Abstract
Background
Updated population‐based estimates on incidence and prevalence of chronic pancreatitis are scarce.
Methods
We used nationwide healthcare registries to identify all Danish patients diagnosed with chronic pancreatitis and computed crude and standardised incidence rates and prevalence estimates in 1994–2018. Incidence and prevalence were evaluated in relation to patients age and gender, aetiology (alcoholic vs. non‐alcoholic) and smoking and alcohol consumption in the general Danish population.
Results
The mean incidence rate of chronic pancreatitis during the study period was 12.6 per 100,000 person years for the total population, for women it was 8.6 per 100,000 person years and for men it was 16.7 per 100,000 person years. The standardised incidence rate was stable from 1994 to 2018, remaining at 12.5 per 100,000 person years in the last observation period (2014–2018). The point prevalence of chronic pancreatitis in 2016 was 153.9 per 100,000 persons. A gradual increase in standardised prevalence estimates was observed during the study period from 126.6 in 1996 to 153.9 in 2016. The mean age at chronic pancreatitis diagnosis increased from 52.1 to 60.0 years during the study period.
Conclusion
The prevalence of chronic pancreatitis is increasing in the Danish population despite a stable incidence level. Improved management strategies and changes in the underlying patient population may explain these observations.
Keywords: alcohol, chronic pancreatitis, epidemiology, incidence, prevalence, smoking
Key Summary
Summarise the established knowledge on this subject
Chronic pancreatitis (CP) is a severe and debilitating disease with a dismal prognosis.
Updated population‐based estimates on incidence and prevalence of CP are scarce.
What are the significant and/or new findings of this study?
Over a 25‐year observation period (1994–2018) the prevalence of CP was increasing in the Danish population, while the incidence remained stable.
During the observation period the mean age at CP diagnosis increased by almost a decade.
Improved management strategies and changes in the underlying patient population may explain these observations.
1. INTRODUCTION
Chronic pancreatitis (CP) is a progressive fibro‐inflammatory disease resulting in gradual replacement of the pancreatic gland with fibrosis. 1 Both genetic and environmental factors are thought to contribute to the pathogenesis of CP. In addition to excessive alcohol consumption smoking has been identified as an independent risk factor. 1 , 2 , 3 In most patients, serious complications evolve as the disease progresses including exocrine pancreatic insufficiency, diabetes mellitus and pancreatic cancer. 4 , 5 , 6 Current treatment strategies are directed at mitigation of these complications, although no treatments available can effectively alter the course of disease. This is reflected in a significantly reduced quality of life and life expectancy of affected individuals. 6 , 7 , 8
Notwithstanding the severity and unfortunate prognosis of CP, epidemiological data on incidence and prevalence of this entity are scarce and recent studies are limited by being mostly derived from insurance claim databases or hospital‐based surveys. 9 , 10 , 11 , 12 These studies may not be representative for the full CP population as patients with less severe disease manifestations may not seek in‐hospital care. Also, older epidemiological studies are likely to provide outdated incidence and prevalence estimates due to changes in patterns of smoking and alcohol consumption over recent decades along with an increased use of modern cross‐sectional imaging that can detect CP at earlier stages than previous diagnostic methods. 13 , 14 Taken together, such changes in risk factor exposure and diagnostic practice may result in changes in incidence and prevalence. This information is important to inform healthcare decision‐makers and to project resource allocation for patient management and research. Consequently, updated population‐based studies on CP epidemiology have been identified as an important and unmet need in the field. 15
We conducted a nationwide population‐based study on time trends of CP incidence and prevalence in Denmark, including every hospitalised patient from 1994 to 2018. Time trends were evaluated in relation to (a) age and gender distributions, (b) aetiology of CP (alcoholic vs. nonalcoholic) and (c) patterns of smoking and alcohol consumption in the general Danish population.
