 5.5 months.22 Recently, initial data from early trials showed encouraging signs of activity of SG combined with rucaparib in TNBC and as single agent in patients with BC brain metastases.35,36 Table 2 provides an overview of 13 currently ongoing studies of SG in patients with BC. Besides a rollover study, SG is under phase III evaluation versus TPC in patients with advanced HR+/HER2− BC (TROPiCS-02, NCT03901339) and in patients with early HER2− BC presenting with residual disease after neoadjuvant treatment (SASCIA, NCT04595565). In advanced TNBC, SG is mainly under evaluation in combination with ICIs (atezolizumab, pembrolizumab) and PARPi (rucaparib, talazoparib), while SG as single agent is being investigated in the neoadjuvant setting in the phase II NeoSTAR study (NCT04230109). SG is under investigation in several other solid tumors, has obtained FDA fast-track designation for metastatic urothelial carcinoma and non-small-cell lung cancer (NSCLC)22 and was granted orphan drug status for small-cell lung cancer, pancreatic cancer and glioblastoma.37, 38, 39
Table 2.
Ongoing clinical trials with sacituzumab govitecan for patients with breast cancer
| Trial | Phase | Treatment setting | Breast cancer subtype | Patient population | Design | Experimental arm | Control arm | Sample size | NCT number | Recruitment status (locations) |
|---|---|---|---|---|---|---|---|---|---|---|
| Unresectable locally advanced/metastatic setting | ||||||||||
| TROPiCS-02 | III | Advanced | HR+/HER2− | After ≥2 and ≤4 prior chemotherapy regimens for metastatic disease | Open-label RCT | SG | Capecitabine, eribulin, gemcitabine or vinorelbine | 520 | NCT03901339 | Recruitment closed |
| EVER-132-002 | III | Metastatic | HR+/HER2− | After ≥2 and ≤4 prior chemotherapy regimens for metastatic disease | Open-label RCT | SG | Capecitabine, eribulin, gemcitabine or vinorelbine | 330 | NCT04639986 | Recruiting (Republic of Korea, Taiwan) |
| IMMU-132-14 | III | Metastatic | TNBC HR+/HER2− HER2+ |
Metastatic solid tumors, rollover from parent SG studies | Single-arm rollover study | SG | NA | 200 | NCT04319198 | On invitation |
| Saci-IO TNBC | II | Metastatic | TNBC | mTNBC, PD-L1 negative | Open-label RCT | SG + pembrolizumab | SG | 110 | NCT04468061 | Recruiting (United States) |
| Saci-IO HR+ | II | Metastatic | HR+/HER2− | HR+/HER2− mBC | Open-label RCT | SG + pembrolizumab | SG | 110 | NCT04448886 | Recruiting (United States) |
| EVER-132-001 | IIb | Metastatic | TNBC | After ≥2 prior chemotherapy | Single arm | SG | NA | 80 | NCT04454437 | Recruiting (China) |
| S2007 | II | Brain metastases | TNBC HR+/HER2− |
Patients with brain metastases with CNS progression after previous CNS-directed therapy | Single arm | SG | NA | 44 | NCT04647916 | Recruiting (United States) |
| SEASTAR | Ib/II | Advanced | TNBC Other subtypesa |
After progression on standard treatment | Dose finding and expansion | SG + rucaparib | NA | 329 | NCT03992131 | Recruiting (United States) |
| Morpheus-TNBC | Ib/II | Advanced | TNBC | First-line advanced setting | Open-label, randomized umbrella study | SG + atezolizumab | SG + nab-paclitaxel | 280 | NCT03424005 | Recruiting (Australia, France, Germany, Israel, Republic of Korea, Spain, UK, United States) |
| 19-239 | Ib/II | Metastatic | TNBC | All treatment lines | Dose finding and expansion | SG + talazoparib | NA | 75 | NCT04039230 | Recruiting (United States) |
| Neuro/SG/breast Brain | 0 | Brain metastases | TNBC HR+/HER2− HER2+ |
Breast cancer with known/suspected brain metastases, planned to undergo craniotomy | Single arm | SG | NA | 20 | NCT03995706 | Recruiting (United States) |
| Early/curative setting | ||||||||||
| SASCIA | III | Post-neoadjuvant | TNBC HR+/HER2− |
Residual disease after neoadjuvant chemotherapy | Open-label RCT | SG | Capecitabine, carboplatin or cisplatin | 1200 | NCT04595565 | Recruiting (Germany) |
| NeoSTAR | II | Early or locally advanced | TNBC | Neoadjuvant setting, previously untreated TNBC | Single arm | SG + pembrolizumab | SG | 50 | NCT04230109 | Recruiting (United States) |
Source: ClinicalTrials.gov: March 2021.
CNS, central nervous system; HER2, human epidermal growth factor receptor-2; HR, hormone receptor; mBC, metastatic breast cancer; mTNBC, metastatic triple-negative breast cancer; NA, not applicable; NCT, National Clinical Trial; PD-L1, programmed death-ligand 1; RCT, randomized, controlled trial; SG, sacituzumab govitecan-hziy; TPC, treatment of physician choice.
If BRCA1/2, PALB2, RAD51C or RAD51D deleterious mutation.
