Abstract
Background
Gastric intestinal metaplasia is a pre‐cancerous condition associated with multiple factors.
Objective
We evaluated whether cumulative proton pump inhibitor dose is associated with the diagnosis of gastric intestinal metaplasia while controlling for multiple variables.
Methods
We retrospectively identified patients who underwent upper endoscopy with gastric biopsy between 2005 and 2014. Covariate data retrieved included age, sex, ethnicity, smoking status, Helicobacter pylori status (based on clarithromycin‐amoxicillin‐proton pump inhibitor issued), cumulative proton pump inhibitor issued within 10 years (quartiles [PPI‐Q1–4] of daily drug dose), anti‐parietal cell antibodies, body mass index and comorbidity index.
Results
Of the 14,147 included patients (median age 63.4 years; women 54.4%; Helicobacter pylori‐positive 29.0%), 1244 (8.8%) had gastric intestinal metaplasia. Increasing age, Helicobacter pylori infection, smoking, anti‐parietal cell antibodies and proton pump inhibitor use were all associated with the diagnosis of gastric intestinal metaplasia. Upper quartiles of cumulative proton pump inhibitor doses (PPI‐Q4 and PPI‐Q3 vs. PPI‐Q1) were associated with the diagnosis of gastric intestinal metaplasia: adjusted odds ratios 1.32 (95% confidence interval [CI] 1.111.57) and 1.27 (95% CI 1.07–1.52), respectively, for the whole cohort (Ptotal 0.007, Ptrend 0.013), 1.69 (95% CI 1.23–2.33) and 1.40 (95% CI 1.04–1.89), respectively, for Helicobacter pylori‐positive patients (Ptotal 0.004, Ptrend 0.005) and 1.21 (95% CI 0.98–1.49) and 1.20 (95% CI 0.96–1.49), respectively, for Helicobacter pylori‐negative patients (Ptotal 0.288, Ptrend 0.018). Upper quartiles of proton pump inhibitor dose were associated with a 5–10‐fold increased risk of low‐grade dysplasia.
Conclusions
Among Helicobacter pylori‐positive patients, proton pump inhibitor use appears to be associated with a dose‐dependent increased likelihood of gastric intestinal metaplasia.
Keywords: gastric intestinal metaplasia, Helicobacter pylori, proton pump inhibitors
Key Summary
The established knowledge on this subject:
Data regarding the effects of long‐term PPI use on the development of pre‐cancerous gastric lesions such as gastric atrophy and GIM, especially in patients treated for H. pylori infection, are confounding
What are the significant findings of this study?
Among H. pylori‐positive patients, upper quartiles of cumulative PPI doses are significantly associated with the diagnosis of GIM in a dose‐dependent manner
Upper quartiles of PPI use are also associated with 5–10‐fold increased odds for the diagnosis of GIM with dysplasia
INTRODUCTION
Interest in the pathogenesis, diagnosis and management of gastric intestinal metaplasia (GIM), a precursor lesion to gastric dysplasia and cancer, is increasing. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 Emerging evidence from several observational studies suggests that the long‐term use of proton pump inhibitors (PPIs) is associated with a higher risk of gastric cancer development, especially in patients who have undergone Helicobacter pylori eradication. 9 , 10 , 11 , 12 , 13
On the other hand, data regarding the effect of long term PPI use on the development of GIM are conflicting. Previously, two randomised controlled trials have shown that long‐term PPI use was not associated with gastric atrophy or GIM. 14 , 15 More recently, a Cochrane systematic review of four randomised controlled trials reported a non‐significant increase in the risk of atrophic gastritis and GIM in patients with long‐term PPI use. 16 A systematic review that pooled 16 studies (1920 patients) concluded that H. pylori‐positive patients who were receiving long‐term PPI therapy were exposed to a higher risk of corpus atrophy than H. pylori ‐negative patients. 17
However, understanding the association of PPI use with the development of GIM is complex, as histological examination is required and other multiple risk factors are associated with the diagnosis of GIM, such as H. pylori infection, increasing age, cigarette smoking, autoimmune gastropathy, alcohol consumption, bile reflux, a diet low in fruits and vegetables, obesity, high body mass index (BMI) and high salt intake. 2 , 18 , 19 , 20 , 21
Hence, to clarify further the association between PPI use and the development of GIM, we conducted a retrospective endoscopic pathology‐based study to assess whether PPI use is associated with the diagnosis of GIM while controlling for quantitative PPI use, H. pylori infection and multiple other risk factors.
METHODS
Patient selection
This retrospective study was conducted at Rabin Medical Center, an academic referral centre of the Clalit Health Services (CHS), the largest health maintenance organisation (HMO) in Israel. 22 Patients were eligible for inclusion if they were members of the CHS for at least 10 consecutive years before undergoing endoscopy, aged over 18 years, underwent upper endoscopy with a stomach biopsy between January 2005 and December 2014 at Rabin Medical Center, and did not have evidence of previous GIM. They were identified from the electronic database of Rabin Medical Center. Patients were excluded from the study if they had undergone an upper gastrointestinal surgery or had been diagnosed as having gastric cancer, had a previous diagnosis of GIM or cancer predisposition syndromes (Lynch syndrome or familial polyposis).
Endoscopic and pathological data
Endoscopy results were extracted from the patients' electronic medical records (EMRs). EMRs were linked to the pathology database for the diagnosis of GIM (International Classification of Disease, version 10 code K318). All pathology reports with GIM were manually reviewed, and GIM was classified for dysplasia according to the Vienna system (absent, low‐grade dysplasia (LGD), or high‐grade dysplasia) and the extent of intestinal metaplasia (extensive [the involvement of both the antrum and body], focal [the involvement of the antrum or stomach body], or unspecified [indeterminate]). 23 , 24 All cases with GIM were manually reviewed for evidence of prior GIM in the pathology archive of the CHS (updated from January 2000).
Cumulative proton pump inhibitor use
PPI use was defined in accordance with PPI prescriptions issued within 10 years before endoscopy. The cumulative PPI dose (number of daily drug doses [DDD]) within 10 years (prior to endoscopy) was calculated in accordance with the anatomical therapeutic chemical (ATC) classification system 21 (omeprazole, 20 mg; pantoprazole, 40 mg; lansoprazole, 20 mg; and esomeprazole, 30 mg). The total DDD was divided into quartiles (Q1: 0–19, Q2: 20–89, Q3: 90–503, Q4: ≥504).