2. METHODS
2.1. Study population
This was a nationwide registry‐based study conducted in Denmark, which has 5.8 m residents (2018) who are ethnically homogenous with more than 95% Caucasian individuals. All Danish residents are provided tax‐supported free access to general practitioners and hospitals. The study included the total Danish population in the period 1 January 1994–31 December 2018, as identified by the Danish Central Office of Civil Registration. In this registry, the vital status, immigration and emigration of each resident are monitored continuously. Each Dane has a unique personal identifier issued at birth or immigration, and this enables linkage of individual‐level data across registers. We identified cases of CP using linked information from the Danish National Patient Registry. This is a nationwide registry that covers all nonpsychiatric hospital admissions since 1977 and all outpatient and emergency room visits since 1995. Data include relevant dates and discharge diagnoses coded in accordance with the International Classification of Diseases, edition 8 (ICD‐8) from 1977 to 1993 and edition 10 (ICD‐10) from 1994 and onwards. The following ICD‐8/ICD‐ 10 codes were used to identify alcoholic CP (ICD‐8: 577.10 alcohol‐induced chronic pancreatitis; ICD‐10: K86.0 alcohol‐induced chronic pancreatitis) and nonalcoholic CP (ICD‐8: 577.11 nonalcoholic chronic pancreatitis, 577.19 recurrent chronic pancreatitis; ICD‐10: K86.1 other chronic pancreatitis) with CP defined as any of the two subtypes. We identified CP cases based on the diagnostic codes in both primary or secondary positions in hospital discharge summaries. The positive predictive value of CP diagnoses in the Danish National Patient Registry is 80% using ICD 10 codes. 16
2.2. Study design
This was a nationwide population‐based study. Incident cases were defined as patients who during 1994–2018 had a first‐time diagnosis of CP; patients with CP diagnosed before January 1994 were excluded from incidence estimate computations. Prevalent cases were defined as patients who had a diagnosis at some time‐point (before or after 1994) and were still alive at the time of the prevalence assessment in question during 1994–2018. Risk time was calculated as the sum of individual risk time for all residents in the study period, combined for the CP outcomes such that individuals with alcoholic CP were not considered at risk for non‐alcoholic CP and vice versa.
Incidence rates were calculated for 5‐year periods between 1994 and 2018 as the number of incident CP cases divided by the time at risk (in 100,000 person years). The choice of 5‐year periods was convenient as we studied a 25‐year period and 1‐year periods gave too much random variation from year to year. Also, to assess how much of the change in incidence rate over the study period was attributable to change of the age‐distribution during this period, we standardised to the age‐distribution of the Danish population 2014–2018 using direct standardisation.
Point prevalence estimates were calculated for five time‐points during the observation period and we choose 1 July in 1996, 2001, 2006, 2011 and 2016, dates which correspond to the middle of the incidence time periods. Point prevalence estimates were calculated as the number of CP patients under observation in our cohort on 1 July in the specific year divided by the population size on the same date and reported per 100,000 persons. In addition, standardised prevalence estimates were computed as described for incidence rates.
Information on total alcohol sales per Danish resident 18 years or older during 1980–2018 was obtained from Statistics Denmark. 17 Information on daily smoking was obtained from the Danish National Cohorts studies which are nationally representative surveys conducted in 1987, 1994, 2000, 2005, 2010, 2013 and 2017. 18 , 19
2.3. Statistics
Data management and computations were performed using Stata version 16.1 (StataCorp) and R version 3.6.1 (R Foundation for Statistical Computing; https://www.R‐project.org/). Since this was a nationwide population‐based study including all residents in Denmark during the observation period no confidence intervals were provided for incidence rates or prevalence estimates.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. According to Danish legislation, approval from an ethics committee was not required as this was a register‐based study using national Danish health registries. Likewise, our study did not require written/informed consent.
3. RESULTS
During the study period from 1994 to 2018, 17,160 individuals were diagnosed with CP in Denmark of whom 11,248 (65.5%) were men. The mean age at diagnosis was 55.9 years.
3.1. Incidence
The mean incidence rate of CP from 1994 to 2018 was 12.6 per 100,000 person years for the total population, for women it was 8.6 per 100,000 person years and for men it was 16.7 per 100,000 person years. Incidence rates by age group and gender are reported in Table 1. The highest incidence rates of CP were observed in individuals older than 50 years, for both men and women.
TABLE 1.
Mean incidence rates (per 100,000 person‐years) of chronic pancreatitis in Denmark from 1994 to 2018, by age group and gender
| Women | Men | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age group | Number of cases | Person‐years | IR/100,000 | Number of cases | Person‐years | IR/100,000 | Number of cases | Person‐years | IR/100,000 |
| 0–29 | 214 | 24,403,636 | 0.9 | 333 | 25,488,460 | 1.3 | 547 | 49,892,096 | 1.1 |
| 30–39 | 442 | 9297,685 | 4.8 | 1112 | 9544,948 | 11.7 | 1554 | 18,842,632 | 8.2 |
| 40–49 | 1034 | 9614,915 | 10.8 | 2531 | 9823,664 | 25.8 | 3565 | 19,438,580 | 18.3 |
| 50–59 | 1416 | 9027,758 | 15.7 | 3053 | 9084,559 | 33.6 | 4469 | 18,112,316 | 24.7 |
| 60–69 | 1400 | 7452,546 | 18.8 | 2442 | 7112,307 | 34.3 | 3842 | 14,564,852 | 26.4 |
| 70–79 | 938 | 5399,029 | 17.4 | 1363 | 4443,983 | 30.7 | 2301 | 9843,012 | 23.4 |
| 80+ | 468 | 3630,063 | 12.9 | 414 | 1957,990 | 21.1 | 882 | 5588,053 | 15.8 |
Abbreviation: IR, incidence rate.
