Abstract
Background
Persistent organ failure (POF) is the strongest determinant of mortality in acute pancreatitis (AP). There is a paucity of data regarding the impact of different POF attributes on mortality and the role of different characteristics of systemic inflammatory response syndrome (SIRS) in the risk of developing POF.
Objective
We aimed to assess the association of POF dynamic features with mortality and SIRS characteristics with POF.
Methods
We studied 1544 AP subjects prospectively enrolled at 22 international centers (APPRENTICE consortium). First, we estimated the association of onset, duration, and maximal score of SIRS with POF. Then, we evaluated the risk of mortality based on POF onset, duration, number, type, and sequence of organs affected. Analyses were adjusted for potential confounders.
Results
58% had SIRS, 11% developed POF, and 2.5% died. Early SIRS, persistent SIRS, and maximal SIRS score ≥ 3 were independently associated with higher risk of POF (p < 0.05). Mortality risk in POF was higher with two (33%, odds ratio [OR] = 10.8, 3.3–34.9) and three (48%, OR = 20.2, 5.9–68.6) organs failing, in comparison to single POF (4%). In subjects with multiple POF, mortality was higher when the cardiovascular and respiratory systems failed first or concurrently as compared to when the renal system failed first or concurrently with other organ (p < 0.05). In multivariate regression model, the number and sequence of organs affected in POF were associated with mortality (p < 0.05). Onset and duration of POF had no impact mortality.
Conclusion
In AP patients with POF, the risk of mortality is influenced by the number, type, and sequence of organs affected. These results are useful for future revisions of AP severity classification systems.
Keywords: acute pancreatitis; mortality; natural history; organ failure; systemic inflammatory response syndrome, severe acute pancreatitis
Key Summary
What is known?
Persistent organ failure (POF; >48 h) is the strongest determinant of mortality in acute pancreatitis (AP).
There is lack of evidence on the impact of different attributes of failing organs on AP mortality.
The association of systemic inflammatory response syndrome (SIRS) characteristics with POF, has not been well studied.
What is new here?
Mortality risk in AP patients with POF is determined by the number, type, and sequence of organ systems affected.
Multiple POF affecting the cardiovascular and respiratory systems first or concurrently carries the highest mortality in AP compared to the renal system.
Involvement of the renal system as the first failing organ or concurrently with other organs during multiple POF is associated with lower mortality than respiratoy or cardiovascular systems as first failing organs.
Onset and duration of POF are not associated with mortality in AP patients.
SIRS on admission, persistent SIRS, and three to four SIRS criteria, are independently associated with higher risk of POF.
INTRODUCTION
The clinical course of acute pancreatitis (AP) is highly variable, ranging from rapid recovery in most, to death in 1%–3% of patients. 1 Several studies have demonstrated that persistent organ failure (POF) lasting more than 48 h is the strongest determinant of mortality in AP. 2 , 3 , 4 , 5 As a consequence, both the Revised Atlanta and Determinant‐Based severity classification systems grades AP patients with POF in the severe category. 6 , 7 However, both classifications used a dichotomous definition of POF, which assumes that all patients with POF have the same prognosis, and disregard the impact of specific features of POF on mortality. Better understanding of the prognosis of AP patients with different POF features is relevant so as to guide end of life discussions, to decide transfer to a higher level of care, and to inform future severity classification systems.
Given its importance, multiple studies have looked at the influence of the onset, duration, organ type, and the number of organs affected during POF. 3 , 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Overall, these studies have consistently reported that multiple organ failure (MOF) is associated with higher mortality than single POF 5 , 8 , 10 , 11 , 14 ; although, previous studies did not systematically adjust for confounding. Furthermore, in critically ill patients with MOF the sequence of organ dysfunction and the first organ failing have been recognized as important determinants of mortality; however, this aspect has not been studied in AP. 15 , 16 Moreover, the association of the timing of onset, duration, and organ type of POF with mortality has been inconsistent across studies. 5 , 9 , 11 , 12 , 14 , 17 Potential reasons for this inconsistency include single center retrospective designs, lack of adjustment for key confounding variables, restrictive eligibility criteria, and heterogeneous definitions of organ failure (OF). Therefore, large, multicenter, prospective studies that assess these associations and that overcome the limitations of prior research are needed.
The systemic inflammatory response syndrome (SIRS) is an easy to calculate, four‐point scoring system, which is pathophysiologically associated with POF, and compares favorably with more complex clinical scores in predicting POF. 18 , 19 Previous studies have suggested that early onset (first day), persistent (>48 h), and three to four criteria of SIRS are associated with higher risk of POF and mortality. 8 , 20 , 21 Validating these associations and determining the accuracy of SIRS characteristics in a large international cohort is important for improving the generalizability of prior results.
The Acute Pancreatitis Patients Registry to Examine Novel Therapies in Clinical Experience (APPRENTICE) is a multinational study that prospectively enrolled over 1500 AP subjects from 22 international centers in four continents. 22 Systemically collected data on disease phenotype, severity, and outcomes, provided us with an opportunity to study the impact of POF features on AP mortality and of SIRS characteristics on POF risk. Thus, our primary objective was to evaluate the effect of POF onset, duration, number, organ type, and sequence of organs on AP mortality. As a secondary objective, the current study aimed to assess the association and predictive value of the onset, duration, and maximal score of SIRS, on development of POF.
