Abstract
Adenomatous polyposis (AP) diseases, including familial adenomatous polyposis (FAP), attenuated FAP (AFAP), and MUTYH‐associated polyposis (MAP), are the second most common hereditary causes of colorectal cancer. A frequent extra‐colonic manifestation of AP disease is duodenal polyposis, which may lead to duodenal cancer in up to 18% of AP patients. Endoscopic surveillance is recommended at 0.5‐ to 5‐year intervals depending on the extent of polyp growth and histological progression. Although the Spigelman classification is traditionally used to determine surveillance intervals, it lacks information on the (peri‐)ampullary site, where 50% of duodenal carcinomas are located. Hence, information on the papilla has recently been added as a prognostic marker. Patients with duodenal adenoma(s) ≥10 mm and ampullary adenomas of any size are suggested to be referred to an expert center for endoscopic therapy, particularly endoscopic mucosal resection and endoscopic ampullectomy. Nonetheless, despite the logic of this approach, the long‐term efficacy of endoscopic therapy is still to be demonstrated.
Keywords: duodenal adenomatosis, duodenal cancer, endoscopic management, familial adenomatous polyposis (FAP), surveillance
INTRODUCTION
With a prevalence of 1 in 10,000 individuals, familial adenomatous polyposis (FAP) is the second most common inherited colorectal cancer (CRC) syndrome. 1 , 2 FAP is caused by an autosomal dominant, highly penetrant mutation in the adenomatous polyposis coli (APC) gene. 3 APC encodes for a tumor suppressor gene, and a mutation in this gene induces the formation of ≥100 synchronous polyps distributed throughout the gastrointestinal tract. Initially, the vast majority of these polyps emerge in the colon, causing CRC if left untreated in virtually all FAP patients at a mean age of 35–45 years. 2 Since the introduction of colonic screening and prophylactic colorectal surgery, mortality from CRC has almost completely been eliminated in FAP patients, and duodenal or ampullary cancer has become the leading cause of cancer‐related mortality in FAP patients.
Two hereditary polyposis syndromes are closely related to FAP. First, attenuated FAP (AFAP) is seen in approximately 10% of cases with a known APC mutation and is characterized by less extended synchronous colorectal polyposis compared to FAP patients. 4 For clinical management, it is important to realize that colorectal polyposis has a right‐sided predominance in AFAP, in contrast to FAP. 5 Furthermore, colorectal polyposis and CRC usually develop later in life in AFAP with an approximate delay of 10 and 15 years, respectively. 6 , 7 Second, MUTYH‐associated polyposis (MAP) is a condition that resembles the clinical characteristics of AFAP but is caused by a recessive mutation of the MUTYH gene. Colorectal polyposis in MAP patients develops even later in life, at a mean age of 40. 1 Both AFAP and MAP have a lower prevalence of duodenal adenomatosis compared to FAP. Nonetheless, the risk of developing duodenal cancer is comparable to that in FAP patients. 6 , 8 , 9
For the prevention of duodenal cancer development, various guidelines recommend starting duodenal screening at the age of 25–35 in FAP, AFAP, and MAP patients (collectively referred to as AP patients). 10 , 11 , 12 , 13 The frequency of surveillance is mostly determined by the Spigelman classification, which measures the extent of duodenal polyposis as a predictor of duodenal cancer risk. 14 However, studies on the clinical management of duodenal adenomatosis are still scarce, which explains why guidelines on the management of duodenaladenomas in AP are mainly based on expert opinion.
In this review, a clinical case is presented to discuss the clinical management of duodenal adenomatosis in AP patients based on the latest insights from the literature. The aim is to assist clinicians in the management of duodenal adenomatosis in AP patients.
CASE
We present a 50‐year‐old male who had undergone subtotal colectomy with ileorectal anastomosis at the age of 13, shortly after a diagnosis of FAP. Two years later, additional ileoanal pouch surgery was performed because of the presence of numerous rectal adenomas. He is under duodenal surveillance since 2010, when he was referred to our hospital at the age of 40 to participate in a randomized trial investigating the prophylactic effect of celecoxib and ursodeoxycholic acid co‐treatment on duodenal adenomatosis in FAP. 15 Since 2010, he has undergone seven duodenoscopies with a median interval of 1 year (range 0.5–3). He had insisted on undergoing as few as possible surveillance duodenoscopies, and when scheduled, canceled his appointments many times.
During the first duodenoscopy, a Spigelman stage of III was found, which, over the years, increased to stage IV, but decreased to stage III again in 2019 without performing any endoscopic intervention (see Figure 1). In 2017, the modified Spigelman classification was implemented, meaning that duodenal lesions were no longer biopsied to prevent scarring, which could potentially complicate performing endoscopic mucosal resection (EMR). As part of this changed policy, we also started performing EMR of duodenal adenomas larger than 10 mm. As no longer biopsies are taken since 2017, the Spigelman score is calculated based on the previous histopathological outcomes as no mucosal tissue is available. Additionally, we agreed to increase the Spigelman classification by one stage in the presence of endoscopically papillary adenoma.
FIGURE 1.
