Abstract
Introduction
Recently, based on data from the PREDICT study, the European Foundation for the Study of Chronic Liver Failure (EF‐CLIF) consortium proposed pathophysiological/prognostic groups in hospitalized patients with cirrhosis: stable decompensated cirrhosis (SDC), unstable decompensated cirrhosis (UDC), pre‐acute‐on‐chronic liver failure (pre‐ACLF), and ACLF. We evaluated the outcomes of these subgroups in a real‐life cohort of hospitalized patients with cirrhosis.
Methods
Patients with cirrhosis developing first AD between 09/2010 and 12/2017 at the Vienna General Hospital were evaluated for this retrospective analysis.
Results
Two hundred and ten patients with cirrhosis (aged 57.6 ± 11.8 years) including n = 45 (21.4%) SDC, n = 100 (47.6%) UDC, n = 28 (13.3%) pre‐ACLF, and n = 37 (17.6%) with ACLF were considered. The proposed AD subgroups discriminated between patients with favorable (1‐year mortality: SDC: 6.7% and UDC: 19.6%) and dismal prognosis (90‐day mortality: pre‐ACLF: 42.9%). Interestingly, systemic inflammation gradually increased (e.g., C‐reactive protein, SDC: 0.9 mg/dl, vs. UDC: 2.0 mg/dl vs. pre‐ACLF: 3.2 mg/dl, p < 0.001) while renal function was progressively deteriorating (creatinine levels, SDC: 0.8 mg/dl vs. UDC: 0.9 mg/dl vs. pre‐ACLF: 1.2 mg/dl, p < 0.001) across prognostic subgroups in patients with cirrhosis.
Discussion
The recently proposed pathophysiological/prognostic EF‐CLIF subgroups are also reproduceable in a real‐life cohort of cirrhotic patients. As ACLF is a common and important complication, patients at risk of pre‐ACLF at index AD should be evaluated and if disease proceeds, been treated early and aggressively to avoid excessive mortality.
Keywords: ACLF, acute decompensation, acute‐on‐chronic liver failure, cirrhosis, natural course
INTRODUCTION
cute‐on‐chronic liver failure (ACLF) is a recently defined syndrome occurring in patients with cirrhosis. 1 According to the European Foundation for the Study of Chronic Liver Failure (EF‐CLIF) consortium, ACLF is distinct from acute decompensation (AD) and is characterized by pronounced systemic inflammation and development of extrahepatic organ failures, 1 as defined by the sequential organ failure(s) assessment (SOFA) score. 2
Key Summary.
Summarize the established knowledge on this subject
Acute‐on‐chronic liver failure (ACLF) is characterized by pronounced systemic inflammation and development of extrahepatic organ failures, leading to high short‐term mortality rates.
Based on the PREDICT study, the EF‐CLIF consortium proposed 3 prognostic groups in hospitalized patients with cirrhosis: stable decompensated cirrhosis (SDC), unstable decompensated cirrhosis (UDC) and pre‐ACLF.
What are the significant findings of this study?
In patients with pre‐ACLF, development of circulatory dysfunction was common, and these patients had highest levels of systemic inflammation. As a result, mortality was high and even comparable to patients who presented with ACLF at first acute decompensation (AD).
In contrast, patients with UDC had a more benign course and 1‐year mortality in patients with SDC was only 7%.
Resolution of ACLF is rare, therefore, close monitoring of patients at risk to prevent ACLF is essential. If ACLF develops, early and aggressive treatment as well as evaluation for liver transplantation are essential.
Potential precipitating events triggering AD and/or organ failures in patients with cirrhosis include hepatotoxic injury caused by alcohol abuse or drug‐induced liver injury, bacterial, fungal, or viral infections (including SARS‐CoV‐2 3 ), flares of autoimmune liver diseases, invasive procedures, or major bleedings. 4 , 5 , 6 However, next to these identifiable triggers, no precipitating triggers can be found in up to 44% of ACLF cases. 4 , 6 Nevertheless, systemic inflammation due to release of pathogen‐associated molecular patterns 7 resulting from bacterial translocation or release of danger‐associated molecular patterns due to tissue injury or cellular necrosis/necroapoptosis 8 play an important role in the development of AD and/or ACLF. 9 , 10 The clinical course of ACLF is very dynamic with potentially rapid worsening or improvement. Even though ACLF most commonly develops in patients with previous decompensation, ACLF may also occur during the first episode of AD. 4 , 11 Moreover, ACLF development is the strongest predictor of mortality in patients with acute variceal bleeding. 12
Due to the high clinical relevance of ACLF, research in this area has been strongly supported by international consortia leading to the establishment of the EF‐CLIF definition in 2013 following the CANONIC trial. 4 Just recently, first results of the second trial supported by the EF‐CLIF consortium, the PREDICT (Predicting Acute‐on‐Chronic Liver Failure in Cirrhosis) study, have been published: Within this study, the investigators were able to distinguish between three distinct clinical courses following AD, which differ with regards to pathophysiology and outcomes. The first group, termed pre‐ACLF, showed a high short‐term mortality and were characterized by pronounced systemic inflammation. The second group, patients with unstable decompensated cirrhosis (UDC), were characterized by a high number of hospital re‐admissions and complications associated with severe portal hypertension (PH). The last group, termed stable decompensated cirrhosis (SDC), included patients with a lower risk of hospital admissions and mortality. 13 Importantly, these three clinical courses have not been validated in other cohorts with longer follow‐up (FU) and more extensive patient characterization with regard to hemodynamic state and severity of PH. Therefore, the aim of this study was to retrospectively describe the clinical course of patients who developed first AD in a real‐world setting at a tertiary care center, stratified by prognostic groups. Moreover, patients initially presenting with ACLF were included as a comparator.
MATERIALS AND METHODS
Patients and definitions
Hospitalized patients with liver cirrhosis developing AD between 09/2010 and 12/2017 at the Vienna General Hospital were considered for this retrospective analysis. Advanced chronic liver disease was defined as (i) liver biopsy indicating advanced fibrosis (F3) or cirrhosis (F4), (ii) hepatic venous pressure gradient (HVPG) measurement ≥6 mmHg, or (iii) liver stiffness ≥10 kPa 14 on transient elastography. Patients were included if information on the clinical course, as well as the incidence and severity of ACLF were available. According to the primary study aim, baseline was defined as the date of first documented AD. Patients were excluded if they suffered from non‐hepatocellular carcinoma (HCC)‐malignancies or HCC out of Milan criteria, or if they were younger than 18 years. Patient demographics, clinical data, and information regarding development of further hepatic decompensation, HCC, transjugular intrahepatic portosystemic shunt (TIPS) placement, 15 orthotopic liver transplantation (OLT), and liver‐related mortality were extracted from patients' medical records. ACLF was defined according to the EF‐CLIF criteria. 4 Organ failure and organ dysfunction were defined according to the CLIF SOFA score. 16 Clinical data on potential precipitating factors/events were collected at the time of first AD and of ACLF development. Significant alcohol consumption was defined as ≥3 drinks per day in men and ≥2 drinks per day in women. 17 Severe alcoholic hepatitis (ASH) was defined by a significant alcohol consumption combined with a CLIF‐C AD score ≥50, serum bilirubin values ≥3 mg/dl and AST >50 IU/L. 6 Major bleeding was diagnosed in patients presenting with a hemoglobin decrease of ≥2 g/dl or patients requiring blood products. Life‐threatening gastrointestinal bleeding was diagnosed in patients with major bleeding accompanied by hemorrhagic shock and need for vasopressor therapy. 18 Septic shock was diagnosed in patients with proven bacteremia, circulatory dysfunction and need for vasopressor therapy. 6
Prognostic patient groups
Patients with AD were stratified according to the PREDICT study 13 into four prognostic categories: SDC included patients who were neither readmitted, nor developed ACLF during short‐term FU (3 months). UDC patients had ≥1 readmission but did not develop ACLF within 3 months. Pre‐ACLF patients developed ACLF within the next 3 months. ACLF group included patients who developed ACLF at the time of their first episode of AD. SDC patients were screened for the occurrence of re‐compensation. Re‐compensation was defined as no need for diuretic or hepatic encephalopathy (HE) treatment as well as no further decompensating events during long‐term FU.