Trastuzumab deruxtecan
Approximately 15%-20% of all BCs are classified as HER2+ based on high HER2 protein expression by immunohistochemistry (IHC 3+) and/or ERBB2 amplification by in situ hybridization (ISH).40 Overexpression of HER2 is biologically associated with a worse prognosis, but its strong predictive value for benefit from HER2-targeted agents led to a significant improvement in outcomes for patients with HER2+ BC.41, 42, 43 Additionally, more than half of BCs may classify as HER2 low, defined as BC with a HER2 IHC score of 1+ or 2+ with negative ISH assay.44 Besides HER2-targeted ADCs, including T-DXd, several other anti-HER2-targeted agents have recently granted approval or are under development.44
T-DXd (DS-8201a, Enhertu®) is composed of an anti-HER2 immunoglobulin G1 antibody and a topoisomerase I inhibitor payload, coupled by a cleavable tetrapeptide-based linker.43,45 It has a high drug-to-antibody ratio of 8 and a potent bystander effect.18 In December 2019, T-DXd gained accelerated FDA approval for adult patients with unresectable or metastatic HER2+ BC who have received two or more prior anti-HER2-based regimens in the metastatic setting.46 This accelerated approval was based on the results of the DESTINY-Breast01 trial that showed impressive antitumor activity of T-DXd in heavily pretreated patients.18,47 In January 2021, T-DXd received a conditional marketing authorization by the EMA for HER2+ BC after two or more prior anti-HER2-based regimens.48,49 A timeline of development and regulatory milestones of T-DXd in BC is shown in Figure 1B.
In the phase II DESTINY-Breast01 trial, 184 patients with HER2+ metastatic BC with a median of 6 prior lines received the recommended dose of 5.4 mg/kg T-DXd intravenously once every 3 weeks.18 Updated study results at a median follow-up of 20.5 months showed an ORR of 61.4% (median duration of response: 20.8 months) and a median PFS of 19.4 months [95% confidence interval (CI): 14.1 months to not estimable].50 The landmark analysis estimations of OS at 12 and 18 months were 85% and 74%, respectively. In an earlier presented subgroup analysis, the median PFS in 24 patients with stable brain metastases at diagnosis was 18.1 months (95% CI: 6.7-18.1). Intracranial response was observed, and six of eight recurrences in this subgroup occurred outside the central nervous system.51 These updated results confirm the robust efficacy of T-DXd in HER2+ pretreated metastatic BC.
Grade 3 or higher AEs occurred in 61.4% of patients. Nausea, alopecia, fatigue, vomiting, neutropenia, anemia, thrombocytopenia and ILD/pneumonitis were the most common AEs.18,50 TRAEs observed in the DESTINY-Breast01 clinical trial are summarized in Table 3. At the updated safety analysis, grade 1/2, grade 3/4 and grade 5 ILD events were reported in 12%, 0.5% and 2.7%, respectively. Dose interruption, reduction and discontinuation associated with treatment emergent adverse events occurred in 40.8%, 23.9% and 18.5% of patients, respectively.50 Left ventricular ejection fraction decrease was reported in <1% of patients.18 The substantial risk for ILD in patients treated with T-DXd highlights the importance of raising awareness for potential symptoms of ILD or pneumonitis among patients and primary care physicians. Symptoms that require caution are cough, dyspnea, fever or other new or worsening respiratory symptoms. Prompt clinical and imaging evaluation of potential signs and symptoms is warranted to investigate evidence of pulmonary toxicity. T-DXd should be interrupted from grade 1 onwards (asymptomatic suspicious findings at imaging), and permanently discontinued for any symptomatic (grade ≥2) ILD or pneumonitis.48,52 Further evaluation consists of a high-resolution computed tomography, testing of pulmonary function, oxygen saturation and consultation with a pneumologist. Corticosteroid treatment should be considered as soon as ILD/pneumonitis is suspected, and promptly initiated for any grade ≥2 pulmonary toxicity.53 More research is required to establish the pathophysiology, risk factors and optimal treatment of ILD and pneumonitis associated with T-DXd.54,55 Current data do not suggest an increased risk with higher cumulative doses and rather show a plateau of cumulative ILD probability with the highest risk of onset in the first 12 months of exposure and a median time to onset of ∼6 months.50 Patients of Japanese ethnicity and patients with BC seemed to be more likely to develop ILD after treatment with T-DXd.56
Table 3.