Comparing the first 5 years (1994–1999) to the last 5 years (2014–2018) of the study period, the standardised incidence rate of CP remained relatively stable from 14.3 to 12.5 per 100,000 years. The crude standardised incidence rates stratified by gender and study periods are shown in Figure 1.
FIGURE 1.
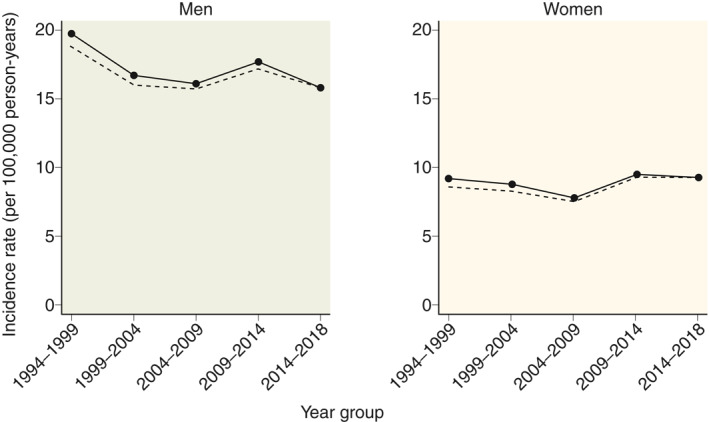
Mean incidence rates (per 100,000 person‐years) of chronic pancreatitis in Denmark from 1994 to 2018, by gender. Solid lines represent incidence rates standardised to the age distribution in 2014–2018, dashed lines represent crude incidence rates
3.2. Distribution of aetiology
An alcoholic aetiology was registered in 6761 of the 17,160 (39.4%) individuals diagnosed with CP. The mean incidence rate of alcoholic CP was 5.0 per 100,000 person years and the mean incidence rate of nonalcoholic CP was 7.6 per 100,000 person years. Incidence rates of CP by age group and aetiology are reported in Table S1. The incidence of alcoholic CP peaked in the age group 50–59 years, while the incidence of nonalcoholic CP peaked later in life with the highest incidence rate observed for the age group 70–79 years.
Comparing the first 5 years (1994–1999) to the last 5 years (2014–2018) of the study period, the standardised incidence rate of alcoholic CP decreased from 9.8 to 5.2 per 100,000 person years for men, while it remained stable for women (Figure 2). In contrast, the standardised incidence rate of nonalcoholic CP increased slightly from 9.8 to 10.6 per 100,000 person years for men and from 6.6 to 7.2 per 100,000 person years for women (Figure 2).
FIGURE 2.
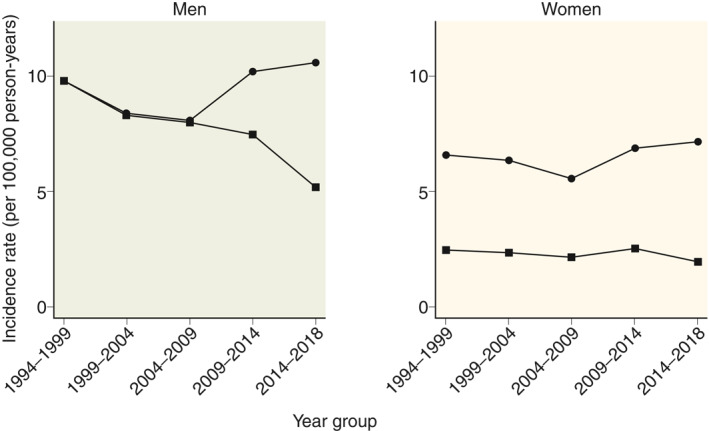
Mean incidence rates (per 100,000 person‐years) of chronic pancreatitis in Denmark from 1994 to 2018, by gender and aetiology. Circles represent nonalcoholic chronic pancreatitis and squares represent alcoholic chronic pancreatitis. Incidence rates standardised to the age distribution in 2014–2018 are shown
3.3. Prevalence
The point prevalence of CP in 2016 was 153.9 per 100,000 individuals, for women it was 112.5 per 100,000 individuals and for men it was 195.7 per 100,000 individuals. The crude and standardised prevalence estimates stratified by gender are shown in Figure 3. A gradual increase in standardised prevalence estimates was observed during the study period from 126.6 per 100,000 individuals in 1996 to 153.9 per 100,000 individuals in 2016 for the total population. For men, the increase in standardised prevalence was 169.2 to 195.7 per 100,000 individuals and for women it was 85.4 to 112.5 per 100,000 individuals.