Measurement of hepatic venous pressure gradient
Hepatic venous pressure gradient (HVPG) measurements were obtained at the Vienna Hepatic Hemodynamic Lab according to a standardized protocol 19 using a specifically designed balloon catheter (Pejcl Medizintechnik) 20 as previously described. 21 Transjugular liver biopsy specimens were either obtained by using an aspiration or the TruCut biopsy set. 19 , 22 The results of HVPG measurements were only considered for this study if they were obtained within 6 months of the date of AD.
Definition of hepatic decompensation and liver‐related mortality
Hepatic decompensation was defined as the development of ascites, HE, or variceal bleeding for the first time. 23 Among others, infections and ASH were considered as precipitating events of AD. Survival was evaluated using data obtained from the National Death Registry provided by Statistics Austria. This data set included information on the date and reason (ICD‐10 codes) of death as stated on the death certificate as well as the date of the last hospitalization in an Austrian hospital. Based on these data and additional information obtained by chart review, the cause of death was likely attributable to liver disease (i.e., liver‐related death) or excluded to be liver‐related.
Statistics
Statistical analyses were performed using IBM SPSS Statistics 26 (SPSS Inc.) and GraphPad Prism 8.0 (GraphPad Software, Inc.). Continuous variables were reported as mean ± standard deviation or median (interquartile range), while categorical variables were reported as number of patients with/without (proportion of patients with) the certain characteristic. Student's t‐test was used for group comparisons of normally distributed variables and Mann–Whitney‐U‐test for non‐normally distributed variables. Group comparisons of categorical variables were performed using either Pearson's chi‐squared, Fisher's Exact, or Kruskal–Wallis test. Clinical outcomes were evaluated using Kaplan–Meier curves and compared using log‐rank‐test. Patients lost to FU were censored at the date of the last visit. Transplant‐free survival was calculated by censoring patients at the date of OLT. Median (potential) survival was calculated using the reverse Kaplan–Meier method. A p‐value ≤0.05 was considered as statistically significant.
Ethics
This study was performed in accordance with the Helsinki Declaration and approved by the local ethics committee of the Medical University of Vienna (MUV‐EK‐1774/2019). Due to the retrospective design of this study, the need for written‐informed consent was waived by the ethics committee.
RESULTS
Study population and patient characteristics
In total, 222 patients with ACLD and AD/ACLF within the study period were identified (Figure 1). Of these, 12 patients were diagnosed with HCC out of Milan or had undergone OLT prior to the date of first evaluable hepatic decompensation and were therefore excluded. Finally, 210 patients were included in this retrospective analysis (173 patients with AD/37 patients with ACLF). While information on first AD was available in 160, further decompensation (i.e., the first sufficiently documented AD episode) was considered as baseline in 50 patients (Figure 1; Table 1).
FIGURE 1.
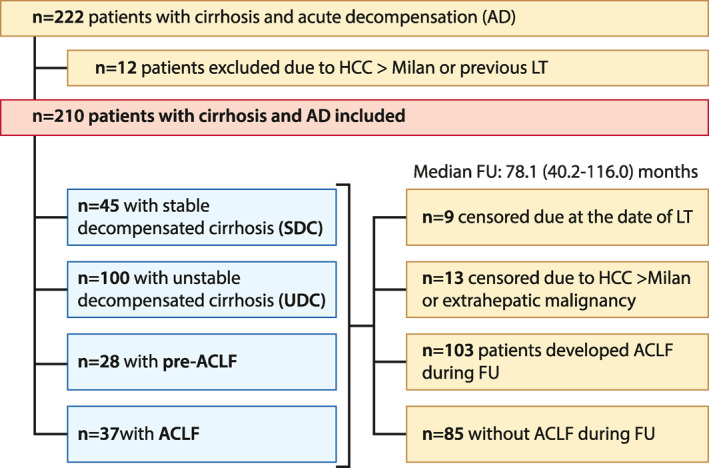
Study flow chart. AD, acute decompensation; ACLF, acute‐on‐chronic liver failure; FU, follow‐up; HCC, hepatocellular carcinoma; LT, liver transplantation; SDC, stable decompensated cirrhosis; UDC, unstable decompensated cirrhosis
TABLE 1.