Treatment-related adverse events of trastuzumab deruxtecan (all grade, grade 3/4 or grade 5)
| TRAE | All grade (%) | Grade 3/4 (%) | Grade 5 (%) | |
|---|---|---|---|---|
| Hematologic | Decreased neutrophil count | 34.8 | 20.7 | 0 |
| Anemia | 29.9 | 8.7 | 0 | |
| Decreased white cell count | 21.2 | 6.5 | 0 | |
| Decreased platelet count | 21.2 | 4.3 | 0 | |
| Decreased lymphocyte count | 14.1 | 6.5 | 0 | |
| Gastrointestinal | Diarrhea | 29.3 | 2.7 | 0 |
| Constipation | 35.9 | 0.5 | 0 | |
| Nausea | 77.7 | 7.6 | 0 | |
| Vomiting | 45.7 | 4.3 | 0 | |
| Decreased appetite | 31.0 | 1.6 | 0 | |
| Abdominal pain | 16.8 | 1.1 | 0 | |
| Lung | Interstitial lung disease | 15.2 | 0.5 | 2.7 |
| Cough | 19 | 0 | 0 | |
| Heart | Prolonged QT interval | 4.9 | 1.1 | 0 |
| Decreased LV ejection fraction | 1.6 | 0.5 | 0 | |
| Other | Fatigue | 49.5 | 6.0 | 0 |
| Alopecia | 48.4 | 0.5 | 0 | |
| Headache | 19.6 | 0 | 0 |
Similar to SG, T-DXd also has shown initial auspicious results and is under phase III evaluation in BC subtypes beyond the current label. In a phase I expansion cohort of 54 patients with heavily pretreated advanced HER2-low BC (IHC 1+ or IHC 2+ with negative ISH), treatment with T-DXd (at 5.4 or 6.4 mg/kg) resulted in an ORR of 37% by ICR, with a median duration of response of 10.4 months.57 The HER2-low subclass presents 40%-50% of BCs and there is high interest in several HER2-targeted agents, among which are ADCs, to enlarge the treatment landscape and improve the outcomes of patients with this BC subtype.44,47,57 Interestingly, in a recent interim analysis of a phase Ib trial investigating the combination of T-DXd and nivolumab in pretreated HER2-expressing metastatic BC, the confirmed ORR by ICR was 59.4% and 37.5% in the HER2+ (amplified or IHC 3+) and HER2-low cohorts, respectively.58 Notably, the proportion of patients with grade 1/2 and grade 5 treatment-related ILD was 8.3% and 2.1%, respectively. Although caution is warranted given the low sample size and cross-trial comparison, current data do not suggest an increased risk of pulmonary toxicity when combining T-DXd with an ICI.
Table 4 provides an overview of all currently ongoing studies with T-DXd in BC. In HER2+ BC, T-DXd is under phase III investigation in the advanced/metastatic setting in the first, second and third or more treatment lines. DESTINY-Breast09 compares T-DXd with or without pertuzumab with the current standard of taxane, pertuzumab and trastuzumab as first-line treatment. DESTINY-Breast03 evaluates T-DXd as single agent versus T-DM1 in the second line, while DESTINY-BREAST02 evaluates the value of T-DXd after prior T-DM1. Several trials with T-DXd recruit patients with untreated clinically asymptomatic central nervous system metastases. Two single-arm trials specifically focus on T-DXd as single agent in patients with treated or untreated brain metastases (including leptomeningeal disease) from HER2+ BC, while HER2-CLIMB-04 evaluates the combination of T-DXd and tucatinib after ≥2 prior anti-HER2 treatments in the advanced setting. Several other combinations of T-DXd and other anticancer agents (ICIs, HER2-targeted agents, chemotherapy ± ICI, endocrine therapy, ATR inhibitor) in HER2+ aBC are ongoing. In the curative setting, T-DXd is under phase III investigation versus T-DM1 for patients with HER2+ BC and residual disease after neoadjuvant chemotherapy and trastuzumab ± pertuzumab (DESTINY-Breast05).
Table 4.
Ongoing clinical trials with trastuzumab deruxtecan for patients with breast cancer
| Trial | Phase | Treatment setting | Breast cancer subtype | Patient population | Design | Experimental arm | Control arm | Sample size | NCT number | Recruitment status (locations) |
|---|---|---|---|---|---|---|---|---|---|---|
| Unresectable locally advanced/metastatic setting | ||||||||||
| DESTINY-Breast12 | IV | Advanced | HER2+ | After trastuzumab, pertuzumab or T-DM1; ≤2 prior regimens for metastatic disease | Single arm | T-DXd | NA | 500 | NCT04739761 | Not yet recruiting |
| DESTINY-Breast09 | III | Advanced | HER2+ | First-line advanced setting | Open-label, three-arm RCT | Arm A: T-DXd with placebo Arm B: T-DXd with pertuzumab |
Taxane + pertuzumab + trastuzumab | 1134 | NCT04784715 | Not yet recruiting |
| DESTINY-Breast02 | III | Advanced | HER2+ | After prior T-DM1 | Open-label RCT | T-DXd | Capecitabine + trastuzumab or capecitabine + lapatinib | 600 | NCT03523585 | Recruitment closed |
| DESTINY-Breast03 | III | Advanced | HER2+ | After prior trastuzumab and taxane | Open-label RCT | T-DXd | T-DM1 | 500 | NCT03529110 | Recruitment closed |
| DESTINY-Breast06 | III | Advanced | HER2-low/HR+ | After progression on prior endocrine treatment(s), no prior chemotherapy for advanced disease | Open-label RCT | T-DXd | Capecitabine, paclitaxel or nab-paclitaxel | 850 | NCT04494425 | Recruiting (across 25 countries worldwide) |