FIGURE 3.
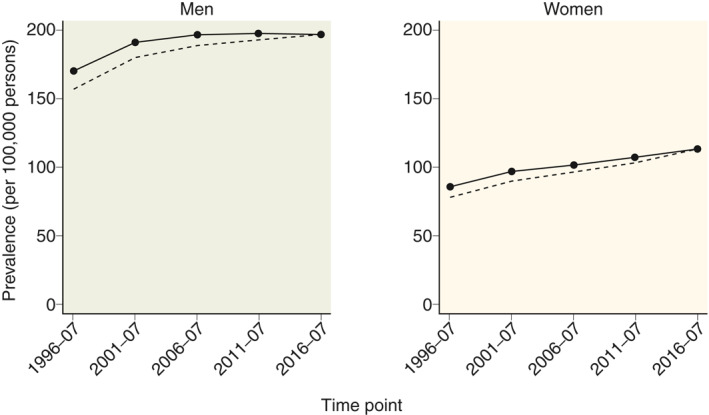
Prevalence (per 100,000 individuals) of chronic pancreatitis in Denmark from 1994 to 2018, by gender. Point estimates are calculated for the middle of the five periods. Solid lines represent prevalence estimates standardised to the age distribution in 2014–2018, dashed lines represent crude prevalence estimates
Standardised prevalence estimates stratified by gender and aetiology are shown in Figure 4. The prevalence of alcoholic CP was increasing for both men and women during the observation period, despite a decreasing incidence rate. The prevalence of nonalcoholic CP appears was increasing in women and remained stable in men.
FIGURE 4.
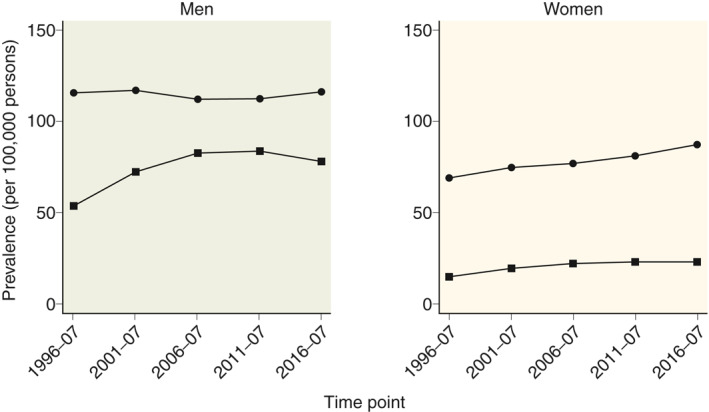
Prevalence (per 100,000 individuals) of chronic pancreatitis in Denmark from 1994 to 2018, by gender and aetiology. Circles represent nonalcoholic chronic pancreatitis and squares represent alcoholic chronic pancreatitis. Prevalence estimates were calculated as point estimates for the middle of the five periods and standardised to the age distribution in 2014–2018
3.4. Age at diagnosis
The age at CP diagnosis was increasing from 52.1 years in 1994–1999 to 60.0 years in 2014–2018. The increase in age at diagnosis was both observed for patient with alcoholic and nonalcoholic aetiology (Figure 5).
FIGURE 5.