METHODS
Study population
We used data from APPRENTICE for this study. Adults hospitalized with AP at any of the participating centers (United States: 8, Europe: 6, Latin America: 5, and India: 3), between November 2015 and January 2018, were eligible for inclusion. Diagnosis of AP was based on presence of at least two of the three diagnostic criteria according to the American College of Gastroenterology (Table S1). 23 Subjects with chronic pancreatitis or pancreatic cancer were excluded. Eligible subjects were invited to participate. Informed consent was obtained from all subjects based on local requirements of the institutional review board (IRB).
The University of Pittsburgh served as the coordinating center and an umbrella IRB approved the study protocol (PRO15040389; approval date: 14 July 2015). Ethical committee approvals were obtained from local IRBs at all participating centers. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. The study was registered in Clinicaltrials.gov (NCT03075618). The detailed methodology has been previously reported. 22 , 24
Data collection
Data were prospectively collected during the hospital admission through direct patient interview and medical chart review. Information on demographics, etiology, severity according to Revised Atlanta classification (Table S2), laboratory biomarkers, radiologic findings, treatment strategies, and hospital course was organized into 260 variables. Infected necrosis was defined as a positive culture from the necrotic bed, or the presence of gas in the necrotic collection on cross‐sectional images. Deidentified data were entered by local study investigators to a central electronic database (REDCap) and managed by a data specialist at the coordinating center. To optimize data quality, a monitoring team verified data completeness, identified discrepancies, eliminated duplicate entries, and reconciled data directly with the participating centers.
SIRS, OF, and death
SIRS was defined by the presence of a score ≥2 (Table S3). 25 The timing of SIRS onset in relation to initial admission was categorized into five groups: on admission, Day 1, Day 2, Day 3, and Day 4 or thereafter. Duration of SIRS was classified as transient (≤48 h) or persistent (>48 h). The maximal SIRS score during the hospital admission was recorded as 2, 3, or 4.
OF was defined as a score ≥2 for cardiovascular (CV), respiratory or renal systems, using the modified Marshall scoring system (Table S4). 26 Involvement of two to three organ systems was defined as MOF. The date and time of initial OF detection was recorded. OF was assessed on a daily basis by local investigators, and the last day with any failing organ was used to calculate OF duration. Subjects with POF (>48 h) were further stratified based on the number of failed organs (one, two, three) and organ type affected. Furthermore, we categorized the time of onset (admission or Day 1, Day 2–3, Day 4–7, and after 7 days) and duration of POF (≤7, 8–14, and >14 days) into different intervals given the lack of data collinearity and guided by prior research using those cutoffs. 5 , 12 , 14 In patients with persistent MOF, the first organ failing was recorded. Given the small number of subjects with CV failure in the cohort, the three categories for organ type affected and the first failing organ were collapsed into two groups: respiratory or CV versus renal. Mortality was recorded during the hospitalization related to study enrollment.
Statistical analysis
Descriptive statistics were reported as proportions for categorical data and as mean ± standard deviation (SD) or median (interquartile range [IQR]) for continuous data. Univariate comparisons were performed using χ 2 test (or Fisher's exact test as appropriate) for categorical variables, and the Wilcoxon rank‐sum test for continuous variables.
Among patients with SIRS, the risk of POF was compared across various SIRS characteristics. These comparisons were then adjusted for age, sex, etiology, Charlson Comorbidity Index (CCI), transfer status, and obesity using multivariate logistic regression models. To assess the performance of combining SIRS characteristics in prediction of POF, a multivariate logistic regression model was constructed and the area under the receiver operating characteristic curve (AUC) was generated.
Mortality of subjects with different POF characteristics was then compared. Multivariate logistic regression models were used to determine the independent effect of POF characteristics on mortality. Only features significantly associated with mortality on univariate analyses were considered. The type of organ affected was not included because it could not be outlined in patients with three‐organ persistent MOF. Age, sex, etiology, CCI, transfer status, and geographic location were selected a priori for inclusion in the model due to their clinical relevance. Cross‐sectional imaging was not performed in 22/166 subjects with POF, in whom data on pancreatic necrosis was lacking, and thus, these covariates were not included in the final model. Instead, a sensitivity analysis that incorporated sterile and infected pancreatic necrosis, and pancreatic interventions as model covariates, was conducted among 144/166 subjects with POF and radiologic data available. Goodness of fit of the model was tested with the Hosmer–Lemeshow test. Comparisons are presented using odds ratios (ORs) and 95% confidence intervals (CIs). p‐values < 0.05 were considered statistically significant. Statistical analyses were performed using R software (Version 3.5.1; R Foundation).