Patient and disease characteristics at the date of first decompensation as well as comparison of these characteristics among different subgroups
| Characteristics | All patients, n = 173 (100%) | SDC, n = 45 (26.0%) | UDC, n = 100 (57.8%) | Pre‐ACLF, n = 28 (16.2%) | p |
|---|---|---|---|---|---|
| Clinical data | |||||
| Age, year, mean ± SD | 57.6 ± 11.8 | 55.7 ± 11.0 | 58.1 ± 11.3 | 58.7 ± 14.7 | 0.466 |
| Male sex, n (%) | 123 (71.1%) | 38 (84.4%) | 66 (66.0%) | 19 (67.9%) | 0.070 |
| BMI (kg/m2) | 27.3 ± 6.8 | 26.0 ± 4.5 | 28.0 ± 7.6 | 26.9 ± 6.5 | 0.232 |
| MAP (mmHg) | 86.8 ± 13.3 | 87.6 ± 11.4 | 86.8 ± 13.9 | 85.6 ± 14.5 | 0.823 |
| Etiology of cirrhosis, n (%) | |||||
| ALD | 98 (56.6%) | 32 (71.1%) | 53 (53.0%) | 13 (46.4%) | 0.503 |
| Viral hepatitis | 21 (12.1%) | 4 (8.9%) | 14 (14.0%) | 3 (10.7%) | |
| ALD + Viral | 8 (4.6%) | 2 (4.4%) | 4 (4.0%) | 2 (7.1%) | |
| NASH | 11 (6.4%) | 1 (2.2%) | 8 (8.0%) | 2 (7.1%) | |
| Other | 35 (20.2%) | 6 (13.3%) | 21 (21.0%) | 8 (28.6%) | |
| Laboratory parameters | |||||
| Bilirubin, mg/dl, median (IQR) | 2.1 (1.1–3.9) | 1.7 (1.2–3.4) | 2.4 (1.2–4.6) | 1.9 (1.0–2.7) | 0.179 |
| Albumin, g/dl, mean ± SD | 30.2 ± 5.8 | 32.4 ± 6.1 | 29.4 ± 5.5 | 29.9 ± 5.8 | 0.014 |
| INR, median (IQR) | 1.4 (1.3–1.6) | 1.4 (1.2–1.6) | 1.4 (1.3–1.7) | 1.4 (1.2–1.7) | 0.339 |
| Creatinine, mg/dl, median (IQR) | 0.9 (0.7–1.2) | 0.8 (0.7–1.0) | 0.9 (0.7–1.1) | 1.2 (0.9–1.4) | <0.001 |
| Na, mEq/L, mean ± SD | 135.3 ± 5.9 | 136.2 ± 4.8 | 134.9 ± 5.8 | 135.0 ± 7.5 | 0.440 |
| Biomarkers of systemic inflammation, median (IQR), and diagnosed bacterial infections, n (%) | |||||
| White blood count, ×109/L | 7.0 (4.9–9.8) | 7.8 (4.6–8.8) | 6.7 (4.7–9.8) | 7.8 (5.7–11.3) | 0.629 |
| CRP, mg/dl | 1.9 (0.7–3.6) | 0.9 (0.5–1.9) | 2.0 (0.8–3.7) | 3.2 (1.5–5.4) | <0.001 |
| Bacterial infection | 47 (27.2%) | 6 (13.3%) | 28 (28.0%) | 13 (46.4%) | 0.008 |
| Disease severity scores, mean ± SD | |||||
| Child‐Pugh score | 9.3 ± 1.9 | 8.8 ± 2.0 | 9.6 ± 1.8 | 9.5 ± 1.9 | 0.067 |
| MELD | 14.9 ± 4.6 | 13.4 ± 4.5 | 15.4 ± 4.7 | 15.6 ± 4.4 | 0.037 |
| MELD‐Na | 16.9 ± 5.6 | 15.2 ± 5.6 | 17.5 ± 5.6 | 17.5 ± 5.5 | 0.062 |
| CLIF‐C AD | 53.7 ± 7.5 | 51.2 ± 7.5 | 54.2 ± 7.3 | 55.7 ± 7.7 | 0.025 |
| First decompensation event, n (%) (some patients had ≥1 event at baseline) | |||||
| Ascites | 125 (72.3%) | 32 (71.1%) | 69 (69.0%) | 24 (85.7%) | 0.214 |
| Hepatic encephalopathy | 19 (11.0%) | 3 (6.7%) | 13 (13.0%) | 3 (10.7%) | 0.528 |
| Variceal bleeding | 35 (20.2%) | 9 (20.0%) | 23 (23.0%) | 3 (10.7%) | 0.359 |
| Indicators of portal hypertension severity | |||||
| Presence of gastroesophageal varices, n (%) | 108/150 (72.0%) | 29/42 (69.0%) | 63/83 (75.9%) | 16/25 (64.0%) | 0.322 |
| vWF levels, percentage, median (IQR) | 412.0 (331.0–420.0) | 381.5 (329.8–420.0) | 420.0 (372.5–420.0) | 392.0 (271.5–420.0) | 0.098 |
| VITRO score, median (IQR) | 3.0 (2.0–4.5) | 2.8 (1.7–4.1) | 3.2 (2.2–4.7) | 3.0 (1.8–4.3) | 0.230 |
| HVPG (mmHg), median (IQR) | 21.0 (17.0–24.0) | 19.5 (17.0–22.8) | 22.0 (18.0–25.0) | 21.5 (16.5–31.3) | 0.591 |
Abbreviations: ACLF, acute‐on‐chronic liver failure; AD, acute decompensation; CRP, C‐reactive protein; HCC, hepatocellular carcinoma; HVPG, hepatic venous pressure gradient; IQR, interquartile range; MELD, model of end stage liver disease; SD, standard deviation; SDC, stable decompensated cirrhosis; UDC, unstable decompensated cirrhosis; VITRO, von‐Willebrand factor antigen/platelet ratio; VWF, von‐Willebrand factor antigen.
Alcohol‐related liver disease was the most common etiology (n = 98, 56.6%). Development of ascites was the most common AD event occurring in 125 patients (72.3%), variceal bleeding was diagnosed in n = 35 (20.2%) and HE in n = 19 (11.0%). The majority of patients had varices (72.0%, n = 108), and HVPG measurement was available in 39 patients (22.5%) with a median value of 21.0 (IQR: 17.0–24.0) mmHg.
Patient stratification to the proposed subgroups at the date of first AD
While 26.0% of patients (n = 45) were retrospectively assigned to the SDC group, more than half of patients had UDC (n = 100, 57.8%) and 16.2% of patients (n = 28) were classified as pre‐ACLF. Detailed patient characteristics are displayed in Table 1. Age, etiology of cirrhosis, mean arterial pressure, and von‐Willebrand factor antigen levels were comparable between patients in different strata. UDC and pre‐ACLF patients had significantly higher MELD (pre‐ACLF: 15.6 ± 4.4 vs. UDC: 15.4 ± 4.7 vs. SDC: 13.4 ± 4.5, p = 0.037) and CLIF‐C AD (pre‐ACLF: 55.7 ± 7.7 vs. UDC: 54.2 ± 7.3 vs. SDC: 51.2 ± 7.5, p = 0.025) as well as lower mean albumin levels (pre‐ACLF: 29.9 ± 5.8 vs. UDC: 29.4 ± 5.5 vs. SDC: 32.4 ± 6.1, p = 0.014) at baseline. However, the absolute differences were rather low. Additionally, systemic inflammation was most pronounced in pre‐ACLF patients, as indicated by significantly elevated C‐reactive protein values (CRP, pre‐ACLF: 3.2 (1.5–5.4) versus UDC: 2.0 (0.8–3.7) versus SDC: 0.9 (0.5–1.9) mg/dl, p < 0.001). Furthermore, creatinine levels were highest in pre‐ACLF patients (pre‐ACLF: 1.2 (0.9–1.4) versus UDC: 0.9 (0.7–1.1) versus SDC: 0.8 (0.7–1.0), p < 0.001) (Table 1; Table S1).
Main reasons for hospitalization were ascites in 67.1% (n = 116), followed by gastrointestinal bleeding in 16.2% (n = 28), overt HE in 8.7% (n = 15), bacterial infections in 5.2% (n = 9), and other reasons in 2.9% (n = 5), as displayed in Supplementary Table S1.
Baseline characteristics of patients in the ACLF group
As a comparator, we also included a cohort of patients who developed ACLF during first AD. At baseline, the ACLF group had worst organ functions as indicated by significantly higher disease severity scores (Child–Pugh score, ACLF: 10.9 ± 1.9 versus pre‐ACLF: 9.5 ± 1.9 versus UDC: 9.6 ± 1.8 versus SDC: 8.8 ± 2.0, p < 0.001) and MELD score (ACLF: 25.6 ± 7.3 vs. pre‐ACLF: 15.6 ± 4.4 vs. UDC: 15.4 ± 4.7 vs. SDC: 13.4 ± 4.5, p < 0.001). Furthermore, prevalence of overt HE was highest in ACLF patients (ACLF: 40.5% vs. pre‐ACLF: 10.7% vs. UDC: 13.0% vs. SDC: 6.7%, p < 0.001).