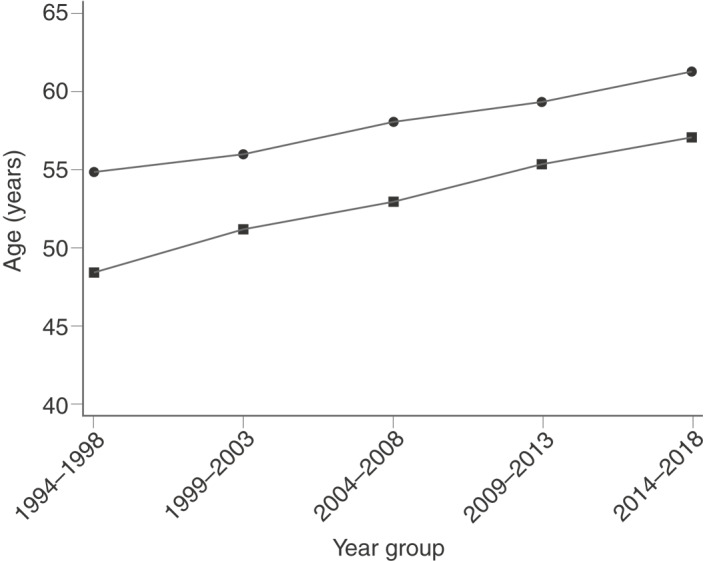
Mean age at time of diagnosis of chronic pancreatitis in Denmark from 1994 to 2018, by aetiology. Circles represent nonalcoholic chronic pancreatitis and squares represent alcoholic chronic pancreatitis
3.5. Population‐based patterns of smoking and alcohol consumption
Alcohol consumption per capita and prevalence of smokers in Denmark from 1980 to 2018 are shown in Figure 6. During the observation period, the annual alcohol consumption decreased from 12.4 to 9.7 L of pure alcohol per capita and the prevalence of smokers decreased from 44.3% to 16.7%.
FIGURE 6.
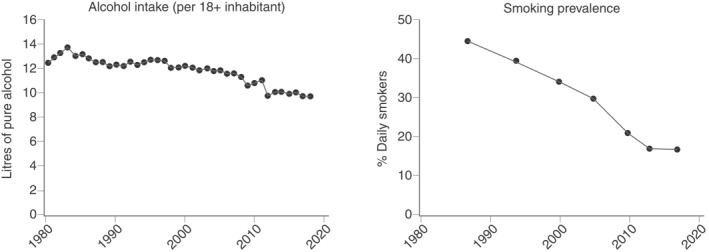
Alcohol consumption per capita and prevalence of smokers in Denmark from 1980 to 2018
4. DISCUSSION
We conducted a nationwide population‐based study on time trends in incidence and prevalence of CP in Denmark from 1994 to 2018. We observed a gradually increasing prevalence of CP during the study period with a point prevalence of 153.9 per 100,000 persons in 2016. In contrast, the incidence rate remained stable during the study period with a mean incidence rate of 12.6 per 100,000 person years. The incidence rate of alcohol‐related CP decreased, while the incidence of nonalcoholic CP was increasing. Our findings were paralleled by decreasing alcohol consumption and smoking prevalence in the Danish population during the same observation period. Finally, the mean age at CP diagnosis increased by almost a decade during the study period.
The mean incidence rate of CP was 12.6/100,000 person years in our study. A meta‐analysis estimating the global incidence rate of CP reported an incidence of 9.2/100,000 person years, but only included two studies. One of these studies was a population‐based study from Olmsted County in Minnesota, USA, which reported an incidence rate of CP of 4.4/100,000 person years. 10 However, in that study, only patients with a definitive diagnosis of CP verified by detailed chart reviews were included, which is in opposition to the epidemiological approach used for the present study. Also, the CP population in the Olmsted County study was limited to patients with end‐stage CP based on the Mayo clinic criteria. 10 The estimate from the meta‐analysis is lower compared to results from a recent study from the USA reporting and incidence of 24.7/100,000 person years in 2014. 9 However, this study was restricted to the adult population and excluded patients older than 65 years. In addition to these US‐based studies, a survey among gastroenterologist in France reported a CP incidence rate of 7.8/100,000 person years 12 but was limited by a relatively low response rate (23% of invited providers) and did not include cases handled in primary care. Taken together, a direct comparison of incidence estimates between studies is difficult due to differences in patient cohorts, settings and case definition. Of particular note, studies based on register data rather than individual case assessment may overestimate the incidence rate by 20%–30% as the positive predictive value of a CP diagnosis using register data has been estimated to be 70%–80%. 16 , 20
Prevalence estimates of CP have mostly been limited to studies based on non‐population‐based settings with relatively short observation periods. We observed the highest prevalence of CP reported to date in Europe (153.9/100,000 persons in 2016), which is considerably higher than prevalence estimates previously reported. Accordingly, Machicado et al. 11 reported a period prevalence (2001–2013) of 98.7/100,000 persons, which is fairly similar to the prevalence estimate reported from the aforementioned study by Sellers et al. 9 (91.9/100,000 persons in 2014). However, both of the US‐based studies were based on insurance claim databases as compared to our nationwide cohort including all patients diagnosed with CP in Denmark. In further contrast to our findings, a Japanese study reported a prevalence of 52.0/100,000 persons based on a nationwide survey in 2011, which is close to prevalence estimates reported from the southern Europe including Spain (49.0/100,000 persons) and Italy (44.0/100,000 persons). 21 , 22 , 23 The lower prevalence observed in southern Europe and Japan compared to the register‐based studies from the USA and our estimates are likely explained by differences in study design (surveys vs. register‐based studies) but may also represent a true difference in CP prevalence across regions. However, a direct comparison of prevalence estimates between studies is difficult for the same reasons as those discussed above. Future studies should aim to adopt comparable methodology, and the high prevalence of CP observed in our study needs confirmation from other population‐based studies.