| DESTINY-Breast04 | III | Advanced | HER2-low | After 1-2 lines of prior chemotherapy for metastatic disease | Open-label RCT | T-DXd | Capecitabine, eribulin, gemcitabine, paclitaxel or nab-paclitaxel | 557 | NCT03734029 | Recruitment closed |
| DAISY | II | Advanced | HER2+ HER2-low HER2 IHC0 |
After progression on standard treatment | Single arm, multicohort | T-DXd | NA | 162 | NCT04132960 | Recruitment closed |
| HER2CLIMB-04 | II | Advanced | HER2+ | After ≥2 prior anti-HER2 regimens for metastatic disease | Single arm | T-DXd + tucatinib | NA | 70 | NCT04539938 | Recruiting (United States) |
| DEBBRAH | II | Brain metastases | HER2+ HER2-low |
Untreated or treated brain or leptomeningeal metastases, after standard treatment | Single arm, multicohort | T-DXd | NA | 39 | NCT04420598 | Recruiting (Spain) Not yet (Portugal) |
| TUXEDO-1 | II | Brain metastases | HER2+ | Untreated or treated brain metastases, after trastuzumab and pertuzumab ± T-DM1 | Single arm | T-DXd | NA | 15 | NCT04752059 | Recruiting (Austria) |
| DESTINY-Breast07 | Ib/II | Advanced | HER2+ | Dose-finding phases: in second line or later Dose-expansion phases: first-line advanced setting Two modules for patients with active brain metastases |
Modular dose finding and expansion | T-DXd single agent or with
|
NA | 450 | NCT04538742 | Recruiting (across 15 countries worldwide) |
| BEGONIA | Ib/II | Metastatic | HER2-low/HR− | First-line treatment | Open-label, multi-arm | Arm 6: T-DXd + durvalumab | NA | 57 (arm 6) | NCT03742102 | Recruiting (United States, Canada, Korea, Poland, Taiwan, UK) |
| DESTINY-Breast08 | Ib | Advanced | HER2-low/HR+ | Dose-finding phases: in second line or later Dose-expansion phases: first- or second-line advanced setting |
Modular dose finding and expansion | T-DXd +
|
NA | 185 | NCT04556773 | Recruiting (United States, Australia, Korea, Taiwan, Canada) |
| DS8201-A-U106 | Ib | Advanced | HER2+ HER2-low |
Disease progression after standard treatment, including T-DM1 for HER2+ | Dose finding and expansion | T-DXd + pembrolizumab | NA | 115 | NCT04042701 | Recruiting (United States, France) |
| DS8201-A-U105 | Ib | Advanced | HER2+ HER2-low |
Disease progression after standard treatment, including T-DM1 for HER2+ | Dose finding and expansion | T-DXd + nivolumab | NA | 99 | NCT03523572 | Recruitment closed |
| DASH trial | I/Ib | Advanced | HER2+ HER2-low |
Disease progression after ≥1 prior chemotherapy, including 1 anti-HER2 regimen | Dose finding and expansiona | T-DXd + ceralasertib | NA | 15 | NCT04704661 | Not yet recruiting |
| DS8201-A-J102 | I | Advanced | HER2+ HER2-low |
Disease progression after standard treatment | Single-arm, safety study (QT interval, PK, AE) | T-DXd | NA | 51 | NCT03366428 | Recruitment closed |
| DS8201-A-A104 | I | Advanced | HER2+ HER2-low |
Disease progression after ≥1 prior chemotherapy | Single-sequence, crossover safety study (DDI, AE) | T-DXd + ritonavir or itraconazole | NA | 40 | NCT03383692 | Recruitment closed |
| NCI-2020-01206 | I | Advanced | HER2+ HER2-low |
Disease progression after standard treatment | Single-arm, safety study (PD, AE) | T-DXd | NA | 28 | NCT04294628 | Recruiting (United States) |
| DS8201-A-A103 | I | Advanced | HER2+ HER2-low |
Disease progression after standard treatment | Single-arm, safety study (AE, PK) | T-DXd | NA | 12 | NCT03368196 | Recruitment closed |
| Early/curative setting | ||||||||||
| DESTINY-Breast05 | III | Post-neoadjuvant | HER2+ | Residual disease after neoadjuvant chemotherapy and HER2-directed treatment | Open-label RCT | T-DXd | T-DM1 | 1600 | NCT04622319 | Recruiting (across 31 countries worldwide) |
| 20-001275 | II | Early or locally advanced | HER2-low/HR+ | Neoadjuvant setting, previously untreated | Open-label RCT | T-DXd + anastrozole | T-DXd | 88 | NCT04553770 | Recruiting (United States) |
| Translational/non-interventional studies | ||||||||||
| HER2-PREDICT | — | Advanced | HER2+ HER2-low |
Patients treated with T-DXd in a clinical trial | Non-interventional translational | Tumor and blood sample collection | NA | 180 | NCT04257162 | Recruiting (Spain) |
Source: ClinicalTrials.gov: March 2021.
AE, adverse events; BM, brain metastases; DDI, drug–drug interactions; HER2, human epidermal growth factor receptor-2; HR, hormone receptor; IHC, immunohistochemistry; LMC, leptomeningeal carcinomatosis; NA, not applicable; NCT, National Clinical Trial; RCT, randomized, controlled trial; T-DM1, ado-trastuzumab emtansine; PD, pharmacodynamics; PK, pharmacokinetics; SG, sacituzumab govitecan-hziy; T-DXd, trastuzumab deruxtecan; TNBC, triple-negative breast cancer; TPC, treatment of physician's choice.