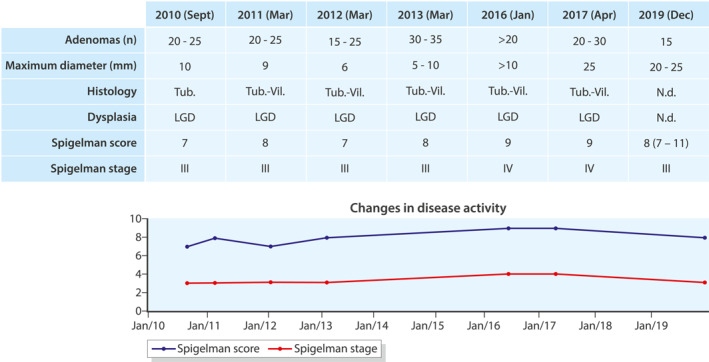
Duodenal disease activity throughout the years. Tub, tubular; Vil, villous; LGD, low‐grade dysplasia; n.d., not done; *participation in RCT
Over 10 years (2010–2020), the papilla appeared normal, but in 2011 and 2012, histological examination of periampullary adenomatous‐like tissue showed a tubulovillous adenoma with low‐grade dysplasia (LGD). At this time, no further action was taken.
LITERATURE SEARCH
In order to summarize the latest findings on endoscopic surveillance and treatment of duodenal adenomatosis in FAP, a PubMed search was performed with the MeSH term “familial adenomatous polyposis” or this wording in the title field. Our search was limited to articles written in English and published in the last 10 years at the time of the search (June 2020). This resulted in a total of 2277 articles that were screened for title and abstract. Of the studies included, reference lists were screened to identify additional papers. Only articles that focused on (endoscopic) surveillance and management of duodenal adenomatosis were included. A total of 137 articles were identified that met our inclusion criteria.
WHY SURVEIL DUODENAL ADENOMATOSIS?
AP patients are at an increased risk of developing duodenal polyposis and duodenal cancer. The cumulative incidence of duodenal polyposis in FAP patients is 50%–90% at age 70. The severity of duodenal polyposis increases with age, with a cumulative risk of approximately 50% of Spigelman stage IV at age 70. 16 , 17 Nonetheless, this cumulative risk is presumably an overestimation since increasing Spigelman scores over the years can (at least) partly be explained by improvements in endoscopic technology. 18 The cumulative incidence of duodenal cancer in FAP patients is 2%–5% at age 55 which increases to 18% at age 75. 16 , 19 , 20 , 21 As already previously stated, despite a lower incidence of duodenal adenomatosis in AFAP and MAP patients (12% and 17%–34%, respectively), the risk of duodenal carcinoma is similar to FAP patients. 4 , 8 , 9 , 16 , 22
Studies have shown a beneficial effect of upper GI endoscopic surveillance with regard to survival in AP patients. A study by Vasen et al. concluded that endoscopic surveillance resulted in an increased life expectancy of 7 months. 23 Furthermore, a more recent study reported that the prognosis of duodenal cancer is much better when an asymptomatic duodenal carcinoma was detected by screening endoscopy than when cancer had already become symptomatic (8 vs. 0.8 years, respectively; p < 0.0001). 21
WHO SHOULD WE SURVEIL?
All AP patients should be surveilled for duodenal neoplasia, but some delay in initiating surveillance is justified in the case of MAP. It is recommended to start surveillance at ages 20–30 and 25 for (A)FAP patients, according to the US and European guidelines, respectively. For MAP patients, this can be delayed to ages 30–35 and 35, respectively. 10 , 11 , 12 , 13 The delay in initiation of surveillance in MAP patients is based on a European study including 92 MAP patients that demonstrated that duodenal polyposis in patients with MAP develops less frequently and at a later age compared to patients with FAP. 9
HOW SHOULD WE SURVEIL?
During each duodenoscopy, the duodenum and the ampullary site should be thoroughly assessed and documented. Guidelines recommend to complement standard forward‐viewing duodenoscopy with a side‐viewing duodenoscope or with cap‐assisted forward‐viewing endoscopy. 10 , 11 , 12 , 13 The latter is based on a study that showed that a short, transparent cap at the tip of a gastroscope allows visualizing the duodenum and the ampulla in 95% of FAP patients. 24 Thorough endoscopic assessment of the ampulla is important since approximately 50% of duodenal cancers are located in this region. 25 Although chromoendoscopy, in general, may increase adenoma detection, its use in duodenal surveillance is not recommended. 26 It is still debated whether the detection of more adenomas actually reflects a higher cancer risk. This is at least partly based on recent data that suggests that large polyp size (>10 mm), rather than a high number of adenomas, is associated with duodenal cancer risk in FAP patients. 27 Endoscopic ultrasonography (EUS) is also not generally recommended, but may be considered for large or suspicious (peri‐)ampullary lesions, mainly to better differentiate benign from invasive growth. 11 Although one study reported that in 10/28 patients, disease severity changed after EUS examination due to a difference in the estimated lesion size; this was not confirmed in two other studies in high‐risk patients. 28 , 29 , 30
HOW OFTEN SHOULD WE SURVEIL?