We observed a stable incidence rate of CP during our observation period. Interestingly, subanalysis revealed a decreasing incidence rate of alcohol‐related CP in the male subpopulation with an almost 50% decrease in the standardised incidence rate. This finding was paralleled by decreasing alcohol consumption and smoking prevalence in the Danish population during the same observation period. The decreasing incidence of alcohol‐related CP was counterbalanced by a slightly increasing incidence rate of non‐alcoholic CP. These time trends are difficult to compare to past studies as incidence estimates stratified by aetiology were not previously reported. A decreasing incidence rate of adult CP was observed in the study by Sellers et al. 9 while the aforementioned study from Olmsted County and epidemiological surveys from Japan reported increasing incidence rates. 10 , 24 , 25
In contrast to the stable incidence rate of CP, the prevalence of CP gradually increased during our observation period. This may imply that the overall prognosis and life expectancy of affected individuals is improving. An explanation for this may be found in optimised treatment strategies and implementation of evidence‐based guidelines. 26 However, our observation may also reflect a change in the underlying patient population with fewer patients being diagnosed with alcohol‐related CP which has a poor prognosis compared to non‐alcoholic CP. 27 Also, an increasing number of patients are likely diagnosed at earlier disease stages than previously, due to an increased awareness of “early CP” and use of more sensitive imaging modalities including endoscopic ultrasound (EUS) and modern cross‐sectional imaging methods. 28 , 29 For example, over a 20‐year period, a US‐based study with time intervals overlapping to our study (1994–2013) reported an increase in imaging utilisation of 312% for magnetic resonance imaging and 151% for computed tomography, and these are the cross‐sectional imaging modalities typically used to diagnose CP. 14 , 30 In addition, hereditary and autoimmune pancreatitis have emerged as independent disease entities during the study period and, in particular, autoimmune pancreatitis has an excellent prognosis. 31 , 32 As these entities lack distinct ICD codes, they cannot be captured at present in the registers and consequently many of these cases may be coded as “nonalcoholic CP”.
The mean age at diagnosis increased by almost a decade during the observation period. Although our study was not designed to elucidate the source of this observation, we suspect that increased utilisation of modern imaging modalities may also explain this phenomenon. Hence, the majority of imaging studies are performed in elderly people and this may lead to increased diagnosis of CP in this population which, again, could explain the observed increase in mean age at CP diagnosis. The possible implication is that some patients may be diagnosed “incidentally” solely based on morphological features, but with no symptoms or medical history suggestive of CP. This introduces a risk of detection and lead time bias, which is particularly relevant for patients with minor morphological changes indicative of CP as the specificity of such findings has been questioned. 33 , 34
The prevalence and incidence of CP was higher in men compared to women, in particular for alcoholic CP. This is in keeping with findings from most previous studies and may be explained by an overall increased exposure to alcohol and smoking in the male population. 15 Also, an increased genetic susceptibility to alcoholic CP is seen in men due to the CLDN2 risk allele associated with atypical localisation of claudin‐2 in pancreatic acinar cells. Accordingly, the hemizygous CLDN2 genotype confers an increased risk of alcoholic pancreatitis in men, while only women with the homozygous genotype are at increased risk. As the male hemizygote frequency (0.26) is much higher than the female homozygote frequency (0.07), this could partly explain the male predominance of alcohol‐related pancreatitis. 35
The strengths of this study include the use of nationwide register data enabling complete identification and follow‐up of all subjects as well as inclusion of both outpatient and inpatient CP cases. In addition, our study had a long follow‐up period of 25 years, which is considerably longer than previous population‐based studies. 9 This allowed for a detailed examination of time trends and makes our estimates less prone to random short‐term variations.