RESULTS
Study participants
A total of 1612 subjects with AP were enrolled in APPRENTICE. Sixty‐eight (4%) subjects were excluded due to lack of data on OF, resulting in a final study population of 1544 (Figure S5). Mean age was 49.6 years (SD, 18.5), 52% were male, 63% were Caucasian, and 34% were transferred from another institution. The median interval from pain onset to admission was 8 h (IQR, 3–25). Most subjects had biliary etiology (45%), experienced mild interstitial AP (66%), and were enrolled during their first episode of AP (75%). Pancreatic necrosis was diagnosed in 311 (20%) participants, and became infected in 45 (3%). In this cohort, 892 (58%) met ≥2 SIRS criteria, 354 (23%) had moderately severe AP, 166 (11%) developed POF (severe AP), 86 (5%) had MOF, and 39 (2.5%) died. Table 1 depicts the characteristics of subjects with and without POF.
TABLE 1.
Characteristics of AP patients with and without POF
| Characteristics | POF (n = 166) | No POF (n = 1378) | p‐Value |
|---|---|---|---|
| Age, mean ± SD | 48.1 ± 17.4 | 49.8 ± 18.6 | 0.26 |
| Male sex, n (%) | 118 (71.1) | 689 (50.0) | <0.001 |
| Race or ethnicity, n (%) | |||
| Caucasian | 64 (38.6) | 705 (51.2) | |
| Hispanic or Latino | 15 (9.0) | 299 (21.7) | |
| Asian Indian | 81 (48.8) | 285 (20.7) | <0.001 |
| African American | 5 (3.0) | 75 (5.4) | |
| Other | 1 (0.6) | 14 (1.0) | |
| Geographic region, n (%) | |||
| Europe | 41 (24.7) | 355 (25.8) | <0.001 |
| India | 81 (48.8) | 280 (20.3) | |
| Latin America | 14 (8.4) | 285 (20.7) | |
| North America | 30 (18.1) | 458 (33.3) | |
| Obesity, n (%) | 40 (24.1) | 387 (28.1) | 0.24 |
| Comorbidities, n (%) | |||
| No comorbidity | 78 (47.0) | 612 (44.4) | |
| CCI = 1 | 36 (21.7) | 248 (18.0) | 0.24 |
| CCI ≥ 2 | 52 (31.3) | 518 (37.6) | |
| Transferred from other hospital, n (%) | 113 (68.1) | 415 (30.1) | <0.001 |
| Etiology, n (%) | |||
| Gallstone | 55 (33.1) | 642 (46.6) | |
| Alcohol | 69 (41.6) | 263 (19.1) | |
| Idiopathic | 21 (12.7) | 228 (16.5) | <0.001 |
| Hypertriglyceridemia | 11 (6.6) | 58 (4.2) | |
| Post‐ERCP | 5 (3.0) | 126 (9.1) | |
| Others | 5 (3.0) | 61 (4.4) | |
| First episode of AP, n (%) | 134 (80.7) | 1018 (73.9) | 0.07 |
| Pancreatic necrosis, n (%) | 109 (65.7) | 202 (14.7) | <0.001 |
| Extent of pancreatic necrosis a | |||
| <30% | 29 (27.4) | 112 (58.3) | <0.001 |
| 30%–50% | 30 (28.3) | 48 (25.0) | |
| >50% | 47 (44.3) | 32 (16.7) | |
| Peripancreatic necrosis, n (%) | 89 (53.6) | 119 (8.6) | <0.001 |
| Infected pancreatic necrosis, n (%) | 24 (14.5) | 21 (1.5) | <0.001 |
| Pancreatic interventions, n (%) | 71 (42.8) | 58 (4.2) | <0.001 |
| Endoscopic and/or percutaneous approach, n (%) | 61 (36.7) | 47 (3.4) | <0.001 |
| Surgical intervention, n (%) | 24 (14.5) | 11 (0.8) | <0.001 |
| Total LOS, median days (IQR) | 20 (12, 30) | 7 (5, 12) | <0.001 |
| ICU admission, n (%) | 136 (81.9) | 121 (8.8) | <0.001 |
| ICU LOS, median days (IQR) | 7 (5, 12) | 3 (2, 6) | <0.001 |
| Mortality, n (%) | 34 (20.5) | 5 (0.4) | <0.001 |
Abbreviations: AP, acute pancreatitis; CCI, Charlson Comorbidity Index; ERCP, endoscopic retrograde cholangiopancreatography; ICU, intensive care unit; LOS, length of stay; POF, persistent organ failure.
Data on the extent of pancreatic necrosis were missing for 3 patients with POF and 10 patients without POF.
Characteristics of SIRS
Figure 1 shows the frequency distribution of SIRS by onset, duration, and maximal score, in subjects with and without POF. The majority of subjects with SIRS had onset on admission (71%), persistent duration (57% based on 708 subjects with duration recorded), and reached maximal score of 2–3 (81%). SIRS was present in 43% of subjects with mild AP (445/1024), 79% of subjects with moderately severe AP (281/354), and all subjects with POF (166/166). Among subjects with POF, 84% had SIRS on admission, 93% developed persistent SIRS, and 56% reached a maximal score of 4.
FIGURE 1.
