Abstract
Background and Aim
Cold snare polypectomy (CSP) is growing in popularity due to its safety and convenience. Its indication is benign tumours such as adenoma and sessile serrated lesions (SSLs) <10 mm in size. CSP for SSLs ≥10 mm in size has not been well examined. In this study, we aimed the feasibility of this treatment regarding therapeutic results and local recurrence.
Methods
This was a single‐centre retrospective cohort study. We reviewed SSLs with or without dysplasia of 10–20 mm that were resected by CSP from 2014 to 2020. All tumours were diagnosed endoscopically as SSLs without dysplasia before CSP with the help of magnifying narrow band imaging or blue laser imaging. We analysed the lesion characteristics, en bloc resection, histopathological diagnosis, adverse events and local recurrence. We analysed risk factors for recurrence, comparing recurrent lesions to non‐recurrent lesions. We also compared risk factors for lesions 10–14 mm in size to those for lesions 15–20 mm in size.
Results
We analysed 160 lesions in 100 patients (M age ± SD = 67.7 ± 10.1 years). The polyp size (M ± SD) was 11.8 ± 2.8 mm, and the en bloc resection rate was 60.0% (96 cases). The rates of massive perioperative haemorrhage, postoperative haemorrhage and perforation were 1.3%, 0% and 0%, respectively. Regarding histopathological diagnosis, two (1.2%) cases showed SSLs with high‐grade dysplasia. The recurrence rate in 101 lesions with a median follow‐up period of 18 months (interquartile range 12–24 months) was 5.0%. There were no significant risk factors such as tumour size, location, morphology and so on in terms of recurrence. All recurrent cases could be resected by repeat CSP. The recurrence rates of lesions 10–14 mm in size and 15–20 mm in size were 4.7% and 6.3%, respectively (p = 0.713).
Conclusion
CSP of SSLs ≥10 mm in size according to magnifying endoscopic diagnosis was safe and promising, but the rate of recurrence was slightly high, meaning that close follow‐up is required.
Keywords: cold snare polypectomy, colorectal polyps, local recurrence, sessile serrated lesions
1. INTRODUCTION
Cold snare polypectomy (CSP) is an endoscopic treatment which does not need an electrosurgical unit, and its use is spreading rapidly across the world because of its safeness and convenience, as CSP does not cause perforation and is associated with a low risk of postoperative haemorrhage, even in patients taking antith‐rombotic drugs. 1 , 2 , 3 , 4 , 5 However, the indications for CSP are limited to benign lesions <10 mm in size, such as sessile serrated lesions (SSLs) and adenomas, because tumours ≥10 mm in size are more likely to be cancerous lesions than tumours <10 mm in size. 2 , 6 , 7 Operators have to differentiate benign lesions from cancerous lesions by white light image (WLI) and magnifying narrow‐band imaging (NBI) or blue laser imaging (BLI). 2 , 8 , 9 Additionally, our previous study showed that the rate of local recurrence after CSP for polyps ≥10 mm was higher than that for polyps <10 mm. 10 Thus, CSP is not regularly recommended for neoplastic lesions ≥10 mm.
SSL is known as a pre‐malignant lesion that sometimes develops into cancer. Thus, throughout the world, SSL is resected to prevent colorectal cancer and is a good indication for CSP. However, it sometimes includes high‐grade dysplasia, and this kind of lesion is not an indication of CSP. Several papers have demonstrated that SSL with dysplasia (SSLD) can be diagnosed with WLI and imaged‐enhanced endoscopy, such as NBI and BLI. 11 , 12 , 13 SSLs without dysplasia 10–20 mm in size are sometimes resected with piecemeal CSP in Western countries, and some papers have demonstrated the efficacy of piecemeal CSP for SSLs ≥10 mm. 14 , 15 However, there is insufficient evidence with regard to the diagnostic accuracy of SSL ≥10 mm without dysplasia and detailed therapeutic results of those lesions, including recurrence rate, to support the use of CSP for resecting SSLs ≥10 mm. In the present study, we aimed the feasibility of CSP for SSLs 10–20 mm in size, including diagnostic accuracy, adverse events, local recurrence and risk factors for recurrence.