International guidelines slightly differ on how frequent surveillance should be performed (see Table 1). The American College of Gastroenterology (ACG) guidelines are more conservative compared to other guidelines. All guidelines use (at least partly) the Spigelman classification to determine the frequency of surveillance. The Spigelman classification focuses on the extent of duodenal polyposis, but lacks information about the ampullary region. 14 Various studies have shown that neither the Spigelman classification nor individual components predict the development of ampullary carcinoma, although the ampulla is a known high‐risk region in AP patients. 25 , 27 Moreover, a recent study by Sourrouille et al. showed that an abnormal‐looking ampulla was an independent risk factor for duodenal high‐grade dysplasia. 31 Until 2019, international guidelines recommended inspection of the ampulla, but no guidance was provided on how to act when ampullary abnormalities were found. As a consequence, even in more experienced centers, adenomatosis of the ampulla is underreported in endoscopy documentation. 27 In 2019, the European Society of Gastrointestinal Endoscopy (ESGE) was the first to incorporate assessment of ampullary polyp appearance in the decision on the frequency of surveillance. 11 The British Society of Gastroenterology (BSG) adopted this strategy in 2020. 12 A surveillance interval of 5 years is proposed if the ampulla is normal, 3 years when adenomatous changes <10 mm are present, and 1 year for adenomatous changes ≥10 mm. Surveillance is therefore adapted to the shortest interval based on the Spigelman stage and the evaluation of the ampulla.
TABLE 1.
Spigelman classification and ampullary disease classification per guideline
| Surveillance interval according to ACG guideline | Surveillance interval according to ASGE guideline | Surveillance interval according to BSG guideline | Surveillance interval according to ESGE guideline | |
|---|---|---|---|---|
| Spigelman stage | ||||
| 0 | 4 years | 5 years | 5 years | 5 years |
| I | 2–3 years | 5 years | 5 years | 5 years |
| II | 1–3 years | 3 years | 3 years | 3 years |
| III | 6–12 months | 6–12 months | 1 year, consider endoscopic therapy | 1 year |
| IV | 3–6 months, surgical evaluation, and surgical intervention if papilla is involved | 3–6 months, surgical evaluation | 6–12 months and consider endoscopic or surgical therapy | 6 months, consider (endoscopic or surgical) therapy |
| Ampullary disease | ||||
| Normal ampulla | ‐ | ‐ | 5 years | 5 years |
| Adenomatous changes,ampulla < 10mm | ‐ | ‐ | 3 years a | 3 years |
| Adenomatous changes, ampulla ≥ 10 mm | ‐ | ‐ | 1 year b | 1year |
Abbreviations: ACG, American College of Gastroenterology; ASGE, American Society for Gastrointestinal Endoscopy; BSG, British Society of Gastroenterology; ESGE, European Society of Gastrointestinal Endoscopy.
Combined with mild dysplasia.
Combined with villous histology and/or moderate or severe dysplasia.
In addition to the advice on optimal surveillance intervals, the ESGE guidelines discourage routine biopsies of suspected lesions since this may interfere with future optical diagnosis and possible endoscopic resection. ESGE recommends to determine the Spigelman stage on the basis of previous pathology reports or on optical diagnosis when endoscopic resection is deemed unnecessary because the adenoma is smaller than 10 mm and/or not suspect for invasive growth. 11 This is in line with the modified Spigelman classification that we are using in our practice since 2017. Additionally, an endoscopic biopsy of the ampulla is associated with a risk of acute pancreatitis. Up to now, four case reports have been published on the development of acute pancreatitis following an endoscopic biopsy of the ampulla. 32 , 33 , 34 , 35 Furthermore, one prospective study in 35 FAP patients revealed an asymptomatic increase in amylase levels (<2 times the normal range) in 30% of available tests. 17 If a biopsy of the ampulla is unavoidable, it has been suggested to take biopsies from 9 to 1 o'clock region of the ampulla, away from the pancreatic orifice. 36
Notwithstanding the reduced frequency and delayed onset of duodenal adenomatosis in MAP patients, various case series have shown that MAP patients may develop duodenal cancer in the absence of advanced duodenal adenomatosis. 8 , 37 One study concluded that duodenal adenomas in MAP patients, in fact, carry a significantly higher burden of somatic mutations than duodenal adenomas of FAP patients, despite showing lower Spigelman stages. 38 Nonetheless, long‐term data on the cancer risk in MAP patients with apparent less severe disease are hardly available, and surveillance intervals are still in accordance with the (A)FAP patients.
A simple endoscopic treatment algorithm is shown in Figure 2.
FIGURE 2.