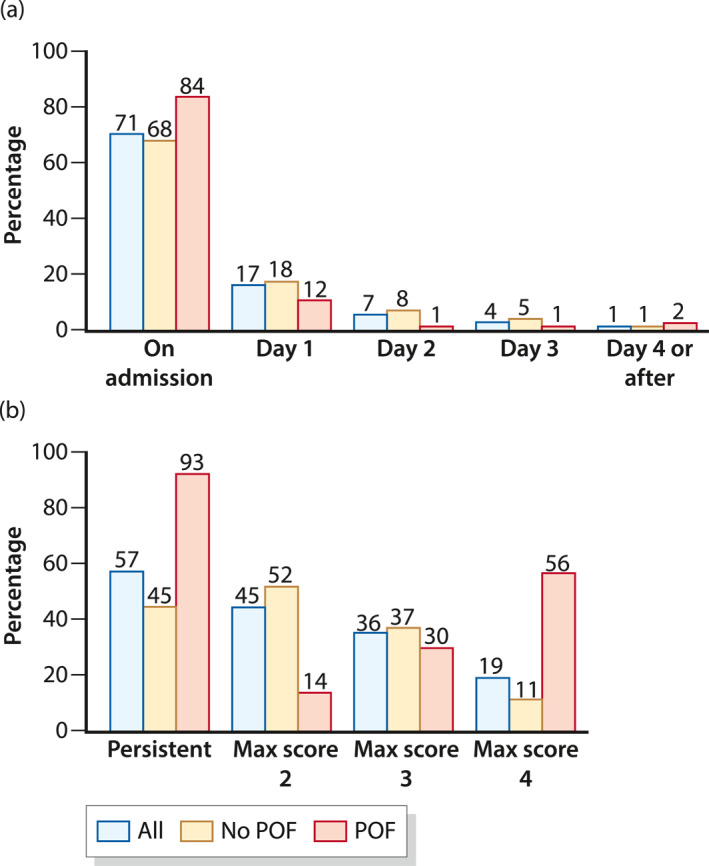
Frequency distribution among acute pancreatitis subjects with systemic inflammatory response syndrome (SIRS; n = 892) and stratified by development of persistent organ failure (POF)—(a) by onset of SIRS and (b) by duration and maximal SIRS score
Impact of SIRS characteristics on POF
SIRS on admission was associated with higher risk of POF compared with delayed onset of SIRS (p < 0.05). Subjects with persistent SIRS had 26.3 greater odds of developing POF than subjects with transient SIRS (95% CI: 12.12–57.20, p < 0.001). Compared to subjects with two SIRS criteria, those with maximal SIRS scores of 3 (OR: 2.84, 95% CI: 1.70–4.73, p < 0.001) and 4 (OR: 17.94, 95% CI: 10.80–29.80, p = 0.02) had higher odds of POF. These results were unchanged after adjusting for potential confounding variables (Table 2). When the SIRS characteristics were combined in a multivariate regression model that predicts POF, the AUC was 0.849 (95% CI: 0.818–0.879; Figure 2). An increase in the SIRS score from admission to 24 h was correlated with higher risk of developing POF (p < 0.001; Table S5).
TABLE 2.
Risk of POF based on different characteristics of SIRS
| Subgroups of SIRS | POF, n (%) | Unadjusted OR (95% CI) | p‐value | Adjusted OR (95% CI) a | p‐Value |
|---|---|---|---|---|---|
| Onset | |||||
| On admission | 139/632 (21.9) | Reference | NA | Reference | NA |
| Day 1 | 20/150 (13.3) | 0.55 (0.31–0.92) | 0.02 | 0.56 (0.32–0.93) | 0.03 |
| Day 2 | 2/61 (3.3) | 0.12 (0.01–0.47) | <0.001 | 0.10 (0.02–0.35) | <0.001 |
| Day 3 | 2/38 (5.3) | 0.20 (0.02–0.78) | 0.01 | 0.17 (0.03–0.59) | 0.02 |
| Day 4 and after | 3/11 (27.3) | 1.33 (0.22–5.63) | 0.71 | 1.20 (0.25–4.54) | 0.80 |
| Duration | |||||
| <48 h | 7/305 (2.3) | Reference | NA | Reference | NA |
| >48 h | 154/403 (38.2) | 26.30 (12.12–57.20) | <0.001 | 26.46 (12.89–64.04) | <0.001 |
| Maximal score | |||||
| 2 | 24/399 (6.0) | Reference | NA | Reference | NA |
| 3 | 49/319 (15.4) | 2.84 (1.70–4.73) | <0.001 | 3.24 (1.91–5.63) | <0.001 |
| 4 | 93/174 (53.4) | 17.94 (10.8–29.8) | <0.001 | 19.65 (11.42–35.07) | <0.001 |
Abbreviations: CI, confidence interval; OR, odds ratio; POF, persistent organ failure; SIRS, systemic inflammatory response syndrome.
Adjusted for age, sex, etiology, comorbidity index, transfer status, and obesity.
FIGURE 2.

Area under the receiver operating characteristic (AUC) curve for prediction of persistent organ failure combining systemic inflammatory response syndrome characteristics
Characteristics of POF
Figure 3 summarizes the characteristics of POF. The median time from admission to onset of POF was 1.3 days (IQR, 0.2–3 days), and 92% developed it within the first week. The median duration of POF was 6 days (IQR, 4–10), and lasted less than 2 weeks in 84% of 160 subjects with data available. Overall, the respiratory system was the most common organ failing (87%). MOF was present in 46% of POF subjects. In two‐organ MOF, 38 subjects had failing respiratory and renal systems (84%), five CV and respiratory systems (11%), and two CV and renal systems (4%). The renal system failed first in 27 (60%) of patients with 2‐organ MOF, and in 17 (55%) of those with 3‐organ MOF.