H. pylori infection status
H. pylori infection status was evaluated on the basis of prescription drug history, available since 1999 at the CHS database. A positive H. pylori infection status was defined when a combination therapy of PPI‐amoxicillin‐clarithromycin was prescribed. 25 Patients who were treated with a combination of PPI‐amoxicillin‐ clarithromycin and had a negative urease breath test (UBT) following the combination treatment were defined as H. pylori‐positive patients with verified eradication. They were sorted according to the time between the endoscopy date and first time negative UBT as follows: 7 or more months before the procedure, within 6 months of the procedure, and 7 or more months after the procedure. A validation set of 100 records of patients who received clarithromycin‐based triple therapy showed that all patients (100%) were indeed treated for H. pylori infection. In 75 cases, the diagnosis of H. pylori infection was done at the time of endoscopy, confirmed by a pathology report or positive rapid urease test result, and in 25 cases, based on the UBT.
Other variables
Other variables included age at endoscopy, sex, ethnicity (Jewish or Arab Israeli), smoking status (current, past or never smoker), 25 presence of anti‐parietal cell antibodies (APCAs) at a threshold of 1/80 or greater, BMI divided into quantiles (<22.86 kg/m2, 22.86–<25.35 kg/m2, 25.35–<27.68 kg/m2, 27.68–<30.83 kg/m2, ≥30.83 kg/m2) and by the World Health Organization (WHO) classification (underweight <18.5 kg/m2, normal weight 18.5–24.9 kg/m2, overweight 25–29.9 kg/m2 and obese ≥30 kg/m2, the age‐adjusted Charlson co‐morbidity index (none, mild, moderate and severe) 26 and a family history of gastrointestinal tract cancer.
Statistical analyses
Binary logistic regression analysis of GIM diagnoses was performed. The variate data included age group, sex, ethnicity, H. pylori infection status (positive or negative), smoking status (current/past or never), BMI (divided into quintiles or WHO classification), age‐adjusted Charlson comorbidity index, family history of gastrointestinal cancer (yes or no), cumulative PPI dose in DDD within 10 years prior to the index endoscopy (divided into quartiles) and the presence APCAs. As both H. pylori positivity and PPI use were associated with the outcome, we repeated the analysis, stratifying according to H. pylori status. For trend analysis, the odds ratios (ORs) were plotted in a linear regression analysis model. In the sensitivity analysis, we repeated the analysis among patients with verified H. pylori eradication up to 6 months after endoscopy. We repeated the analysis with age as a continuous variable (model 2) and performed the analysis for diagnosis of GIM with LGD. Most of the analyses were performed using IBM SPSS v25.
Study oversight and conduct
The Helsinki institutional review board of Rabin Medical Center approved the study and waived the requirement for written informed consent (RMC 544‐17).
RESULTS
Patients
During the study period, 40,178 patients underwent upper gastrointestinal endoscopy. Of the patients, 5888 were excluded (3796 without consecutive CHS membership, 1201 with previous gastric cancer, 705 with current gastric malignancy, 101 with previous GIM and 85 with familial polyposis or Lynch syndrome). Of the remaining 34,391 patients (85.4%), 14,147 (41.4%) had a biopsy taken during endoscopy and were included in the final data set (Figure 1). The patients' median age was 63.4 years. Of the patients, 54.4% were women, 96.8% were Jewish Israeli, 35.5% were current or past smokers and 29% received a clarithromycin‐based triple therapy treatment for H. pylori infection. Of the 14,244 included patients, GIM was detected in 1244 patients (8.8%; Table 1).
FIGURE 1.
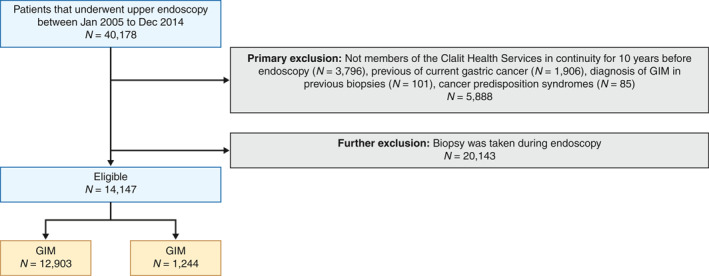
Study flowchart
TABLE 1.
Patient characteristics and cumulative PPI use
| Total, n (%) | 14,147 | (100) |
|---|---|---|
| Age (years), median IQR | 63.3 | 52.1–73.6 |
| Age, group, n (%) | ||
| <50 | 3098 | (21.9) |
| 50–74 | 7974 | (56.4) |
| 75 | 3075 | (21.7) |
| Sex, n (%) | ||
| Women | 7691 | (54.4) |
| Men | 6456 | (45.6) |
| Ethnicity, n (%) | ||
| Jewish | 13,701 | (96.8) |
| Arab | 446 | (3.2) |
| Smoking status, n (%) | ||
| Current/past | 5019 | (35.5) |
| Helicobacter pylori status, n (%) | ||
| Negative | 10,055 | (71.1) |
| Positive | 4092 | (28.9) |
| Anti‐parietal cell antibodies, n (%) | ||
| Yes | 211 | (1.5) |
| PPI cumulative (DDD) a n (%) | ||
| Q1: 0–19 | 3566 | (25.2) |
| Q2: 20–89 | 3652 | (25.8) |
| Q3: 90–503 | 3410 | (24.1) |
| Q4: ≥504 | 3519 | (24.9) |
| Family history b n (%) | ||
| Yes | 120 | (0.8) |
| Charlson comorbidity index | ||
| None | 5298 | (37.4) |
| Mild | 5642 | (39.9) |
| Moderate | 2095 | (14.8) |
| Severe | 1110 | (7.8) |
| Missing | 2 | (0.0) |
| Body mass index quantiles (kg/m2) | ||
| 1st (<22.86) | 2802 | (19.7) |
| 2th (22.86–<25.35) | 2820 | (19.8) |
| 3rd (25.35–<27.68) | 2810 | (19.7) |
| 4th (27.68–30.83) | 2810 | (19.7) |
| 5th (≥30.83) | 2828 | (19.8) |
| Missing | 178 | (1.2) |
Abbreviations: DDD, drug daily dose; IQR, interquartile range; PPI, proton pump inhibitor.