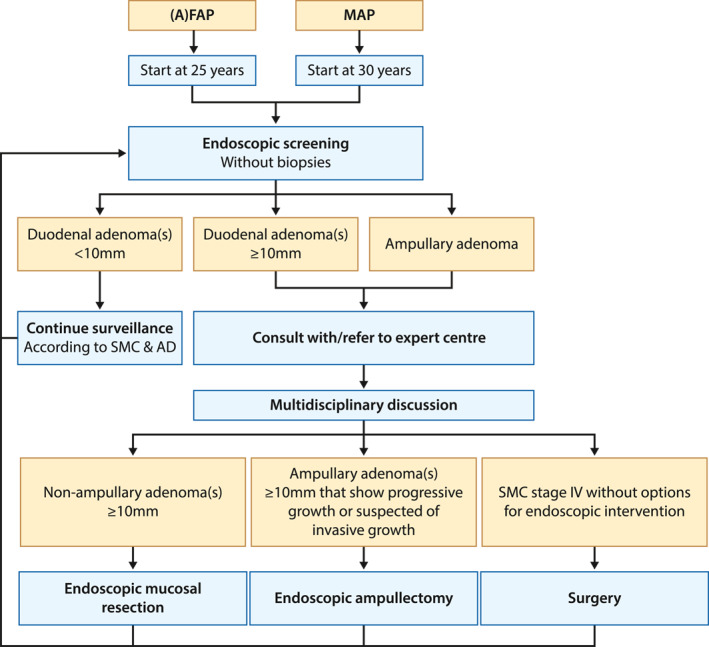
Endoscopic treatment algorithm. (A)FAP, (attenuated) familial adenomatous polyposis; MAP, MUTYH adenomatous polyposis; SMC, Spigelman classification; AD, ampullary disease. Dotted line indicates a possible outcome after multidisciplinary discussion
WHY TREAT DUODENAL ADENOMATOSIS?
Endoscopic therapy has been suggested to downstage the Spigelman classification, but it is inevitable that duodenal adenomatosis will still re‐occur. Moreover, there is no long‐term evidence that endoscopic therapy reduces cancer risk, nor delays or prevents the need for (prophylactic) surgery. Over the last 10 years, seven cohort studies, including a total of 181 FAP patients, reported on the long‐term (range: 3–10 years) effect of endoscopic removal of ampullary and non‐ampullary adenomas. 39 , 40 , 41 , 42 , 43 , 44 , 45 One French study, including 35 patients, recently showed that endoscopic treatment (i.e., APC, mucosectomy, and/or ampullectomy) of duodenal polyposis in Spigelman stage IV patients reduced the Spigelman score with 6 ± 2.2 points (p = 0.002) in 95% of the patients after a median follow‐up period of 9 years. 40 Nevertheless, five other studies reported residual and/or recurrent disease in 78%–100% and 15%–38% of patients with non‐ampullary and ampullary diseases, respectively. 39 , 41 , 42 , 43 , 44 One recent Dutch study (n = 49) reported a duodenal surgery‐free survival of 74% after 89 months and 71% after 71 months after polypectomy and endoscopic papillectomy, respectively. 45 It is noteworthy that the maximum follow‐up was much longer for studies investigating non‐ampullary disease compared to ampullary disease (10 vs. 5.9 years). Only one study reported duodenal adenocarcinoma after endoscopic therapy. 44
It is important to remember that stage IV disease patients should be regarded as high‐risk patients, even after the disease has been downstaged by endoscopic therapy. One retrospective cohort study showed that Spigelman stage IV patients in whom endoscopic therapy caused downstaging of duodenal disease had an increased rate of disease progression compared to patients with primary disease progression. 46
WHEN SHOULD WE INTERVENE?
Patients with the advanced duodenal disease should be discussed with or referred to an expert center, where endoscopic or surgical therapeutic options can be discussed in a multidisciplinary setting. The timing of various therapies is dependent on the degree and location of neoplasia (see Figure 2). First, based on current evidence, EMR should be considered for non‐ampullary adenomas ≥10 mm in all AP patients. However, data regarding the optimal timing of endoscopic therapy are scarce, and only the ESGE guideline provides us with the before mentioned recommendation. 11 Second, endoscopic ampullectomy should be considered for ampullary adenomas ≥10 mm showing progressive growth or when invasive growth is suspected. 11 Third, guidelines recommend considering surgical treatment for patients with Spigelman stage IV disease in whom the duodenal disease is considered not suitable for endoscopic intervention. 10 , 11 , 13 Although one study advocates that an ampullary polyp size >3 cm is a relative indication for surgery, 29 we consider ampullary lesion size as one of the parameters for clinical decision‐making rather than a relative indication.
HOW SHOULD WE INTERVENE?