Expansion cohorts only enroll gastroesophageal and colorectal cancer.
In advanced HER2-low BC, recruitment has closed in the phase III trial DESTINY-Breast04, evaluating T-DXd versus TPC (capecitabine, eribulin, gemcitabine, paclitaxel or nab-paclitaxel) after 1-2 prior chemotherapy lines. In advanced HR+, HER2-low BC, T-DXd is currently under phase III investigation as single agent in the first chemotherapy line setting (DESTINY-Breast06, versus capecitabine, paclitaxel, nab-paclitaxel) and in several drug combinations in multiple phase Ib trials. The combination of T-DXd and durvalumab is being evaluated as first-line treatment for HR−/HER2-low BC in the phase Ib/II BEGONIA trial. In early HR+/HER2-low BC, a phase II evaluation of T-DXd alone or in combination with anastrozole in the neoadjuvant setting is ongoing.
T-DXd also has made the transition to several other solid tumors. In May 2020, the FDA granted breakthrough therapy designation for patients with metastatic NSCLC with HER2 mutations after progression on or after platinum-based treatment.59 In January 2021, T-DXd was granted FDA approval for patients with locally advanced or metastatic HER2+ gastric- or gastroesophageal adenocarcinoma after prior trastuzumab-based treatment.60 Clinical trials in other solid cancers, such as uterine, biliary tract, colorectal and urothelial cancer, are ongoing.45
Discussion
Two novel ADCs containing a topoisomerase I inhibitor payload have recently emerged in the BC treatment landscape. The anti-HER2 ADC T-DXd gained accelerated FDA approval and conditional market authorization by EMA for patients with unresectable or metastatic HER2+ BC who were previously after treatment with two or more anti-HER2 therapies, including T-DM1.46,49 The anti-Trop-2 ADC SG recently received regular FDA approval for patients with advanced TNBC after at least two prior regimens including at least one in the metastatic setting,25 while accelerated review by EMA in this indication is ongoing, and a positive decision is eagerly awaited, hopefully by the end of 2021.28
Both ADCs have shown impressive efficacy in the target population of their current label, representing heavily pretreated patients with advanced HER2+ or TNBC where the efficacy of other available treatment options is modest. Their introduction is expected to substantially impact the treatment standards in these BC subtypes, representing a new standard of care in their current indications. For SG, the approval was based on safety and efficacy data from a phase I/II basket study,19 and a confirmatory phase III trial has recently reported a significant improvement in PFS and OS versus single-agent chemotherapy of physician's choice in patients with mTNBC after at least two prior lines.24 Approval of T-DXd was based on safety and efficacy data of a phase II expansion cohort;18 the confirmatory phase III trial DESTINY-Breast02 (NCT03523585) has completed recruitment with estimated primary completion date in February 2022. Depending on local availability, these new agents are likely to become the preferred treatment option in their current label for the majority of patients. Given the different mechanism of action as compared with other treatment options for aBC, implementation of these new ADCs in standard practice is expected to postpone other available treatments to later lines. Both SG and T-DXd are currently under investigation in earlier treatment lines in the advanced setting for TNBC and HER2+ BC, respectively. In the post-neoadjuvant setting, phase III evaluations are ongoing in patients with residual disease. Incorporation of new ADCs in neoadjuvant regimens serves as another strategy to evaluate the potential of these drugs (e.g. substituting a chemotherapy compound of a current neoadjuvant regimen by a new ADC).
Given that the target Ags of these new ADCs, HER2 and Trop-2, are frequently overexpressed in other BC subtypes, there is an excellent rationale for and high interest in the exploration of the potential of T-DXd and SG beyond HER2+ and TNBC. Early clinical trials have shown promising efficacy of T-DXd and SG in heavily pretreated cohorts of HER2-low and HR+/HER2− aBC, respectively, and both drugs currently are under phase III evaluation in these BC subtypes in the advanced setting. Confirmation of the activity of T-DXd in HER2-low BC could drastically change current clinical–pathological classification of BC subtypes, which would have an important impact on daily practice for oncologists, pathologists and other BC professionals. The preliminary activity of T-DXd and SG beyond patients with high expression on IHC paves the way for the development of other ADCs in tumors with low or moderate Ag expression, which broadens the patient population and applicability of this promising approach.