2. METHODS
This was a single‐centre retrospective study. We reviewed SSLs with or without dysplasia of 10–20 mm that were resected by CSP from April 2014 to March 2020 at our institution. The indication of CSP for a SSL of 10–20 mm was lesions showing neither signs of a dysplasia nor cancer based on both WLI and magnifying endoscopy using either NBI or BLI. Regarding findings of NBI or BLI, SSL was diagnosed based on the presence of either crypts opening and dilated vessels, according to previous reports. 9 , 11
Lesions showing irregular vessel pattern were excluded due to the possibility of dysplasia. Among all lesions, the detail location and the distance from the anal verge were recorded at CSP for follow‐up colonoscopy.
After CSP, scheduled follow‐up colonoscopy was performed at three to six months for lesions with piecemeal resection or lesions with a positive or unclear margin of dysplasia or cancer. The remaining lesions were followed up 1 year after CSP. Afterwards, follow‐up colonoscopy was performed every 1 to 2 years. During follow‐up colonoscopy, local recurrence was evaluated. Recurrence was defined as a lesion on the CSP scar, and was diagnosed with WLI, NBI or BLI. We regularly performed magnified colonoscopy to evaluate local recurrence. When we could not confirm recurrence with magnified colonoscopy, we performed an additional biopsy to confirm it. The evaluation of recurrence was performed by two endoscopists. When the scar was not detected by WLI in the follow‐up colonoscopy, we evaluated the area three to five times with a help of NBI or BLI, according to previous reports. 10 When a scar was not found with these careful observations, we concluded that the case had no recurrence. We analysed the number of undetected scars and did not exclude these cases in order to prevent an over‐estimation of the recurrence rate. Cases in which a recurrent lesion was detected were treated with repeat CSP when a magnifying endoscopy showed no definite dysplasia.
The study outcomes were the various therapeutic results of CSP, including tumour size, tumour morphology, tumour location, antithrombotic drug rate, en bloc resection rate, histopathological complete resection rate (negative horizontal and vertical margins), histopathological diagnosis, histopathological margin and adverse events. We also analysed the follow‐up period, local recurrence and the number of scars detected. Then, all lesions were divided into either the recurrent lesions group or the non‐recurrent lesions group in order to analyse risk factors of recurrence (tumour size, location, morphology, histopathology and histopathological margin). Additionally, treatments for recurrent lesions and further follow‐up after the treatments were analysed.
Polyp locations were divided into three groups: (a) the right‐sided colon (the caecum to the transverse colon), (b) the left‐sided colon (the descending colon to the sigmoid colon) and (c) the rectum. Morphology was divided into non‐polypoid lesions and polypoid lesions according to the Paris classification. 16 With respect to adverse events, massive perioperative haemorrhage was defined as haemorrhage disturbing resection for more than one minute, and postoperative haemorrhage was defined as the development of bloody stool for which endoscopic haemostasis was required.
2.1. Cold snare polypectomy
With respect to bowel preparation, we used high concentrated polyethylene glycol (PEG) according to our previous report. 17 In brief, patients had a low residual diet on the day before colonoscopy, and they took 10 ml picosulfate sodium between 9:00 PM and 10:00 PM on the day. Then, patients took 1.0 L high concentrated PEG and 0.5 L water three hours prior to the examination on the day of colonoscopy. We used the following endoscopes: PCF‐H290AZI (Olympus) and PCF‐Q260AZI, CF‐HQ290 and EC‐ L600ZP7 (Fujifilm). The following snares were used: Captivator II 15 mm and Captivator Cold 9 mm (Boston Scientific), Exacto cold snare 9 mm (US Endoscopy), Snare Master Plus 9 mm (Olympus Co.) and Dualoop M (Medicos Hirata Co.), which has two loops (12 and 25 mm). The snare was pushed to a lesion, and a lesion was resected with en bloc resection or piecemeal resection according to the size of the lesion decided by the operator (Figure 1 and Video S1). When en bloc resection failed, subsequent piecemeal resection was performed. After CSP, we checked for the presence of a residual lesion by magnifying endos‐copy with NBI or BLI. When part of the lesion remained, further CSP was performed to resect the whole lesion. In patients taking antithrombotic drugs, cessation of medication was not required for every CSP, in accordance with previous studies. 3 , 4 , 5 Endoscopic clipping just after resection was performed in each lesion according to the operator's decision when perioperative haemorrhage was severe and did not stop spontaneously. All CSPs were performed by four veteran endoscopists with experience of performing more than 5000 colonoscopies.
FIGURE 1.