In contrast, while bacterial infections were most commonly diagnosed in ACLF patients (ACLF: n = 19 [51.4%] versus pre‐ACLF: n = 13 [46.4%] versus UDC: n = 28 [28.0%] versus SDC: n = 6 [13.3%], p < 0.001), systemic inflammation was most pronounced in pre‐ACLF patients as indicated by significantly elevated CRP values (pre‐ACLF 3.2 [1.5–5.4] versus ACLF: 2.5 [1.3–5.3] versus UDC: 2.0 [0.8–3.7] versus SDC: 0.9 [0.5–1.9] mg/dl, p < 0.001).
Short‐term course of patients allocated to the different prognostic groups
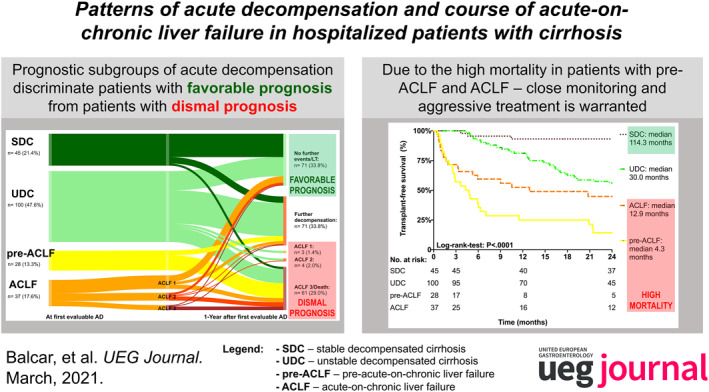
First, we evaluated the outcomes of patients after first evaluable AD at 3 months within the proposed groups (Figure 2). According to the definition of SDC and UDC, these patients did not develop ACLF within 3 months. Additionally, none of these patients died within the first 3 months. However, outcomes in the pre‐ACLF cohort were significantly worse. While 16 patients developed ACLF (35.7%: ACLF 1, 14.3%: ACLF 2, and 7.1%: ACLF 3) and survived for at least 3 months, 12 patients (42.9%) died. In the ACLF cohort, 19 patients (51.4%) were diagnosed with ACLF 1, while 18 (48.6%) had higher ACLF grades. Interestingly, ACLF resolved in only 12 patients (32.4%), and additionally, 3 patients underwent OLT (8.1%). In the remaining 22 patients (59.5%), ACLF did not resolve. Short‐term mortality of ACLF was high (27.0%) and comparable to that of pre‐ACLF patients (42.9%, p = 0.182) (Figure 2).
FIGURE 2.
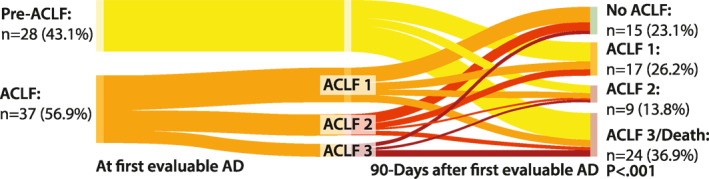
Sankey plot indicating the short‐term (three months) course of patients with pre‐ACLF and ACLF. ACLF, acute‐on‐chronic liver failure; AD, acute decompensation
Long‐term outcomes of patients allocated to the different prognostic groups
Second, we evaluated the outcomes of patients at 12 months after first evaluable decompensation according to the prognostic groups (Figure 3). Overall, 21 patients (10.0%) underwent TIPS implantation, and 8 patients (5.5%) OLT prior to ACLF development, while 58 patients died (1‐year mortality: 29.6%). Until the end of the study period, 137/210 patients died (65.2%). These deaths were mostly liver‐related (n = 115, 83.9%). Detailed information on the causes of deaths are provided in Table S2 (Figures 3 and 4; Table 2; Tables S2–S4).
FIGURE 3.
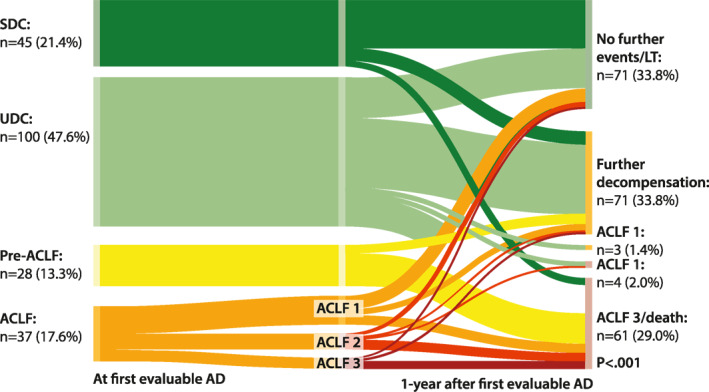
Sankey plot indicating the long‐term course of liver disease at 1 year. AD, acute decompensation; ACLF, acute‐on‐chronic liver failure; LT, liver transplantation; SDC, stable decompensated cirrhosis; UDC, unstable decompensated cirrhosis
FIGURE 4.
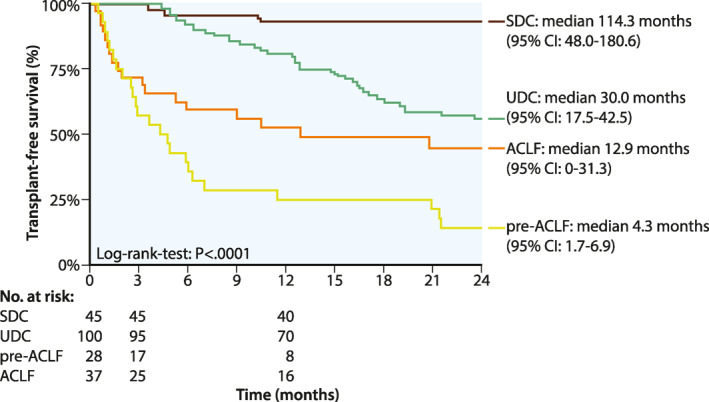
Comparison of LT‐free survival between the different prognostic groups. ACLF, acute‐on‐chronic liver failure; LT, liver transplant; SDC, stable decompensated cirrhosis; UDC, unstable decompensated cirrhosis
TABLE 2.