The inherent limitation in the use of nationwide register data is the inability to verify accurate diagnosis coding of CP. Also, lack of detailed information on risk factor exposure including alcohol and smoking may bias the classification of CP aetiologies. Indeed, the prevalence of alcoholic CP was low in our study (39.4%). This may reflect that registers have a low accuracy for aetiology classification when compared with clinical studies where alcohol aetiology is typically reported in higher proportions of patients. 36 , 37 However, the coding practice during the study period has likely not changed and, as such, the temporal changes in aetiology of CP observed in our study are most likely valid. Another limitation of our study is the lack of data on cross‐sectional imaging and other diagnostic modalities.
In conclusion, we have provided updated nationwide population‐based estimates on incidence and prevalence of CP over a 25‐year period in Denmark.
Overall the prevalence of CP was increasing during the study period, while the incidence remained stable. Improved management strategies and changes in the CP population may explain these observations.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS APPROVAL
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. According to Danish legislation approval from an ethics committee was not required as this was a register‐based study using national Danish health registries.
AUTHOR CONTRIBUTIONS
Study design: Søren S. Olesen, Dhiraj Yadav and Janne S. Tolstrup. Data management and statistical analysis: Laust H. Mortensen, Elisabeth Zinck and Janne S. Tolstrup. Writing committee: Søren S. Olesen and Janne S. Tolstrup. Critical revision of manuscript for important intellectual content and final approval of the manuscript: all authors.
5.
ACKNOWLEDGEMENTS
This work was supported by the Novo Nordisk Foundation, grant identifiers NNF17OC0027594 and NNF17OC0027812.
DATA AVAILABILITY STATEMENT
The data are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Singh VK, Yadav D, Garg PK. Diagnosis and management of chronic pancreatitis: a review. J Am Med Assoc. 2019;322:2422–34. [DOI] [PubMed] [Google Scholar]
- 2. Tolstrup JS, Kristiansen L, Becker U, et al. Smoking and risk of acute and chronic pancreatitis among women and men: a population‐based cohort study. Arch Intern Med. 2009;169:603–9. [DOI] [PubMed] [Google Scholar]
- 3. Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olesen SS, Nøjgaard C, Poulsen JL, et al. Chronic pancreatitis is characterized by distinct complication clusters that associate with etiological risk factors. Am J Gastroenterol. 2019;114:656–64. [DOI] [PubMed] [Google Scholar]
- 5. Olesen SS, Kuhlmann L, Novovic S, et al. Association of multiple patient and disease characteristics with the presence and type of pain in chronic pancreatitis. J Gastroenterol Hepatol. 2020;35:326–33. [DOI] [PubMed] [Google Scholar]
- 6. Bang UC, Benfield T, Hyldstrup L, et al. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched‐cohort study. Gastroenterology. 2014;146:989–94.el. [DOI] [PubMed] [Google Scholar]
- 7. Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain‐associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Machicado JD, Amann ST, Anderson MA, et al. Quality of life in chronic pancreatitis is determined by constant pain, disability/unemployment, current smoking, and associated co‐morbidities. Am J Gastroenterol. 2017;112:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sellers ZM, MacIsaac D, Yu H, et al. Nationwide trends in acute and chronic pancreatitis among privately insured children and non‐elderly adults in the United States, 2007‐2014. Gastroenterology. 2018;155:469–78.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population‐based study. Am J Gastroenterol. 2011;106:2192–9. [DOI] [PubMed] [Google Scholar]
- 11. Machicado JD, Dudekula A, Tang G, et al. Period prevalence of chronic pancreatitis diagnosis from 2001‐2013 in the commercially insured population of the United States. Pancreatology. 2019;19:813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lévy P, Barthet M, Mollard BR, et al. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol. 2006;30:838–44. [DOI] [PubMed] [Google Scholar]
- 13. Bhargavan M, Sunshine JH. Utilization of radiology services in the United States: levels and trends in modalities, regions, and populations. Radiology. 2005;234:824–32. [DOI] [PubMed] [Google Scholar]
- 14. Frøkjær JB, Akisik F, Farooq A, et al. Guidelines for the diagnostic cross sectional imaging and severity scoring of chronic pancreatitis. Pancreatology. 2018;18:764–73. [DOI] [PubMed] [Google Scholar]