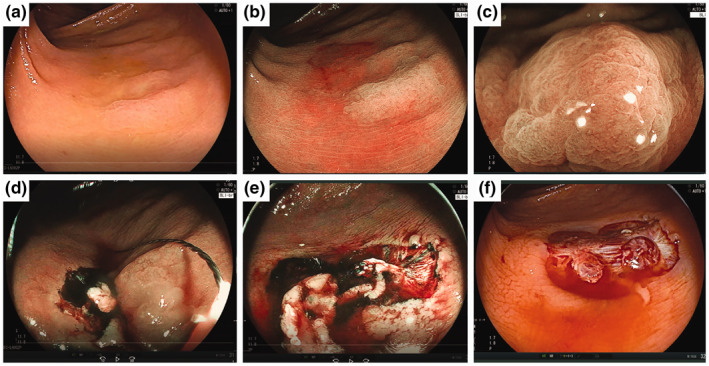
Piecemeal cold snare polypectomy (CSP) for a sessile serrated lesion (SSL) 16 mm in size. (a) A 49‐year‐old woman with a non‐polypoid (IIa) lesion 16 mm in size located in the ascending colon. (b) Blue laser imaging (BLI) clearly showed the margin of the tumour. (c) Magnifying endoscopy with BLI showed a dilated crypt. (d) Piecemeal resection was performed from the oral side in BLI. (e) Final resection (4th resection) was performed after identifying the margin of the lesion. (f) The lesion was resected by piecemeal CSP. A histopathological examination revealed a SSL
All resected specimens were fixed with formalin after resection. To evaluate the margin precisely, pathological technicians fixed the specimens in order to evaluate the vertical margin as much as possible, adjusting the direction of the specimens. At least two slices of the specimen were stained with hematoxylin and eosin, and they were evaluated by two authorized pathologists (Y.M. and M.K.). The histopathological diagnosis of SSL was distinguished from that of hyperplastic polyp according to the Japanese Society for Cancer of the Colon and Rectum criteria as follows: (a) dilatation of ducts, (b) horizontally arranged basal ducts (inverted T shape or L shape) and (c) irregularly branched ducts. SSLs were diagnosed when at least 10% of the lesions had two of these three findings. 18
In this study, the finding of a positive margin was used for both SSL and dysplasia. The definition of a positive margin was made by the detection of a lesion gland and cells on the definite resection margin. The definition of a negative margin was performed by the absence of a lesion gland and cells on the definite resection margin. Lesions with neither positive margin nor negative margin were defined as unclear.
Informed consent was obtained from all patients before the colorectal examination and CSP in this study. This research was approved by the Ethics Committee of Kyoto Prefectural University of Medicine (ERB‐C‐417, approved date: 1 October 2015; ERB‐C‐1600, approved date: 23 December 2019), and was in accordance with the Declaration of Helsinki.
2.2. Statistical analysis
The results were analysed using chi‐square tests, Yates continuity correction and Mann‐Whitney U‐tests. All statistical analyses were performed using IBM SPSS Statistics for Windows v22.0 (IBM Japan Ltd). Continuous variables such as tumour size were analysed using Mann‐Whitney U‐tests. Categorical variables were analysed using chi‐square tests. If the number categorized was <5, Yates continuity correction was also performed. p‐Values <0.05 were considered to indicate statistical significance.
3. RESULTS
We reviewed 160 SSLs 10–20 mm in size resected by CSP in 100 patients. The mean tumour size was 11.8 (SD = 2.8 mm; range 10–20 mm), and 88.1% (n = 141) of the lesions were in the right‐sided colon (Table 1). All lesions were non‐polypoid lesions. The rate of cases with serrated polyposis syndrome due to World Health Organization classification was 15.0% (15/100). 19 The en bloc resection rate was 60.0% (96/160). In the 64 cases in which piecemeal resection was performed, the lesion was resected in two to three pieces in 89.1% of cases and in four to six pieces in 10.9% of cases. Regarding adverse events, perioperative bleeding occurred in two cases, and there were no cases of postoperative haemorrhage. No patients needed readmission due to adverse events. Regarding histopathology, the rates of SSL, SSL with LGD and SSL with HGD were 96.3%, 2.5% and 1.2%, respectively. All lesions with dysplasia had a smooth appearance, without any depression or polypoid area. Among the 101 lesions that were followed up, the local recurrence rate was 5.0% (5/101). Among these 101 lesions, the scar due to CSP was not detected in 12 lesions whose size ranged from 10 to 12 mm. Among 89 lesions in which the scar was detected, the local recurrence rate was 5.6% (5/89).
TABLE 1.