FIGURE 3.
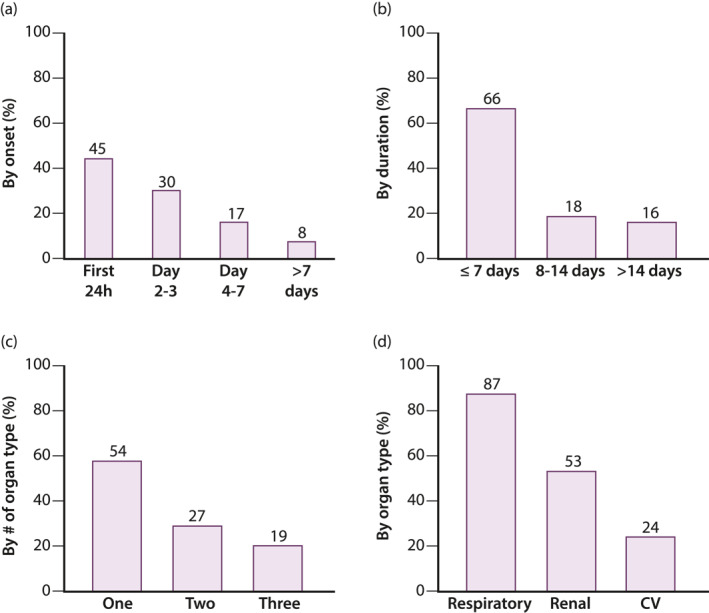
Frequency distribution among acute pancreatitis subjects with persistent organ failure (n = 166) based on—(a) onset, (b) duration, (c) number of organs affected, and (d) type of organ failing
Death occurred at a median of 10.7 days (IQR, 3.7–25.6) from initial admission. In patients with POF who died (n = 34), death occurred at a median of 11.7 days from admission (IQR, 4.1–27), and at a median of 8.7 days (IQR, 3.5–24) after OF onset.
Impact of POF characteristics on mortality
Table 3 shows the association between POF subgroups and mortality. The odds of mortality with POF of 8–14 days (32%) was 2.7‐folds greater than with shorter duration (15%, 95% CI: 1.03–6.92). In contrast, subjects with POF more than 14 days had similar mortality compared to those with POF ≤7 days. Mortality rate was 4% in subjects with single POF (CV or respiratory 6% and renal 0%). In comparison, the odds of mortality were significantly higher with two (33%, OR: 10.8, 95% CI: 3.3–34.9) and three (48%, OR: 20.2, 95% CI: 5.9–68.6) organs failing. In subjects with two‐organ MOF, the combination of CV and respiratory POF carried 10.5 greater odds of mortality (95% CI: 1.1–105) than the combination of renal with either CV or respiratory POF. Presence of CV or respiratory involvement as the first failing system in subjects with two‐organ MOF was associated with higher mortality versus when renal failure occurred first (OR: 9.0, 95% CI: 2.2–37.5). There was no difference in mortality based on the timing of POF onset.
TABLE 3.
Mortality of AP based on POF time of onset, duration, number, organ type, and sequence of organs affected
| Subgroups of POF | Mortality, n (%) | OR (95% CI) | p‐Value |
|---|---|---|---|
| By onset | |||
| Within 24 h | 17/74 (23.0) | Reference | 0.57 |
| Between Day 2–3 | 8/50 (16.0) | 0.64 (0.25–1.62) | |
| Between Day 4–7 | 5/29 (17.2) | 0.70 (0.23–2.11) | |
| After Day 7 | 4/13 (30.8) | 1.49 (0.41–5.45) | |
| By total duration | |||
| ≤7 days | 16/106 (15.1) | Reference | 0.11 |
| 8–14 days | 9/28 (32.1) | 2.66 (1.03–6.92) | |
| >14 days | 6/26 (23.1) | 1.69 (0.59–4.85) | |
| By number of failed organs | |||
| One organ (single POF) | 4/90 (4.4) | Reference | <0.001 |
| Two organ MOF | 15/45 (33.3) | 10.75 (3.31–34.94) | |
| Three organ MOF | 15/31 (48.4) | 20.16 (5.92–68.63) | |
| By organ type in single POF | |||
| Respiratory/CV | 4/73 (5.5) | Reference | 1 |
| Renal | 0/17 (0) | 0 (0–6.67) | |
| By organ type in two‐organ MOF | |||
| Renal with respiratory or CV | 11/40 (27.5) | Reference | 0.04 |
| Respiratory and CV | 4/5 (80.0) | 10.5 (1.1–105) | |
| By organ sequence in two‐organ MOF | |||
| Renal first | 4/27 (14.8) | Reference | 0.003 |
| Respiratory or CV first | 11/18 (61.1) | 9.0 (2.2–37.5) | |
| By organ sequence in three‐organ MOF | |||
| Renal first | 6/17 (35.3) | Reference | 0.16 |
| Respiratory or CV first | 9/14 (64.3) | 3.3 (0.75–14.5) | |
Abbreviations: AP, acute pancreatitis; CI, confidence interval; CV, cardiovascular; MOF, multiple organ failure; OR, odds ratio; POF, persistent organ failure.