Comparison of the outcome of patients allocated to the different prognostic groups
| Outcomes, n (%) | All patients, n = 210 (100%) | SDC, n = 45 (21.4%) | UDC, n = 100 (47.6%) | Pre‐ACLF, n = 28 (13.3%) | ACLF, n = 37 (17.6%) | p |
|---|---|---|---|---|---|---|
| Median number of hospitalizations during 3 months FU | 1 (1–1) | 0 | 1 (1–2) | 1 (1–2) | 1 (1–2) | <0.001 |
| 90‐day mortality rate | 22/204 (10.8%) | 0 | 0 | 12 (42.9%) | 10/34 (29.4%) | <0.001 |
| 1‐year mortality rate | 58/196 (29.6%) | 3 (6.7%) | 18/92 (19.6%) | 21 (75.0%) | 16/31 (51.6%) | <0.001 |
| Liver‐related | 51/58 (87.9%) | 3 (6.7%) | 13 (14.1%) | 20 (71.4%) | 15 (48.4%) | 0.104 |
| Nonliver‐related | 7/58 (12.1%) | 0 | 5 (5.4%) | 1 (3.6%) | 1 (3.2%) | |
| LT within 12 months after enrollment | 14 (6.7%) | 0 | 8 (8.0%) | 0 | 6 (16.2%) | 0.012 |
| TIPS within 12 months after enrollment, n (%) | 21 (10.0%) | 5 (11.1%) | 9 (9.0%) | 4 (14.3%) | 3 (8.1%) | 0.827 |
Abbreviations: ACLF, acute‐on‐chronic liver failure; FU, follow‐up; LT, liver transplantation; SDC, stable decompensated cirrhosis; TIPS, transjugular intrahepatic portosystemic shunt; UDC, unstable decompensated cirrhosis.
Notably, the majority of patients from the SDC group (n = 33, 73.3%) remained clinically stable without any additional liver‐related decompensation event and 26.7% (12/45) of SDC patients showed re‐compensation during FU. While only nine patients (20.0%) were diagnosed with an infection or developed further decompensation, three patients (6.7%) died during FU. Accordingly, survival rates of patients in the SDC group were high (1‐year survival: 93.3%, 5‐year survival: 70.4%). Baseline characteristics were comparable between patients who did versus did not re‐compensate during FU (Table S3). Not surprisingly, re‐compensation led to a longer survival compared to patients who remained decompensated (re‐compensated: 224.2 [95% CI: 101.6–346.9] versus decompensated: 62.5 [95% CI: 39.5–85.4] months, log‐rank‐test: p = 0.012).
In the UDC group, more than half of patients (n = 54, 54.0%) developed further decompensating events (ACLF: n = 7, 7.0%) and only 20 patients (20.0%) remained clinically stable. Additionally, 19 patients (19.0%) died, while 8 patients (8.0%) were transplanted prior to ACLF development resulting in a pretty high 1‐year survival rate of 80.4% (5‐year survival: 18.1%). A subanalysis in the group of UDC patients showed that patients with diagnosed bacterial infections at baseline had the shortest median time to ACLF development compared to patients with gastrointestinal bleeding or other reasons for AD (infection: 10.3 [IQR: 7.5–30.2] versus bleeding: 42.7 [IQR: 17.2–58.2] versus others: 17.6 [IQR: 8.5–38.4] months, p = 0.026). As displayed in Table S4, outcomes tended to be more favorable in UDC patients with GI bleeding as precipitating event (1‐year mortality: bleeding: 5.0% vs. infection: 19.2% vs. others: 22.2%); however, this difference did not attain statistical significance most likely due to the low number of patients (p = 0.172).
Interestingly, pre‐ACLF patients had the worst outcomes: Overall, 21 (75%) patients died within 12 months, and only 7 patients (25%) resolved ACLF but still suffered from decompensating events. This resulted in a median transplant‐free survival of 4.3 months (95% CI: 1.7–6.9 months).
In the ACLF group, 16 patients (51.6%) died within 12 months and 6 patients (16.2%) underwent OLT. Interestingly, eight patients (21.6%) resolved ACLF without any additional liver‐related decompensation event. While short‐term mortality was significantly higher in patients with ACLF at baseline when compared to UDC patients (6‐month survival: ACLF: 59.4% vs. UDC: 92.4%; median transplant‐free survival: ACLF: 12.9 months [95% CI: 0.0–31.3] versus UDC: 30.0 months [95% CI: 17.5–42.5], p < 0.001), survival curves approximated each other during long‐term FU (Figure 4).
Development of ACLF during long‐term follow‐up as well as associated outcomes
Next, we aimed at analyzing the occurrence of ACLF as well as the associated outcomes during long‐term FU in 188 patients. Overall, more than half of patients (n = 103, 54.8%) developed ACLF, while 22 patients had to be censored (n = 9 due to OLT and n = 13 due to HCC out of Milan or other malignancies). Interestingly, one fifth of patients from the initial SDC group (n = 10/45, 22.2%) developed ACLF (median survival of 70.6 months (95% CI: 0.0–226.4)), while 31% (31/100) of UDC patients (median survival: 35.2 months (95% CI: 13.7–56.6)) were diagnosed with ACLF (Figure S1; Table S5).
Figure S1 demonstrates the maximum ACLF grades and the associated clinical outcomes at the end of FU.
Comparison of patient and disease characteristics at the time of ACLF development as well as comparison of potential ACLF triggers
At the time of ACLF development, patients in the pre‐ACLF group more commonly developed circulatory dysfunction/failure (pre‐ACLF: n = 14 (50.0%) versus ACLF: n = 9 (24.3%), p = 0.012). Presence of other organ failures was comparable between the two groups (Table S6).
The same applied to potential triggers of ACLF development—no statistically significant differences could be observed. However, patients developing ACLF at baseline were more commonly affected by more than one potential trigger, compared to pre‐ACLF patients (>1: ACLF: n = 34 [91.9%] versus pre‐ACLF: n = 20 [71.4%], p = 0.045). In the whole cohort, patients who developed ACLF had more often >1 (ACLF: 51/65 [78.5%] versus no ACLF: 93/145 [64.1%], p = 0.038) and >2 potential precipitating events at baseline (ACLF: 29/65 [44.6%] versus no ACLF: 46/145 [31.7%], p = 0.071). The most common triggers for ACLF development were infections (n = 32, 49.2%), followed by severe ASH (n = 30, 46.2%). Overall, significant active alcohol consumption was reported by 26/65 patients (40.0%) in the whole cohort and in 35.7% (n = 10) pre‐ACLF versus 43.2% (n = 16) ACLF patients (p = 0.540). Active drinking in the group of patients with alcoholic cirrhosis was associated with higher ACLF grades (ACLF grade 3: ALD with active alcohol consumption: n = 5/15 (33.3%) versus ALD without active alcohol consumption: n = 1/15 (6.7%), p < 0.001) when compared to patients with alcoholic cirrhosis but without active alcohol consumption. More detailed characteristics are displayed in Table S6.
DISCUSSION
ACLF is an important complication occurring in patients with cirrhosis. This syndrome is characterized by a high short‐term mortality 4 and may develop at any time in patients with compensated or decompensated cirrhosis. 24 Recently, and due to increased scientific efforts in this area, pathophysiological mechanisms are increasingly understood. 25 , 26 , 27 However, prediction of ACLF development remains a challenge in clinical routine 13 and apart from treatment of triggering factors, there are no ACLF‐specific therapeutic options. 28 , 29 Therefore, studies evaluating the natural course of decompensated cirrhosis and ACLF are required.