- 15. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirkegard J, Mortensen MR, Johannsen IR, et al. Positive predictive value of acute and chronic pancreatitis diagnoses in the Danish National Patient Registry: a validation study. Scand J Publ Health. 2020;48:14–9. [DOI] [PubMed] [Google Scholar]
- 17. Statistics Denmark Forbrugerpriser. http://www.dst.dk/da/Statistik/emner/priser‐og‐forbrug/forbrugerpriser. [Google Scholar]
- 18. Ekholm O, Hesse U, Davidsen M, et al. The study design and characteristics of the Danish national health interview surveys. Scand J Publ Health. 2009;37:758–65. [DOI] [PubMed] [Google Scholar]
- 19. Jensen HAR, Ekholm O, Davidsen M, et al. The Danish health and morbidity surveys: study design and participant characteristics. BMC Med Res Methodol. 2019;19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao AY, Tan ML, Plana MN, et al. The use of international classification of diseases codes to identify patients with pancreatitis: a systematic review and meta‐analysis of diagnostic accuracy studies. Clin Transl Gastroenterol. 2018;9:e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Capurso G, Archibugi L, Pasquali P, et al. Prevalence of chronic pancreatitis: results of a primary care physician‐ based population study. Dig Liver Dis. 2017;49:535–9. [DOI] [PubMed] [Google Scholar]
- 22. Domínguez‐Munoz JE, Lucendo A, Carballo LF, et al. A Spanish multicenter study to estimate the prevalence and incidence of chronic pancreatitis and its complications. Rev Esp Enferm Dig. 2014;106:239–45. [PubMed] [Google Scholar]
- 23. Hirota M, Shimosegawa T, Masamune A, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology. 2014;14:490–6. [DOI] [PubMed] [Google Scholar]
- 24. Hirota M, Shimosegawa T, Masamune A, et al. The sixth nationwide epidemiological survey of chronic pancreatitis in Japan. Pancreatology. 2012;12:79–84. [DOI] [PubMed] [Google Scholar]
- 25. Hirota M, Shimosegawa T, Masamune A, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology. 2014;14:490–6. [DOI] [PubMed] [Google Scholar]
- 26. Löhr JM, Dominguez‐Munoz E, Rosendahl J, et al. United European Gastroenterology evidence‐based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United Eur Gastroenterol J. 2017;5:153–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Layer P, Yamamoto H, Kalthoff L, et al. The different courses of early‐ and late‐onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481–7. [DOI] [PubMed] [Google Scholar]
- 28. Whitcomb DC, Shimosegawa T, Chari ST, et al. International consensus statements on early chronic Pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology. American Pancreatic Association, Japan Pancreas Society, PancreasFest Working Group and European Pancreatic Club. Pancreatology. 2018;18:516‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Madzak A, Olesen SS, Wathle GK, et al. Secretin‐stimulated magnetic resonance imaging assessment of the benign pancreatic disorders: systematic review and proposal for a standardized protocol. Pancreas. 2016;45:1092–103. [DOI] [PubMed] [Google Scholar]
- 30. Rosman DA, Duszak R, Wang W, et al. Changing utilization of noninvasive diagnostic imaging over 2 decades: an examination family‐focused analysis of Medicare claims using the Neiman imaging types of service categorization system. AJR Am J Roentgenol. 2018;210:364–8. [DOI] [PubMed] [Google Scholar]
- 31. Löhr J‐M, Beuers U, Vujasinovic M, et al. European guideline on IgG4‐related digestive disease––UEG and SGF evidence‐based recommendations. United Eur Gastroenterol J. 2020;8:637–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology. 2015;149:39–51. [DOI] [PubMed] [Google Scholar]
- 33. Sheel ARG, Baron RD, Sarantitis I, et al. The diagnostic value of Rosemont and Japanese diagnostic criteria for ‘indeterminate’, ‘suggestive’, ‘possible’ and ‘early’ chronic pancreatitis. Pancreatology. 2018;18:774–84. [DOI] [PubMed] [Google Scholar]
- 34. Masamune A, Kikuta K, Kume K, et al. Nationwide epidemiological survey of chronic pancreatitis in Japan: introduction and validation of the new Japanese diagnostic criteria 2019. J Gastroenterol. 2020. 10.1007/s00535-020-01704-9. [DOI] [PubMed] [Google Scholar]
- 35. Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1‐PRSS2 loci alter risk for alcohol‐related and sporadic pancreatitis. Nat Genet. 2012;44:1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Olesen SS, Poulsen JL, Drewes AM, et al. The Scandinavian Baltic Pancreatic Club (SBPC) database: design, rationale and characterisation of the study cohort. Scand J Gastroenterol. 2017;52:909–15. [DOI] [PubMed] [Google Scholar]
- 37. Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology. 2008;8:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available upon reasonable request to the corresponding author.