The number and sequence of organs affected during POF were independently associated with mortality in final multivariate regression model (Table 4). Mortality was highest with three‐organ MOF when the CV or respiratory systems failed first (OR: 601, p < 0.001), followed by two‐organ MOF that started with CV or respiratory failure (OR: 108, p < 0.001), and then by three (OR: 30, p = 0.002) and two‐organ (OR: 10, p = 0.002) MOF with renal failure occurring first. Duration of POF was not associated with mortality in the final model. After adding sterile and infected pancreatic necrosis, and pancreatic interventions as covariates, sensitivity analysis showed slight differences in the estimated effect sizes but were overall consistent with the main model. Infected pancreatic necrosis was associated with mortality (OR: 21, p = 0.04), whereas sterile pancreatic necrosis was not.
TABLE 4.
Multivariate regression model showing the effect of the number, sequence, and duration of POF on AP mortality
| Variable | Reference category | OR (95% CI) | p‐Value |
|---|---|---|---|
| Number and sequence of POF | |||
| Three‐organ MOF with CV or respiratory failure first | Single POF | 601.49 (34.3–21167.75) | <0.001 |
| Three‐organ MOF with renal failure first | 29.98 (4.2–311.93) | 0.002 | |
| Two‐organ MOF with CV or respiratory failure first | 108.34 (16.4–1117.28) | <0.001 | |
| Two‐organ MOF with renal failure first | 9.91 (1.5–78.63) | 0.02 | |
| Duration of POF > 7 days | POF ≤ 7 days | 3.82 (0.9–18.4) | 0.08 |
| Age (years) | – | 0.94 (0.86–1.01) | 0.09 |
| Male sex | Female sex | 1.62 (0.26–9.78) | 0.59 |
| Etiology | |||
| Gallstone | Other | 2.46 (0.4–16.82) | 0.34 |
| Alcohol | 0.91 (0.17–5.13) | 0.91 | |
| CCI ≥ 2 | CCI < 2 | 23.85 (2.41–374.19) | 0.01 |
| Transferred from another institution | Not transferred | 0.18 (0.04–0.74) | 0.02 |
| Geographic location | |||
| India | Europe | 1.25 (0.18–10.15) | 0.82 |
| Latin America | 1.02 (0.08–14.48) | 0.99 | |
| North America | 0.07 (0–0.72) | 0.04 | |
Note: p‐value of Hosmer–Lemeshow goodness of fit test was 0.60, indicating no evidence of poor fit.
Abbreviations: AP, acute pancreatitis; CCI, Charlson Comorbidity Index; CI, confidence interval; CV, cardiovascular; MOF, multiple organ failure; OR, odds ratio; POF, persistent organ failure.
DISCUSSION
There is a range of clinical outcomes based on POF characteristics that is not currently captured by the revised Atlanta classification severe group. In this large, prospective, multicenter study, we found that mortality of AP patients with POF was significantly higher with ≥2 organs failing, especially with the combination of CV and respiratory failure, and when the first organ failing was the CV or respiratory system. This study also underscores that the risk for developing POF is higher when SIRS occurs early, becomes persistent, or has higher scores. Our results provide data to serve as endpoints in future clinical trials, to guide physicians in clinical practice about patient subgroups requiring treatment escalation, and to inform discussions with patients and families about disease prognosis.
Consistent with previous studies, we confirmed that persistent MOF is associated with higher mortality than single POF. 3 , 5 , 8 , 10 , 11 , 14 Furthermore, we demonstrated a biological gradient based on the number of organs affected, and independence of this association from several potential confounding factors, including sterile and infected pancreatic necrosis. This evidence has fundamental clinical and research implications. Our study emphasizes the need to consider refining severity classification systems that differentiate subjects with persistent MOF from those with persistent single OF. While adding a new category to the current classification systems might reduce the number of patients within each stratum, 46% of patients with POF in our cohort would have been reclassified to the most severe category due to multiorgan involvement. Lastly, our findings support the use of persistent MOF as a surrogate endpoint of mortality, or as part of a composite endpoint with mortality in clinical trials.
A novel finding of our study was that mortality in subjects with persistent MOF was determined by the sequence and type of organ failing. Specifically, mortality from persistent MOF was higher when the CV or respiratory systems failed first as compared to when the renal system failed first, even after adjusting for confounders. Moreover, mortality in subjects with two‐organ MOF was higher when the CV and respiratory systems both failed as to when renal failure was combined with another organ. These findings suggest that involvement of the renal system as the first failing organ or concomitantly with other organs during persistent MOF, results in a less detrimental outcome. A less severe disease course in AP subjects with renal failure has also been observed in two recent US studies. 27 , 28 This may be explained by enhanced survival of AP subjects with renal failure over time due to improved fluid resuscitation and dialysis strategies. 28 These results have a prognostic value for AP patients with MOF and inform clinicians about the natural history of this selective group of critically ill patients.