In this study, we applied the recently described natural history subgroups to a large “real‐life” cohort of patients with cirrhosis developing hepatic decompensation. In contrast to the PREDICT study, 13 we aimed at defining the prognostic groups already at the first decompensation event and not at a random event during FU; however, this might not always be possible in clinical reality due to late patient referral to hepatology units. Therefore, we also included a subgroup of patients, in whom the first decompensation event could not be determined. Still, we believe to assess potential differences in the patients' outcomes according to first decompensation or second decompensation after re‐compensation. In addition, and to complete the natural history subgroups proposed by the PREDICT study, we could show that nearly every fifth inpatient in our series (17.6%) fulfilled ACLF criteria at the time of first AD. 4 , 24
The CANONIC trial defined ACLF by a 28‐day mortality rate of ≥15%, which increases with every consecutive organ failure. 4 In the PREDICT study, short‐ and long‐term mortality rates were low in SDC and moderate in UDC patients (90‐day mortality rates: SDC: 0.0% vs. UDC: 21.0%, 1‐year mortality rates: SDC: 9.5% vs. UDC 35.6%), whereas pre‐ACLF patients showed the worst outcomes (90‐day mortality rates: 53.7%, 1‐year mortality rates: 67.4%). 13 This can also be explained by the group definition, as the pre‐ACLF group includes patients who did not improve with initial management, had higher inflammation parameters and developed organ dysfunction within a short period of time. Importantly, the PREDICT findings are comparable to those observed in our study in which we calculated a 90‐day mortality of pre‐ACLF patients at 42.9% and a 1‐year mortality at 75.0%. In contrast, SDC and UDC patients in our cohort had considerably more favorable outcomes (90‐day mortality rates: SDC and UDC: 0%, 1‐year mortality rates: SDC: 6.7% and UDC: 19.6%) compared to the data of the PREDICT study, probably due to the fact that we analyzed the majority of patients after the first decompensation, while the PREDICT study also included a substantial number of patients with already decompensated cirrhosis. An increasingly discussed topic is the impact of re‐compensation on clinical outcomes in patients with decompensated cirrhosis. Monteiro and colleagues 30 showed that re‐compensated patients had higher rates of ACLF development compared to compensated patients who never had a decompensating event before. In our cohort, every forth SDC patient achieved re‐compensation and this was associated with an almost four times longer survival compared to patients without re‐compensation.
Importantly, more pronounced systemic inflammation as indicated by higher CRP levels, an impaired kidney function, lower albumin levels, and a trend to higher clinical scores were the only parameters distinguishing SDC, UDC, and pre‐ACLF at baseline. In line, systemic inflammation, as for example indicated by elevated IL‐6 levels, was a strong predictor of mortality in patients with decompensated cirrhosis 31 and appears to be one of the key contributors to ACLF development in pre‐ACLF patients. Therefore, inflammation may represent an early indicator of ACLF development as well as a potential therapeutic target. 32 Interestingly, HVPG was comparable between patients in the different prognostic groups. These findings are in line with Turco et al. 33 The authors reported that while HVPG strongly increased in earlier clinical stages of cirrhosis (from Baveno stage 1 [compensated ACLD with subclinical portal hypertension] to stage 4 [patients experiencing first decompensation]), HVPG leveled off in later stages (i.e., numerical differences between clinical stages 4 and 5 were rather small). However, HVPG was only available in a small proportion of our patients and larger studies will be needed to confirm these findings.
Early detection and aggressive treatment of complications especially in pre‐ACLF patients is crucial, as these patients have an extremely high short‐term mortality. Interestingly, 90‐day mortality was comparable between patients with pre‐ACLF and ACLF at baseline; however, long‐term outcome was more favorable in patients with ACLF even though these patients were sickest at baseline. We can only speculate on the underlying reasons. First, we believe that ACLF patients at baseline were treated more aggressively due to already present organ failures, and second, in some of these patients, the underlying trigger may have stopped (e.g., alcohol consumption). On the contrary, in pre‐ACLF patients, the triggering event seemed to continue as these patients went on to develop a second event within 3 months after the index decompensation. This could be underlined by the finding that the number of patients with alcoholic liver disease (a triggering factor that may be controlled) was relatively low in the pre‐ACLF group.
As demonstrated by our study, ACLF commonly progresses, while resolution of the syndrome is only rarely observed. In our cohort, only 32% of patients resolved ACLF within 3 months, which was associated with lower ACLF grades at baseline. Therefore, early detection and treatment of potential triggers and other disease‐promoting factors is essential. This includes: choosing the adequate antibiotic treatment in patients with cirrhosis and bacterial infections, 6 , 34 using pre‐emptive TIPS in patients with ACLF and acute variceal bleeding 12 and using adequate plasma expansion with albumin in patients with ACLF and hepatorenal syndrome‐type acute kidney injury. 35 Furthermore, while prophylactic antibiotic treatment did not change systemic inflammation and outcome, 36 , 37 long‐term high dose albumin treatment reduced the risk of infections, systemic inflammation, cardiocirculatory dysfunction, and mortality. 10 , 38 , 39 However, knowledge on correct albumin doses in patients with advanced liver disease is still insufficient among specialists for Gastroenterology/Hepatology at secondary/tertiary care centers, as was demonstrated by a recent questionnaire study by Pfisterer et al. 40 indicating the need for ongoing educational efforts.
Apart from albumin substitution in a particular subgroup of patients, up to date, there is no established disease‐modifying therapy to prevent further progression or the development of complications neither in decompensated cirrhosis nor in ACLF. 29 Additionally, early detection of complications may be difficult in times of the COVID‐19‐pandemic since routine clinical visits are commonly postponed or performed via telemedical contact. 3 Additionally, alcohol consumption likely increased during the pandemic. 41 Not surprisingly, active alcoholism in the group of patients with alcoholic‐related cirrhosis was associated with higher ACLF grades, and patients tended to have worse short‐term outcomes than those without active alcohol consumption. These findings are in line with the results of the CANONIC trial. 4
Until now, liver transplantation remains the only potentially curative treatment in patients with ACLF. While ACLF does not represent a contraindication for OLT, due to the high short‐term mortality and the often long waiting time, many patients die prior to a suitable organ offer. Unfortunately, ACLF patients are still ranked for OLT by the MELD score, although CLIF‐C ACLF‐D score would be better suited as these patients are significantly underserved. 42 In line, only 7% of patients underwent liver transplantation in our study. However, large retrospective and smaller prospective studies suggested that even patients with ACLF 3 are benefiting from OLT, with a 1‐year survival similar to that of patients with a lower ACLF grade. 43 , 44 , 45 Therefore, all suitable patients with ACLF should be listed for OLT, including those with higher grade ACLF 46 and those who resolve their first ACLF episode, as the likelihood for developing a higher ACLF grade in the future is high. 47 In our study, 49% of patients with decompensated cirrhosis developed organ failures at some point, and patients who primarily recovered from ACLF are at high risk of redeveloping organ failures. Notably, we did not find any predictors for the development of ACLF during long‐term FU; however, patients developing ACLF were more commonly affected by more than one potential trigger underlining the role of precipitating events for ACLF development. 6 The risk of further organ failures might be especially high in patients, in whom the triggering factors cannot be controlled. 48
Our study has several limitations
Due to the retrospective design, several patients had to be excluded due to important missing data at baseline or during FU. Additionally, the retrospective design also explains why some parameters of great interest, such as IL‐6, were not available in a substantial number of patients. Therefore, systemic inflammation was only measured by CRP in our study. Furthermore, patients with more advanced stage of liver disease have usually undergone a more detailed diagnostic work‐up. Therefore, we cannot exclude that missing data may have been relevant, and patients with more advanced liver disease might be overrepresented in our cohort. Furthermore, we also included patients who decompensated several years ago, when ACLF was not yet properly recognized as such. However, we could demonstrate that outcome in our cohort was comparable to the more current PREDICT cohort, pointing out that unfortunately treatment of ACLF patients has not significantly improved within the last years, underlining the importance of novel therapeutic approaches.