Family history of malignancy of the gastrointestinal tract.
Q1–4: Quartiles of cumulative PPI dose (in DDD) within 10 years preceding index endoscopy.
Factors associated with the diagnosis of GIM
GIM was evident in 13.8% of those aged 75 years old and older, 9.0% of those aged 50–74 years old and 3.3% of those aged under 50 years old (P < 0.001; Table 2).
TABLE 2.
Univariate and multivariate analysis for diagnosis of GIM
| No GIM | GIM | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N(%) | N(%) | OR | 95% | CI | p value | Adjusted OR | 95% | CI | p value | |
| Total | ||||||||||
| N (%) | 12,903 (91.2) | 1244 (8.8) | ||||||||
| Age group, years | ||||||||||
| <50 | 2997 (96.7) | 101 (3.3) | 1.00 | (Ref) | ‐ | ‐ | 1.00 | (Ref) | ‐ | ‐ |
| 50–74 | 7255 (91.1) | 719 (9.0) | 2.92 | 2.36 | 3.61 | <0.001 | 2.80 | 2.24 | 3.51 | <0.001 |
| 75 | 2651 (86.2) | 424 (13.8) | 4.69 | 3.75 | 5.87 | <0.001 | 4.54 | 3.55 | 5.80 | <0.001 |
| p total | <0.001 | ‐ | ‐ | Ptotal | <0.001 | |||||
| Sex | ||||||||||
| Women | 7056 (91.7) | 635 (8.3) | 1.00 | (Ref) | 1.00 | (Ref) | ‐ | ‐ | ||
| Men | 5847 (90.6) | 609 (9.4) | 1.14 | 1.01 | 1.28 | 0.032 | 1.10 | 0.97 | 1.25 | 0.120 |
| Ethnicity | ||||||||||
| Jewish | 12,591 (91.2) | 1208 (8.8) | 1.10 | 0.78 | 1.56 | 0.587 | 1.27 | 0.89 | 1.81 | 0.190 |
| Arab | 410 (91.9) | 36 (8.1) | 1.00 | (Ref) | ‐ | ‐ | 1.00 | (Ref) | ‐ | ‐ |
| Smoking status | ||||||||||
| Never | 8368 (91.7) | 760 (8.3) | 1.00 | (Ref) | ‐ | ‐ | 1.00 | (Ref) | ‐ | ‐ |
| Current/past | 4535 (90.4) | 484 (9.6) | 1.18 | 1.04 | 1.32 | 0.008 | 1.21 | 1.06 | 1.37 | 0.003 |
| H. pylori status | ||||||||||
| Negative | 9183 (91.3) | 872 (8.7) | 1.00 | (Ref) | ‐ | ‐ | 1.00 | (Ref) | ‐ | ‐ |
| Positive | 3720 (91.3) | 372 (9.1) | 1.18 | 1.03 | 1.34 | 0.013 | 1.22 | 1.07 | 1.40 | 0.003 |
| Anti‐parietal cell antibodies | ||||||||||
| No | 12,749 (91.5) | 1187 (8.5) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| Yes | 154 (73.0) | 57 (27.0) | 4.04 | 2.95 | 5.52 | <0.001 | 4.21 | 3.06 | 5.78 | <0.001 |
| PPI cumulative (DDD) a | ||||||||||
| PPI‐Q1 | 3306 (92.7) | 260 (7.3) | 1.00 | (Ref) | ‐ | ‐ | 1.00 | (Ref) | ‐ | ‐ |
| PPI‐Q2 | 3373 (92.4) | 279 (7.6) | 1.05 | 0.88 | 1.24 | 0.573 | 1.10 | 0.92 | 1.32 | 0.284 |
| PPI‐Q3 | 3093 (90.7) | 388 (9.3) | 1.27 | 1.08 | 1.50 | 0.004 | 1.27 | 1.07 | 1.52 | 0.007 |
| PPI‐Q4 | 3131 (89.0) | 388 (11.0) | 1.60 | 1.37 | 1.88 | <0.001 | 1.32 | 1.11 | 1.57 | 0.002 |
| P total | <0.001 | P total | 0.007 | |||||||
| Family history b | ||||||||||
| No | 12,794 (91.2) | 1233 (8.8) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| Yes | 109 (90.8) | 11 (9.2) | 1.13 | 0.61 | 2.13 | 0.693 | 1.17 | 0.62 | 2.19 | 0.633 |
| Charlson comorbidity index | ||||||||||
| None | 4926 (93.0) | 372 (7.0) | 1.00 | (Ref) | ‐ | ‐ | 1.00 | (Ref) | ||
| Mild | 5128 (90.9) | 514 (9.1) | 1.33 | 1.16 | 1.53 | <0.001 | 1.02 | 0.88 | 1.18 | 0.839 |
| Moderate | 1861 (88.8) | 234 (11.2) | 1.67 | 1.40 | 1.98 | <0.001 | 1.12 | 0.93 | 1.35 | 0.252 |
| Severe | 986 (88.8) | 124 (11.2) | 1.67 | 1.34 | 2.06 | <0.001 | 1.04 | 0.82 | 1.31 | 0.748 |
| ‐ | ‐ | P total | <0.001 | ‐ | ‐ | P total | 0.682 | |||
| BMI quantiles | ||||||||||
| 1st | 2571 (91.8) | 231 (8.2) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| 2th | 2536 (89.9) | 284 (10.1) | 1.24 | 1.03 | 1.48 | 0.018 | 1.02 | 0.84 | 1.24 | 0.797 |
| 3rd | 2516 (89.5) | 294 (10.5) | 1.30 | 1.08 | 1.55 | 0.004 | 0.97 | 0.80 | 1.18 | 0.774 |
| 4th | 2525 (89.9) | 285 (10.1) | 1.25 | 1.04 | 1.50 | 0.014 | 0.92 | 0.76 | 1.13 | 0.477 |
| 5th | 2597 (91.8) | 231 (8.2) | 0.99 | 0.81 | 1.19 | 0.910 | 0.75 | 0.61 | 0.92 | 0.007 |
| ‐ | ‐ | P total | 0.003 | ‐ | ‐ | P total | 0.051 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; DDD, drug daily dose; GIM, gastric intestinal metaplasia; OR, odds ratio; PPI, proton pump inhibitor.