Endoscopic therapy for non‐ampullary adenomas nowadays concentrates on EMR for larger (≥10 mm) sessile adenomas. Previous studies have also focused on APC as a therapeutic option. However, APC has shown to have a high rate of adenoma recurrence in FAP patients. One study showed that 75% (12/16) of FAP patients who were primarily treated with APC had a persistent or recurrent duodenal disease. Moreover, none of the patients showed regression of the primary lesion after 1 year. The same study showed that seven of eight FAP patients who were primarily treated with EMR also had persistent or recurrent disease. 39 This is in contrast to another study, in which seven of nine FAP patients treated with EMR did not have signs of recurrent duodenal disease requiring treatment. 43 Endoscopic therapies have a risk of adverse events, with delayed bleeding (0%–20%) being the most common. The risk of duodenal perforation after EMR is estimated to be 0%–3%. 39 , 40 , 45 , 47
In the case of progressive ampullary disease, endoscopic ampullectomy should be considered. Nevertheless, this procedure also carries a high recurrence rate of 19%–58%. 41 , 44 , 48 In addition, adverse events are also relatively common, with hemorrhage (in 4%–21%), pancreatitis (in 4%–30%), and perforation (in 2%–3%) being the most common. 40 , 41 , 42 , 45 , 48 Because of the risk of adverse events, endoscopic ampullectomy should preferably be performed in a specialized center. 12
When duodenal disease is no longer controllable with endoscopic therapies, two surgical options are available. First, patients with Spigelman stage IV without malignancy are candidates for a preventative pancreas‐preserving duodenectomy (PPD). Second, for patients who have duodenal cancer pancreaticoduodenectomy (PD) is the procedure of choice. 49 In cases of doubt regarding malignancy, an intraoperative frozen section may help in deciding which surgical procedure to perform. Nevertheless, duodenal surgery has high morbidity and mortality rates. One Dutch study reported an in‐hospital morbidity of 49%, regardless of the indication for surgical intervention (benign adenomatosis or cancer). 20 Other studies have reported even higher short‐term morbidity rates (60%–76%). 50 , 51 Long‐term morbidity (>30 days post‐surgery) is also frequent, with pancreatitis (16%–21%) and exocrine pancreatic insufficiency (30%–60%) being the most common after PPD and PD, respectively. 49 , 52 , 53 The 30‐day mortality rates vary from 2% to 29%. 20 , 53 , 54 Even after surgery, adenomas in the neo‐duodenum will eventually be detected in 50%–78% of patients (median follow‐up 75 and 46 months, respectively). 20 , 55 A recent study reported on three patients (6.4%; two gastric, one jejunal) who developed adenocarcinoma after a median follow‐up of 9.25 years. 52 Similarly to endoscopic expertise, affected patients with an indication for upper GI surgery should be referred to a high‐volume specialized center.
FUTURE PROSPECTS
Currently, a Danish observational study is investigating the inter‐ and intra‐observer variability of endoscopic variables of the Spigelman score (NCT03346980). Likewise, a Dutch observational study is investigating the inter‐laboratory variability of the histopathological variables of the Spigelman classification (NL8757). An ongoing therapeutic study aims to assess the safety and efficacy of cryoballoon ablation as treatment for non‐ampullary flat duodenal lesions (NCT03847636).
Long‐term efficacy and safety data of endoscopic therapy in the duodenum and ampullary site are almost not available. Over the last 10 years, six out of seven studies that have been published on the efficacy and/or safety of endoscopic therapy were single‐center, retrospective studies with a maximum of 49 included subjects. 39 , 40 , 41 , 42 , 43 , 44 , 45 The absence of prospective international data makes it difficult to tell what the true effect of endoscopic therapy is on the prevention of duodenal surgery, development of duodenal cancer, and survival. Also, the question remains if endoscopic therapy is preferred over endoscopic surveillance, optionally combined with prophylactic surgery, since comparative studies are nonexistent. Additional, preferably prospective multicenter studies are required that assess whether endoscopic therapy indeed reduces duodenal surgery and/or cancer risk with an acceptable degree of discomfort due to repeated endoscopic examinations and, more importantly, with acceptable adverse event rates. Also, postponing prophylactic surgery to older age may be of interest. Nonetheless, more comorbidities at an older age may impede performing prophylactic surgery, and a balanced interdisciplinary approach is needed to decide on the optimal long‐term beneficial patient outcome. Finally, comparative studies between different endoscopic and surgical therapies are needed.
CASE (CONTINUED)
Considering the extensive duodenal disease in December 2019 (two lesions ≥1 cm, located in the D2 of the duodenum), an EMR was scheduled in May 2020. As the patient decided to postpone it, the EMR has still not been performed. This phenomenon is not uncommon, since 20%–60% of FAP patients are known to be non‐adherent to endoscopic surveillance. Various causes are known to be associated with non‐compliance, that is, older age, a longer time since previous surgery, no history of previous malignancy, perceived self‐efficacy, low perceived benefits of surveillance, insufficient sedation during earlier surveillance endoscopy, and pain after surveillance endoscopy. 56 , 57 , 58 A patient‐reported study showed that AP patients naïve to sedation during surveillance endoscopy were significantly more likely to be uncompliant (Odds ratio 9.23, 95% confidence interval 1.46–58.23, p < 0.05) compared to patients in whom sedation was sometimes or always used. 58 Unfortunately, no differentiation was made between various types of sedation. Considering all possible causes for non‐compliance, patients need extensive counseling focused on the expected disease course of AP, and optimal sedation measures should be implemented during surveillance.
Our case is a good example of an AP patient with moderate to severe duodenal disease, which remained stable over the course of 10 years without the need for endoscopic or surgical intervention. This clinical course is not unusual in AP patients. Based on the current absence of evidence on the long‐term efficacy of endoscopic therapy, it may well be time to reconsider the use of endoscopic therapy in FAP patients. We might even consider and discuss prophylactic PPD in high‐risk patients, not willing or compliant to undergo endoscopic surveillance. Nonetheless, given the lack of evidence for safety and (long‐term) efficacy of endoscopic and surgical therapy, the choice of therapy should be taken in a multidisciplinary setting and in close collaboration with the patient, clearly elucidating the pros and cons of optional treatment decisions.