Clinical trials in the neoadjuvant setting are also ongoing, while there is high interest in combination regimens for both drugs. The majority of combination trials investigate the combination of SG or T-DXd with ICIs, based on the preclinical and clinical data supporting the combination of ADC and ICI.58,61,62 Other ongoing trials evaluate combinations with other anticancer treatments, among which are PARPi, endocrine agents or other HER2-directed therapies. The high interest results in a dynamic clinical trial landscape for both drugs, with currently 11 and 12 recruiting clinical trials for SG and T-DXd, respectively, including two phase III trials for both drugs. Additionally, four phase III trials have recently completed recruitment and positive results in these trials could have potential practice-changing impact with a transition to earlier treatment lines and/or other BC subtypes. Besides SG and T-DXd, several other HER2- or Trop-2-targeted ADCs are or have been under development.9,12,44,63 Interestingly, the anti-Trop-2 ADC DS-1062 combines an anti-Trop-2 antibody datopotamab with deruxtecan as payload has recently shown early signs of promising activity in a phase I cohort of advanced NSCLC unselected for Trop-2 expression,64 which currently enrolls patients into a TNBC cohort (NCT03401385). Several other Ags are considered in the pipeline of ADCs in BC, among which are LIV-1 and HER3.12
Despite the fact that the magnitude of benefit seems to outweigh toxicity in the majority of patients resulting in a favorable benefit/risk profile, awareness of the unique safety profile of both drugs is crucial for optimal management of patients treated with these novel ADCs. Regarding hematological toxicity, almost half of the patients treated with SG in ASCENT received G-CSF, which should be considered when implementing this drug in clinical practice.24 Prompt evaluation and treatment of diarrhea or nausea and if necessary, dose modification are warranted. Patients treated with SG should be informed about the high risk of alopecia. Premedication with antipyretics, antihistamines and antiemetics is recommended for prevention of infusion reactions and nausea.34 For patients treated with T-DXd, treatment-related ILD or pneumonitis is an important identified risk in ∼15% of patients treated with T-DXd, and grade 5 toxicity has been reported in ∼2% at the recommended dose of 5.4 mg/kg.50 Awareness for potential symptoms among physicians and patients as well as prompt evaluation and optimal management are of utmost importance to manage this potential severe AE. A low threshold to interrupt treatment with T-DXd is recommended, even in case of asymptomatic findings on imaging, and T-DXd should be permanently discontinued from symptomatic ILD or pneumonitis (grade 2) onwards.
Regarding disparities in access to these new ADCs, treatment costs will in addition to the regulatory approvals be a critical issue which might hamper reimbursement negotiations and significantly delay the interval between approval and local availability in certain countries. Early access programs might decrease these delays in selected situations. Costs will be even of more importance anticipating future combinations of ADCs with other drugs.
In conclusion, SG and T-DXd are two new promising ADCs with robust single-agent activity that are expected to substantially impact treatment standards, both in and far beyond BC subtypes and their currently approved indications. Several trials are investigating new treatment settings for both drugs, including a transition to earlier treatment lines and combinations with other anticancer treatments such as ICIs, PARPi, endocrine agents or other HER2-directed therapies. Knowledge of the distinct safety profiles and their management is important for optimal care of BC patients receiving these novel drugs.
Acknowledgments
Funding
None declared.
Disclosure
PN's institution received honoraria for advisory boards and research funding from AstraZeneca, Amgen, Eli Lilly, Novartis, Pfizer and Roche (all outside the submitted work). HW received travel support from Roche and Pfizer (all outside the submitted work), institution received consulting fees and honoraria from AstraZeneca, Biocartis, Lilly, Novartis, Pfizer, PUMA Biotechnology, Roche, Sirtex and Daiichii-Sankyo and unrestricted research grants from Roche and Novartis. KP received travel support from AstraZeneca, Pfizer, PharmaMar and Roche (all outside the submitted work), institution received honoraria for advisory/consultancy roles for AstraZeneca, Eli Lilly, Gilead Sciences, Novartis, Pfizer, Pierre Fabre, Roche, Teva and Vifor Pharma; speaker fees for Eli Lilly, Medscape, MSD, Mundi Pharma, Novartis, Pfizer and Roche; and research funding from Sanofi. EA has declared no conflicts of interest.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N., Penault-Llorca F., Cortes J. Breast cancer. Nat Rev Dis Prim. 2019;5(1):66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 3.Caparica R., Lambertini M., de Azambuja E. How I treat metastatic triple-negative breast cancer. ESMO Open. 2019;4:e000504. doi: 10.1136/esmoopen-2019-000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poggio F., Bruzzone M., Ceppi M. Single-agent PARP inhibitors for the treatment of patients with BRCA-mutated HER2-negative metastatic breast cancer: a systematic review and meta-analysis. ESMO Open. 2018;3(4):e000361. doi: 10.1136/esmoopen-2018-000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franzoi M.A., de Azambuja E. Atezolizumab in metastatic triple-negative breast cancer: IMpassion130 and 131 trials—how to explain different results? ESMO Open. 2020;5(6):e001112. doi: 10.1136/esmoopen-2020-001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Food & Drug FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. 2020. fda.govhttps://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple#:∼:text=Approvals and Databases-,FDA grants accelerated approval to pembrolizumab for locally recurren Available at.