Family history of malignant gastrointestinal tract.
Q1‐4: Quartiles of cumulative PPI dose in 10 years preceding index endoscopy.
Age, H. pylori infection (adjusted OR 1.22, 95% confidence interval [CI] 1.07–1.40), current or past smoking (adjusted OR 1.21, 95% CI 1.06–1.37), the presence of APCAs (adjusted OR 4.21, 95% CI 3.06–5.78) and cumulative PPI doses were all significantly associated with a diagnosis of GIM.
As compared to the lower quartile of cumulative PPI doses (PPI‐Q1), PPI‐Q4 and PPI‐Q3 were significantly associated with a diagnosis of GIM: adjusted OR 1.32 (95% CI 1.11–1.57) and 1.27 (95% CI 1.07–1.52), respectively, for the whole cohort; 1.69 (95% CI 1.23–2.33) and 1.40 (95% CI 1.04–1.89), respectively, for H. pylori‐positive patients and 1.21 (95% CI 0.98–1.49) and 1.20 (95% CI 0.96–1.49) for H. pylori‐negative patients (Figure 2, Table 3). In a sensitivity analysis for the group of H. pylori‐positive with verified eradication, the adjusted ORs for PPI‐Q4 and PPI‐Q3 were 2.43 (95% CI 1.44–4.11) and 1.67 (95% CI 1.0–2.78), respectively.
FIGURE 2.
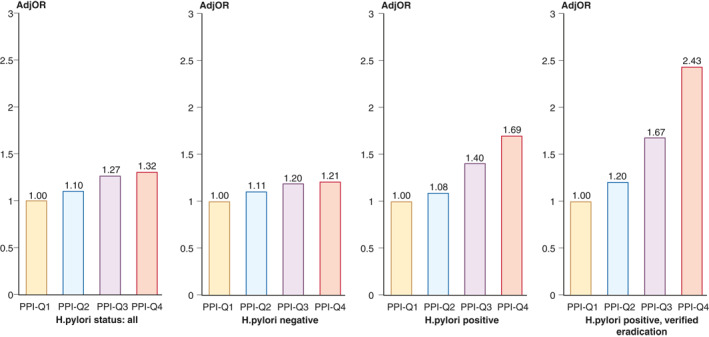
Adjusted odds ratios (ORs) for the association of proton pump inhibitor (PPI) use and the diagnosis of pathologically confirmed gastric intestinal metaplasia (GIM) among the whole cohort, Helicobacter pylori‐negative patients, patients treated for H. pylori infection, and patients with verified H. pylori eradication after treatment (a sensitivity analysis)
TABLE 3.
Univariate and multivariate analysis for diagnosis of GIM with cumulative PPI exposure, stratified by Helicobacter pylori status
| Cohort | PPI quartiles (DDD) a | No GIM | GIM | Univariate | Multivariate (model 1) | Multivariate (model 2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | OR | 95% | CI | p value | Adj OR | 95% | CI | p value | Adj OR | 95% | CI | p value | ||
| H. pylori status: All | PPI‐Q1 | 3306 (92.7) | 260 (7.3) | 1.00 | (Ref) | 1.00 | (Ref) | 1.00 | (Ref) | ||||||
| PPI‐Q2 | 3373 (92.4) | 279 (7.6) | 1.05 | 0.88 | 1.24 | 0.573 | 1.10 | 0.92 | 1.32 | 0.284 | 1.12 | 0.94 | 1.33 | 0.206 | |
| PPI‐Q3 | 3093 (90.7) | 388 (9.3) | 1.27 | 1.08 | 1.50 | 0.004 | 1.27 | 1.07 | 1.52 | 0.007 | 1.26 | 1.06 | 1.49 | 0.010 | |
| PPI‐Q4 | 3131 (89.0) | 388 (11.0) | 1.60 | 1.37 | 1.88 | <0.001 | 1.32 | 1.11 | 1.57 | 0.002 | 1.32 | 1.11 | 1.57 | 0.001 | |
| Total | 12,903 (91.2) | 1244 (8.8) | P total | <0.001 | P total | 0.007 | P total | 0.008 | |||||||
| Trend | R 2: 0.94 | P trend | 0.006 | R 2: 0.90 | P trend | 0.013 | R2: 0.90 | P trend | 0.013 | ||||||
| H. pylori negative | PPI‐Q1 | 1935 (92.8) | 151 (7.2) | 1.00 | (Ref) | 1.00 | (Ref) | 1.00 | (Ref) | ||||||
| PPI‐Q2 | 2258 (92.5) | 184 (7.5) | 1.04 | 0.84 | 1.31 | 0.704 | 1.11 | 0.88 | 1.39 | 0.387 | 1.10 | 0.88 | 1.38 | 0.402 | |
| PPI‐Q3 | 2330 (91.1) | 227 (8.9) | 1.25 | 1.01 | 1.55 | 0.043 | 1.20 | 0.96 | 1.49 | 0.102 | 1.17 | 0.94 | 1.45 | 0.157 | |
| PPI‐Q4 | 2660 (89.6) | 310 (10.4) | 1.49 | 1.22 | 1.83 | 0.000 | 1.21 | 0.98 | 1.49 | 0.079 | 1.21 | 0.98 | 1.49 | 0.074 | |
| Total | 9183 (91.3) | 872 (8.7) | P total | <0.001 | P total | 0.288 | P total | 0.321 | |||||||
| Trend | R 2: 0.93 | P trend | 0.008 | R 2: 0.88 | P trend | 0.018 | R 2: 0.88 | P trend | 0.018 | ||||||
| H. pylori positive | PPI‐Q1 | 1371 (92.6) | 109 (7.4) | 1.00 | (Ref) | 1.00 | (Ref) | 1.00 | (Ref) | ||||||
| PPI‐Q2 | 1115 (92.1) | 95 (7.9) | 1.07 | 0.81 | 1.43 | 0.636 | 1.08 | 0.81 | 1.44 | 0.615 | 1.11 | 0.84 | 1.47 | 0.450 | |
| PPI‐Q3 | 763 (89.4) | 90 (10.6) | 1.48 | 1.11 | 1.99 | 0.008 | 1.40 | 1.04 | 1.89 | 0.026 | 1.39 | 1.04 | 1.85 | 0.028 | |
| PPI‐Q4 | 471 (85.8) | 78 (14.2) | 2.08 | 1.53 | 2.84 | <0.001 | 1.69 | 1.23 | 2.33 | 0.001 | 1.65 | 1.21 | 2.25 | 0.002 | |
| Total | 3720 (90.9) | 372 (9.1) | P total | <0.001 | P total | 0.004 | P total | 0.008 | |||||||
| Trend | R 2: 0.97 | P trend | 0.002 | R 2:0.95 | P trend | 0.005 | R 2: 0.95 | P trend | 0.005 | ||||||
| H. pylori positive and verified eradication | PPI‐Q1 | 444 (92.1) | 38 (7.9) | 1.00 | (Ref) | 1.00 | (Ref) | 1.00 | (Ref) | ||||||
| PPI‐Q2 | 312 (90.7) | 32 (9.3) | 1.20 | 0.73 | 1.96 | 0.471 | 1.20 | 0.73 | 1.97 | 0.477 | 1.22 | 0.74 | 2.03 | 0.432 | |