CONCLUSION
The management of AP patients starts with a thorough surveillance program, which, based on the modified Spigelman classification, should be performed every 0.5 to 5 years. Referral to a specialized center should be considered for patients with duodenal adenoma(s) ≥10 mm in size and with ampullary adenomas of any size. Recent data suggests that MAP patients may have an increased risk of developing duodenal cancer in the absence of severe duodenal adenomatosis, which means that surveillance should be performed even more thoroughly in these patients. Although long‐term (comparative) data on the effect of endoscopic treatment is not yet available, endoscopic treatment is already widely implemented in most expert centers. It is therefore important to consider centralized data collection of endoscopic treatment results at least on a national, but preferably more global level. Future research should focus on optimizing surveillance strategies and determining whether endoscopic therapy indeed reduces the risk of developing duodenal cancer, or at least delays or prevents the need for (prophylactic) surgery without risking more cancer diagnoses.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanth P, Grimmett J, Champine M, Burt R, Samadder JN. Hereditary colorectal polyposis and cancer syndromes: a primer on diagnosis and management. Am J Gastroenterol. 2017;112:1509–25. [DOI] [PubMed] [Google Scholar]
- 3. Bodmer WF, Bailey CJ, Bodmer J, Bussey HJR, Ellis A, Gorman P, et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328:614–6. [DOI] [PubMed] [Google Scholar]
- 4. Knudsen AL, Bisgaard ML, Bulow S. Attenuated familial adenomatous polyposis (AFAP). A review of the literature. Fam Cancer. 2003;2:43–55. [DOI] [PubMed] [Google Scholar]
- 5. Soravia C, Berk T, Madlensky L, Mitri A, Cheng H, Gallinger S, et al. Genotype‐phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet. 1998;62:1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knudsen AL, Bülow S, Tomlinson I, Möslein G, Heinimann K, Christensen IJ. Attenuated familial adenomatous polyposis: results from an international collaborative study. Colorectal Dis. 2010;12:e243–9. [DOI] [PubMed] [Google Scholar]
- 7. Burt RW, Leppert MF, Slattery ML, Samowitz WS, Spirio LN, Kerber RA, et al. Genetic testing and phenotype in a large kindred with attenuated familial adenomatous polyposis. Gastroenterology. 2004;127:444–51. [DOI] [PubMed] [Google Scholar]
- 8. Vogt S, Jones N, Christian D, Engel C, Nielsen M, Kaufmann A, et al. Expanded extracolonic tumor spectrum in MUTYH‐associated polyposis. Gastroenterology. 2009;137:1976–85.e1‐10 [DOI] [PubMed] [Google Scholar]
- 9. Walton S‐J, Kallenberg FGJ, Clark SK, Dekker E, Latchford A. Frequency and features of duodenal adenomas in patients with MUTYH‐associated polyposis. Clin Gastroenterol Hepatol. 2016;14:986–992. [DOI] [PubMed] [Google Scholar]
- 10. Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223–62.quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Leerdam ME, Roos VH, van Hooft JE, Dekker E, Jover R, Kaminski MF, et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51:877–95. [DOI] [PubMed] [Google Scholar]
- 12. Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of great Britain and Ireland (ACPGBI)/United Kingdom cancer genetics group (UKCGG). Gut. 2020;69:411–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Gurudu SR, Koptiuch C, Agarwal D, Buxabaum JL, Fehmi SMA, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020. [DOI] [PubMed] [Google Scholar]
- 14. Spigelman A, Talbot IC, Williams CB, Domizio P, Phillips RKS. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;334:783–5. [DOI] [PubMed] [Google Scholar]
- 15. Van Heumen BW, Roelofs HM, Vink‐Börger M, Dekker E, Mathus‐Vliegen EM, Dees J, et al. Ursodeoxycholic acid counteracts celecoxib in reduction of duodenal polyps in patients with familial adenomatous polyposis: a multicentre, randomized controlled trial. Orphanet J Rare Dis. 2013;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bulow S, Bjork J, Christensen IJ, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saurin J‐C, Gutknecht C, Napoleon B, Chavaillon A, Ecochard R, Scoazec J‐Y, et al. Surveillance of duodenal adenomas in familial adenomatous polyposis reveals high cumulative risk of advanced disease. J Clin Oncol. 2004;22:493–8. [DOI] [PubMed] [Google Scholar]
- 18. Mathus‐Vliegen EMH, Boparai KS, Dekker E, van Geloven N. Progression of duodenal adenomatosis in familial adenomatous polyposis: due to ageing of subjects and advances in technology. Fam Cancer. 2011;10:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Groves CJ, Saunders BP, Spigelman AD, et al. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Heumen BWH, Nieuwenhuis MH, van Goor H, Mathus‐Vliegen LMH, Dekker E, Gouma DJ, et al. Surgical management for advanced duodenal adenomatosis and duodenal cancer in Dutch patients with familial adenomatous polyposis: a nationwide retrospective cohort study. Surgery. 2012;151:681–90. [DOI] [PubMed] [Google Scholar]