Clinical outcomes of CSP for SSLs ≥10 mm in size
| Lesions, n | 160 |
|---|---|
| Patients, n | 100 |
| Age (years), M ± SD (range) | 67.7 ± 10.1 (36–85) |
| Sex, % (n) male/female | 50.0/50.0 (50/50) |
| Tumour size (mm), M ± SD (range) | 11.8 ± 2.8 (10–25) |
| Ratio of polyps (≥15 mm), % (n) | 16.3 (26) |
| Location, % (n) right‐sided/left‐sided | 88.1/11.9 (141/19) |
| Morphology, % (n) polypoid/non‐polypoid, % (n) | 0.0/100.0 (0/218) |
| Serrated polyposis syndrome, % (n) | 15.0 (15) |
| Antithrombotic drugs, % (n) | 20.0 (20) |
| Mean procedure time (minutes), M ± SD (range) | 1.1 ± 0.8 (0.5–6) |
| En bloc resection, % (n) | 60.0 (96) |
| Piecemeal resection, % (n) | 40.0 (64) |
| Number of pieces in piecemeal resection, n 2‐3/4‐6 | 89.1/10.9 57/7 |
| Histopathology, % (n) SSL/SSL with LGD/SSL with HGD | 96.3/2.5/1.2 (154/4/2) |
| Histopathological complete resection, % (n) | 34.4 (55) |
| Margin, % (n) negative/positive/unclear | 25.0/44.4/30.6 (40/71/49) |
| Massive perioperative bleeding | 1.3 (2) |
| Postoperative haemorrhage bleeding, % (n) | 0 (0) |
| Perforation, % (n) | 0 (0) |
| Number of follow‐up lesions, %, (n) | 63.1 (101) |
| Follow‐up period, month, median (IQR) | 18 (12–24) |
| Overall recurrence rate, % (n) | 5.0 (5/101) |
Abbreviations: CSP, cold snare polypectomy; HGD, high‐grade dysplasia; IQR, interquartile range; left‐sided, descending colon to rectum; LGD, low‐grade dysplasia; right‐sided, caecum to transverse colon; SD, standard deviation; SSL, sessile serrated lesion.
The characteristics of SSLs 10–14 and 15–20 mm in size are compared in Table 2. There were no significant differences between these groups in terms of age, sex, location or morphology. The mean procedure time for SSLs 10–14 mm in size was significantly shorter than for SSLs 15–20 mm in size (M ± SD = 0.9 ± 0.4 min vs. 1.9 ± 1.4 min, p < 0.001). The en bloc resection rate for SSLs 10–14 mm in size was higher than that for SSLs ≥15 mm in size (67.9% vs. 19.2%, p < 0.001). Regarding histopathology, the rate of dys‐plasia did not differ between the two groups (3.7% vs. 3.8%, p = 0.592). Among 101 follow‐up cases, the recurrence rate was 4.7% for SSLs 10–14 mm in size, and 6.3% for SSLs 15–20 mm in size (p = 0.713).
TABLE 2.
Comparison of therapeutic results of CSP for SSLs 10–14 mm and ≥15 mm in size
| 10–14 mm | 15–20 mm | p‐Value | |
|---|---|---|---|
| Lesions, n | 134 | 26 | |
| Patients, n | 81 | 19 | |
| Age (years), M ± SD | 66.2 ± 10.5 | 70.0 ± 10.5 | 0.163 |
| Sex, % (n) male/female | 53.1/46.9 (43/38) | 36.8/63.2 (7/12) | 0.307 |
| Tumoir size (mm), M ± SD | 11.0 ± 1.3 | 16.4 ± 2.2 | <0.001 |
| Location, % (n) right‐sided/left‐sided | 87.3/12.7 (117/14) | 92.3/7.7 (24/2) | 0.915 |
| Morphology, % (n) polypoid/non‐polypoid | 0.0/100.0 (30134) | 0.0/100.0 (0/26) | 1.0 |
| Mean procedure time (minutes), M ± SD | 0.9 ± 0.4 | 1.9 ± 1.4 | <0.001 |
| En bloc resection, % (n) | 67.9 (91) | 19.2 (5) | <0.001 |
| Histopathology, SSL/SSL with dysplasia, % (n) | 96.3/3.7 (129/5) | 96.2/3.8 (25/1) | 0.592 |
| Degree of dysplasia, % (n) LGD: HGD | 60.0/40.0 (3/2) | 100.0/0.0 (1/0) | ‐ |
| Histopathological complete resection, % (n) | 29.9 (40) | 0.0 (0) | <0.001 |
| Rates of positive margin, % (n) | 37.3 (50) | 80.8 (21) | <0.001 |
| Rates of unclear margin, % (n) | 32.8 (44) | 23.1 (5) | 0.252 |
| Follow‐up cases | 85 | 16 | |
| Recurrence rate, % (n) | 4.7 (4) | 6.3 (1) | 0.713 |
Regarding the comparison between 96 nonrecurrent lesions and five recurrent lesions, there were not significant differences in terms of age, sex, location, size (M ± SD = 11.4 ± 2.1 mm vs. 12.0 ± 2.3 mm, p = 0.544), en bloc resection rate (60.0% vs. 54.2%, p = 0.837) and histopathological positive margin rate (52.1 vs. 40.0%, p = 0.713). The details of five cases of recurrence after CSP are shown in Table 3. All recurrent cases were diagnosed as SSL without dysplasia by magnifying endoscopy. The recurrent lesions were detected 6–36 months after CSP, and all of them were treated with repeat CSP (Figure 2). After repeat CSP, all five cases received follow‐up colonoscopy, and there were no cases of re‐recurrence.