In summary, we compared thoroughly characterized cirrhotic patients with AD across different prognostic subgroups proposed by the PREDICT study and could validate their findings in a real‐life cohort. We demonstrated that ACLF is a common entity in clinical routine and should be recognized as a severe complication in patients with cirrhosis. While mortality in patients with SDC is low, long‐term outcome is significantly compromised in patients with UDC. Patients with ACLF at first decompensation and especially pre‐ACLF patients have a very high short‐term mortality. Even though there were only few differences in baseline characteristics between the subgroups, pre‐ACLF patients showed highest levels of inflammatory markers and a particularly unstable clinical course. These patients should be identified rapidly and evaluated for early and aggressive treatment in order to prevent and/or detect the development of ACLF. Additionally, these patients should be seen in the outpatient clinic in short intervals allowing initiation of pre‐emptive treatments (e.g., antibiotics) or timely admission for intensified monitoring and therapies. Moreover, transplant evaluation is clearly warranted. Unfortunately, OLT is still the only potentially curative treatment for patients with ACLF and should be evaluated even in patients with higher ACLF grades; however, long waiting lists reduce the chances for transplantation before ACLF development, and lack of alcohol abstinence is often considered a contraindication, limiting liver transplant as a therapeutic option for ACLF patients. Therefore, further trials evaluating specific interventions among patients in different prognostic groups are urgently required.
CONFLICT OF INTEREST
The authors have nothing to disclose regarding the work under consideration for publication. The following authors disclose conflicts of interests outside the submitted work: Lorenz Balcar, Georg Semmler, Katharina Pomej, Rafael Paternostro, David Bauer, and Benedikt Simbrunner have nothing to disclose. Theresa Bucsics received travel support from Gilead, BMS, Roche, Bayer, and AbbVie. Michael Trauner received grant support from Albireo, Cymabay, Falk, Gilead, Intercept, MSD and Takeda, honoraria for consulting from AbbVie, Albireo, Boehringer Ingelheim, BiomX, Falk, Genfit Gilead, Intercept, Janssen, Novartis Regulus and Shire, speaker fees from, Falk, Gilead, Intercept and MSD as well as travel support from Abbvie, Falk, Gilead and Intercept. Mattias Mandorfer has served as a speaker and consultant for AbbVie, BMS, Gilead, Gore, and Janssen. Thomas Reiberger received speaker fees from Boehringer Ingelheim, Roche, W.L. Gore and MSD, grant support from Boehringer Ingelheim, Boston Scientific, Cook Medical, Gilead, Guerbet, Abbvie, Phenex Pharmaceuticals, Philips, W.L. Gore, and MSD, served as a consultant for Abbvie, Bayer, Boehringer Ingelheim, Gilead, Intercept and MSD and received travel support from Gilead, Roche, MSD, and Gore. Bernhard Scheiner received travel support from AbbVie, Ipsen, and Gilead.
AUTHOR CONTRIBUTIONS
Concept of the study: Lorenz Balcar, Mattias Mandorfer, Thomas Reiberger, and Bernhard Scheiner. Data collection: Lorenz Balcar and Bernhard Scheiner. Statistical analysis: Lorenz Balcar and Bernhard Scheiner. Drafting of the manuscript: Lorenz Balcar, Mattias Mandorfer, Thomas Reiberger, and Bernhard Scheiner. Revision for important intellectual content and approval of the final manuscript: All authors.
Supporting information
Supporting Information 1
Supporting Information 2
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Arroyo V, Moreau R, Jalan R, Gines P, EASL‐CLIF Consortium CANONIC Study . Acute‐on‐chronic liver failure: a new syndrome that will re‐classify cirrhosis. J Hepatol. 2015;62:S131–143. [DOI] [PubMed] [Google Scholar]
- 2. Arroyo V, Moreau R, Kamath PS, Jalan R, Gines P, Nevens F, et al. Acute‐on‐chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041 [DOI] [PubMed] [Google Scholar]
- 3. Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep. 2020;2:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–37. [DOI] [PubMed] [Google Scholar]
- 5. Silva PESe, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, et al. Single‐centre validation of the EASL‐CLIF Consortium definition of acute‐on‐chronic liver failure and CLIF‐SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516–23. [DOI] [PubMed] [Google Scholar]
- 6. Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2020;74(5):1097–1108.33227350 [Google Scholar]
- 7. Simbrunner B, Mandorfer M, Trauner M, Reiberger T. Gut‐liver axis signaling in portal hypertension. World J Gastroenterol. 2019;25:5897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022–33. [DOI] [PubMed] [Google Scholar]
- 9. Wiest R, Garcia‐Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–33. [DOI] [PubMed] [Google Scholar]
- 10. Fernández J, Clària J, Amorós A, Aguilar F, Castro M, Casulleras M, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157:149–62. [DOI] [PubMed] [Google Scholar]
- 11. Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, et al. Clinical Course of acute‐on‐chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243–52. [DOI] [PubMed] [Google Scholar]
- 12. Trebicka J, Gu W, Ibáñez‐Samaniego L, Hernández‐Gea V, Pitarch C, Garcia E, et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre‐emptive TIPS. J Hepatol. 2020;73:1082–91. [DOI] [PubMed] [Google Scholar]
- 13. Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73(4):842–54. [DOI] [PubMed] [Google Scholar]
- 14. Reiberger T, Ferlitsch A, Payer BA, Pinter M, Schwabl P, Stift J, et al. Noninvasive screening for liver fibrosis and portal hypertension by transient elastography‐‐a large single center experience. Wien Klin Wochenschr. 2012;124:395–402. [DOI] [PubMed] [Google Scholar]
- 15. Bucsics T, Schoder M, Diermayr M, Feldner‐Busztin M, Goeschl N, Bauer D, et al. Transjugular intrahepatic portosystemic shunts (TIPS) for the prevention of variceal re‐bleeding ‐ a two decades experience. PLoS One. 2018;13:e0189414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, et al. Development and validation of a prognostic score to predict mortality in patients with acute‐on‐chronic liver failure. J Hepatol. 2014;61:1038–47. [DOI] [PubMed] [Google Scholar]
- 17. EASL, EASD, EASO. EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388‐402. [DOI] [PubMed] [Google Scholar]
- 18. de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- 19. Reiberger T, Schwabl P, Trauner M, Peck‐Radosavljevic M, Mandorfer M. Measurement of the hepatic venous pressure gradient and transjugular liver biopsy. JoVE. 2020;160:e58819. [DOI] [PubMed] [Google Scholar]