- 21. Bülow S, Christensen IJ, Højen H, Björk J, Elmberg M, Järvinen H, et al. Duodenal surveillance improves the prognosis after duodenal cancer in familial adenomatous polyposis. Colorectal Dis. 2012;14:947–52. [DOI] [PubMed] [Google Scholar]
- 22. Kadmon M, Tandara A, Herfarth C. Duodenal adenomatosis in familial adenomatous polyposis coli. Int J Colorectal Dis. 2001;16:63–75. [DOI] [PubMed] [Google Scholar]
- 23. Vasen HF, Bülow S, Myrhoj T, Mathus‐Vliegen L, Griffioen G, Buskens E, et al. Decision analysis in the management of duodenal adenomatosis in familial adenomatous polyposis. Gut. 1997;40:716–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kallenberg FGJ, Bastiaansen BAJ, Dekker E. Cap‐assisted forward‐viewing endoscopy to visualize the ampulla of Vater and the duodenum in patients with familial adenomatous polyposis. Endoscopy. 2017;49:181–5. [DOI] [PubMed] [Google Scholar]
- 25. Latchford AR, Neale KF, Spigelman AD, Phillips RKS, Clark SK. Features of duodenal cancer in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2009;7:659–63. [DOI] [PubMed] [Google Scholar]
- 26. Hurley JJ, Thomas LE, Walton S‐J, Thomas‐Gibson S, Haycock A, Suzuki N, et al. The impact of chromoendoscopy for surveillance of the duodenum in patients with MUTYH‐associated polyposis and familial adenomatous polyposis. Gastrointest Endosc. 2018;88:665–73. [DOI] [PubMed] [Google Scholar]
- 27. Thiruvengadam SS, Lopez R, O’Malley M, LaGuardia L, Church JM, Kalady M, et al. Spigelman stage IV duodenal polyposis does not precede most duodenal cancer cases in patients with familial adenomatous polyposis. Gastrointest Endosc. 2019;89:345–54. [DOI] [PubMed] [Google Scholar]
- 28. Azih LC, Broussard BL, Phadnis MA, et al. Endoscopic ultrasound evaluation in the surgical treatment of duodenal and peri‐ampullary adenomas. World J Gastroenterol. 2013;19:511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Labib PLG G, Turbett JP, Skipworth J, Shankar A, Johnson G, Clark S, et al. Endoscopic ultrasound in the assessment of advanced duodenal adenomatosis in familial adenomatous polyposis. BMJ Open Gastroenterol. 2019;6:e000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gluck N, Strul H, Rozner G, Leshno M, Santo E. Endoscopy and EUS are key for effective surveillance and management of duodenal adenomas in familial adenomatous polyposis. Gastrointest Endosc. 2015;81:960–6. [DOI] [PubMed] [Google Scholar]
- 31. Sourrouille I, Lefèvre JH, Shields C, Colas C, Bellanger J, Desaint B, et al. Surveillance of duodenal polyposis in familial adenomatous polyposis: should the Spigelman score Be modified? Dis Colon Rectum. 2017;60:1137–46. [DOI] [PubMed] [Google Scholar]
- 32. Gincul R, Ciocirlan M, Dumortier J, Guerrier B, Comte J, Faure A, et al. Severe acute pancreatitis following endoscopic biopsy of the minor duodenal papilla. Endoscopy. 2009;41 (Suppl 2):E195–E196. [DOI] [PubMed] [Google Scholar]
- 33. Ishida Y, Okabe Y, Tokuyasu H, Kaji R, Sugiyama G, Ushijima T, et al. A case of acute pancreatitis following endoscopic biopsy of the ampulla of Vater. Kurume Med J. 2013;60:67–70. [DOI] [PubMed] [Google Scholar]
- 34. Skelton D, Barnes J, French J. A case of severe necrotising pancreatitis following ampullary biopsy. Ann R Coll Surg Engl. 2015;97:e61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Michopoulos S, Kozompoli D, Ntai S, Kalantzis G, Zampeli E, Petraki K. Acute pancreatitis following endoscopic ampullary biopsies without attempted Cannulation of the ampulla of Vater. Clin Endosc. 2016;49:575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kandler J, Neuhaus H. How to approach a patient with ampullary lesion. Gastroenterology. 2018;155:1670–6. [DOI] [PubMed] [Google Scholar]
- 37. Nielsen M, Poley JW, Verhoef S, van Puijenbroek M, Weiss MM, Burger GT, et al. Duodenal carcinoma in MUTYH‐associated polyposis. J Clin Pathol. 2006;59:1212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas LE, Hurley JJ, Meuser E, Jose S, Ashelford KE, Mort M, et al. Burden and profile of somatic mutation in duodenal adenomas from patients with familial adenomatous‐ and MUTYH‐associated polyposis. Clin Cancer Res. 2017;23:6721–32. [DOI] [PubMed] [Google Scholar]
- 39. Jaganmohan S, Lynch PM, Raju RP, Ross WA, Lee JE, Raju GS, et al. Endoscopic management of duodenal adenomas in familial adenomatous polyposis‐A single‐center experience. Dig Dis Sci. 2012;57:732–737. [DOI] [PubMed] [Google Scholar]