TABLE 3.
Recurrent cases after CSP for SSLs >10 mm in size
| NO. | Age | Sex | Location | Size (mm) | Morphology | En bloc resection | Histopathology | Histopathological margin | Follow‐up period until recurrence (months) | Size of recurrent lesion (mm) | Treatment for recurrence | Histopathology | Follow‐up period after treatment of recurrence (months) | Re‐ recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | F | C | 10 | Non‐poly poid | 2 pieces | SSL | Positive | 12 | 5 | Re‐CSP | SSL | 48 | — |
| 2 | 64 | F | T | 12 | Non‐poly poid | 3 pieces | SSL | Negative | 24 | 4 | Re‐CSP | SSL | 36 | — |
| 3 | 75 | F | A | 10 | Non‐poly poid | En bloc | SSL | Positive | 6 | 8 | Re‐CSP | SSL with LGD | 6 | — |
| 4 | 67 | F | A | 10 | Non‐poly poid | En bloc | SSL | Positive | 36 | 5 | Re‐CSP | SSL | 24 | — |
| 5 | 62 | M | T | 15 | Non‐poly poid | En bloc | SSL | Positive | 12 | 6 | Re‐CSP | SSL | 24 | — |
Abbreviations: A, ascending colon; C, caecum; F, female; M, male; Re‐CSP, repeat CSP; T, transverse colon.
FIGURE 2.
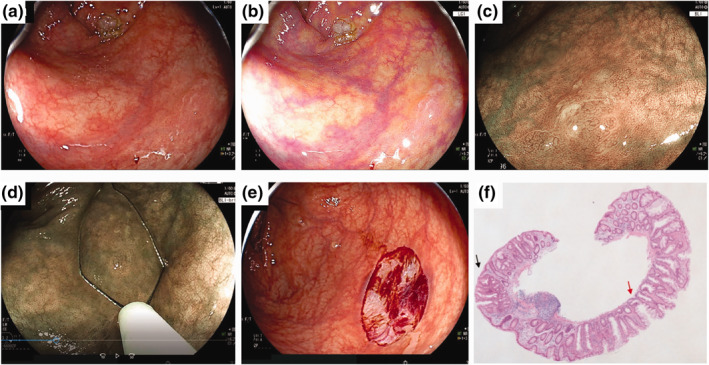
Repeat cold snare polypectomy (CSP) for a recurrent sessile serrated lesion (SSL). (a) A non‐polypoid SSL 10 mm in size located in the ascending colon was detected by LED endoscopy (no. 3 in Table 3). (b) Linked colour imaging detected a clear lesion. (c) The lesion showed dilated crypts on magnifying endoscopy with blue laser imaging (BLI). A minor network was seen, which might have represented a small amount of dysplasia. (d) Repeat CSP was performed with a dedicated snare. (e) The lesion was resected en bloc. (f) Histopathology showed SSL with low‐grade dysplasia (black arrow). The horizontal margin of the lesion was negative, but the vertical margin was unclear (red arrow)
4. DISCUSSION
Many studies have reported the therapeutic results and adverse events of CSP. 1 , 2 , 3 , 4 , 5 , 6 However, there have been few reports about follow‐up after CSP for lesions ≥10 mm in size. In a previous study, we showed that the rates of recurrence after CSP for 480 lesions <10 mm and 74 lesions 10–14 mm were 1.4% and 5.4%, respectively (p = 0.06). 10 In the study, there were only seven SSL in lesions 10–14 mm in size, and other histology included 61 low‐grade adenomas and six lesions with high‐grade dysplasia (intramucosal cancer in Japan). Additionally, we tried to achieve en bloc resection for all lesions so that enough margins were not obtained compared to piecemeal CSP. The rates of recurrence after hot polypectomy, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are reported to be 0.3%, 1.4% and 2.3%, respectively. 20 , 21 Regarding recurrence after CSP for lesions of any histology ≥10 mm in size, a systematic review of eight studies for which CSP was indicated for adenoma and SSL was reported. 22 There were five reports of piecemeal CSP from the USA, the UK and Australia, focusing on SSLs ≥10 mm in size, 14 , 15 , 23 , 24 , 25 although two studies included 33.3% and 51.4% low‐grade adenoma. 23 , 25 The rates of recurrence and median follow‐up periods were reported 0% (6 months), 14 0.6% (5 months), 15 3.4% (8.6 months) 24 and 20% (≤6 months). 23 One unique study did follow‐up analysis twice: (a) for early recurrence 150 days after CSP and (b) for late recurrence 18 months after CSP. 25 The rates of early recurrence (5.5%) and late recurrence (3.5%) were almost the same as our rate (5.0%). We experienced five cases of recurrence. In four of these, recurrence occurred ≥12 months after initial CSP. However, the remaining case occurred six months after CSP. Thus, we recommend that the first follow‐up after CSP for SSLs ≥10 mm in size should be after six months, and that subsequent follow‐up examinations be performed every 1 to 2 years thereafter. Regarding treatment of recurrence due to CSP, all recurrence could be resected with repeat CSP, as done in some other studies. 24 , 25 We assumed scars due to CSP were not hard and were different from other endoscopic resections using electrosurgical units such as EMR and ESD, although further studies should be performed to prove this.