- 20. Ferlitsch A, Bota S, Paternostro R, Reiberger T, Mandorfer M, Heinisch B, et al. Evaluation of a new balloon occlusion catheter specifically designed for measurement of hepatic venous pressure gradient. Liver Int. 2015;35:2115–20. [DOI] [PubMed] [Google Scholar]
- 21. Reiberger T, Ferlitsch A, Payer BA, Pinter M, Homoncik M, Peck‐Radosavljevic M. Vienna Hepatic Hemodynamic L. Non‐selective beta‐blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J Gastroenterol. 2012;47:561–8. [DOI] [PubMed] [Google Scholar]
- 22. Stift J, Semmler G, Walzel C, Mandorfer M, Schwarzer R, Schwabl P, et al. Transjugular aspiration liver biopsy performed by hepatologists trained in HVPG measurements is safe and provides important diagnostic information. Dig Liver Dis. 2019;51:1144–51. [DOI] [PubMed] [Google Scholar]
- 23. Scheiner B, Steininger L, Semmler G, Unger LW, Schwabl P, Bucsics T, et al. Controlled attenuation parameter does not predict hepatic decompensation in patients with advanced chronic liver disease. Liver Int. 2019;39:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563–76. [DOI] [PubMed] [Google Scholar]
- 25. Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, et al. Bacterial and fungal infections in acute‐on‐chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870–80. [DOI] [PubMed] [Google Scholar]
- 26. Triantafyllou E, Woollard KJ, McPhail MJW, Antoniades CG, Possamai LA. The role of monocytes and macrophages in acute and acute‐on‐chronic liver failure. Front Immunol. 2018;9:2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang T, Sun K, Wang Y, Huang L, Lang R, Jiang W. Disruption of the gut‐liver axis in the pathogenesis of acute‐on‐chronic liver failure. Eur J Gastroenterol Hepatol. 2018;30:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arroyo V, Moreau R, Jalan R. Acute‐on‐Chronic liver failure. N Engl J Med. 2020;382:2137–45. [DOI] [PubMed] [Google Scholar]
- 29. Solà E, Pose E, Campion D, Piano S, Roux O, Simon‐Talero M, et al. Endpoints and design of clinical trials in patients with decompensated cirrhosis: position paper of the LiverHope Consortium. J Hepatol. 2020;74(1):200–19. [DOI] [PubMed] [Google Scholar]
- 30. Monteiro S, Grandt J, Uschner FE, Kimer N, Madsen JL, Schierwagen R, et al. Differential inflammasome activation predisposes to acute‐on‐chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut. 2021;70:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Costa D, Simbrunner B, Jachs M, Hartl L, Bauer D, Paternostro R, et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J Hepatol. 2020;74(4):819–28. [DOI] [PubMed] [Google Scholar]
- 32. Jachs M, Hartl L, Schaufler D, Desbalmes C, Simbrunner B, Eigenbauer E, et al. Amelioration of systemic inflammation in advanced chronic liver disease upon beta‐blocker therapy translates into improved clinical outcomes. Gut. 2020. 10.1136/gutjnl-2020-322712. [DOI] [PubMed] [Google Scholar]
- 33. Turco L, Garcia‐Tsao G, Magnani I, Bianchini M, Costetti M, Caporali C, et al. Cardiopulmonary hemodynamics and C‐reactive protein as prognostic indicators in compensated and decompensated cirrhosis. J Hepatol. 2018;68:949–58. [DOI] [PubMed] [Google Scholar]
- 34. Piano S, Brocca A, Mareso S, Angeli P. Infections complicating cirrhosis. Liver Int. 2018;38:126–33. [DOI] [PubMed] [Google Scholar]
- 35. Reiberger T, Püspök A, Schoder M, Baumann‐Durchschein F, Bucsics T, Datz C, et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr. 2017;129:135–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernández J, Tandon P, Mensa J, Garcia‐Tsao G. Antibiotic prophylaxis in cirrhosis: good and bad. Hepatology. 2016;63:2019–31. [DOI] [PubMed] [Google Scholar]
- 37. Moreau R, Elkrief L, Bureau C, Perarnau JM, Thevenot T, Saliba F, et al. Effects of long‐term norfloxacin therapy in patients with advanced cirrhosis. Gastroenterology. 2018;155:1816–27. [DOI] [PubMed] [Google Scholar]
- 38. Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long‐term albumin administration in decompensated cirrhosis (ANSWER): an open‐label randomised trial. Lancet. 2018;391:2417–29. [DOI] [PubMed] [Google Scholar]
- 39. Di Pascoli M, Fasolato S, Piano S, Bolognesi M, Angeli P. Long‐term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019;39:98–105. [DOI] [PubMed] [Google Scholar]
- 40. Pfisterer N, Schmidbauer C, Riedl F, Stadlbauer‐Köllner V, Maieron A, Gschwantler M, et al. Real‐life perceptions on the use of albumin in patients with liver cirrhosis in specialized gastroenterology units in Austria. Z Gastroenterol. 2020;58:e94–e95. [Google Scholar]
- 41. Koopmann A, Georgiadou E, Kiefer F, Hillemacher T. Did the general population in Germany drink more alcohol during the COVID‐19 pandemic lockdown? Alcohol Alcohol. 2020;55:698–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barosa R, Roque Ramos L, Patita M, Nunes G, Fonseca J. CLIF‐C ACLF score is a better mortality predictor than MELD, MELD‐Na and CTP in patients with Acute on chronic liver failure admitted to the ward. Rev Esp Enferm Dig. 2017;109:399–405. [DOI] [PubMed] [Google Scholar]
- 43. Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic‐Bedoya J, et al. Liver transplantation in the most severely ill cirrhotic patients: a multicenter study in acute‐on‐chronic liver failure grade 3. J Hepatol. 2017;67:708–15. [DOI] [PubMed] [Google Scholar]
- 44. Finkenstedt A, Nachbaur K, Zoller H, Joannidis M, Pratschke J, Graziadei IW, et al. Acute‐on‐chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 2013;19:879–86. [DOI] [PubMed] [Google Scholar]
- 45. Sundaram V, Jalan R, Wu T, Volk ML, Asrani SK, Klein AS, et al. Factors associated with survival of patients with severe acute‐on‐chronic liver failure before and after liver transplantation. Gastroenterology. 2019;156:1381–91. [DOI] [PubMed] [Google Scholar]
- 46. Trebicka J, Sundaram V, Moreau R, Jalan R, Arroyo V. Liver transplantation for acute‐on‐chronic liver failure: science or fiction? Liver Transplant. 2020;26:906–15. [DOI] [PubMed] [Google Scholar]
- 47. Mahmud N, Sundaram V, Kaplan DE, Taddei TH, Goldberg DS. Grade 1 acute on chronic liver failure is a predictor for subsequent grade 3 failure. Hepatology. 2020;72:230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muntaner L, Altamirano JT, Augustin S, González A, Esteban R, Guardia J, et al. High doses of beta‐blockers and alcohol abstinence improve long‐term rebleeding and mortality in cirrhotic patients after an acute variceal bleeding. Liver Int. 2010;30:1123–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Supporting Information 2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