| PPI‐Q3 | 199 (86.5) | 31 (13.5) | 1.82 | 1.10 | 3.01 | 0.020 | 1.67 | 1.00 | 2.78 | 0.050 | 1.69 | 1.00 | 2.85 | 0.048 | |
| PPI‐Q4 | 128 (80.0) | 32 (20.0) | 2.92 | 1.76 | 4.86 | <0.001 | 2.43 | 1.44 | 4.11 | 0.001 | 2.21 | 1.29 | 3.81 | 0.004 | |
| Total | 1083 (89.1) | 133 (10.9) | P total | <0.001 | P total | 0.006 | P total | 0.023 | |||||||
| R 2: 0.98 | P trend | 0.001 | R 2: 0.98 | P trend | 0.001 | R 2: 0.98 | P trend | 0.001 | |||||||
Note: Model 1: age as a categorical variable; Model 2: age as a continuous variable; R 2:R square value of the regression model.
Abbreviations: CI, confidence interval; DDD, drug daily dose; GIM, gastric intestinal metaplasia; OR, odds ratio; PPI, proton pump inhibitor.
Ql–4: Quartiles of cumulative PPI dose in 10 years preceding index endoscopy.
As can be seen in Table 3, these associations remained stable when age was used as either a continuous variable or a categorical variable. As can be seen in Table 3, the trend analysis yielded a positive dose‐response with a strong fit (R 2 > 0.95) for the H. pylori‐positive group and the H. pylori‐positive with verified eradication.
After adjusting for multiple variates, gender, ethnicity and the comorbidity index were not significantly associated with the diagnosis of GIM. Analysis of the association between BMI and diagnosis of GIM showed that after adjusting for all variables, as compared to the first quintile, the fifth quintile was associated with reduced odds for a diagnosis of GIM (adjusted OR, 0.75%, 95% 0.61–0.92). Repeated analysis with BMI classified according to WHO classification yielded consistent results (obese: adjusted OR 0.76, 95% CI 0.64–0.89; normal weight: referent; see Table S1).
GIM with LGD was diagnosed in 43 patients (0.3%). In multivariable analysis, independent variables associated with the diagnosis of GIM with LGD were increasing age, the presence of APCAs (adjusted OR 6.76, 95% CI 2.02–22.6), and upper quartiles (PPI‐Q4 and PPI‐Q3) of PPI use (adjusted OR 10.1, 95% CI 2.32–44.1 and adjusted OR 5.66, 95% CI 1.24–25.83, respectively; PPI‐Q1: reference; Table 4).
TABLE 4.
Univariate and multivariate analysis for diagnosis of GIM with LGD
| No GIM‐LGD | GIM‐LGD | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | OR | 95% | CI | p value | Adj OR | 95% | CI | p value | |
| Total | 14,248 (99.7) | 43 (0.3) | ||||||||
| Age group, years | ||||||||||
| <50 | 3102 (100) | 1 (0.0) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| 50–74 | 8020 (99.8) | 16 (0.2) | 6.18 | 0.82 | 46.6 | 0.077 | 5.28 | 0.68 | 41.02 | 0.112 |
| 75 | 3083 (99.2) | 26 (0.8) | 26.1 | 3.54 | 192.0 | 0.001 | 22.30 | 2.85 | 174.32 | 0.003 |
| P total | < 0.001 | P total | < 0.001 | |||||||
| Sex | ||||||||||
| Women | 7726 (99.7) | 23 (0.3) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| Men | 6479 (99.7) | 20 (0.3) | 1.03 | 0.56 | 1.89 | 0.906 | 1.20 | 0.64 | 2.25 | 0.567 |
| Ethnicity | ||||||||||
| Jewish | 13,757 (99.7) | 42 (0.3) | 1.36 | 0.18 | 9.96 | 0.757 | 1.55 | 0.21 | 11.66 | 0.670 |
| Arab | 448 (99.8) | 1 (0.2) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| Smoking status | ||||||||||
| Never | 9164 (99.7) | 31 (0.3) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| Current/past | 5041 (99.8) | 12 (0.2) | 0.70 | 0.36 | 1.37 | 0.302 | 0.81 | 0.40 | 1.64 | 0.572 |
| Helicobacter pylori status | ||||||||||
| Negative | 10,084 (99.7) | 34 (0.3) | 1.00 | (Ref) | 1.00 | |||||
| Positive | 4121 (99.8) | 9 (0.2) | 0.65 | 0.31 | 1.35 | 0.247 | 1.05 | 0.49 | 2.23 | 0.894 |
| Anti‐parietal cell antibodies | ||||||||||
| No | 13,995 (99.7) | 40 (0.3) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| Yes | 210 (98.6) | 3 (1.4) | 4.98 | 1.53 | 16.28 | 0.008 | 6.76 | 2.02 | 22.6 | 0.002 |
| PPI cumulative (DDD) a | ||||||||||
| PPI‐Q1 | 3585 (99.9) | 2 (0.1) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| PPI‐Q2 | 3668 (99.8) | 6 (0.2) | 2.93 | 0.59 | 14.5 | 0.188 | 3.25 | 0.65 | 16.20 | 0.151 |
| PPI‐Q3 | 3417 (99.7) | 11 (0.3) | 5.77 | 1.28 | 26.0 | 0.023 | 5.66 | 1.24 | 25.83 | 0.025 |
| PPI‐Q4 | 3535 (88.8) | 24 (0.7) | 12.1 | 2.87 | 51.5 | 0.001 | 10.12 | 2.32 | 44.10 | 0.002 |
| P total | < 0.001 | P total | 0.004 | |||||||
| Family history b | ||||||||||
| No | 14,086 (99.7) | 42 (0.3) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| Yes | 119 (99.2) | 1 (0.8) | 2.81 | 0.38 | 20.6 | 0.308 | 4.65 | 0.61 | 35.17 | 0.137 |