- 40. Moussata D, Napoleon B, Lepilliez V, Klich A, Ecochard R, Lapalus M‐G, et al. Endoscopic treatment of severe duodenal polyposis as an alternative to surgery for patients with familial adenomatous polyposis. Gastrointest Endosc. 2014;80:817–25. [DOI] [PubMed] [Google Scholar]
- 41. Ismail S, Marianne U, Heikki J, Jorma H, Leena K. Endoscopic papillectomy, single‐centre experience. Surg Endosc. 2014;28:3234–9. [DOI] [PubMed] [Google Scholar]
- 42. Ridtitid W, Tan D, Schmidt SE, Fogel EL, McHenry L, Watkins JL, et al. Endoscopic papillectomy: risk factors for incomplete resection and recurrence during long‐term follow‐up. Gastrointest Endosc. 2014;79:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yachida T, Nakajima T, Nonaka S, Nakamura K, Suzuki H, Yoshinaga S, et al. Characteristics and clinical outcomes of duodenal neoplasia in Japanese patients with familial adenomatous polyposis. J Clin Gastroenterol. 2016. [DOI] [PubMed] [Google Scholar]
- 44. Angsuwatcharakan P, Ahmed O, Lynch PM, Lum P, Gonzalez GN, Weston B, et al. Management of ampullary adenomas in familial adenomatous polyposis syndrome: 16 years of experience from a tertiary cancer center. Gastrointest Endosc. 2020. [DOI] [PubMed] [Google Scholar]
- 45. Roos VH, Bastiaansen BA, Kallenberg FGJ, Aelvoet AS, Bossuyt PMM, Fockens P, et al. Endoscopic management of duodenal adenomas in patients with familial adenomatous polyposis. Gastrointest Endosc. 2020. [DOI] [PubMed] [Google Scholar]
- 46. Balmforth DC, Phillips RKS, Clark SK. Advanced duodenal disease in familial adenomatous polyposis: how frequently should patients be followed up after successful therapy? Fam Cancer. 2012;11:553–7. [DOI] [PubMed] [Google Scholar]
- 47. Valli PV, Mertens JC, Sonnenberg A, Bauerfeind P. Nonampullary duodenal adenomas rarely recur after Complete endoscopic resection: a Swiss experience including a literature review. Digestion. 2017;96:149–57. [DOI] [PubMed] [Google Scholar]
- 48. Ma T, Jang EJ, Zukerberg LR, Odze R, Gala MK, Kelsey PB, et al. Recurrences are common after endoscopic ampullectomy for adenoma in the familial adenomatous polyposis (FAP) syndrome. Surg Endosc. 2014;28:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parc Y, Mabrut J‐Y, Shields C. Surgical management of the duodenal manifestations of familial adenomatous polyposis. Br J Surg. 2011;98:480–84. [DOI] [PubMed] [Google Scholar]
- 50. Watanabe Y, Ishida H, Baba H, Iwama T, Kudo A, Tanabe M, et al. Pancreas‐sparing total duodenectomy for Spigelman stage IV duodenal polyposis associated with familial adenomatous polyposis: experience of 10 cases at a single institution. Fam Cancer. 2017;16:91–8. [DOI] [PubMed] [Google Scholar]
- 51. Skipworth JRA, Morkane C, Raptis DA, Vyas S, Olde Damink SW, Imber CJ, et al. Pancreaticoduodenectomy for advanced duodenal and ampullary adenomatosis in familial adenomatous polyposis. HPB. 2011;13:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Naples R, Simon R, Moslim M, Augustin T, Church J, Burke CA, et al. Long‐term outcomes of pancreas‐sparing duodenectomy for duodenal polyposis in familial adenomatous polyposis syndrome. J Gastrointest Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 53. Walsh RM, Augustin T, Aleassa EM, Simon R, El‐Hayek KM, Moslim MA, et al. Comparison of pancreas‐sparing duodenectomy (PSD) and pancreatoduodenectomy (PD) for the management of duodenal polyposis syndromes. Surgery. 2019;166:496–502. [DOI] [PubMed] [Google Scholar]
- 54. Campos FG, Martinez CAR, Bustamante Lopez LA, Kanno DT, Nahas SC, Cecconello I. Advanced duodenal neoplasia and carcinoma in familial adenomatous polyposis: outcomes of surgical management. J Gastrointest Oncol. 2017;8:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alderlieste YA, Bastiaansen BA, Mathus‐Vliegen EMH, Gouma DJ, Dekker E. High rate of recurrent adenomatosis during endoscopic surveillance after duodenectomy in patients with familial adenomatous polyposis. Fam Cancer. 2013;12:699–706. [DOI] [PubMed] [Google Scholar]
- 56. James AS, Chisholm P, Wolin KY, Baster M, Kaphingst K, Davidson NO. Screening and health Behaviors among persons diagnosed with familial adenomatous polyposis and their relatives. J Cancer Epidemiol. 2012;2012:506410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eriksson LE, Fritzell K, Rixon L, Björk J, Wettergren L. The role of illness perceptions in adherence to surveillance in patients with familial adenomatous polyposis (FAP). Psycho‐Oncology. 2016;25:699–706. [DOI] [PubMed] [Google Scholar]
- 58. Douma KFL, Bleiker EMA, Aaronson NK, Cats A, Gerritsma MA, Gundy CM, et al. Long‐term compliance with endoscopic surveillance for familial adenomatous polyposis. Colorectal Dis. 2010;12:1198–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