There were two methods of CSP for SSLs ≥10 mm in size: (a) CSP with injection and (b) CSP without injection. 14 , 15 , 23 , 24 , 25 Either 4% succinylated gelatin or 0.1% hyaluronate was used as an injection solution with methylene blue or indigocarmine and epinephrine. 15 , 23 , 24 , 25 They considered that the injection made the lesion margin clear and also enabled the prevention of perioperative bleeding. On the other hand, the injection method makes the procedure time a little longer, and the injection solution and needle are associated with additional costs. We did not use the injection method because we thought we could detect the margin with NBI or BLI, and perioperative bleeding could be controlled. However, when perioperative bleeding is not well controlled, either the injection method or endoscopic clipping should be applied for haemostasis.
Regarding adverse events, no cases of perforations were reported in any studies involving large CSP similar to the current study. 14 , 15 , 23 , 24 , 25 The rates of perioperative haemorrhage ranged from 0% to 2.2%. All could be controlled with endoscopic clipping. Thus, the safety of CSP for lesions ≥10 mm seems acceptable. However, postoperative haemorrhage can develop after CSP, especially in patients treated with anticoagulants, although the rate in our study showed 0% without any cessation of antithrombotic drugs. However, one randomized controlled study of CSP for patients treated with anticoagulants demonstrated a relatively high rate of postoperative haemorrhage (4.7%). 26
The multivariate analysis of our previous study identified a positive margin as the only risk factor (odds ratio = 16.600, 95% confidence interval 3.707–74.331, p < 0.001). 11 However, in cases of piecemeal resection by CSP, it is not possible to prevent a positive margin, and we have to minimize and manage residual lesions. For this purpose, it is important to know the unique features of SSLs. First, it is difficult to determine the edges of a SSL with WLI because the colour of SSLs is often similar to that of the surrounding normal mucosa. Image‐enhanced endoscopy, such as NBI, BLI or linked colour imaging, should probably be used to detect the tumour margins of SSLs. 9 , 13 , 27 Second, SSLs regularly have a flat morphology, and are more difficult to resect by CSP in comparison to polypoid lesions. 4 Thus, appropriate resection using thin dedicated snares should be used to snare flat lesions. During resection, we must obtain a sufficient lateral margin. Wider resection was recommended in previous studies in order to prevent residual lesions and recurrence. 14 , 15 In the current study, our lateral margin may not have been enough, especially until 2017, because we were afraid of postoperative haemorrhage due to a large defect of CSP. However, we achieved sufficient margin and prevented recurrence after 2017. In fact, three cases (cases 1, 2 and 5) in Table 3 were recurrences due to CSP from before 2017. Further studies should be performed to evaluate this hypothesis.