| Charlson comorbidity index | ||||||||||
| None | 5286 (99.8) | 12 (0.2) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| Mild | 5619 (99.6) | 23 (0.4) | 1.80 | 0.90 | 3.63 | 0.098 | 0.94 | 0.46 | 1.92 | 0.855 |
| Moderate | 2088 (99.7) | 7 (0.3) | 1.48 | 0.58 | 3.76 | 0.413 | 0.54 | 0.20 | 1.43 | 0.216 |
| Severe | 1109 (99.9) | 1 (0.1) | 0.40 | 0.05 | 3.06 | 0.375 | 0.12 | 0.02 | 0.92 | 0.041 |
| P total | 0.221 | P total | 0.132 | |||||||
| BMI quantiles | ||||||||||
| 1st | 2788 (99.5) | 14 (0.5) | 1.00 | (Ref) | 1.00 | (Ref) | ||||
| 2th | 2814 (99.8) | 6 (0.2) | 0.42 | 0.16 | 1.10 | 0.080 | 0.30 | 0.11 | 0.79 | 0.015 |
| 3rd | 2797 (99.5) | 13 (0.5) | 0.92 | 0.43 | 1.97 | 0.841 | 0.58 | 0.26 | 1.25 | 0.166 |
| 4th | 2806 (99.9) | 13 (0.5) | 0.28 | 0.09 | 0.86 | 0.027 | 0.18 | 0.60 | 0.56 | 0.003 |
| 5th | 2822 (99.8) | 6 (0.2) | 0.43 | 0.16 | 1.10 | 0.079 | 0.33 | 0.12 | 0.87 | 0.026 |
| P total | 0.061 | P total | 0.011 |
Abbreviations: BMI, body mass index; CI, confidence interval; DDD, drug daily dose; GIM, gastric intestinal metaplasia; LGD, low‐grade dysplasia; OR, odds ratio; PPI, proton pump inhibitor.
Family history of malignant gastrointestinal tract.
Q1–4: Quartiles of cumulative PPI dose in 10 years preceding index endoscopy.
Extensive GIM was evident in 232 of the 804 subjects with GIM and separate biopsies that were obtained from both the antrum and stomach body (28.9%). After adjusting for age, only the presence of APCAs was significantly associated with extensive GIM (adjusted OR 2.05, 95% CI 1.14–3.70).
DISCUSSION
Our findings show a dose‐dependent association of cumulative PPI dose with the diagnosis of GIM. We have also shown that the association of cumulative PPI use with the diagnosis of GIM is dependent on H. pylori status: from 1.7–1.4‐fold for the cohort of patients who were treated for H. pylori infection to a non‐significant association for the cohort of H. pylori‐negative patients. Furthermore, analysis of the factors associated with the diagnosis of GIM with LGD revealed that apart from increasing age and the presence of APCAs, upper quartiles of PPI use were associated with a 5–10‐fold increased odds. In a sensitivity analysis, we found the magnitude of association of PPI dose with GIM was even higher. It is unclear why the risk of GIM appears to be higher in patients with confirmed H. pylori eradication compared to patients treated for H. pylori but without confirmed eradication. This may be related to better compliance in this subset of patients, not only for performing a follow‐up breath test to confirm eradication but also for taking the PPI prescribed. We cannot rule out bias due to the smaller sample size group and false negative eradication results due to continuous PPI use before performing the breath test to verify eradication.
As stated earlier, data regarding the effects of long‐term PPI use on the development of pre‐cancerous gastric lesions such as gastric atrophy or GIM are conflicting. 12 , 27 While two randomised controlled trials reported no association between PPI use and gastric atrophy or GIM, 14 , 15 a recent Cochrane review of four randomised controlled trials reported non‐significant increases in the risks of atrophic gastritis and GIM in patients with long‐term PPI use. 16 Finally, a systematic review that pooled 16 studies concluded that H. pylori‐positive patients who were receiving long‐term PPI therapy were exposed to a higher risk of corpus atrophy than H. pylori‐negative patients. 17
As guidelines are conflicting regarding routine H. pylori testing before PPI use, 28 , 29 , 30 our findings suggest that determining H. pylori infection status before commencing long‐term PPI treatment should be considered. Several clinical decision tools as well as the American Gastroenterological Association (AGA) guidelines have also recently been published regarding the optimal management of GIM 8 , 31 , 32 ; however, none has addressed PPI use as a risk factor for GIM development. Given our findings, it may be advisable to minimise PPI exposure as much as clinically possible in patients with GIM, just as it is advisable to stop smoking and eradicate H. pylori.