- 7.Ehrlich P. Die Wertbemessung des Diphterie-heilserums und deren theoretische Grundlagen. Klin Jahrb. 1897;6:299–326. [Google Scholar]
- 8.Strebhardt K., Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8(6):473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 9.Nagayama A., Vidula N., Ellisen L., Bardia A. Novel antibody–drug conjugates for triple negative breast cancer. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920915980. 1758835920915980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennell C.A., Erickson H.A. Designing immunotoxins for cancer therapy. Immunol Res. 2002;25(2):177–191. doi: 10.1385/IR:25:2:177. [DOI] [PubMed] [Google Scholar]
- 11.Pondé N., Aftimos P., Piccart M. Antibody-drug conjugates in breast cancer: a comprehensive review. Curr Treat Options Oncol. 2019;20(5):37. doi: 10.1007/s11864-019-0633-6. [DOI] [PubMed] [Google Scholar]
- 12.Barroso-Sousa R., Tolaney S.M. Clinical development of new antibody–drug conjugates in breast cancer: to infinity and beyond. BioDrugs. 2021;35(2):159–174. doi: 10.1007/s40259-021-00472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trail P.A., Dubowchik G.M., Lowinger T.B. Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design. Pharmacol Ther. 2018;181:126–142. doi: 10.1016/j.pharmthera.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Criscitiello C., Morganti S., Curigliano G. Antibody–drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol. 2021;14(1):20. doi: 10.1186/s13045-021-01035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma S., Miles D., Gianni L. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krop I.E., Kim S.-B., Martin A.G. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743–754. doi: 10.1016/S1470-2045(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G., Huang C.-S., Mano M.S. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 18.Modi S., Saura C., Yamashita T. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardia A., Mayer I.A., Vahdat L.T. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 20.Syed Y.Y. Sacituzumab govitecan: first approval. Drugs. 2020;80(10):1019–1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrogi F., Fornili M., Boracchi P. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9(5):e96993. doi: 10.1371/journal.pone.0096993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalinsky K., Diamond J.R., Vahdat L.T. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020;31(12):1709–1718. doi: 10.1016/j.annonc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Fenn K.M., Kalinsky K. Sacituzumab govitecan: antibody-drug conjugate in triple-negative breast cancer and other solid tumors. Drugs Today (Barc) 2019;55(9):575–585. doi: 10.1358/dot.2019.55.9.3039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardia A., Hurvitz S.A., Tolaney S.M. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Food & Drug FDA grants accelerated approval to sacituzumab govitecan-hziy for metastatic triple negative breast cancer. 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-sacituzumab-govitecan-hziy-metastatic-triple-negative-breast-cancer Available at.
- 26.Wahby S., Fashoyin-Aje L., Osgood C.L. FDA approval summary: accelerated approval of sacituzumab govitecan-hziy for third line treatment of metastatic triple-negative breast cancer (mTNBC) Clin Cancer Res. 2021;27:1850–1854. doi: 10.1158/1078-0432.CCR-20-3119. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food & Drug FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. FDA. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer Available at.
- 28.Gilead Sciences Inc European medicines agency validates marketing authorization application for Sacituzumab Govitecan-Hziy for the treatment of metastatic triple-negative breast cancer. 2021. https://www.gilead.com/news-and-press/press-room/press-releases/2021/3/european-medicines-agency-validates-marketing-authorization-application-for-sacituzumab-govitecan-hziy-for-the-treatment-of-metastatic-triple-negative Available at.
- 29.Hurvitz S., Tolaney S., Punie K. Biomarker evaluation in the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium. Cancer Res. 2021;81(suppl 4) https://cancerres.aacrjournals.org/content/81/4_Supplement/GS3-06 Abstract nr GS3-06. Available at. [Google Scholar]
- 30.Bardia A., Tolaney S.M., Punie K. Biomarker analyses in the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021 doi: 10.1016/j.annonc.2021.06.002. In press. [DOI] [PubMed] [Google Scholar]
- 31.Diéras V., Weaver R., Tolaney S. Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer [abstract]. Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium. Am Assoc Cancer Res. 2021;81(suppl 4) https://cancerres.aacrjournals.org/content/81/4_Supplement/PD13-07 Available at. [Google Scholar]
- 32.Rugo H.S., Tolaney S.M., Loirat D. Impact of UGT1A1 status on the safety profile of sacituzumab govitecan in the phase 3 ASCENT study in patients with metastatic triple-negative breast cancer [abstract]. Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium. Cancer Res. 2021;81(suppl 4) https://cancerres.aacrjournals.org/content/81/4_Supplement/PS11-09 Abstract nr PS11-09. Available at. [Google Scholar]
- 33.FDA Highlights of prescribing information, TRODELVY for injection, for intravenous use. FDA. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761115s000lbl.pdf Available at.
- 34.Bardia A., Messersmith W.A., Kio E.A. Sacituzumab govitecan, a trop-2–directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase 1/2 IMMU-132-01 basket trial. Ann Oncol. 2021;32:746–756. doi: 10.1016/j.annonc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Yap T.A., Hamilton E.P., Bauer T.M. 547P Rucaparib + sacituzumab govitecan (SG): initial data from the phase Ib/II SEASTAR study ( NCT03992131) Ann Oncol. 2020;31(S4):S476–S477. [Google Scholar]
- 36.Brenner A.J., Pandey R., Chiou J. 373MO—Delivery and activity of SN-38 by sacituzumab govitecan in CNS tumours. Ann Oncol. 2020;31(suppl 4):S396–S408. [Google Scholar]
- 37.U.S. Food & Drug Search orphan drug designations and approvals: sacituzumab govitecan in treatment of malignant glioma. FDA. 2020. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=769520 Available at.