The impact of the onset and duration of POF on mortality has been controversial. Previous studies suggested that early POF within the first 48 h carried higher mortality than later onset POF. 9 , 11 , 12 , 17 Furthermore, Shi et al. 14 reported higher mortality in patients with prolonged duration of POF over 2 weeks, compared to those with shorter duration. More recently, the study of Schepers et al. 5 challenged these findings and found that mortality was not different based on the onset and duration of POF onset. Our study supports the lack of association between the onset and duration of POF with mortality. The discrepant results between early and more recent studies may be a result of improvements in critical care of POF over time, and transition from early, open surgery to less‐invasive, step‐up approaches in necrotizing pancreatitis. 29
The development of SIRS is central to the pathophysiology of POF and mortality. 30 In APPRENTICE, all patients with POF were also found to have SIRS. After adjusting for potential confounders, we demonstrated that AP patients with SIRS on admission, persistent SIRS, and maximal SIRS score of 3–4 had higher risk of POF. This validates the findings from Singh et al., 21 using a larger multicenter cohort, and applying a more robust approach to account for confounding. Our predictive model also suggests that the addition of SIRS characteristics may allow better prognostication of POF (AUC: 0.85) compared to the traditional dichotomous approach of SIRS “positive versus negative” (AUC: 0.70). 18 , 19
Our study has some limitations. Most participants were enrolled from tertiary‐care centers with expertise in pancreatic disorders, so there may be potential for selection bias; however, the distribution of disease severity and mortality approximates to that observed in population‐based studies. 1 , 31 , 32 Ascertainment of death occurred during hospitalization; thus, it is possible that some subjects considered as survivors may have actually died later in the disease course, introducing potential misclassification bias. The lack of association of POF onset and mortality may be biased by the inability to define POF in very early deaths less than 48 h, and potential underrepresentation of late onset POF more than 7 days. Unmeasured aspects of POF such as the severity gradation for each individual organ failing, the sequence of all failing organs, and the precise cause of POF may have caused residual confounding. Although we used the Marshall scoring system as suggested by the revised Atlanta classification, 6 this limited our ability to ascertain POF beyond the CV, respiratory, and renal systems. Despite our efforts in data quality assurance, duration of SIRS and POF was not recorded in 184 and 6 patients, respectively. These patients were excluded from the analyses of these characteristics and might have biased the associations of SIRS and POF duration. Finally, screening methods, recruitment strategies, ancillary testing, imaging interpretation, and treatment strategies varied from site to site based on available resources and local clinical practices. 24
Our study has many strengths. First, it was conducted in a large, multiethnic population of AP subjects, prospectively enrolled at four different continents, regardless of their predicted severity or imaging changes, allowing for better external validity and generalizability. Selective inclusion of patients with necrotizing pancreatitis and POF would have resulted in exclusion of 57/166 patients with POF who had no cross‐sectional imaging or no pancreatic necrosis on imaging. Second, to optimize the internal validity of our study, we used logistic regression models to adjust for several potential confounders such as transfer status, geographic location, and sterile/infected pancreatic necrosis. Finally, local investigators prospectively monitored and recorded OF parameters on a daily basis, which resulted in only 4% of patients with incomplete data. Future observational studies that evaluate the prognostic role of other characteristics of POF unmeasured in our study are needed. Such studies should consider using alternative OF scoring methods that could also measure the impact of POF of other important systems such as the liver and nervous system.
In summary, this large multicenter, international, prospective study showed that specific characteristics and dynamic features of SIRS and POF impact the clinical course and mortality of AP. A novel contribution of our study is that involvement of the CV and respiratory systems, either concurrently or as the first failing organ during persistent MOF, carries the highest mortality in AP as compared to when the renal system failed first or concurrently with other organ. Future refinement of severity classification systems should consider incorporating an additional category for patients with persistent MOF, which is independently associated with the highest risk of mortality in AP.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Study concept and design: Jorge D. Machicado, Amir Gougol, and Georgios I. Papachristou. Data analysis: Xiaoqing Tan, Xiaotian Gao, and Gong Tang. Drafting of the manuscript: Jorge D. Machicado and Georgios I. Papachristou. Generation and collection of data, data interpretation, critical revision of the manuscript for important intellectual content, final approval of the manuscript: all authors.