A recent large Korean study reported that obesity was independently associated with an increased incidence of GIM. 21 In our study, the univariate analysis did show an association between increasing BMI and a diagnosis of GIM. However, after adjusting for multiple variables, this association was no longer observed, and a negative association was observed for the obese (as defined by either the WHO classification and by quintiles). Our findings are consistent with a recent study performed among US veterans, which found that smoking is associated with a diagnosis of GIM. 20
The precise mechanism by which PPIs may induce gastric cancer is unclear, although several mechanisms have been proposed. PPIs induce hypergastrinemia and hypochlorhydria, which may contribute to enterochromaffin‐like cell hyperplasia and proliferation of gastric mucosa. PPI‐induced hypergastrinemia occurs due to inhibition of the somatostatin‐mediated negative feedback of gastrin release on antral G cells. 17 Similarly, gastrin may exert a direct trophic effect on the oxyntic mucosa. It has also been hypothesised that PPI‐induced alterations of the gastric microbiome may play a role in carcinogenesis. However, a recent study showed that PPI‐treated patients showed similar microbial diversity compared with normal subjects, while patients with H. pylori‐induced atrophic gastritis manifested a lower bacterial abundance and diversity. This finding suggests that PPIs do not significantly alter gastric microbiota nor do they contribute significantly to the development of gastric cancer. 33
The main strength of our study is that it is a large‐scale endoscopic pathology‐based study conducted in an HMO. We verified continuous membership, quantified PPI use for up to 10 years before endoscopy, defined H. pylori infection status according to a clarithromycin‐based triple therapy and UBT result, validated the treatment regimen from the patient records and performed a sensitivity analysis for patients with verified H. pylori eradication up to 6 months after endoscopy. We also verified that the patients with a GIM diagnosis did not have a diagnosis in the pathology database of the HMO. Also, the associations of increasing age, treatment for H. pylori infection and APCAs with the incidence of GIM observed in our study are in accordance with those reported in other studies 2 , 3 , 18 , 19 , 20 and validate our findings.
As a retrospective association study, the main limitation of this study is that it did not clarify whether the association between PPI use and the diagnosis of GIM is causative. Also, our sample size was not large enough to allow stratification according to the timing of H. pylori eradication. We also did not have information about the indications for PPI use, adherence to the operative link on the GIM assessment protocol for gastric biopsies, 34 indication for performing endoscopy and the use of over‐the‐counter (OTC) PPI use. Nevertheless, OTC PPI use was unlikely to be significant because PPI therapy is only reimbursed when the drug is purchased at CHS member pharmacies. As the diagnosis of H. pylori infection was inferred from dispensed drugs with clarithromycin‐based triple therapy, some misclassification of H. pylori‐negative patients may have occurred, as patients who were allergic to penicillin were treated with non‐clarithromycin‐based therapies and therefore incorrectly categorised as H. pylori negative. Nevertheless, this does not affect the H. pylori‐positive cohort, and the effect was probably limited in size among the total H. pylori‐negative cohort. It has also been shown that in our region during the study period 93% of primary care physicians used clarithromycin‐based triple therapy for first‐line treatment of H. pylori infection. 35 Finally, dietary habits which may play a role in GIM have not been taken into account and histological subtyping of GIM as complete or incomplete was not performed, and there are still considerable barriers to the application of this diagnostic tool. 36
In summary, we report a dose‐dependent association of PPI doses with the diagnosis of GIM among patients treated for H. pylori infection but not in H. pylori‐negative patients. We also report that the upper quartiles of PPI use were associated with a 5–10‐fold increased risk of GIM with LGD. As already suggested, 27 larger prospective studies with repeated endoscopy are needed to monitor the incidence of GIM according to H. pylori infection status and PPI use. Translational studies to elucidate further the mechanisms involved in PPI‐induced carcinogenesis are also necessary. In the meantime, we suggest that healthcare professionals should be mindful of the association we describe and administer the minimum effective PPI dose. Healthcare professionals should consider evaluating patients' H. pylori infection status before initiating long‐term PPI treatment.
AUTHOR CONTRIBUTIONS
Specific author contributions: conceptualisation and design: Yifat Snir, Zohar Levi and Arnon D Cohen; data extraction: Ilan Feldhamer and Haim Leibovitzh; analysis and interpretation of data: Ilan Feldhamer, Alex Vilkin and Tzippy Shochat; drafting of the manuscript: Yifat Snir and Yaara Leibovici‐Weissman; critical revision of the manuscript for valuable intellectual content: Doron Boltin, Yaron Niv and Iris Dotan; final approval of the paper: Zohar Levi and Doron Boltin; guarantor of the article: Zohar Levi. All authors approved the final version of the paper.
ETHICS APPROVAL
The Helsinki institutional review board of Rabin Medical Center approved the study.
INFORMED CONSENT
The Helsinki institutional review board of Rabin Medical Center approved the study and waived the requirement for written informed consent (RMC 544‐17).
Supporting information
Supplementary Material
ACKNOWLEDGEMENT
This study received financial support from the Rabin Medical Center Research Fund.
Doron Boltin and Zohar Levi contributed equally.