In the current study, 3.7% of cases had dysplasia, even though they were initially diagnosed as SSL without dysplasia. Two other two studies, which only investigated SSLs ≥10 mm, also unintentionally included cases with dysplasia (7.3% [3/41]; 1.2% [2/163]). 14 , 15 We suggest there were two reasons for this. One possibility is that the dysplastic area cannot always be seen due to the presence of folds and mucus. The other is that these subtle findings might be missed, even with magnifying endoscopy. However, none of these lesions had submucosal invasion, and our cases with dysplasia did not develop recurrence. Additionally, the rate of high‐grade dysplasia was only 1.2%, and we suggest this low frequency may be acceptable. A longer follow‐up period and a large number of cases should be examined in a multicentre study.
There were some limitations to this study. This was a retrospective single‐centre study. Various snares or image‐enhanced endoscopy were used, and those can preclude generalizability. We had some cases with minor haemorrhage in which haemorrhage stopped spontaneously until the day after CSP. However, we could not analyse the exact number. Scar assessment was based not on biopsy sampling but on endoscopic images. We could not detect some of the scars, which would have been potential sites of recurrence. Accordingly, our recurrence rate may have been underestimated.
In conclusion, we revealed the feasibility of CSP for SSLs 10–20 mm in size, including its safety. Due to careful diagnosis by magnifying endoscopy, we could identify most SSL without dysplasia from SSLD. The rate of recurrence was 5.0%. Close and long follow‐up is required, even though all cases were treated with repeat CSP.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICS APPROVAL
This research was approved by the Ethics Committee of Kyoto Prefectural University of Medicine (ERB‐C‐417, approved date: 1 October 2015; ERB‐C‐1600, approved date: 23 December 2019), and was in accordance with the Declaration of Helsinki.
INFORMED CONSENT
All patients have given written informed consent for the endoscopic treatment. This study was retrospective in setting, and opt out of the study was performed in our institution, using a website and posting the notice at the posting area.
ACKNOWLEDGEMENTS
We appreciated all the members of the Department of Molecular Gastroenterology and Hepatology, Kyoto Prefectural University of Medicine regarding their helps for this study. The authors received no financial support for the research, authorship and/or publication of this article.
REFERENCES
- 1. Repici A, Hassan C, Vitetta E, et al. Safety of cold poly‐ pectomy for <10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44:27–31. [DOI] [PubMed] [Google Scholar]
- 2. Uraoka T, Oka S, Ichihara S, et al. Endoscopic management of colorectal tumors less than 10 mm in size: current status and future perspectives in Japan from a questionnaire survey. Dig Endosc. 2018;30:36–40. [DOI] [PubMed] [Google Scholar]
- 3. Horiuchi A, Nakayama Y, Kajiyama M, et al. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc. 2014;79:417–23. [DOI] [PubMed] [Google Scholar]
- 4. Hirose R, Yoshida N, Murakami T, et al. Histopathological analysis of cold snare polypectomy and its indication for colorectal polyps 10‐14 mm in diameter. Dig Endosc. 2017;29:594–601. [DOI] [PubMed] [Google Scholar]
- 5. Kawamura T, Takeuchi Y, Asai S, et al. A comparison of the resection rate for cold and hot snare polypectomy for 4‐9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study). Gut. 2018;67:1950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferlitsch M, Moss A, Hassan C, et al. Colorectal poly‐pectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270–97. [DOI] [PubMed] [Google Scholar]
- 7. Pai RK, Makinen MJ, Rosty C. Colorectal serrated lesions and polyps. In: Nagtegaal ID, Arends MJ, Odze RD, Lam AK, editors. WHO classification of tumours of the digestive system. 5th ed, Vol. 1. Lyon: IARC Press; 2019. p. 163–9. [Google Scholar]
- 8. Sano Y, Tanaka S, Kudo SE, et al. Narrow‐band imaging (NBI) magnifying endoscopic classification of colo‐ rectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526–33. [DOI] [PubMed] [Google Scholar]