Supporting information
Supporting Information 1
DATA AVAILABILITY
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Munigala S, Yadav D. Case‐fatality from acute pancreatitis is decreasing but its population mortality shows little change. Pancreatology. 2016;16(4):542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petrov MS, Shanbhag S, Chakraborty M, Phillips ARJ, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813–20. [DOI] [PubMed] [Google Scholar]
- 3. Sternby H, Bolado F, Canaval‐Zuleta HJ, Marra‐López C, Hernando‐Alonso AI, del‐Val‐Antoñana A, et al. Determinants of severity in acute pancreatitis. Ann Surg. 2019;270(2):348–55. [DOI] [PubMed] [Google Scholar]
- 4. Padhan RK, Jain S, Agarwal S, Harikrishnan S, Vadiraja P, Behera S, et al. Primary and secondary organ failures cause mortality differentially in acute pancreatitis and should be distinguished. Pancreas. 2018;47(3):302–7. [DOI] [PubMed] [Google Scholar]
- 5. Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68(6):1044–51. [DOI] [PubMed] [Google Scholar]
- 6. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis‐2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11. [DOI] [PubMed] [Google Scholar]
- 7. Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví‐Poma E, Petrov MS, et al. Determinant‐based classification of acute pancreatitis severity. Ann Surg. 2012;256(6):875–80. [DOI] [PubMed] [Google Scholar]
- 8. Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89(3):298–302. [DOI] [PubMed] [Google Scholar]
- 9. Johnson CD, Abu‐Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53(9):1340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaheen MA, Akhtar AJ. Organ failure associated with acute pancreatitis in African‐American and Hispanic patients. J Natl Med Assoc. 2007;99(12):1402–6. [PMC free article] [PubMed] [Google Scholar]
- 11. Thandassery RB, Yadav TD, Dutta U, Appasani S, Singh K, Kochhar R. Dynamic nature of organ failure in severe acute pancreatitis: the impact of persistent and deteriorating organ failure. HPB (Oxford). 2013;15(7):523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma M, Banerjee D, Garg PK. Characterization of newer subgroups of fulminant and subfulminant pancreatitis associated with a high early mortality. Am J Gastroenterol. 2007;102(12):2688–95. [DOI] [PubMed] [Google Scholar]
- 13. Wang M, Lei R. Organ dysfunction in the course of severe acute pancreatitis. Pancreas. 2016;45(1):e5‐7. [DOI] [PubMed] [Google Scholar]
- 14. Shi N, Liu T, de la Iglesia‐Garcia D, Deng L, Jin T, Lan L, et al. Duration of organ failure impacts mortality in acute pancreatitis. Gut. 2019;69(3):604–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandri M, Berchialla P, Baldi I, Gregori D, De Blasi RA. Dynamic Bayesian Networks to predict sequences of organ failures in patients admitted to ICU. J Biomed Inf. 2014;48:106–13. [DOI] [PubMed] [Google Scholar]
- 16. Das SL, Papachristou GI, De Campos T, Panek, J , Prim IP, Serrablo A, et al. Individual patient data meta‐analysis of organ failure in acute pancreatitis: protocol of the PANCREA II study. JOP. 2013;14(5):475–83. [DOI] [PubMed] [Google Scholar]
- 17. /Lytras D , Manes K, Triantopoulou C, Paraskeva C, Delis S, Avgerinos C, et al. Persistent early organ failure. Pancreas. 2008;36(3):249–54. [DOI] [PubMed] [Google Scholar]
- 18. Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142(7):1476–82. [DOI] [PubMed] [Google Scholar]
- 19. Di M‐Y, Liu H, Yang Z‐Y, Bonis PAL, Tang J‐L, Lau J. Prediction models of mortality in acute pancreatitis in adults. Ann Intern Med. 2016;165(7):482–90. [DOI] [PubMed] [Google Scholar]
- 20. Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93(6):738–44. [DOI] [PubMed] [Google Scholar]
- 21. Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Mortele KJ, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7(11):1247–51. [DOI] [PubMed] [Google Scholar]
- 22. Papachristou GI, Machicado JD, Stevens T, Goenka MK, Ferreira M, Gutierrez SC, et al. Acute pancreatitis patient registry to examine novel therapies in clinical experience (APPRENTICE): an international, multicenter consortium for the study of acute pancreatitis. Ann Gastroenterol. 2017;30(1):106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–15. [DOI] [PubMed] [Google Scholar]
- 24. Matta B, Gougol A, Gao X, Reddy N, Talukdar R, Kochhar R, et al. Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clin Gastroenterol Hepatol. 2019;18(7):1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55. [DOI] [PubMed] [Google Scholar]
- 26. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score. Crit Care Med. 1995;23(10):1638–52. [DOI] [PubMed] [Google Scholar]
- 27. Gougol A, Dugum M, Dudekula A, Greer P, Slivka A, Whitcomb DC, et al. Clinical outcomes of isolated renal failure compared to other forms of organ failure in patients with severe acute pancreatitis. World J Gastroenterol. 2017;23(29):5431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Devani K, Charilaou P, Radadiya D, Brahmbhatt B, Young M, Reddy C. Acute pancreatitis: trends in outcomes and the role of acute kidney injury in mortality—A propensity‐matched analysis. Pancreatology. 2018;18(8):870–7. [DOI] [PubMed] [Google Scholar]
- 29. van Santvoort HC, Besselink MG, Bakker OJ, SijbrandHofker H, Boermeester MA, Dejong CH, et al. A step‐up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–502. [DOI] [PubMed] [Google Scholar]
- 30. Andersson R, Andersson B, Andersson E, Axelsson J, Eckerwall G, Tingstedt B. Acute pancreatitis—from cellular signalling to complicated clinical course. HPB (Oxford). 2007;9(6):414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamada S, Masamune A, Kikuta K, Hirota M, Tsuji I, Shimosegawa T. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas. 2014;43(8):1244–8. [DOI] [PubMed] [Google Scholar]
- 32. Kamal A, Sinha A, Hutfless SM, Afghani E, Faghih M, Khashab MA, et al. Hospital admission volume does not impact the in‐hospital mortality of acute pancreatitis. HPB (Oxford). 2017;19(1):21–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