REFERENCES
- 1. Graham DY, Rugge M, Genta RM. Diagnosis: gastric intestinal metaplasia—what to do next? Curr Opin Gastroenterol. 2019;35:535–43. 10.1097/mog.0000000000000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang RJ, Ende AR, Singla A, et al. Prevalence, risk factors, and surveillance patterns for gastric intestinal metaplasia among patients undergoing upper endoscopy with biopsy. Gastrointest Endosc. 2019. 10.1016/j.gie.2019.07.038. [DOI] [PubMed] [Google Scholar]
- 3. Pimentel‐Nunes P, Libanio D, Marcos‐Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European society of gastrointestinal endoscopy (ESGE), European Helicobacter and microbiota study group (EHMSG), European society of pathology (ESP), and sociedade Portuguesa de endoscopia digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365–88. 10.1055/a-0859-1883. [DOI] [PubMed] [Google Scholar]
- 4. Akbari M, Tabrizi R, Kardeh S, et al. Gastric cancer in patients with gastric atrophy and intestinal metaplasia: a systematic review and meta‐analysis. PLoS One. 2019;14:e0219865. 10.1371/journal.pone.0219865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lahner E, Zagari RM, Zullo A, et al. Chronic atrophic gastritis: natural history, diagnosis and therapeutic management. A position paper by the Italian society of hospital gastroenterologists and digestive endoscopists [AIGO], the Italian society of digestive endoscopy [SIED], the Italian society of Gastroenterology [SIGE], and the Italian society of Internal medicine [SIMI]. Dig Liver Dis. 2019;51:1621–32. 10.1016/j.dld.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 6. Dhingra R, Natov NS, Daaboul Y, et al. Increased risk of progression to gastric adenocarcinoma in patients with non‐dysplastic gastric intestinal metaplasia versus a control population. Dig Dis Sci. 2020. 10.1007/s10620-019-06031-5. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen TH, Tan MC, Liu Y, et al. Prevalence of gastric intestinal metaplasia in a multi‐ethnic United States veterans population. Clin Gastroenterol Hepatol. 2020;S1542‐3565 (20):30325–6. 10.1016/j.cgh.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altayar O, Davitkov P, Shah SC, et al. AGA Technical review on gastric intestinal metaplasia‐epidemiology and risk factors. Gastroenterology. 2020;158:732–44.e716. 10.1053/j.gastro.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tran‐Duy A, Spaetgens B, Hoes AW, et al. Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2016;14:1706–19.e1705. 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 10. Brusselaers N, Wahlin K, Engstrand L, et al. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: a nationwide population‐based cohort study in Sweden. BMJ Open. 2017;7:e017739. 10.1136/bmjopen-2017-017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang K, Jiang X, Wen Y, et al. Relationship between long‐term use of proton pump inhibitors and risk of gastric cancer: a systematic analysis. J Gastroenterol Hepatol. 2019. 10.1111/jgh.14759. [DOI] [PubMed] [Google Scholar]
- 12. Joo MK, Park JJ, Chun HJ. Proton pump inhibitor: the dual role in gastric cancer. World J Gastroenterol. 2019;25:2058–70. 10.3748/wjg.v25.i17.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheung KS, Chan EW, Wong AYS, et al. Long‐term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population‐based study. Gut. 2018;67:28–35. 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
- 14. Lundell L, Miettinen P, Myrvold HE, et al. Lack of effect of acid suppression therapy on gastric atrophy. Nordic Gerd Study GroupGastroenterology. 1999;117:319–26. 10.1053/gast.1999.0029900319. [DOI] [PubMed] [Google Scholar]
- 15. Lundell L, Havu N, Miettinen P, et al. Changes of gastric mucosal architecture during long‐term omeprazole therapy: results of a randomized clinical trial. Aliment Pharmacol Ther. 2006;23:639–47. 10.1111/j.1365-2036.2006.02792.x. [DOI] [PubMed] [Google Scholar]
- 16. Song H, Zhu J, Lu D. Long‐term proton pump inhibitor (PPI) use and the development of gastric premalignant lesions. Cochrane Database Syst Rev. 2014;12:Cd010623. 10.1002/14651858.CD010623.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lundell L, Vieth M, Gibson F, et al. Systematic review: the effects of long‐term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42:649–63. 10.1111/apt.13324. [DOI] [PubMed] [Google Scholar]
- 18. Shao L, Li P, Ye J, et al. Risk of gastric cancer among patients with gastric intestinal metaplasia. Int J Canc. 2018. 10.1002/ijc.31571. [DOI] [PubMed] [Google Scholar]
- 19. Liu KS, Wong IO, Leung WK. Helicobacter pylori associated gastric intestinal metaplasia: treatment and surveillance. World J Gastroenterol. 2016;22:1311–20. 10.3748/wjg.v22.i3.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan MC, Mallepally N, Liu Y, et al. Demographic and lifestyle risk factors for gastric intestinal metaplasia among US veterans. Am J Gastroenterol. 2020. 10.14309/ajg.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 21. Kim K, Chang Y, Ahn J, et al. Body mass index and risk of intestinal metaplasia: a cohort study. Cancer Epidemiol Biomarkers Prev. 2019;28:789–97. 10.1158/1055-9965.Epi-18-0733. [DOI] [PubMed] [Google Scholar]
- 22. Wikipedia. Clalit Health Services. 2018. https://en.wiki pedia.org/wiki/Clalit_Health_Services. Accessed 4 Aug 2020. [Google Scholar]
- 23. Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–8. 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 25. Itskoviz D, Boltin D, Leibovitzh H, et al. Smoking increases the likelihood of Helicobacter pylori treatment failure. Dig Liver Dis. 2017;49:764–8. 10.1016/j.dld.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 26. Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 27. Cheung KS, Leung WK. Risk of gastric cancer development after eradication of Helicobacter pylori . World J Gastrointest Oncol. 2018;10:115–23. 10.4251/wjgo.v10.i5.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection—the maastricht V/florence consensus report. Gut. 2017;66:6–30. 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 29. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection ‐ the maastricht IV/florence consensus report. Gut. 2012;61:646–64. 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 30. Zagari RM, Romano M, Ojetti V, et al. Guidelines for the management of Helicobacter pylori infection in Italy: the III working group consensus report 2015. Dig Liver Dis. 2015;47:903–12. 10.1016/j.dld.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 31. Shah SC, Gawron AJ, Li D. Surveillance of gastric intestinal metaplasia. Am J Gastroenterol. 2020. 10.14309/ajg.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dinis‐Ribeiro M, Kuipers EJ. How to manage a patient with gastric intestinal metaplasia. Gastroenterology. 2020. 10.1053/j.gastro.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 33. Parsons BN, Ijaz UZ, D'Amore R, et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori‐induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLOS Pathogens. 2017;13:e1006653. 10.1371/journal.ppat.1006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta‐analysis. Gastric Cancer. 2018. 10.1007/s10120-018-0812-3. [DOI] [PubMed] [Google Scholar]
- 35. Boltin D, Kimchi N, Dickman R, et al. Attitudes and practice related to Helicobacter pylori infection among primary care physicians. Eur J Gastroenterol Hepatol. 2016;28:1035–40. 10.1097/MEG.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 36. Shah SC, Gawron AJ, Mustafa RA, et al. Histologic subtyping of gastric intestinal metaplasia: overview and considerations for clinical practice. Gastroenterology. 2020;158:745–50. 10.1053/j.gastro.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