- 38.U.S. Food & Drug Search orphan drug designations and approvals: sacituzumab govitecan in treatment of pancreatic cancer. FDA. 2014. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=433514 Available at.
- 39.U.S. Food & Drug Search orphan drug designations and approvals: sacituzumab govitecan in treatment of small cell lung cancer. FDA. 2013. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=415413 Available at.
- 40.Wolff A.C., Hammond M.E.H., Allison K.H. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 41.Mendes D., Alves C., Afonso N. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer—a systematic review. Breast Cancer Res. 2015;17(1):140. doi: 10.1186/s13058-015-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deluche E., Antoine A., Bachelot T. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Kunte S., Abraham J., Montero A.J. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer. 2020;126(19):4278–4288. doi: 10.1002/cncr.33102. [DOI] [PubMed] [Google Scholar]
- 44.Tarantino P., Hamilton E., Tolaney S.M. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38(17):1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 45.Keam S.J. Trastuzumab deruxtecan: first approval. Drugs. 2020;80(5):501–508. doi: 10.1007/s40265-020-01281-4. [DOI] [PubMed] [Google Scholar]
- 46.FDA FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2-positive-breast-cancer Available at.
- 47.Tesch M.E., Gelmon K.A. Targeting HER2 in breast cancer: latest developments on treatment sequencing and the introduction of biosimilars. Drugs. 2020;80:1811–1830. doi: 10.1007/s40265-020-01411-y. [DOI] [PubMed] [Google Scholar]
- 48.EMA CHMP assessment report Enhertu, INN-trastuzumab deruxtecan. 10 December 2020; 2020:210. https://www.ema.europa.eu/en/documents/assessment-report/enhertu-epar-public-assessment-report_en.pdf Available at.
- 49.European Medicines Agency Enhertu. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/enhertu Available at.
- 50.Modi S., Saura C., Yamashita T. Updated results from DESTINY-breast01, a phase 2 trial of trastuzumab deruxtecan (T-DXd) in HER2 positive metastatic breast cancer. San Antonio Breast Cancer Symposium. 2020. https://www.abstractsonline.com/pp8/#!/9223/presentation/797 Available at.
- 51.Jerusalem G., Park Y.H., Yamashita T. 138O—CNS metastases in HER2-positive metastatic breast cancer treated with trastuzumab deruxtecan: DESTINY-Breast01 subgroup analyses. Ann Oncol. 2020;31(suppl 2):S62–S82. [Google Scholar]
- 52.EMA Enhertu, summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/enhertu-epar-product-information_en.pdf Available at.
- 53.FDA Highlights of prescribing information, ENHERTU for injection, for intravenous use. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761139s000lbl.pdf Available at.
- 54.Andrikopoulou A., Zografos E., Liontos M., Koutsoukos K., Dimopoulos M.-A., Zagouri F. Trastuzumab deruxtecan (DS-8201a): the latest research and advances in breast cancer. Clin Breast Cancer. 2021;21(3):e212–e219. doi: 10.1016/j.clbc.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Hackshaw M.D., Danysh H.E., Singh J. Incidence of pneumonitis/interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2020;183(1):23–39. doi: 10.1007/s10549-020-05754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powell C.A., Camidge D.R., Modi S. 289P Risk factors for interstitial lung disease in patients treated with trastuzumab deruxtecan from two interventional studies. Ann Oncol. 2020;31:S357–S358. [Google Scholar]
- 57.Modi S., Park H., Murthy R.K. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamilton E., Shapiro C.L., Petrylak D. Abstract PD3-07: trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: a 2-part, phase 1b, multicenter, open-label study. Am Assoc Cancer Res. 2021;81(4 suppl) http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.SABCS20-PD3-07 Abstract nr PD3-07. Available at. [Google Scholar]
- 59.Smit E.F., Nakagawa K., Nagasaka M. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. J Clin Oncol. 2020;38(suppl 15):9504. [Google Scholar]
- 60.U.S. Food & Drug FDA approves fam-trastuzumab deruxtecan-nxki for HER2-positive gastric adenocarcinomas. FDA. 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-positive-gastric-adenocarcinomas#:∼:text=On January 15%2C 2021%2C the,a prior trastuzumab-based regimen Available at.
- 61.Gerber H.-P., Sapra P., Loganzo F., May C. Combining antibody–drug conjugates and immune-mediated cancer therapy: what to expect? Biochem Pharmacol. 2016;102:1–6. doi: 10.1016/j.bcp.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Han H.S., Alemany C.A., Brown-Glaberman U.A. SGNLVA-002: single-arm, open label phase Ib/II study of ladiratuzumab vedotin (LV) in combination with pembrolizumab for first-line treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer. J Clin Oncol. 2019;37(suppl 15):TPS1110. [Google Scholar]
- 63.Rinnerthaler G., Gampenrieder S., Greil R. HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer. Int J Mol Sci. 2019;20(5):1115. doi: 10.3390/ijms20051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spira A., Lisberg A., Sands J. OA03.03 Datopotamab deruxtecan (Dato-DXd; DS-1062), a TROP2 ADC, in patients with advanced NSCLC: updated results of TROPION-PanTumor01 phase 1 study. J Thorac Oncol. 2021;16(3):S106–S107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.