- 9. Yoshida N, Dohi O, Inoue K, et al. Blue laser imaging, blue light imaging, and linked color imaging for the detection and characterization of colorectal tumors. Gut Liver. 2019;13:140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murakami T, Yoshida N, Yasuda R, et al. Local recurrence and its risk factors after cold snare polypectomy of colorectal polyps. Surg Endosc. 2020;34:2918–2925. [DOI] [PubMed] [Google Scholar]
- 11. Yamashina T, Takeuchi Y, Uedo N, et al. Diagnostic features of sessile serrated adenoma/polyps on magnifying narrow band imaging: a prospective study of diagnostic accuracy. J Gastroenterol Hepatol. 2015;30:117–23. [DOI] [PubMed] [Google Scholar]
- 12. Burgess NG, Pellise M, Nanda KS, et al. Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps. Gut. 2016;65:437–46. [DOI] [PubMed] [Google Scholar]
- 13. Sano W, Fujimori T, Ichikawa K, et al. Clinical and endoscopic evaluations of sessile serrated adenoma/polyps with cytological dysplasia. J Gastroenterol Hepatol. 2018;33:1454–60. [DOI] [PubMed] [Google Scholar]
- 14. Tate DJ, Awadie H, Bahin FF, et al. Wide‐field piecemeal cold snare polypectomy of large sessile serrated polyps without a submucosal injection is safe. Endoscopy. 2018;50:248–52. [DOI] [PubMed] [Google Scholar]
- 15. Tutticci NJ, Hewett DG. Cold EMR of large sessile serrated polyps at colonoscopy (with video). Gastrointest Endosc. 2018;87:837–42. [DOI] [PubMed] [Google Scholar]
- 16. The Paris endoscopic classification of superficial neoplas‐ tic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2013; 58: S3–S43. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida N, Naito Y, Murakami T, et al. Safety and efficacy of a same‐day low‐volume 1 L PEG bowel preparation in colonoscopy for the elderly people and people with renal dysfunction. Dig Dis Sci. 2016;61:3229–3235. [DOI] [PubMed] [Google Scholar]
- 18. Japanese Society for Cancer of the Colon and Rectum . Japanese classification of colorectal carcinoma. 8th ed. Tokyo: Kanehara; 2009. [Google Scholar]
- 19. Rosty C, Brosens LAA, Dekker E, et al. Serrated polyposis. WHO classification of tumours: digestive system tumours. 5th ed. Lyon: IARC Press; 2019. p. 532–4. [Google Scholar]
- 20. Oka S, Tanaka S, Kanao H, et al. Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc. 2010;22:376–80. [DOI] [PubMed] [Google Scholar]
- 21. Yoshida N, Inoue K, Dohi O, et al. Efficacy of precutting endoscopic mucosal resection with full or partial circumferential incision using a snare tip for difficult colorectal lesions. Endoscopy. 2019;51:871–6. [DOI] [PubMed] [Google Scholar]
- 22. Thoguluva Chandrasekar V, Spadaccini M, Aziz M, et al. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm: a systematic review and pooled‐analysis. Gastrointest Endosc. 2019;89:929–36.e3. [DOI] [PubMed] [Google Scholar]
- 23. Muniraj T, Sahakian A, Ciarleglio MM, et al. Cold snare polypectomy for large sessile colonic polyps: a single‐center experience. Gastroenterol Res Pract. 2015;2015:175959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rameshshanker R, Tsiamoulos Z, Latchford A, et al. Resection of large sessile serrated polyps by cold piecemeal endoscopic mucosal resection: serrated COld Piecemeal Endoscopic mucosal resection (SCOPE). Endoscopy. 2018;50:E165–167. [DOI] [PubMed] [Google Scholar]
- 25. Mangira D, Cameron K, Simons K, et al. Cold snare piecemeal EMR of large sessile colonic polyps ≥20 mm (with video). Gastrointest Endosc. 2020;91:1343–52. [DOI] [PubMed] [Google Scholar]
- 26. Takeuchi Y, Mabe K, Shimodate Y, et al. Continuous anticoagulation and cold snare polypectomy versus hep‐ arin bridging and hot snare polypectomy in patients on anticoagulants with subcentimeter polyps: a randomized controlled trial. Ann Intern Med. 2019;171:229–37. [DOI] [PubMed] [Google Scholar]
- 27. Yamada M, Sakamoto T, Otake Y, et al. Investigating endoscopic features of sessile serrated adenomas/polyps by using narrow‐band imaging with optical magnification. Gastrointest Endosc. 2015;82:108–117. [DOI] [PubMed] [Google Scholar]


